ABSTRACT
The RV3-BB human neonatal rotavirus vaccine aims to provide protection from severe rotavirus disease from birth. A phase IIa safety and immunogenicity trial was undertaken in Dunedin, New Zealand between January 2012 and April 2014. Healthy, full-term (≥ 36 weeks gestation) babies, who were 0–5 d old were randomly assigned (1:1:1) to receive 3 doses of oral RV3-BB vaccine with the first dose given at 0–5 d after birth (neonatal schedule), or the first dose given at about 8 weeks after birth (infant schedule), or to receive placebo (placebo schedule). Vaccine take (serum immune response or stool shedding of vaccine virus after any dose) was detected after 3 doses of RV3-BB vaccine in >90% of participants when the first dose was administered in the neonatal and infant schedules. The aim of the current study was to characterize RV3-BB shedding and virus replication following administration of RV3-BB in a neonatal and infant vaccination schedule. Shedding was defined as detection of rotavirus by VP6 reverse transcription polymerase chain reaction (RT-PCR) in stool on days 3–7 after administration of RV3-BB. Shedding of rotavirus was highest following vaccination at 8 weeks of age in both neonatal and infant schedules (19/30 and 17/27, respectively). Rotavirus was detected in stool on days 3–7, after at least one dose of RV3-BB, in 70% (21/30) of neonate, 78% (21/27) of infant and 3% (1/32) placebo participants. In participants who shed RV3-BB, rotavirus was detectable in stool on day 1 following RV3-BB administration and remained positive until day 4–5 after administration. The distinct pattern of RV3-BB stool viral load demonstrated using a NSP3 quantitative qRT-PCR in participants who shed RV3-BB, suggests that detection of RV3-BB at day 3–7 was the result of replication rather than passage through the gastrointestinal tract.
KEYWORDS: diarrhea, neonates, rotavirus, vaccines, viral load
Introduction
Rotavirus is the most common cause of severe diarrhea in children under 5 y of age. Globally, rotavirus infection causes an estimated 215,000 annual deaths.1 Two rotavirus vaccines, Rotarix (GSK, Rixensart, Belgium) and RotaTeq (Merck, Whitehouse Station, USA) have reduced hospital admissions and child deaths from diarrhea in settings where vaccine has been introduced in national immunisation programs.2-7 However, Rotarix and RotaTeq have lower vaccine effectiveness in low income countries when compared with high-income countries.8-10 Several host and environmental factors have been suggested to play a role in the lower vaccine effectiveness. Rotavirus disease can occur earlier in life in low-income settings, with 56% of children in a birth cohort from Vellore infected by 6 months11 compared with 27% in Philadelphia.12 The administration of Rotarix and RotaTeq is recommended from 6 weeks of age, with completion of a 2- or 3-dose schedule at 4–6 months which may be too late to provide protection in some infants.
The RV3-BB vaccine is currently in clinical development with a birth dose vaccination schedule.13 A birth dose of a rotavirus vaccine, given before the intestinal microbiota has fully established and when breast milk intake is limited may help to overcome some of the restrictions on vaccine take. The RV3-BB vaccine is based on a naturally attenuated neonatal G3P[6] rotavirus strain, first identified in Melbourne obstetric hospitals in the 1970s. Neonatal strains replicate well in the immature newborn intestine.14 Natural infection with this strain was shown to protect against severe disease during reinfection with heterotypic rotavirus strains during the first 3 y of life.14 A phase IIa immunogenicity study demonstrated vaccine take (serum immune response or stool shedding of vaccine virus after any dose) after administration of 3 doses of RV3-BB vaccine in >90% of participants when the first dose was administered at 0–5 d after birth (neonatal schedule) or at ∼8 weeks of age (infant schedule).13
Several large epidemiological studies have demonstrated that natural infection provides protection from subsequent severe disease.14,15 The mechanisms responsible for generating protective immunity following vaccination or natural infection are not completely understood. Serological responses such as serum neutralizing antibodies and anti-rotavirus antibodies are important host responses following infection and vaccination,16 but are imperfect correlates of protection against disease. Therefore using RV3-BB shedding as a surrogate for virus replication may be useful in understanding protection following rotavirus vaccination. The phase IIa immunogenicity trial provided an opportunity to investigate the characteristics of replication and shedding of this novel human neonatal vaccine. In the present study, we used a rotavirus specific quantitative reverse-transcription polymerase chain reaction (qRT-PCR) to investigate if the schedule or dose of vaccine was associated with differences in stool viral load and if there was a relationship between stool viral load and serological response. In addition, we investigated the kinetics of rotavirus shedding to provide insights into the replication of RV3-BB in the intestine following vaccination.
