ABSTRACT
The killed bivalent (O1 and O139) whole cell oral cholera vaccine (OCV) (Shanchol™) was first licensed in India in 2009 and World Health Organization pre-qualified in 2011. We assessed the safety and immunogenicity of this OCV in the Philippines. This was a phase IV, single-arm, descriptive, open-label study. We recruited 336 participants from 2 centers: 112 participants in each age group (1–4, 5–14 and ≥ 15 years). Participants received 2 OCV doses 14 d apart. Safety was monitored throughout the trial. Blood samples were collected at baseline (pre-vaccination) and 14 d after each dose. Serum vibriocidal antibody titers to V. cholerae O1 (El Tor Inaba and El Tor Ogawa) and O139 strains were assessed, with seroconversion defined as ≥ 4-fold increase from baseline in titers. No immediate unsolicited systemic adverse events/reactions were observed. Unsolicited systemic adverse events were mostly grade 1 intensity. One serious adverse event occurred after the first dose, but was unrelated to vaccination. High seroconversion rates (range 69–92%) were achieved against the O1 serotypes with a trend toward higher rates in the 1–4 y (86–92%) and 5–14 y (86–88%) age groups than the ≥ 15 y age group (69–83%). Lower seroconversion rates were achieved against the O139 serotype (35–70%), particularly in those aged ≥ 15 y (35–42%). The 2-dose regimen of the killed bivalent whole cell OCV was well-tolerated in this study conducted in the Philippines, a cholera-endemic country. Robust immune responses were observed even after a single-dose.
KEYWORDS: cholera vaccine, immunogenicity, Shanchol, safety, The Philippines
Introduction
Cholera is endemic in over 60 countries with recent global estimates of 2.9 million cases and 95,000 associated deaths annually.1 Endemic countries are primarily located in Sub-Saharan Africa and South-East Asia, which together account for about 90% of the global burden of cholera cases. The disease is transmitted via contaminated water and food, and is often prevalent in overcrowded areas with poor sanitation. Although all age groups are affected, the disease burden may be highest in children aged under 5 y.2
In the Philippines, there were 42,071 suspected cholera cases reported from 2008 to 2013, of which 5,006 were confirmed.3 The annual incidence of suspected and confirmed cholera cases during this period was estimated at about 1 per 10,000 population. About half of the provinces and metropolitan regions reported at least one confirmed case. The overall case fatality was 0.62%, which increased to up to 2% during outbreaks, with the highest fatality rates among children aged under 5 y.
The killed bivalent (O1 and O139) whole cell oral cholera vaccine (OCV) (Shanchol™, Shantha Biotechnics Pvt LTD, Hyderabad, India) was developed and licensed in India in 2009. Licensure of this OCV was based on studies that demonstrated an acceptable safety profile, robust immune responses and efficacy up to 3 y in adults and children in India, a historically cholera-endemic area.4-7 Over the longer-term, a 2-dose regimen of OCV was shown to provide 65% protective efficacy against cholera up to 5 y.8 A study assessing the immunogenicity of OCV in Haiti, where cholera was introduced in 2010, found that the 2-dose OCV regimen was highly immunogenic.9 Although vibriocidal geometric mean titers (GMTs) after the first dose of OCV were lower in Haitian individuals than age- and blood group-matched individuals from Bangladesh, a historically cholera-endemic area, these did not differ between the 2 groups after the second OCV dose. Moreover, during a campaign that included both water and sanitation improvements and vaccination in poor Haitian urban slums in 2012,10 OCV was shown to be 97.5% effective in reducing the number of culture-confirmed cholera cases in the 37 months post-vaccination.
