Abstract
The virtual immersive gaming to optimize recovery (VIGOR) study is a randomized controlled trial of the effects of virtual reality games to encourage lumbar spine flexion among individuals with chronic low back pain and fear of movement. Whereas traditional graded activity or graded exposure therapies for chronic low back pain have high attrition and poor long-term efficacy, we believe that virtual reality games have distinct advantages that can enhance adherence and clinical outcomes. First, they are engaging and enjoyable activities that can distract from pain and fear of harm. In addition, because they gradually reinforce increases in lumbar spine flexion to achieve game objectives, continued engagement over time is expected to promote recovery through restoration of normal spinal motion. The study design includes two treatment groups which differ in the amount of lumbar flexion required to achieve the game objectives. All participants will play the games for nine weeks, and pre-treatment to 1-week post-treatment changes in pain and disability will serve as the co-primary clinical outcomes. In addition, changes in lumbar flexion and expectations of pain/harm will be examined as potential treatment outcome mediators. Maintenance of treatment outcomes will also be assessed for up to 48-weeks post-treatment. In brief, we hypothesize that the virtual reality games will reduce pain and disability by promoting spinal motion and allowing participants to develop an implicit understanding that they are capable of engaging in significant lumbar spine motion in their daily lives without a risk of injury to their back.
Keywords: virtual reality, low back pain, kinesiophobia, clinical trial
1. Introduction
Kinesiophobia, which is a fear of movement due to expectations of pain and harm, is one of the strongest predictors of chronic low back pain (CLBP) development.[1–4] Whereas avoidance of movement may benefit individuals with back pain in the short-term by reducing anxiety, in the long-term limited activity may contribute to shortening of spinal peri-articular connective tissues, changes in surrounding muscles,[5–7] and risk for chronicity.
Common approaches to CLBP include graded activity and graded exposure therapies. Graded activity focuses on restoring function regardless of pain, and emphasizes the positive effects of physical activity and the negative effects of inactivity on well-being. Treatment relies on a combination of activity quotas and positive reinforcement for increased activity over time. Graded exposure focuses on reducing fear of pain and expectations of harm upon movement, and emphasizes fear as an impediment to recovery. Treatment relies on development of an individualized hierarchy of feared movements, which guides systematic exposure to feared movements and the accompanying opportunities to confront and correct misperceptions of expected harm. Randomized controlled trials indicate that both of these approaches produce significant reductions in pain and disability.[8, 9] At the same time, however, a systematic literature review concluded that graded activity is no more effective than other forms of exercise and that graded exposure is no better than wait-list or usual care controls.[10] A potential reason that these treatments fail to outperform exercise or usual-care is that patients can complete the prescribed tasks with restricted lumbar spine motion simply by increasing motion at the ankles, knees, and hips. For example, we have consistently shown that pain-related fear is associated with restricted lumbar flexion among individuals with subacute LBP,[11, 12] individuals with CLBP,[13] 3) asymptomatic individuals who have recently recovered from LBP,[14] and healthy individuals with experimentally-induced back pain.[15] Hence, continued restriction of lumbar spine motion may be a key impediment to optimal restoration of function.
To address this problem, we developed virtual reality games that encourage gradual increases in lumbar flexion to achieve game objectives. Our games do not have an explicit focus on psychological factors related to avoidance behavior; rather, they offer a combination of acute distraction from pain, reinforcement of movement, and graded increases in expectations of lumbar flexion. In a phase I clinical trial,[16] we demonstrated that three daily sessions of virtual dodgeball was safe for individuals with CLBP, did not exacerbate existing back pain, was rated positively, and increased lumbar flexion during gameplay. Encouraged by these findings, we developed a Phase II randomized controlled trial of a 9–week course of treatment called Virtual Immersive Gaming to Optimize Recovery (VIGOR).
2. Design and Methods
Using a between-subjects design, 230 CLBP participants will be randomly assigned to one of two treatment arms. Those assigned to the experimental group will play our immersive video games that encourage participants to produce progressively larger lumbar flexion excursions at each game level and across treatment sessions. Those assigned to the control group will play the same immersive games, but the parameters will be modified such that less lumbar flexion is required to achieve the same game objectives. Treatment frequency and duration is based on existing evidence that graded activity and graded exposure interventions, typically lasting 6–12 weeks with 8–18 treatments sessions, result in significant reductions in disability.[10, 17] Accordingly, participants in this study will complete 18 intervention visits over 9 weeks with the number of sessions tapered across weeks (i.e., 3 sessions/week in weeks 1–3, 2 sessions/week in weeks 4–6, and 1 session/week in weeks 7–9). Our co-primary outcome variables will be change in pain and change in disability from baseline to 1-week post-treatment (Aim 1). We will also examine changes in expectations of pain/harm and lumbar flexion as potential mechanisms of change in pain and disability (Aim 2). Finally, we will examine maintenance of treatment gains at 1-, 6-, 12-, 24-, and 48-weeks post-treatment (Aim 3).
2.1. Aims and Hypotheses
Aim 1
Examine immediate clinical outcomes as a function of treatment. We hypothesize that, relative to the control group, participants in the experimental group will show greater reductions in pain and disability at post-treatment relative to pre-treatment baseline (Hypothesis 1).
Aim 2
Examine potential mechanisms of pre- to post-treatment changes in clinical outcomes. We hypothesize that participants in the experimental group will exhibit greater pre- to post-treatment decreases in pain/harm expectancy and increases in lumbar flexion as compared to the control group (Hypothesis 2.1). We further posit that decreases in pain/harm expectancy and increases in lumbar flexion will be positively related to pre- to post-treatment reductions in pain and disability (Hypothesis 2.2).
Aim 3
Examine maintenance of treatment gains. We hypothesize that, relative to the control group, participants in the experimental group will continue to show lower levels of pain and disability at 1-, 6-, 12-, 24-, and 48-weeks after the last treatment session, as well as larger increases in physical activity in their natural environment (Hypothesis 3).