Results
Rotavirus shedding in stool following administration of RV3-BB
In the neonatal schedule group, RV3-BB was administered at 0–5 days, 8 weeks and 15 weeks (Fig. 1). The proportion of tested stool samples that were rotavirus positive by RT-PCR was 66/277 (24%). Rotavirus was detected on days 3–7 in stool after at least one vaccine dose in 70% (21/30) participants. The proportion of participants shedding rotavirus at day 3–7 was highest (19/30) following RV3-BB dose 2 (8 weeks) (Table 1). Collectively, 20% (6/30) participants shed rotavirus after any one dose, 40% (12/30) after 2 doses and 10% (3/30) after all 3 doses of RV3-BB.
Figure 1.
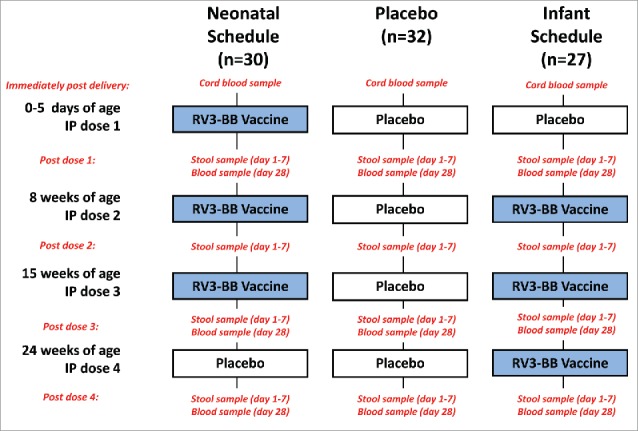
Study design and sample collection. IP, investigational product.
Table 1.
Rotavirus shedding in stool on days 3-7 post administration of RV3-BB or placebo. Neonate and Infant schedule participants received RV3-BB vaccine according the dosing schedule described in Material and methods.
| IP dose 1 (0-5 days) | IP dose 2 (8 weeks) | IP dose 3 (15 weeks) | IP dose 4 (24 weeks) | |
|---|---|---|---|---|
| Neonate Schedule | 13.3% (4/30) | 63.3% (19/30) | 53.3% (16/30) | 0% (0/30)* |
| Infant Schedule | 0% (0/27)* | 62.9% (17/27) | 48.1% (13/27) | 37.0% (10/27) |
| Placebo | 0% (0/32)* | 0% (0/32)* | 3.1% (1/32)* | 0% (0/32)* |
Placebo given at IP dose.
In the infant schedule group, RV3-BB was administered at 8 weeks, 15 weeks and 24 weeks (Fig. 1). The proportion of tested stool samples that were rotavirus positive by RT-PCR was 66/258 (26%). Rotavirus shedding was detected at days 3–7 following each dose of RV3-BB, with the highest proportion (17/27) of shedding detected at RV3-BB dose 1 (8 weeks) (Table 1). Rotavirus shedding occurred after any one dose of RV3-BB in 26% (7/27) participants, 33% (9/27) after any 2 doses and 19% (5/27) after all 3 doses. Rotavirus was detected in stool after at least one vaccine dose in 78% (21/27) of infant schedule participants. In the placebo group, the proportion of tested stool samples that were rotavirus positive by RT-PCR was 2/307 (0.65%). Rotavirus shedding was detected in a single participant at IP dose 3 (15 weeks).
Rotavirus shedding was lower in the neonatal schedule following RV3-BB dose 1 (age 0–5 days) when compared with dose 1 in the infant schedule (8 weeks) (4/30 vs 17/27, difference in proportions 0.49, 95% CI 0.32–0.74, p = 0.0001). The proportion of participants who shed rotavirus following RV3-BB dose 2 in the neonatal (8 weeks) and infant (15 weeks) schedules was not significantly different (19/30 vs 13/27, difference in proportions 0.15, 95% CI −0.10 to 0.38, p = 0.292). Similarly, there was no significant difference in the proportion of participants who shed rotavirus following RV3-BB dose 3 in the neonate (15 weeks) and infant schedules (24 weeks) (16/30 vs 10/27, difference in proportions 0.16, 95% CI −0.11 to 0.41, p = 0.289) (Table 1).
Sequence analysis of VP6 RT-PCR amplicons was performed on 71 stool samples from participants who shed rotavirus at day 3–7 post IP administration. The RV3-BB vaccine VP6 sequence was detected in 69/71 episodes of rotavirus shedding tested (data not shown). Wild-type rotavirus was detected in stool following IP dose 4 in 2 participants from the infant schedule group. Sequencing was unsuccessful on the rotavirus detected following IP dose 3 in the placebo group.
Kinetics of rotavirus shedding following administration of RV3-BB
Rotavirus viral load was quantitated in stool collected on days 1–7 following vaccine administration in a subset of participants from the neonatal and infant schedules. In participants who shed RV3-BB on days 3–7, rotavirus viral load was highest (range 106.1 – 108.5 NSP3 copies/g) on day 1 following RV3-BB administration and remained positive until day 4–5 after administration (Fig. 2a, b). In contrast, participants who did not shed RV3-BB either had undetectable viral load at days 1–7 or a viral load at day 1 which became undetectable after day 2 (Fig. 2a, b). Similar trends were observed in participants from both the neonatal and infant schedules.
