Abstract
Objective
To test the hypothesis that SCNA can adequately estimate the cardiac sympathetic tone and the effects of cryoablation of the stellate ganglion in dogs with pacing-induced HF.
Background
Recording of subcutaneous nerve activity (SCNA) is new method to estimate sympathetic tone in dogs. Heart failure (HF) is known to increase sympathetic tone and atrial arrhythmias.
Methods
Twelve dogs with pacing-induced heart failure were studied using implanted radiotransmitters to record the stellate ganglia nerve activity (SGNA), vagal nerve activity (VNA) and SCNA. Among them, 6 dogs (Ablation Group) underwent bilateral stellate ganglia cryoablation before the rapid ventricular pacing and the remaining 6 dogs (Control Group) had rapid ventricular pacing only. In both groups, SCNA was compared to SGNA and the occurrence of arrhythmias.
Results
SCNA invariably increased preceding 360 identified atrial tachyarrhythmia episodes in the 6 control dogs before and after induction of HF. SCNA and SGNA correlated in all dogs with an average correlation coefficient of 0.64 (95% CI 0.58–0.70). Cryoablation of bilateral stellate ganglia significantly reduced SCNA from 0.34 ± 0.033 µV to 0.25 ± 0.028 µV (p=0.03) and eliminated all atrial tachyarrhythmias.
Conclusions
SCNA can be used to estimate cardiac sympathetic tone in dogs with pacing-induced HF. Cryoablation of the stellate ganglia reduced SCNA and arrhythmia vulnerability.
Keywords: Autonomic Nervous System, Vagus Nerve, Stellate ganglion, heart failure, arrhythmias
Introduction
Sympathetic tone measured by stellate ganglion nerve activity (SGNA) has been shown to influence atrial electrophysiology and the onset of atrial arrhythmias. We(1–3) recently proposed a new method (neuECG) to simultaneously record the electrocardiogram (ECG) and subcutaneous nerve activity (SCNA) using bipolar electrodes placed under the skin. The electrical signals were low pass filtered to optimize ECG and high pass filtered to reveal SCNA. Because a good correlation has been shown between SCNA and the simultaneously recorded stellate ganglia nerve activity (SGNA),(1) we proposed that the SCNA may be used to estimate cardiac sympathetic tone. The non-invasive estimation of sympathetic tone may provide new insights into the mechanisms of cardiac arrhythmias and may also provide a direct method to measure the success of neuromodulation procedures. Ogawa et al(4,5) had previously performed two studies in dogs with pacing-induced heart failure (HF). In the first study, it was shown that HF increases SGNA and occurrence of cardiac arrhythmias.(4) In a second study, using the same HF model, cryoablation of the caudal half of the left and right stellate ganglia and T2-T4 thoracic sympathetic ganglia was performed. It was shown that cryoablation significantly reduced both SGNA and paroxysmal atrial tachycardia (PAT) episodes.(5) The data from these studies have not been analyzed to determine if SCNA increased after induction of HF and decreased after cryoablation of the stellate ganglion. We retrieved the data from both studies and manually analyzed de novo the SGNA and atrial tachyarrhythmia episodes. We also performed analyses of SCNA using the methods previously reported.(1) The purpose of the present study was to test the hypothesis that (1) HF increases both SCNA and SGNA, (2) both SCNA and SGNA preceded the onset of PAT at baseline and after induction of HF and (3) cryoablation of the stellate ganglion can reduce both SCNA and SGNA in ambulatory dogs.
Methods
Original data from two previously published reports(4,5) were retrieved and manually analyzed. The research protocols were approved by the Institutional Animal Use and Care Committees of the Cedars-Sinai Medical Center, Los Angeles, California, and the Indiana University School of Medicine at the Methodist Research Institute, Indianapolis, Indiana.
Surgical preparations
The surgical preparations have been described previously. Briefly, all surgeries were performed under isoflurane anesthesia. Subcutaneous bipolar electrodes were placed in the left thorax of dogs, 6–10 cm apart to record SCNA. The electrodes were stainless steel wires with the terminal 5mm of the wires stripped of their insulation and used for electrical recording. The leads were attached to a Data Science International D70-EEE radiotransmitter (DSI; St. Paul, Minnesota) which was also implanted in the subcutaneous space. In the same procedure, through a left thoracotomy, a pair of bipolar electrodes from the DSI radiotransmitter was sutured onto the left stellate ganglion (LSG) to record SGNA. Another set of bipolar electrodes were placed on the left thoracic vagal nerve to record vagal nerve activity (VNA).
Experimental protocols
In all dogs, a pacing lead was implanted in the right ventricular (RV) apex and connected to a Medtronic Itrel neurostimulator for high rate ventricular pacing. After recovery from the initial surgery, the dogs had 2 weeks of continuous baseline ambulatory monitoring. After the baseline recording, the first group of 6 dogs (Group 1) underwent RV pacing at 150 bpm for 3 days, at 200 bpm for 3 days, and then at 250 bpm for 3 weeks to induce HF which was confirmed by Echocardiographic data.(6) The pacemakers were then turned off to allow ambulatory monitoring for additional two weeks. In the second group of 6 dogs (Group 2), the caudal half of the left stellate ganglion and T2-T4 thoracic sympathetic ganglia were cryoablated through the left thoracotomy using a 7-cm SurgiFrost probe (CryoCath Technologies, Inc., Montreal, Canada) during the same surgery where the DSI radiotransmitter was implanted for monitoring of SCNA, SGNA, and VNA. The dogs then underwent the same surgery and protocol for implantation of the Medtronic Itrel neurostimulator for subsequent high rate ventricular pacing to induce HF. These dogs were also monitored for two weeks before and after induction of HF.