The Shanchol™ vaccine became WHO prequalified in 2011, becoming the second OCV to be prequalified since 2001.11 In addition to India, it is licensed in 23 other countries in Asia, Africa and Latin America. Prequalification enabled the vaccine to be procured by United Nations' agencies and included in the OCV stockpile for epidemic response against cholera globally. As part of the standard requirements for pre-qualified vaccines, the WHO requested that additional studies to assess the safety and immunogenicity of the Shanchol™ vaccine be undertaken in Asian countries other than India and Bangladesh. Here we present the results of a study undertaken to assess the safety and immunogenicity of this OCV in the Philippines.
Results
Study population
A total of 336 participants were recruited: 112 participants in each age group (1–4, 5–14, and ≥ 15 y age groups) (Fig. 1). All participants received both doses of the OCV primary vaccination series; the scheduled interval of 14 (+2 d) between vaccinations was exceeded for 5 participants (3 in the 1–4 y age group, and 1 each in the 5–14 and ≥ 15 y age groups), ranging 18 to 28 d. The participants' characteristics are summarized in Table 1. Only one participant discontinued from the study, in the ≥ 15 y age group, due to non-attendance for 2 of the scheduled study visits (visits 3 and 4).
Figure 1.
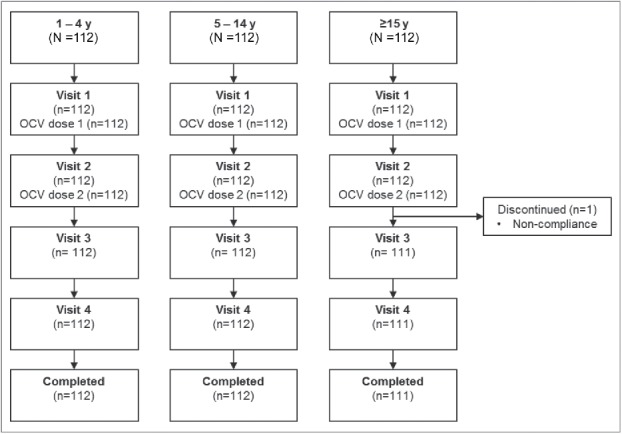
Flow of participants through the study. Visit 1 (Day 0), Visit 2 (D14), Visit 3 (D28), Visit 4 (D44) and study completion. Blood samples were drawn at Visit 1, 2 and 3 (n=112 at Visit 1, 2, and 3 for ages 1–4 and 5–14 years; n=112 at Visit 1 and 2, and n=111 at Visit 3 for ≥15 years).
Table 1.
Participants' characteristics (full analysis set).
| 1–4 y | 5–14 y | ≥ 15 y | Overall | |
|---|---|---|---|---|
| (N = 112) | (N = 112) | (N = 112) | (N = 336) | |
| Sex, n (%) | ||||
| Female | 57 (50.9) | 55 (49.1) | 80 (71.4) | 192 (57.1) |
| Age, years | ||||
| Mean (SD) | 2.83 (1.06) | 8.90 (2.85) | 37.2 (11.7) | 16.3 (16.5) |
| Racial origin, n (%) | ||||
| Asian | 112 (100) | 112 (100) | 112 (100) | 336 (100) |
Reactogenicity and safety
There were no immediate unsolicited adverse events or reactions after any dose of the vaccine in any age group. Solicited systemic reactions tended to be more frequently reported after the first OCV dose than after the second dose, and were more frequent in children aged 1–4 y than the older age groups post-dose 1 and post-dose 2. The most frequent solicited reactions were: cough in the 1–4 y age group (17.9% of children post-any dose); vomiting, abdominal pain and diarrhea in the 5–14 y age group (3.6%, 3.6% and 2.7%, respectively); and diarrhea in the ≥ 15 y age group (5.4% post-any dose) (Table 2). Most solicited systemic reactions were Grade 1 intensity. No Grade 4 solicited systemic reactions were reported during the 7 d after vaccination. There were no vaccine-related SAEs, and no withdrawals for adverse events. No death occurred during the study.
Table 2.