Figure 2.
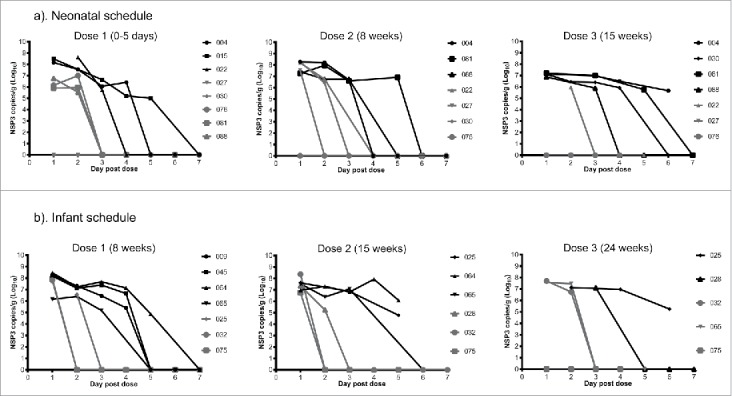
Kinetics of rotavirus shedding in stool following RV3-BB vaccination of participants from the neonatal (a) and infant (b) schedules. Participants who shed RV3-BB on day 3–7 post vaccination are shown in black. Participants who did not shed RV3-BB on day 3–7 post vaccination are shown in gray. Data points represent the mean assay duplicates. Figure legend identifies unique trial participant number.
Stool rotavirus viral load following administration of RV3-BB
We next sought to understand if vaccination schedule or dosing time was associated with differences in rotavirus viral load at days 3–7 in participants which shed rotavirus (Fig. 3a, b). The maximum viral load detected on day 3–7 identified for each participant was used in the analysis. The subset of participants who shed rotavirus (VP6 RT-PCR positive) which had a viral load undetectable in the NSP3 qRT-PCR and were assigned an arbitrary value of 10 copies/g stool. In the neonate schedule the median rotavirus viral load was similar following RV3-BB dose 1 (105.9 NSP3 copies/g), dose 2 (106.7 NSP3 copies/g) and dose 3 (106.9 NSP3 copies/g). In the infant schedule group, the median rotavirus viral load was similar following RV3-BB dose 1 (106.0 NSP3 copies/g), dose 2 (106.8NSP2 copies/g) and dose 3 (106.6 copies/g). In both the neonatal and infant schedules the maximum viral load was variable post RV3-BB administration. However, there was no significant difference in the maximum viral load post RV3-BB dose 1, 2 or 3 when given in either the neonatal or infant vaccination schedule.
Figure 3.
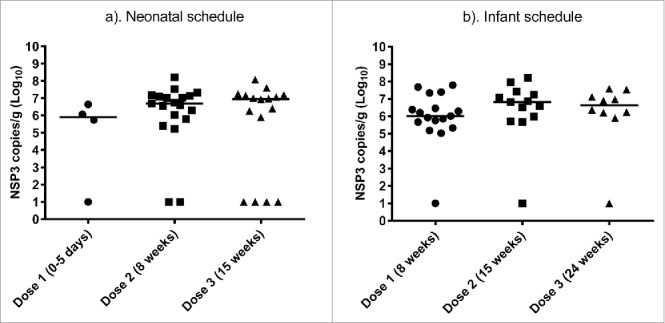
Viral load in stool of participants who shed rotavirus on day 3–7 following each dose of RV3-BB in neonatal (a) and infant (b) schedules. Data points represent the mean of assay duplicates, horizontal line represents the median value. Participants who shed rotavirus (VP6 RT-PCR positive) which had a viral load undetectable in the NSP3 qRT-PCR and were arbitrary assigned a value of 10 copies/g stool.
Relationship between stool viral load and serological response following administration of 3 doses of RV3-BB
The maximum detected rotavirus viral load on day 3–7 following any dose of RV3-BB vaccine was not associated with a serological response (Fig. 4a, b). In both the neonatal and infant vaccine schedules, there were a subset of participants who demonstrated an IgA serological response following 3 doses of RV3-BB despite not shedding detectable RV3-BB. Conversely, a subset of participants who shed RV3-BB did not demonstrate an IgA serological response following 3 doses of RV3-BB. Similar data was demonstrated in a comparison of maximum detected viral load and IgG serological response in both vaccine schedules (data not shown).
Figure 4.
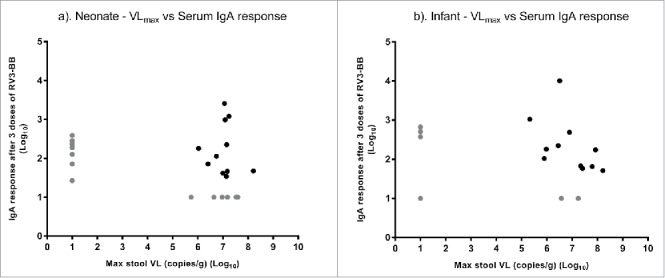
Scatter plot of maximum stool viral load on day 3–7 after any dose of RV3-BB against serum IgA response after 3 doses of RV3-BB (log scale) in neonatal (a) and infant (b) schedules. Participants with a viral load undetectable in the NSP3 qRT-PCR and were arbitrary assigned a value of 10 copies/g stool. Participants without an IgA serological response were assigned a titer of 10 U/ml. Gray circle identify participants without serological and/or stool viral load.