Data analyses
Identifying Paroxysmal Atrial Tachyarrhythmias
Recordings from the bipolar electrodes connected to the radiotransmitter were analyzed to obtain SCNA, SGNA, VNA, and ECG using custom-written software. The impedance of the electrodes was 0.7 – 0.8 Ω and the recordings were amplified 10000 times and sampled at 1000Hz. We obtained an ECG for analyses by applying a bandpass filter (5–100 Hz) on the vagus nerve channel or subcutaneous nerve channel of the radiotransmitter. SCNA, SGNA, and VNA were obtained by high pass filtering at 150 Hz. The SGNA and VNA were rectified and integrated over 1 minute. The SCNA was also rectified and integrated over the same minute after adjusting the SCNA with wavelet analysis as described previously.(7) In particular, spike-triggered averaging was performed to allow removal of the ventricular electrogram from the SCNA by subtracting a ventricular electrogram template obtained from averaged ventricular electrograms in the observational window. Finally, the ECG was used to calculate the heart rate over the same minute. The sum of all digitized nerve activity was then divided by the total number of samples during the same time period to obtain averaged SGNA (aSGNA), averaged SCNS (aSCNA) and averaged VNA (aVNA) per sample. Nerve activity was also quantified by determining the number of spikes and bursts within the nerve activity.(8) The filtered and rectified signals from a 24-hour recording were integrated over a 100 ms sliding window. Within this integrated signal, nerve spikes and bursts of nerve activity were detected. A spike was defined as the continuous time period where the amplitude is above the threshold value. Each spike’s duration and average amplitude were measured within that time period. The threshold value was calculated as 3 times the baseline noise level, which was considered to be the lowest 10th percentile of all values during the 24-hour recording. Spikes with an inter-spike interval of less than 5 seconds were grouped into bursts.
The aSGNA, aSCNA, aVNA, and heart rate were calculated every hour of a 24-hour period (2:00 pm to 2:00 pm the next day) before pacing at baseline and of every hour of the 24-hour period (2:00 pm to 2:00 pm the next day) immediately after pacing was stopped. In order to avoid periods of dropped radiotransmission or artifactual noise caused by movement of the dog, which was greater than nerve activity, the first 10 analyzable minutes of each hour were used for the calculation of the averaged nerve activity and heart rate.
Paroxysmal atrial tachyarrhythmias were defined as episodes of tachycardia (≥ 175 bpm) with abrupt onset (>50 bpm/sec) and a duration of > 5 s. Of these episodes, half were randomly selected for P wave analysis. This analysis involved comparing the P waves before the initiation of the tachycardia to the P waves during the tachycardia episode and analyzing the changes in P wave morphology and PR interval.
Statistical Analysis
The averaged nerve activities were compared between the control dogs and cryoablated dogs using one-sided student’s t-tests both before pacing and after pacing. In addition, the aSCNA for each dog was used to look at the effect of ventricular pacing on both control and cryoablated dogs by one-sided paired t-tests. The data were presented as mean ± standard deviation. In order to test for circadian variation, generalized additive mixed-effects models were fitted to the longitudinal data. A Pearson Correlation Coefficient was used to compare the SCNA and SGNA between the Control Group and the Ablation Group for 24 hours before and after pacing. A p value of ≤ 0.05 was considered to be significant.