Summary of safety and reactogenicity. Data are presented as n (%) (Safety analysis set). For solicited systemic reactions, n represents the number of participants experiencing a reaction (note: some participants experienced the same reaction after both doses 1 and 2, hence the numbers of PD-1 and PD-2 reactions do not necessarily add up).
| 1–4 y (N = 112) | 5–14 y (N = 112) | ≥ 15 y (N = 112) | All groups (N = 336) | |
|---|---|---|---|---|
| Solicited systemic reaction (PD-1)# | 26 (23.2) | 5 (4.5) | 16 (14.3) | 47 (14.0) |
| Solicited systemic reaction (PD-2)# | 16 (14.3) | 4 (3.6) | 7 (6.3) | 27 (8.1) |
| Solicited systemic reaction (Any dose)# | 36 (32.1) | 9 (8.0) | 19 (17.0) | 64 (19.0) |
| Fever | 12 (10.7) | 1 (0.9) | 2 (1.8) | 15 (4.5) |
| Nausea | 3 (2.7) | 1 (0.9) | 2 (1.8) | 6 (1.8) |
| Vomiting | 6 (5.4) | 4 (3.6) | 0 | 10 (3.0) |
| Diarrhea | 10 (8.9) | 3 (2.7) | 6 (5.4) | 19 (5.7) |
| Abdominal pain | 10 (8.9) | 4 (3.6) | 4 (3.6) | 18 (5.4) |
| Itching | 3 (2.7) | 1 (0.9) | 3 (2.7) | 7 (2.1) |
| Rash | 5 (4.5) | 0 | 3 (2.7) | 8 (2.4) |
| Weakness | 8 (7.1) | 1 (0.9) | 1 (0.9) | 10 (3.0) |
| Cough | 20 (17.9) | 0 | 4 (3.6) | 24 (7.1) |
| Vertigo | 2 (1.8) | 1 (0.9) | 4 (3.6) | 7 (2.1) |
| Dry mouth | 3 (2.7) | 0 | 4 (3.6) | 7 (2.1) |
| Solicited Grade 3/4 systemic reaction (Any dose) #,† | 4 (3.6) | 1 (0.9) | 0 | 5 (1.5) |
| Unsolicited systemic adverse event (Any dose) | 39 (34.8) | 17 (15.2) | 10 (8.9) | 66 (19.6) |
| Serious adverse event | 1 (0.9) | 0 | 0 | 1 (0.3) |
Solicited systemic reactions within 7 d after each vaccination.
No Grade 4 solicited systemic reactions were reported
PD-1, post-dose 1; PD-2, post-dose 2.
One participant experienced a SAE (pneumonia) between 7 and 15 d after the first dose of vaccine, requiring hospitalization between d 11 and 13. A chest X-ray confirmed a diagnosis of pneumonia in this patient. The participant recovered without sequelae and completed the study.
Few unsolicited systemic adverse events were assessed as related to vaccination by study investigators: 2 (1.8%) participants in the 1 to 4 y age group (both Grade 1 gastroenteritis; one occurring within 14 days' post-dose 1 and one within 30 days' post-dose 2); and 1 (0.9%) participant in the ≥ 15 y age group (grade 1 flatulence occurring within 14 days' post-dose 1).
Immunogenicity
Immune responses to V. cholerae O1 Inaba, O1 Ogawa, and O139 after 1 and 2 doses of OCV are summarized in Table 3 and Fig. 2. Baseline GMTs increased with age against all serotypes assessed. The percentages of participants with baseline titers ≥ 80 1/dil increased with age for O1 Inaba (8.0% [1−4 y olds], 38.4% [5–14 y olds] and 50.0% [≥ 15 y olds]) and O1 Ogawa (8%, 36.6% and 58.9%, respectively). For the O139 serotype, the lowest percentage was observed for the youngest age group, with similar percentages for the 5–14 and ≥ 15 y age groups (8.9%, 18.8% and 17.0%).
Table 3.