2.2 Overall study design
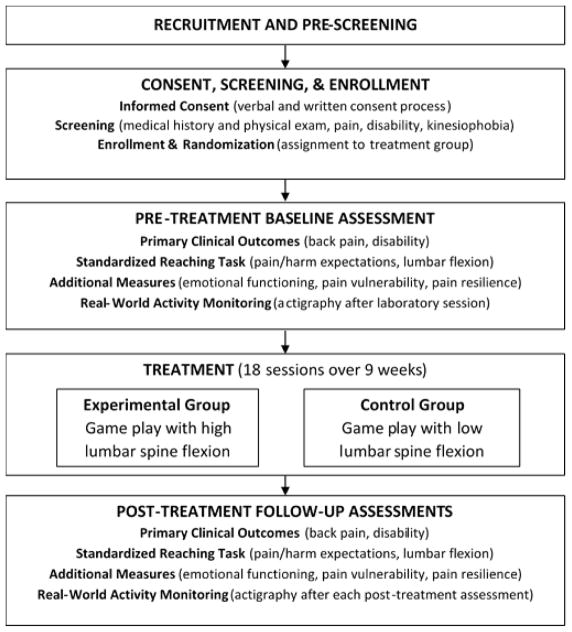
This is a single site study being conducted by Ohio University (Athens, OH, USA). Study candidates include individuals with chronic low back pain who indicate a fear of movement due to risk of injury. As shown in Figure 1, participants proceed through the study in a series of steps that include 1) recruitment and pre-screening, 2) consent, screening, and enrollment, 3) pre-treatment baseline assessment, 4) treatment, and 5) post-treatment follow-up assessments. The study protocol was approved by the Ohio University Institutional Review Board (17F11) and is registered with ClinicalTrials.gov (NCT03463824).
Figure 1.
Study protocol flowchart.
2.3. Participant recruitment and pre-screening
We will recruit 230 participants between the ages of 18–60 and who report no health condition(s) that may restrict movement or preclude safe participation. Participants will be recruited through advertisements and flyers posted in the local community, and via a combination of electronic, radio, print, and possibly television announcements in the local and surrounding communities. We may also recruit from local clinics and using ResearchMatch.org. Interested individuals who respond to the recruitment efforts will be directed to complete a pre-screening survey using REDCap [18], which is a secure online survey and database management application (or a telephone interview, if needed). This pre-screening survey will cover the main inclusion and exclusion criteria (see Table 1), including a numeric pain rating scale (24 hour and 7 day recall), the Roland-Morris Disability Questionnaire,[19, 20] a fear of physical activity question, and medical history related to back pain. Based on their responses, individuals who continue to meet the inclusion/exclusion criteria will be invited to a full screening session scheduled to occur within 30 days of completion of the pre-screening.
Table 1.
Participant inclusion and exclusion criteria.
| Participants must meet the following inclusion criteria: |
|
| Candidates must not meet any of the following exclusion criteria at baseline: |
|
2.4. Participant consent, screening, and enrollment (visit 0)
The goals of the screening session are to 1) describe the study protocol to candidates and begin the informed consent process, and 2) determine if study candidates will qualify for the study. To begin, the study coordinator will verbally describe the study to the potential participant and answer any questions. The consent process will be conducted in a quiet, private room. During the consent process, potential participants will be informed about the study purpose and procedures and be given the opportunity to ask questions. They will be shown a video of the virtual reality games to help them with the process. If they wish to continue, they will be asked to read and sign an informed consent document. Those who provide informed consent will then complete a series of screening surveys (see Table 2), including a repeat of those completed as part of the pre-screening.
Table 2.
Schedule of assessments.
| Pre-screen | Consent, Screen, Enroll | Baseline | Treatment | Post-Treatment | ||
|---|---|---|---|---|---|---|
| Assessment | Visit 0 |
Visit 1 |
Visits 2–19 |
Visit 20 |
Visits 21–24 |
|
| Numeric Pain Rating (7-day/24hr)[22] | x | x | x | xa | x | x |
| Roland-Morris Disability Questionnaire [19],[20] | x | x | x | x | x | |
| Fear Question[22] | x | x | ||||
| Medical History - Back Pain | x | x | ||||
| Medication Log | x | x | x | x | x | |
| Informed Consent Form | x | |||||
| Inclusion/Exclusion Criteria | x | |||||
| Enrollment/Randomization | x | |||||
| Medical History | x | |||||
| Physical Exam Form | x | |||||
| Adverse Events | x | x | x | x | ||
| Numeric Pain Rating (current) | x | x | x | x | ||
| Standardized Reaching Paradigm • Pain & Harm Expectancy • Lumbar Spine Flexion |
x | xa | x | x | ||
| Tampa Scale for Kinesiophobia [23, 24] | x | x | x | |||
| Center for Epidemiologic Studies – Depression [25] | x | x | x | |||
| Pain Catastrophizing Scale [26] | x | x | x | |||
| Pain Resilience Scale [27, 28] | x | x | x | |||
| Pain Self-efficacy Questionnaire[29] | x | x | x | |||
| Brief Pain Inventory - Short Form [30] | x | x | x | |||
| PROMIS - Anxiety [31, 32] | x | x | x | |||
| PROMIS - Depression[31, 32] | x | x | x | |||
| PROMIS - Positive Affect [31, 32] | x | x | x | |||
| PROMIS - Meaning and Purpose Scale [33] [31] | x | x | x | |||
| Life Fulfillment Scale [34] | x | x | x | |||
| Profile of Mood States[35] | x | x | x | |||
| Treatment Evaluation Inventory [36] | x | x | ||||
| Real World Activity Monitoring | x | x | x | |||
| Patient Global Impression of Change [37] | x | x | ||||
Assessed at the first visit of each week.
Based on their responses to the screening surveys, individuals who continue to meet the inclusion/exclusion criteria will then undergo a physical exam by a licensed physical therapist or physician. Participants who remain eligible following the physical exam will be formally enrolled into the study. They will be randomly assigned into a treatment group using a randomization table created by the study statistician prior to study onset, with block randomization within sex to ensure relatively equal numbers of men and women in each group. They will then proceed to a pre-treatment baseline assessment (visit 1), which may occur on the same day as the screening assessment but must be conducted within seven days of the screening visit (or else the participants will be re-screened).