Discussion
Our findings show significant rotavirus shedding in both neonatal and infant schedules following vaccination with RV3-BB, with cumulative vaccine shedding detected in >70% of vaccine recipients in either schedule. In this trial, detection of RV3-BB in stool on days 3 or later after vaccine administration was considered evidence of vaccine virus replication rather than passage of the vaccine through the gut. This assumption is based on the reported stool frequency and the normal physiology of gastrointestinal transit in newborns and young infants.17-19 Using these parameters an oral dose of vaccine is likely to be passed within 24 hours. We have accounted for a greater than doubling of the mean transit time by defining replication at day 3 (> 72 hours) and/or later. The distinct pattern of prolonged detectable rotavirus stool viral load in participants who shed RV3-BB on day 3 post vaccination when compared with participants who did not shed RV3-BB supports the assumption that this reflects vaccine virus replication.
In the neonatal and infant schedules vaccine shedding was highest when a dose was given at 8 weeks of age. However, vaccine shedding was noted in a subset of neonatal schedule participants following a birth dose (IP dose 1, 0–5 days). This demonstrates that the RV3-BB vaccine strain is capable of replication in the neonatal and the infant intestine. Asymptomatic neonatal infection with the progenitor strain of the RV3-BB vaccine conferred protection against clinically severe rotavirus disease during the first 3 y of life.14 This may suggest that infection or transient passage with the RV3-BB strain in a neonate may act to prime the immune response at this early stage to protect against subsequent severe disease. Demonstration of anti-rotavirus serum IgA response in 10% participants following a single birth dose of RV3-BB supports this conclusion.13
The proportion of participants shedding the vaccine virus was higher when compared with clinical trials for Rotarix and RotaTeq where ELISA or plaque assay detection of stool shedding was used (reviewed in ref. 20). In the Rotavirus Efficacy and Safety Trial, RotaTeq was shed in the stools 12.7% of vaccine recipients tested after dose 1, 0% vaccine recipients tested after dose 2; and 0% of vaccine recipients after dose 3.21 Most pre-licensure studies of Rotarix detected shedding in approximately 50% (range 21–80%, dependent on dose and study parameters) following the first dose and approximately 15% following second dose (range 11–21%) (reviewed in20). However, our RT-PCR method is more sensitive when compared the ELISA or plaque assays. A post-licensure study comparing vaccine shedding on days 2–28 following Rotarix or RotaTeq vaccination using RT-PCR demonstrated shedding in 33/35 and 17/18 of participants following dose 1 in both groups respectively, with shedding peaking on day 4–5.22 The maximum stool viral of RV3-BB was more than 10-fold lower when compared with the studies examining the shedding of Rotarix and RotaTeq.22,23 In addition, the duration of shedding of RV3-BB was shorter than in previous studies with Rotarix and RotaTeq, with several reporting a large proportion of participants shedding vaccine strain after day 7.22-25 This suggests that a similar proportion of participants shed rotavirus following vaccination, however, the duration of shedding and maximum stool viral load of RV3-BB may be less when compared with Rotarix and RotaTeq.
Study limitations
Our study was undertaken in a Dunedin, New Zealand in neonates and infants with access to a high standard of healthcare and low infant mortality. The vaccine shedding and viral load data may not be directly applicable to the use of RV3-BB in other socioeconomic and geographic settings. As in the development of other rotavirus vaccines,25 a combination of serological (IgA, IgG or SNA) and stool shedding endpoints were used to assess RV3-BB vaccine take. However, we were unable to demonstrate a clear correlation between vaccine shedding or viral load and serological response following vaccination. A subset of both neonates and infants without detectable shedding demonstrated an IgA serological response. Similar data has also been reported in immunogenicity trials of Rotarix, with higher combined vaccine take than IgA serological response alone.25,26 The lack of understanding of serological correlates of protection and immaturity of the neonatal immune system present a challenge to the assessment of immune response after a birth dose. In addition, our study was limited by the small sample size which provided limited power to identify an association between viral load and immunological responses. Further studies, including the results of an efficacy trial of RV3-BB vaccine recently completed in Indonesia (ACTRN12612001282875), will assist in addressing these important questions.
Conclusions
Our findings show virus shedding in >70% following administration of the human neonatal rotavirus vaccine (RV3-BB) delivered in either the neonatal and infant administration schedules. The distinct pattern of RV3-BB stool viral load noted in participants who shed RV3-BB, suggests that detection of RV3-BB at day 3–7 was the result of replication rather than passage through the gastrointestinal tract. The duration of shedding and stool viral load of RV3-BB was lower when compared with previous studies using Rotarix and RotaTeq.