Results
Effect of Stellate Ganglion Cryoablation on Nerve Activity
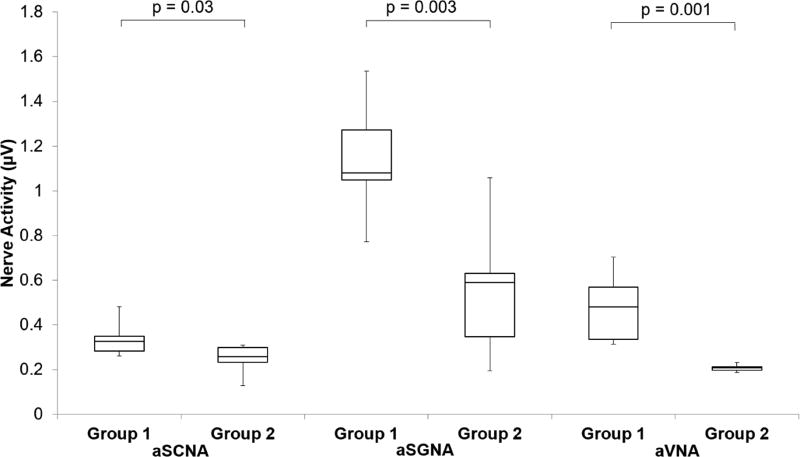
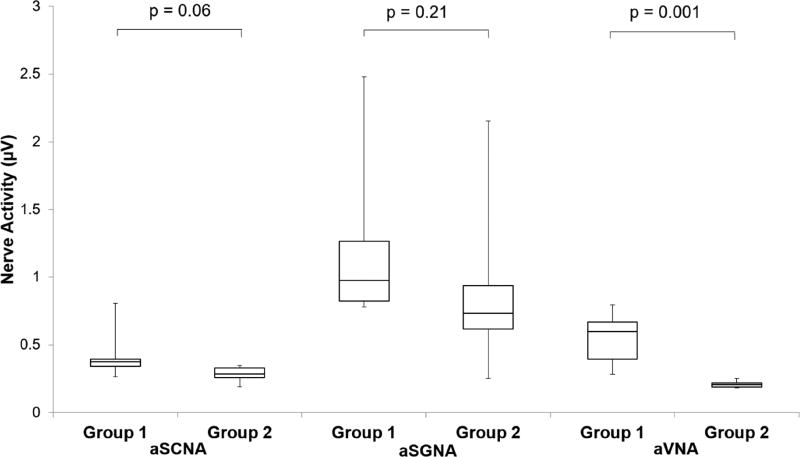
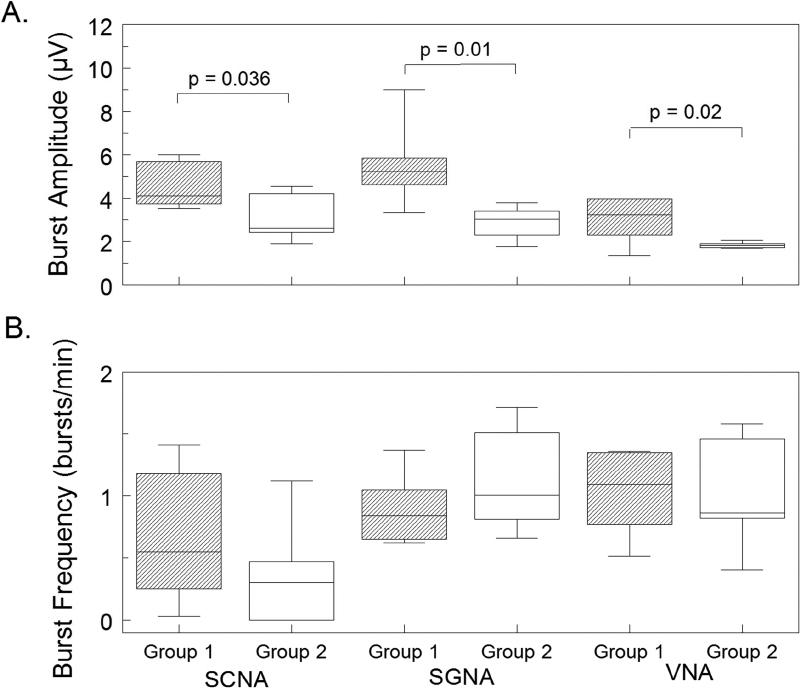
The Group 1 dogs did not have ablation while the Group 2 dogs had stellate ganglion ablation during the first surgery. Figure 1 shows that at baseline (before rapid ventricular pacing), aSGNA was significantly lower in the Group 2 dogs (0.56 ± 0.13 µV) than Group 1 dogs (1.14 µV ± 0.11 µV) (p = 0.003), indicating ablation significantly reduced aSGNA. aSCNA was also significantly lower in Group 2 versus Group 1 dogs (0.25 µV ± 0.028 µV vs 0.34 µV ± 0.033 µV, p =0.03). The aVNA was lower in Group 2 (0.21 µV ± 0.0068 µV) than in Group 1 (0.48 µV± 0.066 µV, p=0.001) dogs. The heart rate (HR) was also lower in Group 2 (88 ± 7.9 bpm) than Group 1 (109 ± 4.0 bpm, p=0.02). Consistent with the previous report,(4) rapid ventricular pacing induced HF increased the nerve activities in both groups of dogs. Figure 2 shows the differences between Group 1 and Group 2 dogs after HF had been induced. As shown in that figure, the Group 2 dogs had lower nerve activities than Group 1 dogs, but the differences did not reach statistical significance for aSCNA and aSGNA. The aSCNA was 0.28 ± 0.024 µV in Group 2 and 0.43 ± 0.079 µV in Group 1 (p=0.06). The aSGNA was 0.91 ± 0.27 µV for Group 2 and 1.23 ± 0.27 µV for Group 1 (p=0.21). The VNA was 0.21 µV ± 0.011 µV for Group 2 and 0.55 µV ± 0.082 µV for Group 1 (p=0.001). The HR was 87 ± 4.9 bpm for Group 2 and 96 ± 3.9 bpm for Group 1 (p=0.11). The nerve activity was also quantified by detecting the spikes and bursts of activity within the nerve recording. Figure 3 shows that the amplitude of the bursts was significantly decreased after cryoablation for each type of nerve recording (SCNA, SGNA, and VNA). While there was no significant change in the burst frequency, due to the significant reduction in burst amplitude total nerve activity is reduced.