2.5. Pre-treatment baseline assessment (visit 1)
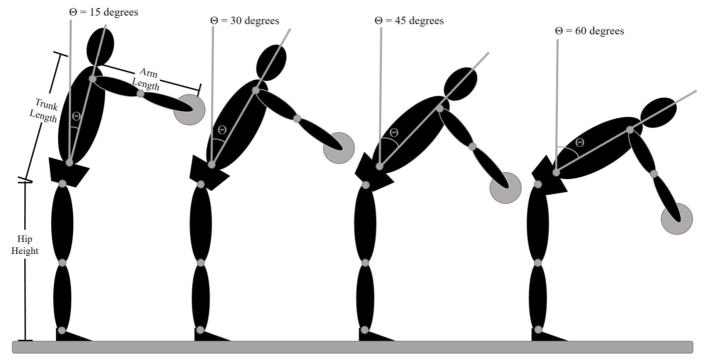
The pre-treatment baseline assessment will include a series of survey measures, participation in a standardized reaching task, and real world activity monitoring. As shown in Table 2, the survey measures will include numeric pain rating scales (Current, 24 hour and 7 day recall), the Roland-Morris Disability Questionnaire,[19, 20] a medication log, and a range of psychological measures (e.g., Tampa Scale for Kinesiophobia,[23, 24] Center for Epidemiologic Studies – Depression,[25] Pain Catastrophizing Scale,[26] Pain Resilience Scale,[27, 28] etc.). For the standardized reaching task, participants will wear a head-mounted display and point to virtual targets while movement of light-reflective marker clusters attached to their head, upper arms, forearms, hands, trunk, pelvis, thighs, shanks, and feet are recorded using a 12-camera Vicon Bonita system. This optoelectric-based kinematic system can track the three-dimensional coordinates of light reflective marker clusters attached to the participant with a spatial resolution of 0.1 mm. Kinematic data will be sampled at 100Hz. Participants will point with their hand to 4 virtual targets co-located in the mid-sagittal plane. As shown in Figure 2, target locations are adjusted to participant anthropometrics to allow for comparison of movement patterns across individuals in a task that requires progressive increases in lumbar spine flexion.[11] Participants will perform five reaching trials to each virtual target location with each hand, pause at the target for 2 seconds, and then return to an upright posture. Instructions will emphasize that participants reach for the targets as fast as possible in a way that is “natural and comfortable for them.” This instruction is used to avoid biasing participants with a perceived correct way to move, and a rapid pace challenges the participant by increasing the loading required to perform the task. While forward excursions of the trunk must be counterbalanced by backward movement of the lower extremities, the targets are located such that they do not require an individual to move anywhere near the limits of available range of motion of the lumbar spine, pelvis, knee, and ankle. Thus, participants can reach the targets using an infinite combination of joint excursions. Even though the reaching task requires no lifting and the loads on the lumbar spine are small, we have shown that individuals with elevated levels of kinesiophobia exhibit reduced lumbar spine flexion at this combination of target height and reaching speed.[11, 12, 38] The time series joint angle data are calculated from the 3-D segment coordinate data using an Euler angle sequence of: 1) flexion-extension, 2) lateral bending, and 3) axial rotation using Motion Monitor software.[39] The standardized reaching paradigm will be used to assess three dependent measures associated with Aim 2 (i.e., lumbar flexion and pain/harm expectancy). Lumbar flexion will be defined as the change in joint angle during each reach (i.e., the difference between the joint angle at the beginning of the trial before the go signal and the joint angle recorded 100 ms after target contact). Consistent with our prior work,[15, 40–42] expectations of pain and harm will also be measured during standardized reaches. For each target height, prior to the first reaching trial, participants will rate the level of “expected pain” and “expected harm” using a visual analog scale displayed through the head mounted display. The scale will consist of a 10 cm horizontal line with no numbers, marks, or descriptive vocabulary along its length. For expected pain ratings, the scale will be anchored with the descriptors “No pain” and “Worst pain imaginable”, respectively, at each end of the line. For expected harm, the scale will be anchored with “Not at all concerned” and “Extremely concerned” regarding potential harm to the back during task performance. Participants will indicate their response by moving a virtual sliding scale.
Figure 2.
Target locations are determined mathematically based on participant hip height, trunk length, and arm length. The high target is located such that the subject could, in theory, reach the target by flexing the hips 15° with the shoulder flexed 90° and the elbow extended. Similarly, the lowest target could be reached by flexing the hips 60°.
After completing all pre-treatment assessments, participants will view a brief (11.5 minute) treatment rationale video that includes a chronic pain educational component designed to explain how pain persists without underlying pathology, and to describe the interaction between biological, psychological, and social factors in maintaining chronic back pain and related disability. Following the treatment rationale video, participants will be introduced to the virtual video games for the first time and have an opportunity to play a practice level to review basic game constructs (e.g., scoring metrics, moving the avatar, sound and visual cues). They will then complete the Treatment Evaluation Inventory to assess their perceptions and expectations regarding the proposed treatment.
At the end of the pre-treatment baseline visit, participants will be asked to wear an ambulatory activity monitor on their non-dominant wrist for one week and then to mail it back to the laboratory. Data from the monitor will be used to assess participants’ total number of steps per day in their natural environment, and will be compared to similar data obtained during follow-up assessments.
2.6. Treatment (visits 2 through 19)
As shown in Table 3, Participants will complete 18 intervention visits over 9 weeks with the number of sessions tapered across weeks (i.e., 3 sessions/week in weeks 1–3, 2 sessions/week in weeks 4–6, and 1 session/week in weeks 7–9).
Table 3.
Schedule of treatment sessions.
| Treatment Session | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Game | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 |
| Matchality | 1,2,3 | ||||||||
| Fishality | 4,5,6 | ||||||||
| Dodgeball Cannon | 7,8 | ||||||||
| Dodgeball Day | 9 | 10 | |||||||
| Dodgeball Night | 11 | 12,13 | |||||||
| Dodgeball Space | 14,15 | 16 | 17 | 18 | |||||
A head mounted display will be used to present the virtual games with a screen refresh rate of 90 Hz. The environmental parameters are controlled by custom software developed in the Unity game engine to manipulate and control all presented graphics and audio. During the initial testing session (visit 1) and at again each post treatment follow-up sessions (i.e., visits 20 through 24), kinematic data from the clusters of light reflective markers placed on the participant will be streamed to the game environment at 100 Hz using Vicon Tracker software. This allows for near real-time presentation of the participant’s avatar. MotionMonitor software sets up bi-directional communication with the Unity game engine and records all kinematic data (e.g., joint excursions, joint moments). During the treatment phase (visits 2 through 19), presentation of the participant’s avatar is controlled through the position data from the head mounted display, hand controllers, and Vive™ trackers attached to the participant’s thorax and pelvis.