Materials and methods
Study design and participants
The study design and recruitment of the Phase IIa safety and immunogenicity of RV3-BB vaccine has been described previously.13 Briefly, a randomized, double-blind, 3-arm, placebo-controlled trial in 96 participants at Dunedin Hospital, New Zealand. The trial had a 2-stage recruitment process. Pregnant women were approached and preliminary written informed consent was obtained for eligibility assessment and cord blood collection. Final written informed consent was confirmed after verification of eligibility status by the site doctor after birth. Babies were eligible if they were healthy, full term (≥ 36 weeks gestation), weighed at least 2500 g, and were 0–5 d old at the time of randomization. Eligible participants were randomly assigned into one of 3 treatment groups (neonatal vaccine schedule group, infant vaccine schedule group, or placebo) in a ratio of 1:1:1 according to a concealed block randomization schedule. During the recruitment process, 231 pregnant women were approached, 187 gave preliminary consent, 100 gave final consent and screened for eligibility, 96 neonates were randomized. The final primary analysis included 30 participants in the neonatal schedule, 27 in the infant schedule and 32 in the placebo schedule.
The investigational product (IP) consisted of RV3-BB vaccine (∼8.3 × 106 FCFU/ml) or Placebo (cell culture medium, DMEM). The primary objective was to assess cumulative vaccine take after administration of 3 doses of RV3-BB vaccine. A positive vaccine take was defined as a serum immune response of anti-rotavirus IgA or serum neutralising antibodies (defined as ≥threefold increase in titer from baseline) 28 d after administration of vaccine or placebo; or detection of RV3-BB virus in stool, on days 3–7 after administration of vaccine or placebo. Participants received 4 oral doses of IP (either RV3-BB vaccine or placebo) according to the treatment group randomization schedule, with doses administered at 0–5 d (dose 1), 8 weeks (dose 2), 14 weeks (dose 3) and 22 weeks of age (dose 4). In the neonatal arm, dose 1, 2 and 3 consisted of RV3-BB vaccine while dose 4 was placebo. In the infant arm dose 1 was placebo while dose 2, 3 and 4 consisted of RV3-BB vaccine. In the placebo arm all 4 doses given were placebo (Fig. 1). Each dose of IP was given orally as a 1 ml dose. IP dose 2, 3 and 4 were administered separately from routine immunisations by at least 10 d.
Stool specimens and viral RNA extraction
Stool samples were collected routinely following each dose of IP once per day on day 1–7 (Fig. 1). Stool samples were obtained using faecal spatulas from the skin or nappy, and placed into a specimen container and frozen in a domestic freezer. Stool specimens were subsequently aliquoted, frozen at −70°C and shipped on dry ice to the enteric virus group at the Murdoch Childrens Research Institute (MCRI). Stool suspensions (20% wt/vol) were prepared in virus dilution buffer (0.01 M Tris-HCl pH7.5, 0.15 M NaCl, 0.01 M CaCl2). Viral RNA extracted using the QIAamp® Viral RNA Mini Kit (QIAGEN Cat# 52906) as according to the manufacturer's instructions. To validate the RNA extraction an internal RNA control, RNA Extraction Control 670 (Bioline Cat# BIO-38040), was added to each sample during the extraction process.
VP6 RT-PCR
Rotavirus shedding was assessed in stool samples, collected on days 3–7 following IP dose administration by a rotavirus VP6 specific reverse transcription polymerase chain reaction (RT-PCR). Briefly, 5 µl of RNA was denatured at 97°C for 4 min and quenched on ice. The denatured RNA, was added to a 20 µl PCR mixture containing 1x Reaction mix, 1 µl SuperScript II RT/Platinum Taq (Invitrogen Cat# 12574026) and 0.2 µM Rot3 and Rot5 oligonucleotide primers.27 RT-PCR conditions consisted of reverse transcription at 40°C for 30 min, denaturation at 94°C for 2 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 30 s, extension 68°C for 30 s. A final extension at 68°C for 5 min was included. PCR products were analyzed by electrophoresis in a 1.2% (wt/vol) agarose/TBE gel and Red Safe (Intron Biotechnology Cat# 21141). Rotavirus positive samples were identified with a 308 bp amplicon. A total of 1889 stool samples were collected following IP administration, 609 from neonatal, 601 from infant and 679 placebo schedule participants. VP6 RT-PCR was performed on 842 samples collected on days 3–7, 277 from neonatal, 258 from infant and 307 from placebo schedule.
Nucleotide sequencing
The presence of rotavirus in stool samples was confirmed by nucleotide sequence analysis. The VP6 RT-PCR products were purified using QIAquick gel extraction kit according to the manufacturer's protocol (QIAGEN Cat# 28706). The purified DNA was sequenced in both directions using Rot3 and Rot5 oligonucleotide primers. Purified DNA and primers were sent to the Australian Genome Research Facility, Melbourne, for sequence analysis using an Applied Biosystems 3730xl DNA Analyser (Applied Biosystems, Foster City, CA, USA). The resulting sequence was analyzed and contigs generated using the Sequencher® Software program version 4.1 (Gene Codes Corp Inc., An Arbor, MI, US) and identity determined using the BLAST server on the GenBank database at the National Centre for Biotechnology Information, USA (www.ncbi.nlm.nih.gov). RV3-BB vaccine VP6 sequences were reported with >99% nucleotide identity to RV3 VP6 (GenBank ID: FJ998275.1).