Geometric mean titers, individual ratio titers and seroconversion rates for V. cholera serogroups, by age group (full analysis set).
| 1–4 y | 5–14 y | ≥ 15 y | |||||
|---|---|---|---|---|---|---|---|
| (N = 112) |
(N = 112) |
(N = 112) |
|||||
| M or n/M | Mean titer, ratio or % (95% CI) | M or n/M | Mean titer, ratio or % (95% CI) | M or n/M | Mean titer, ratio or % (95% CI) | ||
| O1 Inaba | |||||||
| GMT, 1/dil | |||||||
| D0 | 112 | 2.74 (2.03; 3.71) | 112 | 18.7 (11.9; 29.4) | 112 | 35.6 (23.2; 54.6) | |
| D14 | 112 | 193 (118; 316) | 112 | 1103 (805; 1513) | 112 | 894 (672; 1189) | |
| D28 | 112 | 202 (132; 310) | 112 | 922 (707; 1202) | 111 | 636 (502; 807) | |
| Individual ratio titers | |||||||
| D14/ D0 | 112 | 70.2 (45.9; 108) | 112 | 59.1 (38.6; 90.4) | 112 | 25.1 (16.6; 38.1) | |
| D28/ D0 | 112 | 73.8 (49.9; 109) | 112 | 49.4 (32.8; 74.3) | 111 | 17.4 (11.7; 25.6) | |
| Seroconversion (≥ 4-fold rise) | |||||||
| D14/D0 | 98/112 | 87.5 (79.9; 93.0) | 99/112 | 88.4 (81.0; 93.7) | 93/112 | 83.0 (74.8; 89.5) | |
| D28/D0 | 100/112 | 89.3 (82.0; 94.3) | 97/112 | 86.6 (78.9; 92.3) | 87/111 | 78.4 (69.6; 85.6) | |
| O1 Ogawa | |||||||
| GMT (1/dil) | |||||||
| D0 | 112 | 2.56 (1.90; 3.45) | 112 | 18.2 (11.6; 28.7) | 112 | 68.5 (44.3; 106) | |
| D14 | 112 | 183 (115; 292) | 112 | 856 (637;1151) | 112 | 975 (764; 1244) | |
| D28 | 112 | 247 (169; 360) | 112 | 766 (593; 989) | 111 | 748 (600; 932) | |
| Individual ratio titers | |||||||
| D14/ D0 | 112 | 71.5 (46.4; 110) | 112 | 47.0 (30.4; 72.6) | 112 | 14.2 (9.64; 21.0) | |
| D28/ D0 | 112 | 96.3 (66.3; 140) | 112 | 42.0 (27.6; 64.0) | 111 | 10.5 (7.24; 15.3) | |
| Seroconversion (≥ 4-fold rise) | |||||||
| D14/D0 | 96/112 | 85.7 (77.8; 91.6) | 96/112 | 85.7 (77.8; 91.6) | 87/112 | 77.7 (68.8; 85.0) | |
| D28/D0 | 103/112 | 92.0 (85.3; 96.3) | 99/112 | 88.4 (81.0; 93.7) | 76/111 | 68.5 (59.0; 77.0) | |
| O139 | |||||||
| GMT (1/dil) | |||||||
| D0 | 112 | 3.36 (2.42; 4.66) | 112 | 5.31 (3.64; 7.74) | 112 | 7.68 (5.19; 11.4) | |
| D14 | 112 | 64.2 (46.9; 87.9) | 112 | 68.5 (51.5; 91.0) | 112 | 39.0 (28.1; 54.2) | |
| D28 | 112 | 20.5 (14.1; 29.9) | 112 | 57.8 (44.1; 75.8) | 111 | 29.7 (20.8; 42.3) | |
| Individual ratio titers | |||||||
| D14/ D0 | 112 | 19.1 (12.7; 28.8) | 112 | 12.9 (8.74; 19.1) | 112 | 5.08 (3.54; 7.29) | |
| D28/ D0 | 112 | 6.11 (4.15; 9.01) | 112 | 10.9 (7.45; 15.9) | 111 | 3.80 (2.69; 5.38) | |
| Seroconversion (≥ 4-fold rise) | |||||||
| D14/D0 | 78/112 | 69.6 (60.2; 78.0) | 70/112 | 62.5 (52.9; 71.5) | 47/112 | 42.0 (32.7; 51.7) | |
| D28/D0 | 56/112 | 50.0 (40.4; 59.6) | 69/112 | 61.6 (51.9; 70.6) | 39/111 | 35.1 (26.3; 44.8) | |
CI: confidence interval. M: number of participants with available data. n: number of participants with ≥ 4-fold rise in titers at the specified timepoint.