2.6.2 Virtual Reality Games
The immersive games will vary across the 9 weeks of treatment to provide a graded increase in challenge with respect to lumbar spine motion, encourage player engagement, and prevent player boredom. There will be three virtual reality games, each played from a first-person perspective, including Matchality, Fishality, and Dodgeality.
2.6.2.1 Matchality
In week one, participants will play Matchality three times, each in a different virtual environment including earth, on a platform orbiting earth, and on an alien planet (see Figure 3). The game requires players to reach to a static set of cubes, arranged in a 4 × 4 grid, at a self-selected pace. The locations of the cubes in the virtual space are such that the participant could touch the highest row of cubes with 15 degrees of lumbar flexion, the second through fourth rows would necessitate 30, 45, and 60 degrees of lumbar flexion, respectively. The game begins with a sequence of illumination of two cubes, for 100 ms each, in a randomized sequence. The player must then move such that their avatar touches the previously lit cubes in the same sequence as presented. After each successful completion, an additional cube added to create a longer sequence. Sequence length continues to increase until the player is unable to correctly match the sequence, and then the game reverts back to a sequence of only two cubes. This is a time-based game that lasts 90 seconds per set. There are two sets per level and three levels per game. In level 1, only the top two rows of cubes are illuminated in the presented sequences. In level 2, the top three rows are included in the lighted sequences. In level 3, all four rows can be included in the lighted sequences. Thus each level necessitates greater amounts of lumbar flexion to complete the task. However, because the targets are static and the reaches are completed at a self-selected movement speed, this game is the least physically demanding of the three virtual reality games.
Figure 3.
Column 1 shows a participant with the head-mounted display and instrumented with the marker clusters. Column 2 shows images from the “Matchality” game environments, including day one on earth (top), day two in earth orbit (middle), and day 3 on an alien planet (bottom). Column 3 shows images from the “Fishality” game environment. Column 4 shows images from the “Dodgeality” game environment, including inside an arena (top, middle) and on an alien planet (bottom).
2.6.2.2 Fishality
In week two, participants will play Fishality three times. The game requires the player to move their avatar so that it reaches with a virtual net to catch fish that are jumping in slow parabolic trajectories at the edge of a dock on a lake (see Figure 3). Virtual fish can be seen swimming in the lake and then launching out of the water towards the player’s avatar. The goal is to net the fish and place them in a holding tank located to the right of the participant’s avatar. The trajectory of the launched fish is set to the anthropometric characteristics of the individual player to necessitate a specific amount of lumbar flexion to net the fish. As part of the game experience, at random intervals, a large virtual shark is launched toward the avatar’s head. The player is instructed to duck in this condition. The score is assessed by the number of fish successfully netted in a given set. There are 15 fish launch events in each set of game play, 2 sets per level, and three levels per game. The game becomes more challenging across the three days of game play by increasing the number of fish launched (e.g., one fish at a time in session 1, two fish simultaneously in session 2, and three simultaneously in session 3) while requiring the player to net only the bright colored fish to score points. In addition, the location of the intercept point is adjusted across days to encourage increased lumbar flexion.
2.6.2.3 Dodgeality
For weeks three through nine, participants will play variants of virtual dodgeball (see Figure 3). All versions consist of launched virtual balls that are directed at the participant’s avatar. The goal is to either block the launched virtual ball with a virtual ball held by the participant’s avatar or to duck the launched virtual ball if it changes color and is accompanied by a quacking sound. In all variants of Dodgeality there are 15 launched virtual balls per set, 2 sets per level, and 3 levels per game. Performance is updated in real-time and displayed on a virtual scoreboard. In week three, participants will play two sessions of a version of the game where the balls are launched in slow arcs by a cannon located inside an arena (i.e., Dodgeball Cannon). This is the least physically challenging version of Dodgeality as the balls start from one location and the launch trajectory has a high parabolic flight pattern to make interception of the launched virtual ball less challenging. At the end of week three and beginning of week four, participants will play two sessions of a traditional dodgeball game within the same virtual arena environment (i.e., Dodgeball Day). In this version they compete against four opponent avatars who randomly take turns throwing balls using a normal human motion. In weeks four and five participants will play two sessions of the same traditional dodgeball game, except that the arena lights have been removed and the opposing avatars and launched virtual balls glow in the dark (i.e., Dodgeball Night). Finally, in weeks five through nine participants will play weekly sessions of traditional dodgeball that takes place on the surface of an alien planet (i.e., Dodgeball Space). The opposing players are aliens and the physics of the ball launches are adjusted to reflect the reduced gravity of the moon. During weeks five through nine, the launch velocity of virtual balls will be increased by 5% per week. Thus the initial launch velocity will increase from 45 m/s to 57.4 m/s from week five to week nine. Progressively increasing the initial launch velocity will necessitate more rapid movements of the participants to successfully intercept the launched virtual balls and thereby increasing the difficulty of the tasks.
Each of the virtual reality games involve three dimensional sound elements to enhance the immersive quality of the game. Each game also includes scoring for performance, and these scores are clearly displayed within the virtual environment such that players are always aware of their performance. To further promote active engagement, at the end of each week participants will receive printed feedback indicating their game performance (e.g., matches made, fish caught, dodgeball hits/misses) as well as monetary incentives in direct proportion to the points that they earn during gameplay.
2.6.3. Treatment groups
2.6.3.1. Experimental group
In the experimental group the location and presentation of static and dynamic virtual targets (e.g., cubes, fishes, and dodgeballs) will be manipulated to maximize lumbar flexion in the experimental group. The location of the virtual objects are set to necessitate 15, 30, 45, & 60 degrees lumbar spine motion. After week 1 of gameplay, visual gain will be manipulated such that virtual objects will be farther away and at a lower height (5% adjustment) to necessitate greater lumbar flexion to successfully intercept the virtual objects. In week 2 there will be a 10% adjustment (i.e, farther away and at a lower height). In week 3 there will be a 15% adjustment that will continue until the end of treatment.
2.6.3.2. Control group
In the control group, to ensure that lumbar flexion is minimized while playing the virtual reality games, we will manipulate the presentation of virtual targets (i.e., boxes in Matchality, fish in Fishality, and balls in Dodgeality) such that the participant will only need to flex the spine 15 degrees to successfully intersect the virtual objects.