Figure 1.
Effect of cryoablation on nerve activity before ventricular pacing. Nerve activity was quantified by averaging the rectified, high-pass signal every minute for 10 minutes every hour for 24 hours. Data before pacing is shown as a box-and-whisker plot comparing the control group (Group 1) versus dogs that had stellate ganglion cryoablation (Group 2). A significant decrease in nerve activity is seen for the SCNA, SGNA, and VNA in the ablation group.
Figure 2.
Effect of cryoablation on nerve activity after ventricular pacing. Nerve activity was quantified by integrating the rectified, high-pass signal every minute for 10 minutes every hour for 24 hours. Data after pacing is shown as a box-and-whisker plot comparing the control group (Group 1) versus dogs that had stellate ganglion cryoablation (Group 2). A significant decrease in nerve activity is seen for the VNA in the ablation group.
Figure 3.
Effect of cryoablation on nerve activity after ventricular pacing. Nerve activity was quantified by detecting spikes within the integrated signal. Spikes with an inter-spike interval of less than 5 seconds were grouped into bursts. Data after pacing is shown as a box-and-whisker plot comparing the control group (Group 1) versus dogs that had stellate ganglion cryoablation (Group 2). Panel A shows a significant decrease in the amplitude of the burst activity is seen in the ablation group for each type of nerve recording. Panel B shows the results of calculating the frequency of the bursts. Cryoablation of the stellate ganglion did not have a significant effect on the frequency of the bursts of nerve activity.
SCNA and Atrial Tachyarrhythmias
Dogs normally have PAT at baseline.(9) Consistent with that report, we were able to identify a total of 243 PAT episodes in 6 Group 1 dogs during a 24-hour period of monitoring at baseline and 117 PAT episodes after induction of HF by rapid pacing. Our de novo analyses confirmed the previous report that no PAT episodes were present in Group 2 dogs either at baseline or after rapid ventricular pacing.(6)
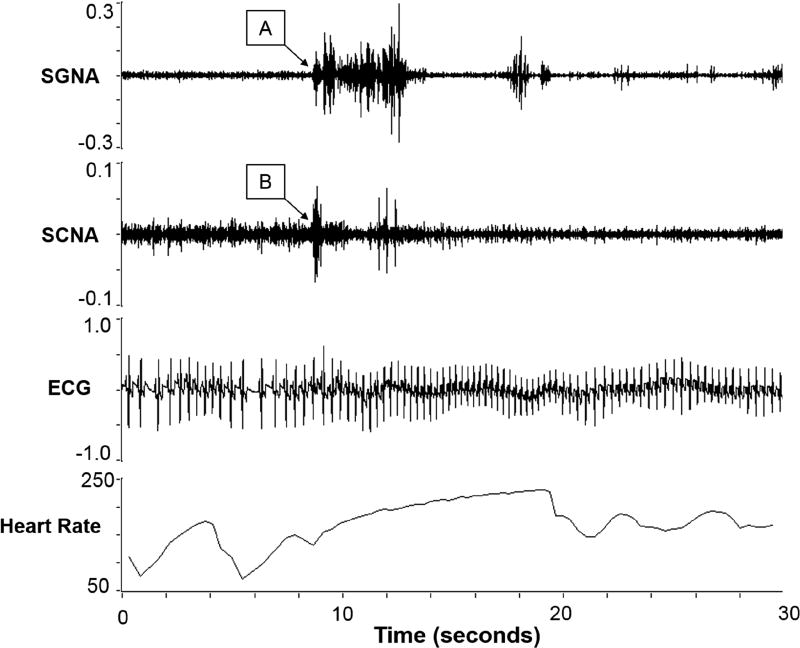
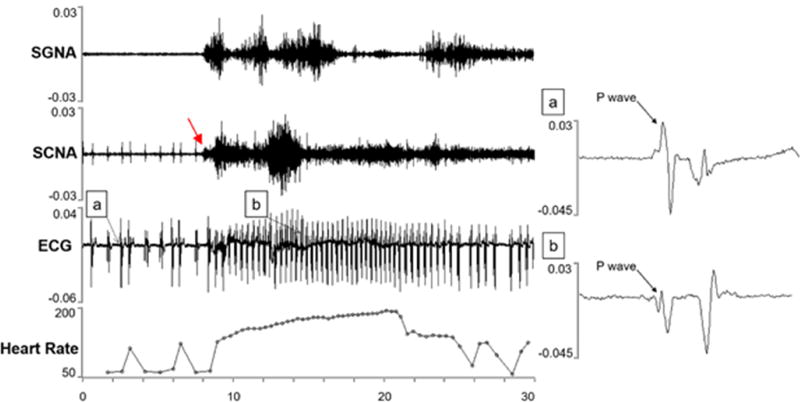
In all PAT episodes, an increase in the SCNA and SGNA correlated with the onset of the tachycardia (Figure 4). At the arrows labeled A and B, the SGNA and SCNA respectively begin to increase dramatically. The heart rate also increased suddenly, increasing at a rate of 87 beats/minute per second (abrupt onset). The heart rate increased to greater than 175 BPM for over 7 seconds, fulfilling the diagnostic criteria for PAT. In all episodes, as in this example, increased SCNA were closely related to increases in SGNA during PAT episodes.