Note that 112 participants had data available for assessment of the immunogenicity endpoints at each time point for 1–4 and 5–14 y age groups, and for D0 and D14 for the ≥ 15 y age group; 111 participants had available data for D28 assessments in the ≥ 15 y age group.
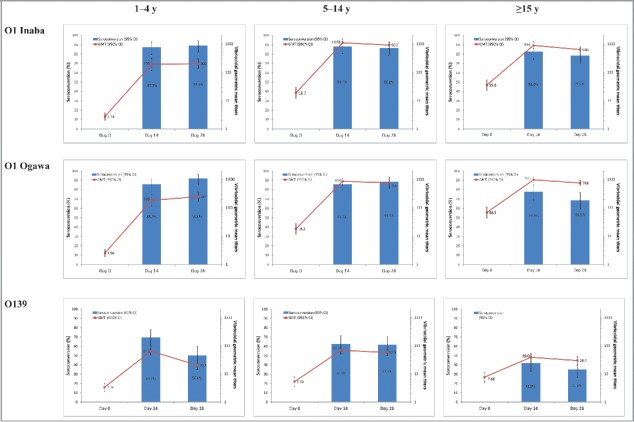
Figure 2.
Vibriocidal antibody titers and proportion of participants with a ≥ 4-fold rise from baseline (seroconversion) (full analysis set).
High seroconversion rates (range 69–92%) were achieved post-dose 1 and post-dose 2 against the O1 serotypes with a trend toward higher rates in the 2 younger age groups (1–4 years, range 86–92%; 5–14 years, range 86–88%) than the ≥ 15 y age group (range 69–83%) (Table 3; Fig. 2). Lower seroconversion rates were achieved against the O139 serotype (range 35–70%) than against the O1 serotypes, particularly in those aged ≥ 15 y (35–42%). Also of note, there was a consistent trend toward lower seroconversion rates against all serotypes post-dose 2 than post-dose 1 in the ≥ 15 years age group, but not in the other age groups.
Post-dose 1, marked increases in GMTs to the O1 Inaba and Ogawa serotypes were observed for all age groups. However, the GMTs post-dose 1 were considerably higher in the 5–14 and ≥ 15 y age groups than in the 1–4 y age group. In the older age groups, GMTs (1/dil) increased from 18–69 pre-vaccination to 856–1103 post-dose 1, whereas for 1–4-year-olds GMTs increased from 3 pre-vaccination to 183 and 193 (1/dil) (O1 Inaba and O1 Ogawa, respectively) post-dose 1. An increase in GMTs, although less pronounced, was also observed against the O139 serotype for all age groups post-dose 1 (Table 3; Fig. 2).
No marked increase in GMTs against O1 Inaba, O1 Ogawa and O139 was observed post-dose 2 relative to post-dose 1 GMTs (Fig. 2). GMTs to O1 Inaba and O1 Ogawa tended to slightly decrease from post-dose 1 to post-dose 2 in the 5–14 y and ≥ 15 y age groups. In contrast, there were slight increases in GMTs against O1 Inaba and O1 Ogawa serotypes in the 1–4 y age group. Overall, GMTs to O139 tended to decrease from post-dose 1 to post-dose 2, the decrease being more marked in the 1–4 y age group.