2.7. Post-treatment follow-up assessments (visits 20 through 24)
After the participants have completed 18 game sessions over nine weeks, post-treatment follow-up sessions (visits 20–24) will be scheduled to assess maintenance of treatment gains at 1-, 6-, 12-, 24-, and 48-weeks after the last treatment session. These follow-up sessions will be identical to the pre-treatment baseline assessments with two exceptions. First, at each of the follow-up visits participants will complete a one-item Patient Global Impression of Change measure[37] to assess their overall sense of improvement as a function of receiving the treatment. Second, participants will repeat the Treatment Evaluation Inventory [36] to assess their acceptance of the virtual reality games as a potential intervention for low back pain a the first follow-up session only (i.e., visit 20).
2.8. Additional design issues
2.8.1. Blinding
The principal investigators, the statistician, and members of the data collection team will remain blinded to intervention assignment throughout the duration of the study. They will be given the identifying codes only at the end of the study when it is necessary to interpret the results. The un-blinded study coordinator, who is responsible for scheduling testing and treatment sessions, will greet participants and escort them to the testing lab; however, the study coordinator will not participate in the assessments of clinical outcomes or testing.
2.8.2. Concomitant interventions
Participants in the study will be permitted to use over the counter pain medications (e.g., Non-Steroidal Anti-Inflammatory Drugs, Acetaminophen, Aspirin), or to apply heat or ice to manage back pain symptoms. Although participants who report using pain interventions other than over-the-counter pain relievers or heat/ice will be allowed to remain in the study, they will be excluded from the per-protocol analyses.
2.8.3. Adherence
For the per-protocol analyses, successful adherence will be defined as >70% attendance (i.e., 13 out of the 18 treatments). To allow for flexibility in participant scheduling due to events that may conflict with scheduled visits (e.g., acute illness), the total length of the 9 week intervention period can be extended by up to 30 days (i.e., total intervention = 9 weeks + 30 days).
2.8.4. Safety assessments
To monitor safety, participants will complete a brief health screening at the beginning of each game session to determine if there are any changes in back pain or radiating symptoms. All negative changes in health status other than back pain will be recorded as an adverse events, logged, and reported per requirements of the Ohio University IRB. In the case of dismissal from the protocol due to negative health changes, the study’s safety committee will meet to determine whether the adverse was caused by the intervention.
2.8.5. Early termination or discontinuation
Participants will be discontinued from the study intervention if a medical condition develops that precludes the continuation of the treatment intervention. If participants discontinue prior to completing all scheduled treatment sessions (regardless of the reason), we will make every attempt to obtain the outcome measurements. If the study participant is unwilling or unable to undergo the laboratory-based tests we will still attempt to obtain the clinical outcome measures. In instances where an adverse event does occur, we will follow-up with participants until the event is resolved or until the IRB deems it unnecessary to continue to follow the participant.
2.9. Outcomes, sample size calculations, and analysis plan
All analyses will first be conducted on an intent-to-treat basis for the original cohort to examine the intervention effects regardless of adherence/attrition. The analyses will then be repeated for all participants demonstrating adherence to their assigned intervention.
2.9.1. Outcomes
The co-primary clinical outcomes are pain and disability, which will be assessed at pre-treatment baseline (visit 1) and at 1-, 6-, 12-, 24-, and 48-weeks post-treatment (i.e., visits 20–24). Secondary outcomes include expected pain, expected harm, and lumbar flexion during standardized reaching, which will be measured at pre-treatment baseline (visit 1), at the beginning of each week during treatment (visits 2, 5, 8, 11, 13, 15, 17, 18, 19), and at 1-, 6-, 12-, 24-, and 48-weeks post-treatment (i.e., visits 20–24).
2.9.2. Sample size calculations
To address study aim 1, power analyses were conducted to determine the sample size needed to achieve clinically important differences in our co-primary clinical outcome measures of pain and disability. Based on the extant literature, we based our analyses on a > 30% decrease in pain ratings[43] (on the 0–10 NRS scale) and a 30% decrease in disability ratings on the RMDQ[44] in the experimental group. Further, we predict a 10% decrease in pain and disability in the control group. The population standard deviations were set at 75% of the population mean values. The pre-post correlation was estimated as r = 0.70. These population parameters translate into an effect size of f = 0.30. Using these parameters, we drew 10,000 samples from a normal population distribution. Based on these parameters, to achieve power of 80% and α = 0.05 will require a total N=78. Separate power analyses were conducted to address study aims 2 and 3. Using the method described by Morris (2008),[45] we calculated effect size estimates from randomized clinical trials on the effects of graded activity or graded exposure interventions on changes in disability among individuals with CLBP.[8, 9, 46] For our power analyses, we adopted the median estimated effect size: δ = .45, which corresponds to what would commonly be described as a medium effect size. Following Raudenbush and Liu’s (2000) recommendation,[47] we set the residual error variance to 1 and estimated the between-subject slope variance to be 0.30. For power equal to .80 and α=0.05, a sample size of 209 participants was indicated. Based on these sample size calculations, using a sex-stratified random allocation table, we will need a minimum sample of 209 participants to address each of our study aims. To allow for 10% attrition from baseline to 48-week follow-up, we will recruit 230 participants.
2.9.3. Analysis plan
We will analyze all outcome variables (pain, disability, pain/harm expectancy, and lumbar flexion) using linear mixed-effects (LME) models with treatment (Experimental, Control) as a between-subject fixed effect and time (pre-treatment, 1, 6, 12, 24, and 48 weeks) as a within-subject fixed effect. Given the expected findings, both linear and quadratic time effects will be tested in every model. We will also include demographic covariates (e.g., age, BMI) and potential confounders (e.g., radiating versus non radiating pain) as well as sex stratification in the LME models. Any variables that differ at baseline will be included in the statistical models as potential confounders. Intention-to-treat analyses are commonly conducted to analyze randomized clinical trial data in the presence of missing values. While various imputation strategies have been proposed to estimate missing data, such as last observation carried forward, use of LME models to analyze longitudinal data renders these strategies largely unnecessary. We will also conduct equivalent per-protocol analyses that will include only successfully adhered participants (i.e., attending > 70% of gaming sessions).