Figure 4.
Atrial tachycardia correlated with an increase in SCNA and SGNA. The arrows indicate the increase in nerve activity for the SGNA (A) and the SCNA (B).
P wave changes were found in most randomly selected episodes of arrhythmias including P wave morphology changes and PR interval shortening as shown in Figure 5. In the atrial tachyarrhythmia in Figure 4, the P wave before the tachycardia started with a biphasic P wave with an initial upward phase followed by a negative phase as shown in panel A, but after the tachycardia, the P wave was initially negative with a small positive phase, then was even more negative as shown in panel B. Furthermore, the PR interval before the tachycardia was 125 ms and during the tachycardia the PR interval shortened to 105 ms. These findings indicate that the PAT episodes are unlikely to be sinus tachycardia.
Figure 5.
Changes in P waves during atrial tachycardia. The onset of tachycardia was preceded by the onset of SCNA (red arrow). A morphology change in the p-wave is shown with the onset of an atrial tachycardia. The p-wave prior to arrhythmia onset is shown in (a), and the p-wave after arrhythmia onset is shown in (b). Also shown is an increase in SGNA and SCNA activity correlating to an increase in heart rate.
Correlation between SGNA and SCNA
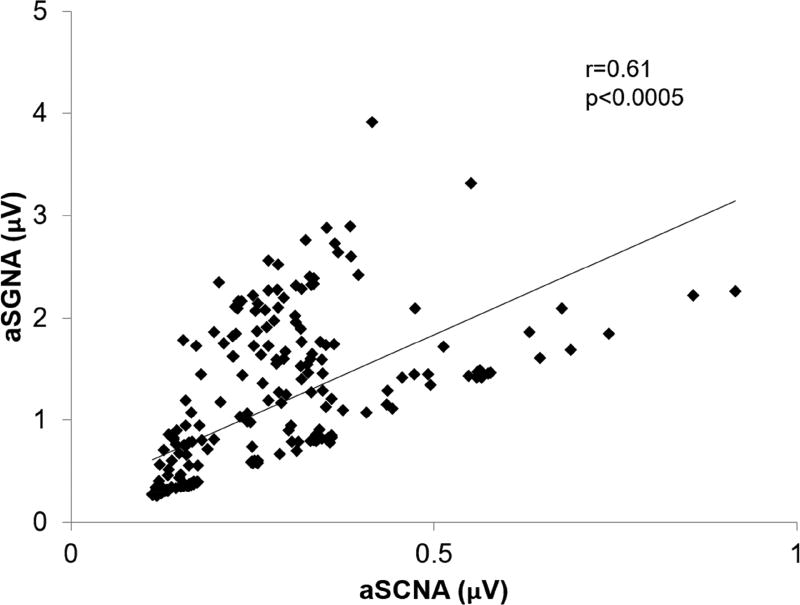
The nerve activity of stellate ganglia and subcutaneous nerves for both Group 1 and Group 2 at baseline and after pacing were compared by plotting SGNA by SCNA. Linear regression was then used to fit the data and calculate the correlation coefficient. Figure 6 shows the results for Dog A including the correlation coefficient of r = 0.61 and p < 0.0005. The correlation coefficient and p value for each dog both before pacing and after pacing is shown in Table 1. In general, SCNA correlated moderately well with SGNA with an average of all the correlation coefficients of all dogs at 0.64 (95% CI 0.58–0.70). To compare the two groups, before pacing the mean difference between the ablation group and the control group (ablation - control) is 0.2567 (p = 0.01). After pacing the mean difference between the ablation group and the control group (ablation - control) is −0.035 (p = NS).
Figure 6.
Correlation of SGNA and SCNA over 24 hours. Relationship between SGNA and the SCNA integrated nerve activity (µV) over a 24 hour period for Dog A.
Table 1.
Pearson Correlation Coefficient Between SCNA and SGNA for the Control Group and the Ablation Group for 24 Hours Before and After Pacing.