Generation of NSP3 ssRNA control
A control RV3-BB NSP3 ssRNA was generated by in vitro transcription. Initially, the NSP3 gene of RV3-BB was amplified using RT-PCR. A 25 µl PCR mixture contained 2.5 µl of denatured RV3 RNA, 1x reaction mix, 1 µl SuperScript III RT/Platinum Taq (Invitrogen Cat# 12574026) and 0.2 µM NSP3F (5′-GGCTTTTAATGCTTTTCAGTG-3′) and NSP3R (5′-ACATAACGCCCCTATAGC-3′). RT-PCR conditions consisted of reverse transcription at 45°C for 30 min, denaturation at 95°C for 15 min, followed by 34 cycles of denaturation at 95°C for 45 s, annealing at 60°C for 45 s, extension 70°C for 60 s. A final extension at 70°C for 7 min was included. PCR product was purified using QIAquick gel extraction kit and cloned into pCR2.1 (Invitrogen Cat#K2000–01) according to the manufacturer's instructions. Purified pCR2.1-NSP3 plasmid was digested with HindIII (NEB Cat# R0104S) and used as a template for in vitro transcription reaction consisting of 1µg HindIII digested pCR2.1-NSP3, 1x transcription buffer (Promega Cat# P118B), 10 U T7 RNA polymerase (Promega Cat# P207B), 20 U RNasin (Promega Cat# N2111), 2.5 mM each rNTP (Roche Cat# 11277057001) and 10 mM DTT (Promega Cat# P1171). Reaction was incubated at 37°C for 2 h and subsequently treated with 10 U recombinant DNase I (Roche). The NSP3 ssRNA was purified by Sodium acetate/ethanol precipitation and resuspended in nuclease free water. Purified NSP3 ssRNA was quantitated using 2200 TapeStation (Agilent Technologies). The calculated MW of NSP3 ssRNA transcript was 386420 g/mol. Copy number was calculated by using the formula:
NSP3 qRT-PCR
Quantitative real-time RT-PCR (qRT-PCR) was used to determine rotavirus NSP3 copy number using TaqMan Fast Virus 1-Step (Life Technologies Cat# 444432) in an Applied Biosystems 7500 HT qPCR system in 96 well format. The assay was multiplexed, with primers and probes used to amplify and detect rotavirus NSP3 as described previously,28,29 and an exogenous control RNA (Bioline Cat# BIO-38040). Extracted RNA template was denatured with NSP3 primers at 95°C for 5 min and cooled on ice. The 25 µl reaction mixture consisted of 1x TaqMan Fast buffer, 0.2 µM NSP3 primers, 0.15 µM NSP3 probe, 1x Control Mix and 5 µl template RNA. The cycling conditions consisted of reverse transcription at 48°C for 30 min, denaturation at 95°C for 20 s, and 45 amplification cycles consisting of 94°C for 15 s and 60°C for 1 min. All samples were tested in duplicate. A 10-fold dilution series (108 to 101 copies/reaction) of the ssRNA standard was prepared in nuclease free water. A standard curve was generated by plotting the log of copy number against Ct values. The average efficiency amplification was calculated as 95.2%. The lower limit of detection was determined as 102 copies/reaction. The final concentration in each sample was calculated as viral NSP3 copies per gram of stool and expressed on a Log10 scale. Samples that were VP6 RT-PCR positive with a viral load <102 copies/reaction were assigned the arbitrary value of 10 NSP3 copies per gram of stool.
Serological assays
The serological assays to assess serum immune response were performed as described previously.13 Blood was collected from the umbilical cord and from all participants 28 d after IP dose 1, 28 d after IP dose 3, and 28 d after IP dose 4 (Fig. 1). Serum samples were stored at –70°C until analyzed. Baseline was defined as cord blood for the neonatal schedule comparison and after IP dose 1 for the infant schedule comparison. Serum rotavirus IgA antibody titres were measured by ELISA, with rabbit anti-RV3 polyclonal antisera as the coating antibody and RV3-BB virus or Vero cell lysate as the capture antigen. The antigen–antibody complexes were detected with biotinylated anti-human IgA and streptavidin-horseradish peroxidase. Concentrations of rotavirus-specific IgA were measured with a standard curve generated from known positive serum samples arbitrarily assigned a titer of 250 000 units (U/mL). The lower detection limit of the assay was 20 U/mL. If rotavirus IgA was not detected in a sample, the concentration assigned corresponded to 50% of the lower limit (i.e., 10 U/mL). Serum rotavirus IgG antibody titres were measured as above except a biotinylated anti-human IgG was used.