2.9.3.1. Aim 1. Examine immediate clinical outcomes as a function of treatment
Relative to the control group, participants in the experimental group will show greater reductions in pain and disability at post-treatment relative to pre-treatment baseline (Hypothesis 1). To test Hypothesis 1, we will examine the Treatment by Time (baseline-post treatment) interaction in a LME model: a greater reduction in pain and disability in the experimental group than in the control group.
2.9.3.2. Aim 2. Examine potential mechanisms of pre- to post-treatment changes in clinical outcomes
Participants in the experimental group will exhibit greater pre- to post-treatment decreases in pain/harm expectancy and increases in lumbar flexion as compared to the control group (Hypothesis 2.1). Decreases in pain/harm expectancy and increases in lumbar flexion will be positively related to pre- to post-treatment reductions in pain and disability (Hypothesis 2.2). To address these hypotheses we will build and test linear mixed-effects models for each of the outcome variables. For hypotheses 2.1, pain, disability, pain/harm expectancy, and lumbar flexion will be the outcome variables, while time (linear and quadratic), treatment group, and the interactions of time and treatment group will be the primary predictor variables. For hypothesis 2.2, pain/harm expectancy and lumbar flexion will serve as the outcome variables, while time (linear and quadratic), treatment group, the time-by-group interactions, pain, and disability will be predictor variables.
2.9.3.3. Aim 3. Examine maintenance of treatment gains at 1-, 6-, 12-, 24-, and 48-weeks post-treatment
Relative to the control group, participants in the experimental group will continue to show lower levels of pain and disability at each time point as well as increased activity in their natural environment (Hypothesis 3). To address hypothesis 3 we will build and test linear mixed-effects models for each of the outcome variables (pain, disability, pain/harm expectancy, lumbar flexion, and activity levels), with time (linear and quadratic), treatment group, and the interactions of time and treatment group as predictor variables.
As noted above, covariates will be added to analyses as needed. The significance level for every omnibus test will be set to 0.05, while Holms procedure will be used to control familywise type-I error rate for post-tests at .05. Assumptions of LME models will be checked by conducting analyses of model residuals. Violations of normality will be addressed by transforming the data, while outliers or influential cases will be handled by conducting sensitivity analyses. In contrast to the standard repeated measures analyses of variance, with their rigid assumptions about the error covariance structure, LME models permit numerous alternative error covariance structures. This allows for modeling of data that exhibit both heteroscedasticity and autocorrelation, which is likely to characterize the data collected for this study.
In addition to these primary analyses, we will use the same framework to analyze additional measures of core outcome domains, including emotional functioning (e.g., Center for Epidemiologic Studies – Depression, Profile of Mood States, PROMIS measures), pain vulnerability (Tampa Scale for Kinesiophobia, Pain Catastrophizing Scale), pain resilience (Pain Resilience Scale, Pain Self-efficacy Questionnaire), and patient global impression of change.
3. Discussion
The fear-avoidance model of chronic low back pain provides a cognitive-behavioral explanation for why some people with acute musculoskeletal pain go on to develop chronic pain and disability, while others do not.[48–51] Central to this model, individuals at highest risk for chronic low back pain are seen as prone to catastrophic thinking (e.g., “It’s not really safe for a person with my back pain to be physically active”). This tendency to focus on pain as a threat leads to a disproportionate emphasis on pain control efforts, an increase in fear and anxiety about movements that are perceived to increase pain or harm (i.e., kinesiophobia), and behavioral adaptations that promote either escape or avoidance of situations and activities that are deemed to pose a threat to the back. Ultimately, this is hypothesized to contribute to a combination of physical deconditioning, negative affect, and disability which complete a vicious cycle as they contribute to a continued focus on pain. In contrast, individuals who are at lower risk for chronic low back pain are believed to have a greater sense of optimism and positive affect, which help to protect them against catastrophic thinking and allows them to prioritize the pursuit of important life goals (e.g., work, leisure, and family) rather than persistent efforts to avoid or control pain. As a consequence, such individuals are viewed as less likely to avoid potential pain-provoking activities and more likely to engage in physical activities that can promote a gradual progress towards recovery.
Despite the fact that the fear-avoidance model of back pain suggests that catastrophic thinking and kinesiophobia lead to reductions in physical performance and deconditioning over time,[48–50] the overall evidence for this hypothesis is mixed with several studies failing to support a relationship with reduced physical performance or cardiovascular deconditioning.[24, 52–55] However, there is consistent evidence that individuals with high pain-related fear engage in a very specific form of avoidance which may not lead to cardiovascular deconditioning, but may lead to physiological adaptations that can maintain or promote continued back. Specifically, we have demonstrated that individuals with low back pain and elevated pain-related fear avoid flexion of the lumbar spine regardless of whether they have acute, subacute, or chronic low back pain. [11–13]} Avoidance of lumbar spine motion may be particularly problematic as it can contribute to shortening of peri-articular connective tissues and adaptive changes in the surrounding musculature.[5–7] These physiological adaptations may, in turn, increase the risk of reinjury when an individual is exposed to unexpected environmental challenges (e.g., a sudden slip or loss of balance) that require rapid postural adjustment. As a result, efforts to encourage a return to normal lumbar spine flexion may be central to the promotion of healthy recovery from acute, chronic, or recurrent low back pain. As an initial test of this hypothesis, the present study will examine a series of virtual reality games that provide an individualized, graded approach to increasing flexion of the lumbar spine among individuals with chronic low back pain and kinesiophobia.