| Control | Ablation | ||||||
|---|---|---|---|---|---|---|---|
| Dog | Before Pacing | After Pacing | Dog | Before Pacing | After Pacing | ||
| A | r | 0.61 | 0.74 | G | r | 0.80 | 0.65 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
| B | r | 0.57 | 0.62 | H | r | 0.76 | 0.81 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
| C | r | 0.35 | 0.83 | I | r | 0.56 | 0.72 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
| D | r | 0.21 | 0.53 | J | r | 0.70 | 0.61 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
| E | r | 0.56 | 0.72 | K | r | 0.84 | 0.59 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
| F | r | 0.58 | 0.74 | L | r | 0.76 | 0.59 |
| P | <0.0005 | <0.0005 | P | <0.0005 | <0.0005 | ||
Circadian Variation
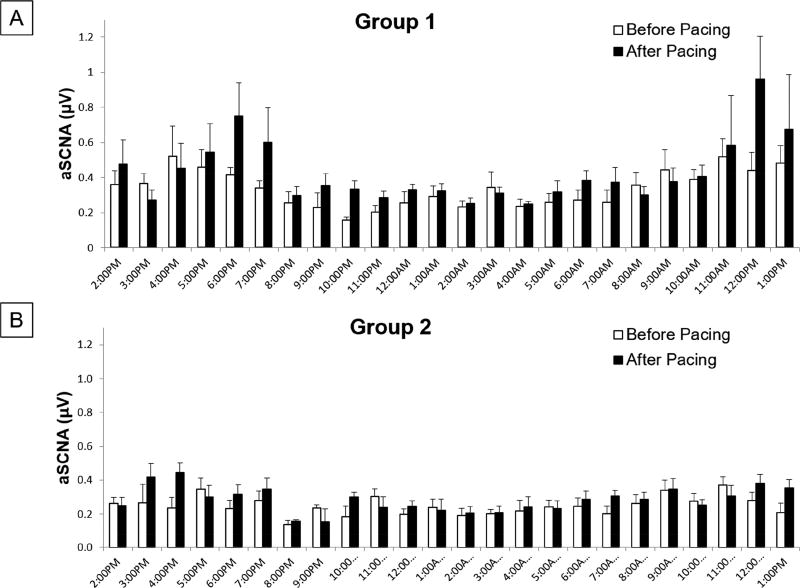
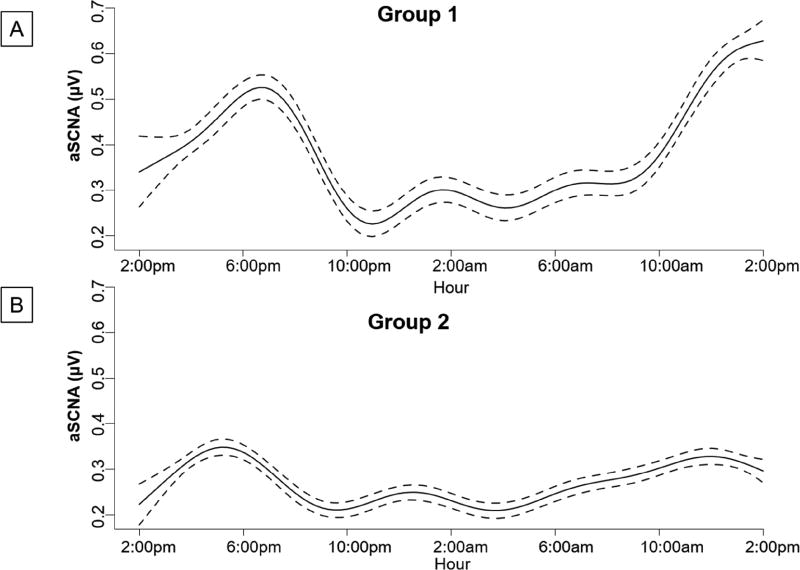
Figure 7 summarizes the SCNA data for all dogs. The SCNA had a significant circadian variation (p<0.001) when fitted to a generalized additive mixed-effects model. Figure 8 shows the generalized additive mixed effect model fitted to the SCNA data. Previous studies have shown a similar significant circadian variation for the SGNA.(5) Compared to the SCNA in Figure 7A, they both show a progressive increase in the morning hours with a secondary increase in the late afternoon.
Figure 7.
Circadian variation in SCNA. Summary data of the averaged integrated nerve activity (µV) for every hour over a 24 hour period for the Control dogs (A) the dogs with cryoablation (B).
Figure 8.
Generalized additive mixed effect model fitted to the SCNA data. Circadian variation of the SCNA in µV for the Control (Group 1) dogs (A) and the dogs with cryoablation (Group 2) (B). Solid lines represent mean integrated SCNA calculated over 1 minute for 10 minutes every hour. Dotted lines represent 95% upper and lower confidence intervals.
Discussion
Using SCNA to measure sympathetic tone
Heart failure is characterized by increased sympathetic tone and cardiac arrhythmias.(4) The sympathetic nerve activity as measured by microneurography is reduced by cardiac resynchronization therapy (CRT) in the responders, but not in non-responders.(10) However, because microneurography techniques are difficult to perform and cannot be used in ambulatory patients, sympathetic nerve activity measurements have not been clinically useful in assessing the effects of CRT or other neuromodulation methods. The results of this study suggest that the SCNA might be a useful method to directly measure sympathetic tone in HF, and determining the effects of HF therapy. A second possible use of SCNA is to determine if cardiac arrhythmias are triggered by sympathetic activation. A previous study showed that SCNA was observed before a majority of episodes of ventricular tachycardia and ventricular fibrillation in an ambulatory canine model of sudden cardiac death.(2) In the present study we have shown that SCNA preceded the onset of all PAT episodes both at baseline and after the induction of HF. These findings suggest that SCNA can be useful in determining whether or not PAT was caused by sympathetic activation.