Statistical methods
Differences in the proportion of participants shedding rotavirus by dose and vaccine schedule were assessed using Fisher's exact, with the 95% confidence interval estimated by Newcombe/Wilson method. Differences in viral load on day 3–7 post dose were compared by Kruskal-Wallis followed by Dunn's post-test. All statistical tests were performed in GraphPad Prism version 7.01.
The analysis of the relationship between maximum viral load, IgA and IgG serological response included participants who received 3 doses of RV3-BB vaccine according to the MCRI-RV3-BB-002 trial protocol, with serological data available post RV3-BB dose 3 and stool excretion following at least one dose of vaccine. A number of participants did not have a detectable serum response (10 U/ml) or viral load (10 NSP3 copies/g) and were arbitrary assigned the minimum assay value. Due to arbitrary assignment it was not appropriate to perform statistical tests on the correlation between variables and data analysis was descriptive only.
Disclosure of potential conflicts of interest
JEB is director of Australian Rotavirus Surveillance Program, which is supported by research grants from the vaccine companies CSL and GlaxoSmithKline, as well as the Commonwealth Department of Health and Aging. JEB is director of the WHO Collaborating Center for Child Health (rotavirus) and a regional reference laboratory. CDK is currently employed as a Senior Program Officer, Enteric and Diarrheal Disease, Bill and Melinda Gates Foundation. CDK and Murdoch Childrens Research Institute hold a provisional patent for the RV3-BB vaccine. All other authors declare no competing interests.
Acknowledgments
We would like to thank the participants and their families for taking part in this study. We would like to thank the study staff from both the Department of Women's and Children's Health, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand and RV3 Rotavirus Vaccine Program, Murdoch Childrens Research Institute, Victoria, Australia. Would also like to thank the midwives and antenatal clinic staff at the Dunedin Hospital for facilitating the invitation to their families to participate.
Funding
This study was supported by the Australian National Health and Medical Research Council through a project grant (ID491239) and the New Zealand Health Research Council International Investment Opportunities Fund Trans-Tasman Clinical Trials Collaborative Initiative (08_T02). The manufacture of the clinical trial lots used in this trial was part funded by PATH. This research was also supported by the Victorian Government's Operational Infrastructure Support Program.
References
- [1].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N . Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis 2016; 62 Suppl 2:S96-S105; PMID:27059362; https://doi.org/ 10.1093/cid/civ1013 [DOI] [PubMed] [Google Scholar]
- [2].Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al.. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299-305; PMID:20107215; https://doi.org/ 10.1056/NEJMoa0905211 [DOI] [PubMed] [Google Scholar]
- [3].Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J 2010; 29:489-94; PMID:20354464 [DOI] [PubMed] [Google Scholar]
- [4].Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, Nolasco JB, De Oliveira LH, Pastor D, Tate JE, et al.. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30:S6-S10; PMID:21048524; https://doi.org/ 10.1097/INF.0b013e3181fefa05 [DOI] [PubMed] [Google Scholar]
- [5].Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:S25-9; PMID:; PMID:21183837; https://doi.org/ 10.1097/INF.0b013e3181fefdee [DOI] [PubMed] [Google Scholar]
- [6].Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, Yen C, Esposito DH, Lopman BA, Patel MM, et al.. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J 2011; 30:S30-4; PMID:21183838; https://doi.org/ 10.1097/INF.0b013e3181ffe3eb [DOI] [PubMed] [Google Scholar]
- [7].Patel M, Pedreira C, De Oliveira LH, Tate J, Leshem E, Mercado J, Umaña J, Balmaceda A, Reyes M, Kerin T, et al.. Effectiveness of Pentavalent Rotavirus Vaccine Against a Diverse Range of Circulating Strains in Nicaragua. Clin Infect Dis 2016; 62 Suppl 2:S127-32; PMID:27059346; https://doi.org/ 10.1093/cid/civ1017 [DOI] [PubMed] [Google Scholar]