Whereas clinical trials of graded activity and graded exposure therapy have been disappointing in terms of long-term pain and disability outcomes,[10] a virtual gaming environment offers several distinct advantages that we believe will help to restore normal movement, encourage protocol adherence, and promote generalization to the natural over time. First, whereas graded activity and graded exposure interventions encourage whole body motion, including motions that may be perceived as threatening the back for those with kinesiophobia, it is still possible for patients to achieve desired movement outcomes while continuing to guard or protect their lumbar spine (e.g., by increasing flexion of the hips and knees). Indeed, we have demonstrated that individuals with pain-related fear are prone to continue to restrict lumbar motion even as they recover from an episode of low back pain.[12] Thus, our virtual reality games are unique in that they continuously measure lumbar spine flexion and gradually reward increases in lumbar motion as a means of achieving game objectives. Second, graded activity and graded exposure therapy may be perceived as aversive for individuals with low back pain; hence, it is not surprising that such interventions have relatively high attrition rates.[8, 46] In this respect virtual reality games may be advantageous in that they can distract from pain, motivate engagement, and encourage treatment completion. Indeed, there is strong evidence that virtual reality games are potent distractors that significantly reduce the perception of pain during uncomfortable medical procedures.[56–59] Virtual reality games may be particularly powerful in this regard as this approach offers a combination of 1) attentional distraction, which can limit cognitive resources available to focus on uncomfortable sensations or thoughts of potential harm, 2) a goal-oriented pursuit that elicits positive affective reactions through achievement of game objectives and in-game rewards, and 3) a progressive challenge that can promote continued engagement.[60] Indeed, we have demonstrated that individuals with low back pain and high fear of movement indicate that they considered our virtual Dodgeball game to be a fun activity that they would voluntarily choose to repeat.[16] Hence, a virtual reality game that encourages graded spinal motion may have a distinct advantage over traditional therapeutic approaches by reducing the pain experience while encouraging continued, active engagement in the intervention process.
In sum, the VIGOR study posits that repeated exposure to virtual reality games that encourage lumbar spine flexion will allow individuals with chronic low back pain and pain-related fear to develop an implicit understanding that lumbar spine motion is not dangerous. We further hypothesize that players will generalize this knowledge to their daily lives, allowing them to resume normal patterns of spinal motion and ultimately a more lasting recovery.
Acknowledgments
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD088417. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
CRF and JST have read and approved the final manuscript and certify that they have no conflicts of interest or financial, personal, or other relationships that could inappropriately influence or be perceived to influence this manuscript.
Clinical Trial Registration: NCT03463824
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher R. France, Department of Psychology, Ohio University, Athens, Ohio, USA
James S. Thomas, Division of Physical Therapy, School of Rehabilitation and Communication Sciences, Ohio University, Athens, Ohio, USA
References
- 1.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA: the journal of the American Medical Association. 2010;303(13):1295–302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 2.Nicholas MK, Linton SJ, Watson PJ, Main CJG. Decade of the Flags” Working, Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91(5):737–53. doi: 10.2522/ptj.20100224. [DOI] [PubMed] [Google Scholar]
- 3.George SZ, Beneciuk JM. Psychological predictors of recovery from low back pain: a prospective study. BMC musculoskeletal disorders. 2015;16:49. doi: 10.1186/s12891-015-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Main CJ, George SZ. Psychologically informed practice for management of low back pain: future directions in practice and research. Phys Ther. 2011;91(5):820–4. doi: 10.2522/ptj.20110060. [DOI] [PubMed] [Google Scholar]
- 5.Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle. Comparison of two different modalities. Spine (Phila Pa 1976) 1995;20(1):54–8. doi: 10.1097/00007632-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine (Phila Pa 1976) 1996;21(23):2763–9. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Lieber RL. Skeletal muscle structure, function, and plasticity: The physiological basis for rehabilitation. Lippincott, Williams & Wikins; Baltimore: 2002. [Google Scholar]
- 8.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136(3):271–80. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Leeuw M, Goossens ME, van Breukelen GJ, de Jong JR, Heuts PH, Smeets RJ, Koke AJ, Vlaeyen JW. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138(1):192–207. doi: 10.1016/j.pain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Macedo LG, Smeets RJ, Maher CG, Latimer J, McAuley JH. Graded activity and graded exposure for persistent nonspecific low back pain: a systematic review. Phys Ther. 2010;90(6):860–79. doi: 10.2522/ptj.20090303. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JS, France CR. Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain. Spine. 2007;32(16):E460–6. doi: 10.1097/BRS.0b013e3180bc1f7b. [DOI] [PubMed] [Google Scholar]
- 12.Thomas JS, France CR. The relationship between pain-related fear and lumbar flexion during natural recovery from low back pain. Eur Spine J. 2008;17(1):97–103. doi: 10.1007/s00586-007-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JS, France CR, Sha D, Vander Wiele N, Moenter S, Swank K. The effect of chronic low back pain on trunk muscle activations in target reaching movements with various loads. Spine. 2007;32(26):E801–8. doi: 10.1097/BRS.0b013e31815d0003. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JS, France CR, Lavender SA, Johnson MR. Effects of fear of movement on spine velocity and acceleration after recovery from low back pain. Spine. 2008;33(5):564–70. doi: 10.1097/BRS.0b013e3181657f1a. [DOI] [PubMed] [Google Scholar]
- 15.Trost Z, France CR, Sullivan MJ, Thomas JS. Pain-related fear predicts reduced spinal motion following experimental back injury. Pain. 2012;153(5):1015–21. doi: 10.1016/j.pain.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Thomas JS, France CR, Applegate ME, Leitkam ST, Walkowski S. Feasibility and Safety of a Virtual Reality Dodgeball Intervention for Chronic Low Back Pain: A Randomized Clinical Trial. J Pain. 2016;17(12):1302–1317. doi: 10.1016/j.jpain.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-de-Uralde-Villanueva I, Munoz-Garcia D, Gil-Martinez A, Pardo-Montero J, Munoz-Plata R, Angulo-Diaz-Parreno S, Gomez-Martinez M, La Touche R. A Systematic Review and Meta-Analysis on the Effectiveness of Graded Activity and Graded Exposure for Chronic Nonspecific Low Back Pain. Pain Med. 2016;17(1):172–88. doi: 10.1111/pme.12882. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8(2):141–4. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Stratford PW, Binkley J, Solomon P, Finch E, Gill C, Moreland J. Defining the minimum level of detectable change for the Roland-Morris questionnaire. Phys Ther. 1996;76(4):359–65. doi: 10.1093/ptj/76.4.359. discussion 366–8. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer W, LeBlanc F, Dupris M. Scientific approach to the assessment and management of activity related spinal diorders. A monograph for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12:S1–S59. [PubMed] [Google Scholar]