Sources of SCNA
Axonal tracer studies have shown that a significant portion of the skin sympathetic nerves of the neck and upper thorax originate from the ipsilateral stellate ganglion.(11,12) Several other studies have demonstrated a strong relationship between sympathetic nerve structures in the chest and skin nerves. Donadio et al have shown in skin biopsies an abundance of sympathetic nerves in arteriovenous anastomosis, arrector pilorum muscles, and arterioles.(13) Baron et al showed in axonal tracer studies that nearly all skin sympathetic nerve somata are located in the middle cervical and stellate ganglia.(11) The middle cervical ganglion and the stellate ganglion are highly interconnected to intrinsic cardiac neurons. In addition, it has been shown that SCNA can be used as an estimate of sympathetic tone,(1) and is more accurate than heart rate variability.(14) The current study confirms that SGNA and SCNA are closely related as they both increase before the onset of paroxysmal atrial tachyarrhythmias and both decrease after cryoablation.
Effects of LSG ablation on SCNA
Previous studies have shown that the right stellate ganglion (RSG) controlled heart rate and only had an effect on blood pressure after the left stellate ganglion was completely resected. This suggests that the RSG innervates the sinus node and plays a role in regulating the heart rate. The left stellate ganglion (LSG) controls blood pressure and is thought to be the main influence in cardiac sympathetic control.(15) In a separate canine study, it was demonstrated that LSG stimulation significantly increased AF inducibility over RSG stimulation. Removing the LSG significantly reduced the incidence of AF.(16) However, in a clinical study, both RSG and LSG block showed similar results in reduced AF inducibility and duration.(17) In addition to controlling atrial arrhythmias in canine models,(18) LSG ablation is known to be effective in decreasing sympathetic outflow and controlling ventricular arrhythmias.(19–22) In animal experiments, it has also been shown that partial LSG ablation can reduce or eliminate PAT in ambulatory canine models.(5,18) Left cardiac sympathetic denervation (LCSD) has been used as a therapeutic option for patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) that have recurrent VF/VT episodes even with β-blocker therapy. Surgical approaches include thoracoscopic, transaxillary, and supraclavicular to expose the left-sided sympathetic chain from T4 – T1. Resection or ablation of the sympathetic ganglia from T1–T2 then has shown to have a positive effect in these patients. A more minimally invasive procedure using video-assisted thoracic surgery to perform LCSD has also proven to be effective in reducing cardiac events. However, even though we have previously shown that LCSD therapy with cryoablation can be used as a therapy for atrial arrhythmias, it is still not a commonly used approach as a clinical therapeutic option for atrial arrhythmias.
If SGNA is a source of SCNA, then partial LSG ablation should reduce the SCNA. The latter findings would further support the validity of SCNA in estimating SGNA. However, as shown in Figure 1, the LSG ablation seems to have more effects on aSGNA than aSCNA. One reason is that the subcutaneous nerves in the thorax may not all come from the LSG. Therefore, the baseline correlation between aSGNA and aSCNA was only moderate. A second reason is that LSG is a large nerve structure that generates strong electrical signals. Because of the high signal to noise ratio, the amplitude of reduction of SGNA after cryoablation is easier to visualize. In contrast, the subcutaneous nerves are small (average < 1 µV at baseline) and have a smaller signal to noise ratio. Given that inherent noise of the recording system is a significant portion of the signal, a reduction of SCNA may not be as apparent after LSG ablation. Monitoring SCNA has many advantages over monitoring SGNA as an indicator of sympathetic nerve activity. Monitoring SGNA or other indicators of sympathetic nerve activity have involved an open chest procedure to implant electrodes on the stellate ganglion or other nerve structures. On the other hand, implantation of subcutaneous electrodes in the chest could be accomplished much less invasively. In addition, the study of subcutaneous nerve activity may lead to the use of surface electrodes for the monitoring of sympathetic nerve activity. Preliminary results have shown that it is possible to record the skin sympathetic nerve activity using ordinary ECG patch electrodes.(3,23) For these reasons, SCNA may be a useful tool in minimally invasively monitoring sympathetic nerve activity involved in the arrhythmogenesis of atrial tachyarrhythmias and in estimating the efficacy of neuromodulation procedures.
Study Limitations
The electrical signals within the recordings from subcutaneous electrodes may come from multiple sources including low frequency motion artifacts, ECG, respiratory muscle activity, and nerve activities. While some noise can be effectively filtered with conventional filters, the ECG can contain high frequency content that is within the range of the frequency bandwidth of the nerve activity. Therefore, we decided to use a wavelet filter to filter out much of the ECG as we have done in previous studies with large ECG signals accompanying the nerve activity of interest.(7) While integrated SCNA showed a significant correlation with integrated SGNA, integrated wavelet SCNA appeared to have a greater correlation with integrated SGNA. The dogs do not have sweat glands in the thorax where the recordings were made. Whether or not sweating affects the SCNA recording cannot be determined in this study.
In addition, our study used widely spaced electrodes to monitor subcutaneous nerve activity. Other studies in our lab have used more narrow spaced electrodes with varying success.(1,3) When using the Data Science International D70-EEE radiotransmitter with narrow spaced electrodes, the bandwidth and sampling rate of the device makes the signal to noise ratio insufficient to accurately monitor subcutaneous nerve activity. However, when using narrow spaced electrodes with recording equipment capable of recording with higher bandwidth and higher sampling rate of 10,000Hz, we were able to high pass filter at 500Hz, improving the signal to noise ratio and increasing the accuracy of monitoring the subcutaneous nerve activity.(1)
Conclusion
Subcutaneous nerve activity correlated with SGNA and the onset of arrhythmias. Cryoablation of the stellate ganglia reduced the SCNA, further validating the use of SCNA to estimate cardiac sympathetic tone. This study suggests that using equipment with an appropriate bandwidth and sampling rate, recording subcutaneous nerve activity with an implantable device may be possible, and the SCNA can be used as a surrogate for SGNA.