- [8].Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al.. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289-98; PMID:20107214; https://doi.org/ 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- [9].Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, Staat MA, Halasa NB, Weinberg GA, Szilagyi PG, Chappell J, et al.. Long-term Consistency in Rotavirus Vaccine Protection: RV5 and RV1 Vaccine Effectiveness in US Children, 2012-2013. Clin Infect Dis 2015; 61:1792-9; PMID:26449565; https://doi.org/ 10.1093/cid/civ872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, Mwansambo C, Costello A, Parashar UD, Heyderman RS, et al.. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015; 15:422-8; PMID:25638521; https://doi.org/ 10.1016/S1473-3099(14)71060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, et al.. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337-46; PMID:21793745; https://doi.org/ 10.1056/NEJMoa1006261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clark HF, Marcello AE, Lawley D, Reilly M, DiNubile MJ. Unexpectedly high burden of rotavirus gastroenteritis in very young infants. BMC Pediatrics 2010; 10:40; PMID:20540748; https://doi.org/ 10.1186/1471-2431-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen MY, Barnes G, et al.. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:1389-97; PMID:26318715; https://doi.org/ 10.1016/S1473-3099(15)00227-3 [DOI] [PubMed] [Google Scholar]
- [14].Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med 1983; 309:72-6; PMID:6304516; https://doi.org/ 10.1056/NEJM198307143090203 [DOI] [PubMed] [Google Scholar]
- [15].Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022-8; PMID:8793926; https://doi.org/ 10.1056/NEJM199610033351404 [DOI] [PubMed] [Google Scholar]
- [16].Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284-94; PMID:23596320; https://doi.org/ 10.1093/infdis/jit166 [DOI] [PubMed] [Google Scholar]
- [17].Tham EB, Nathan R, Davidson GP, Moore DJ. Bowel habits of healthy Australian children aged 0-2 years. J Paediatr Child Health 1996; 32:504-7; PMID:9007780; https://doi.org/ 10.1111/j.1440-1754.1996.tb00963.x [DOI] [PubMed] [Google Scholar]
- [18].Steer CD, Emond AM, Golding J, Sandhu B. The variation in stool patterns from 1 to 42 months: a population-based observational study. Arch Dis Childhood 2009; 94:231-3; PMID:18676434; https://doi.org/ 10.1136/adc.2007.130849 [DOI] [PubMed] [Google Scholar]
- [19].ICRP Human alimentary tract model for radiological protection. ICRP Publication 100. A report of The International Commission on Radiological Protection. Annals of the ICRP 2006; 36:25-327, iii; PMID:17188183; https://doi.org/ 10.1016/j.icrp.2006.03.004 [DOI] [PubMed] [Google Scholar]
- [20].Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 2008; 8:642-9; PMID:18922486; https://doi.org/ 10.1016/S1473-3099(08)70231-7 [DOI] [PubMed] [Google Scholar]
- [21].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23-33; PMID:16394299; https://doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- [22].Hsieh YC, Wu FT, Hsiung CA, Wu HS, Chang KY, Huang YC. Comparison of virus shedding after lived attenuated and pentavalent reassortant rotavirus vaccine. Vaccine 2014; 32:1199-204; PMID:24076325; https://doi.org/ 10.1016/j.vaccine.2013.08.041 [DOI] [PubMed] [Google Scholar]
- [23].Yen C, Jakob K, Esona MD, Peckham X, Rausch J, Hull JJ, Whittier S, Gentsch JR, LaRussa P. Detection of fecal shedding of rotavirus vaccine in infants following their first dose of pentavalent rotavirus vaccine. Vaccine 2011; 29:4151-5; PMID:21477676; https://doi.org/ 10.1016/j.vaccine.2011.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rivera L, Pena LM, Stainier I, Gillard P, Cheuvart B, Smolenov I, Ortega-Barria E, Han HH. Horizontal transmission of a human rotavirus vaccine strain–a randomized, placebo-controlled study in twins. Vaccine 2011; 29:9508-13; PMID:22008819; https://doi.org/ 10.1016/j.vaccine.2011.10.015 [DOI] [PubMed] [Google Scholar]
- [25].Vesikari T, Karvonen A, Korhonen T, Espo M, Lebacq E, Forster J, Zepp F, Delem A, De Vos B. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004; 22:2836-42; PMID:15246619; https://doi.org/ 10.1016/j.vaccine.2004.01.044 [DOI] [PubMed] [Google Scholar]
- [26].Phua KB, Quak SH, Lee BW, Emmanuel SC, Goh P, Han HH, De Vos B, Bock HL. Evaluation of RIX4414, a live, attenuated rotavirus vaccine, in a randomized, double-blind, placebo-controlled phase 2 trial involving 2464 Singaporean infants. J Infect Dis 2005; 192 Suppl 1:S6-S16; PMID:16088807; https://doi.org/ 10.1086/431511 [DOI] [PubMed] [Google Scholar]
- [27].Elschner M, Prudlo J, Hotzel H, Otto P, Sachse K. Nested reverse transcriptase-polymerase chain reaction for the detection of group A rotaviruses. J Veterinary Med B Infect Dis Veterinary Public Health 2002; 49:77-81; PMID:12002423; https://doi.org/ 10.1046/j.1439-0450.2002.00510.x [DOI] [PubMed] [Google Scholar]
- [28].Pang XL, Lee B, Boroumand N, Leblanc B, Preiksaitis JK, Yu Ip CC. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J Med Virol 2004; 72:496-501; PMID:14748075; https://doi.org/ 10.1002/jmv.20009 [DOI] [PubMed] [Google Scholar]
- [29].Pang X, Cao M, Zhang M, Lee B. Increased sensitivity for various rotavirus genotypes in stool specimens by amending three mismatched nucleotides in the forward primer of a real-time RT-PCR assay. J Virol Methods 2011; 172:85-7; PMID:21185331; https://doi.org/ 10.1016/j.jviromet.2010.12.013 [DOI] [PubMed] [Google Scholar]