- 22.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Focus article: report of the NIH Task Force on Research Standards for Chronic Low Back Pain. Eur Spine J. 2014;23(10):2028–45. doi: 10.1007/s00586-014-3540-3. [DOI] [PubMed] [Google Scholar]
- 23.Clark ME, Kori SH, Brockel J. Kinesiophobia and chronic pain: Psychometric characteristics and factor analysis of the Tampa Scale. American Pain Society Abstracts. 1996:77. [Google Scholar]
- 24.French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa scale for kinesiophobia (TSK) Pain. 2007;127(1–2):42–51. doi: 10.1016/j.pain.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: A Self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1(3):385–401. [Google Scholar]
- 26.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;(7):524–532. [Google Scholar]
- 27.Ankawi B, Slepian PM, Himawan LK, France CR. Validation of the Pain Resilience Scale in a Chronic Pain Sample. J Pain. 2017;18(8):984–993. doi: 10.1016/j.jpain.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Slepian PM, Ankawi B, Himawan LK, France CR. Development and Initial Validation of the Pain Resilience Scale. J Pain. 2016;17(4):462–72. doi: 10.1016/j.jpain.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11(2):153–63. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 31.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salsman JM, Lai JS, Hendrie HC, Butt Z, Zill N, Pilkonis PA, Peterson C, Stoney CM, Brouwers P, Cella D. Assessing psychological well-being: self-report instruments for the NIH Toolbox. Qual Life Res. 2014;23(1):205–15. doi: 10.1007/s11136-013-0452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trompetter HR, Ten Klooster PM, Schreurs KM, Fledderus M, Westerhof GJ, Bohlmeijer ET. Measuring values and committed action with the Engaged Living Scale (ELS): psychometric evaluation in a nonclinical sample and a chronic pain sample. Psychol Assess. 2013;25(4):1235–46. doi: 10.1037/a0033813. [DOI] [PubMed] [Google Scholar]
- 35.McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. Educational and Industrial Testing Service; San Diego: 1992. [Google Scholar]
- 36.Kelley ML, Heffer RW, Gresham FM, Elliott SN. Development of a modified treatment evaluation inventory. Journal of Psychopathology and Behavioral Assessment. 1989;11(3):235–247. [Google Scholar]
- 37.Rampakakis E, Ste-Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles MA. Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open. 2015;1(1):e000146. doi: 10.1136/rmdopen-2015-000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas JS, France CR, Sha D, Wiele NV. The influence of pain-related fear on peak muscle activity and force generation during maximal isometric trunk exertions. Spine (Phila Pa 1976) 2008;33(11):E342–8. doi: 10.1097/BRS.0b013e3181719264. [DOI] [PubMed] [Google Scholar]
- 39.McGill SM, Cholewicki J, Peach JP. Methodological considerations for using inductive sensors (3SPACE ISOTRAK) to monitor 3-D orthopaedic joint motion. Clin Biomech (Bristol, Avon) 1997;12(3):190–194. doi: 10.1016/s0268-0033(97)00063-6. [DOI] [PubMed] [Google Scholar]
- 40.Trost Z, France CR, Thomas JS. Exposure to movement in chronic back pain: evidence of successful generalization across a reaching task. Pain. 2008;137(1):26–33. doi: 10.1016/j.pain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Trost Z, France CR, Thomas JS. Examination of the photograph series of daily activities (PHODA) scale in chronic low back pain patients with high and low kinesiophobia. Pain. 2009;141(3):276–82. doi: 10.1016/j.pain.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain. 2011;152(7):1540–7. doi: 10.1016/j.pain.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 43.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland-Morris Disability Questionnaire for low back pain. J Clin Epidemiol. 2006;59(1):45–52. doi: 10.1016/j.jclinepi.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods. 2008;11(2):346–386. [Google Scholar]
- 46.Linton SJ, Boersma K, Jansson M, Overmeer T, Lindblom K, Vlaeyen JW. A randomized controlled trial of exposure in vivo for patients with spinal pain reporting fear of work-related activities. Eur J Pain. 2008;12(6):722–30. doi: 10.1016/j.ejpain.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Raudenbush SW, Liu X. Statistical power and optimal design for multisite randomized trials. Psychol Methods. 2000;5(2):199–213. doi: 10.1037/1082-989x.5.2.199. [DOI] [PubMed] [Google Scholar]
- 48.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 49.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 50.Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain. 2016;157(8):1588–9. doi: 10.1097/j.pain.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 51.Boselie J, Vlaeyen JWS. Broadening the fear-avoidance model of chronic pain? Scand J Pain. 2017;17:176–177. doi: 10.1016/j.sjpain.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Verbunt JA, Smeets RJ, Wittink HM. Cause or effect? Deconditioning and chronic low back pain. Pain. 2010;149(3):428–30. doi: 10.1016/j.pain.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Smeets RJ, van Geel AC, Kester AD, Knottnerus JA. Physical capacity tasks in chronic low back pain: what is the contributing role of cardiovascular capacity, pain and psychological factors? Disabil Rehabil. 2007;29(7):577–86. doi: 10.1080/09638280600925829. [DOI] [PubMed] [Google Scholar]
- 54.Smeets RJ, Wittink H. The deconditioning paradigm for chronic low back pain unmasked? Pain. 2007;130(3):201–2. doi: 10.1016/j.pain.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Smeets RJ, Wade D, Hidding A, Van Leeuwen PJ, Vlaeyen JW, Knottnerus JA. The association of physical deconditioning and chronic low back pain: a hypothesis-oriented systematic review. Disabil Rehabil. 2006;28(11):673–93. doi: 10.1080/09638280500264782. [DOI] [PubMed] [Google Scholar]
- 56.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. 2010;30(8):1011–8. doi: 10.1016/j.cpr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman HG, Patterson DR, Soltani M, Teeley A, Miller W, Sharar SR. Virtual reality pain control during physical therapy range of motion exercises for a patient with multiple blunt force trauma injuries. Cyberpsychol Behav. 2009;12(1):47–9. doi: 10.1089/cpb.2008.0056. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24(4):299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 59.Gold JI, Belmont KA, Thomas DA. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav. 2007;10(4):536–44. doi: 10.1089/cpb.2007.9993. [DOI] [PubMed] [Google Scholar]
- 60.Trost Z, Zielke M, Guck A, Nowlin L, Zakhidov D, France CR, Keefe F. The promise and challenge of virtual gaming technologies for chronic pain: the case of graded exposure for low back pain. Pain Manag. 2015;5(3):197–206. doi: 10.2217/pmt.15.6. [DOI] [PubMed] [Google Scholar]