Perspectives.
Competency in Medical Knowledge
Microneurography is used to directly measure sympathetic nerve activity; however, microneurography techniques are difficult to perform and cannot be used in ambulatory patients. The results of this study suggest that the SCNA might be a useful method to directly measure sympathetic tone in HF, to determine the effects of HF therapy, and to determine if cardiac arrhythmias are triggered by sympathetic activation.
Translational Outlook.
Neuromodulation therapy including the ablation of the ganglionated plexi has been used for controlling AF. Techniques that can be used as a surrogate for microneurography can increase the clinical usefulness of sympathetic nerve activity measurements to help in assessing the efficacy of neuromodulation.
Acknowledgments
Funding Sources:
This study was supported in part by NIH Grants P01HL78931, R0171140, R41HL124741, R42DA043391, a Medtronic-Zipes Endowment, the Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology, and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Abbreviations
- AT
atrial tachycardia
- ECG
electrocardiogram
- HF
heart failure
- HR
heart rate
- HRV
heart rate variability
- LSG
left stellate ganglion
- RV
right ventricle
- SCNA
subcutaneous nerve activity
- SGNA
stellate ganglia nerve activity
- VNA
vagus nerve activity
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of Interest: Shien-Fong Lin, Johnson Wong, and Thomas H. Everett, IV have equity interest in Arrhythmotech, LLC. Cyberonics, Medtronic and St. Jude Medical Inc. donated research equipment to Dr. Chen’s research laboratory.
References
- 1.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J Cardiovasc Electrophysiol. 2015;26:70–8. doi: 10.1111/jce.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doytchinova A, Patel J, Zhou S, et al. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm. 2015;122:612–620. doi: 10.1016/j.hrthm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm. 2015;12:1324–32. doi: 10.1016/j.hrthm.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa M, Tan AY, Song J, et al. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2009;6:1772–1779. doi: 10.1016/j.hrthm.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa M, Tan AY, Song J, et al. Cryoablation of extrinsic cardiac sympathetic nerves markedly reduces atrial arrhythmias in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2008;5:S54. doi: 10.1016/j.hrthm.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HW, Shen MJ, Han S, et al. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–80. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart EC, Head GA, Carter JR, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol. 2017;312:H1031–H1051. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najem B, Unger P, Preumont N, et al. Sympathetic control after cardiac resynchronization therapy: responders versus nonresponders. Am J Physiol Heart Circ Physiol. 2006;291:H2647–52. doi: 10.1152/ajpheart.00373.2006. [DOI] [PubMed] [Google Scholar]
- 11.Baron R, Janig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst. 1995;53:205–14. doi: 10.1016/0165-1838(94)00171-f. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth. 1994;8:441–449. doi: 10.1007/BF02514624. [DOI] [PubMed] [Google Scholar]
- 13.Donadio V, Nolano M, Provitera V, et al. Skin sympathetic adrenergic innervation: an immunofluorescence confocal study. Ann Neurol. 2006;59:376–81. doi: 10.1002/ana.20769. [DOI] [PubMed] [Google Scholar]
- 14.Chan YH, Tsai WC, Shen C, et al. Subcutaneous nerve activity is more accurate than heart rate variability in estimating cardiac sympathetic tone in ambulatory dogs with myocardial infarction. Heart Rhythm. 2015;12:1619–27. doi: 10.1016/j.hrthm.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, DeSimone CV, Suddendorf SH, et al. Effects of stepwise denervation of the stellate ganglion: Novel insights from an acute canine study. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Hu J, Guo Y, et al. Effect of the stellate ganglion on atrial fibrillation and atrial electrophysiological properties and its left-right asymmetry in a canine model. Experimental and clinical cardiology. 2013;18:38–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Leftheriotis D, Flevari P, Kossyvakis C, et al. Acute effects of unilateral, temporary stellate ganglion block on human atrial electrophysiologic properties and atrial fibrillation inducibility. Heart Rhythm. 2016;13:2111–2117. doi: 10.1016/j.hrthm.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss AJ, McDonald J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N Engl J Med. 1971;285:903–904. doi: 10.1056/NEJM197110142851607. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 21.Vaseghi M, Rn Msn NpJG, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: Intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–6. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley U, Yamakawa K, Takamiya T, Andrew Armour J, Shivkumar K, Ardell JL. Targeted stellate decentralization: Implications for sympathetic control of ventricular electrophysiology. Heart Rhythm. 2015;13:282–8. doi: 10.1016/j.hrthm.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doytchinova A, Hassel J, Yuan Y, et al. Using skin sympathetic nerve activity to estimate sympathetic tone in humans. Heart Rhythm. 2015;12:S297. doi: 10.1016/j.hrthm.2015.02.012. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]