In complementary analyses, participants were reclassified based on age groups similar to those used in several previous OCV studies9,12: 1–4, 5–17 and ≥ 18 y. Similar trends were observed, with higher seroconversion rates in the 1–4 (range 86–92%) and the 5–17 y age groups (range 86–89%) than in the ≥ 18 y age group (range 67–83%) (Table S1). There was also a consistent trend toward a lower seroconversion rate post-dose 2 compared with post-dose 1 against the O1 serotypes in the ≥ 18 y age group. GMTs tended to be similar or slightly lower post-dose 2 compared with post-dose 1 in the 2 older age groups (5–17 and ≥ 18 years), consistent with observations for the 5–14 and ≥ 15 y age groups.
Discussion
This study confirms for the first time that the 2-dose OCV (Shanchol™, Shantha Biotechnics Pvt LTD, Hyderabad, India) vaccination regimen is well-tolerated and immunogenic in an Asian setting, outside of India or Bangladesh. The safety profile observed for OCV in this study showed that the vaccine was well-tolerated among all age groups, confirming previous observations in India,4,13 Bangladesh12 and Ethiopia.14 No safety issues were identified during the current study. The greater frequency of solicited systemic reactions after the first dose compared with the second dose of OCV is also consistent with previous studies.
Our findings showed that the vaccine appears to induce a similar immunogenicity pattern in Philippines as previously observed in India, Bangladesh and more recently in Ethiopia.4,12-14 In particular, in this and previous studies, especially those conducted in historically endemic countries like India and Bangladesh, the robust antibody responses induced following the first dose generally do not show a further increase after the second dose. A general trend toward a decrease in GMTs after the second dose compared with the first was observed in the current study for the older age groups (≥ 5 y), consistent with previous data.4,12-14 These observations have been previously suggested to result from the greater antigenic content, and thus increased immunogenicity of OCV, resulting in a stronger response following the first dose compared with the previous generation of OCV (oral whole-cell recombinant B subunit cholera vaccine15) and mitigating a further increase in response after a second dose.13,16 Indeed, the current reformulated OCV has approximately 2 times the lipopolysaccharide (LPS) content of the older version of OCV.
Unlike observations in the older age groups, GMTs against O1 serotypes for the youngest (age 1–4 y) age group tended to be higher or similar to those following the second dose. Similar findings post-dose 2 compared with post-dose 1 were also previously reported for the youngest age group in Bangladesh (1–23 month age group)12 and Ethiopian studies (1–5-year-olds).14 This difference in observations between the younger and older age groups may be due to previous exposure among older individuals in an endemic setting, leading to a booster-like effect after the first dose and high vibriocidal titers. Whereas, in very young age groups, antibody titers may remain sufficiently low to allow a further boost in titers following the second dose.4,13
Baseline titers have been shown to influence serotype-specific vibriocidal responses in both endemic settings like Bangladesh12 and outbreak prone settings like South Sudan17; for each serotype, those with high baseline titers (> 80) tended to have lower fold-increases than those with low titers (≤ 80) irrespective of age. Although this was also the case in our study (Supplementary Table S2 compared with Table 3), the difference in responses between those with high compared with lower baseline titers was less pronounced in the youngest age group compared with the other age groups across all serotypes. The greatest difference in vibriocidal responses between those with high compared with low baseline titers was observed in 5–14-year-olds. The differences in vibriocidal responses between those with high and low baseline titers by age group may be related to host physiology such as maturity of the immune system and/or evolving gut microbiota composition.
Antibody responses against O139 were much lower than those against the O1 serotypes, also consistent with previous data.4,12,14 It should be noted that baseline GMTs reported in Bangladesh12 and Ethiopia14 were much higher than those reported in the current study. This may simply reflect the higher cholera endemicity in those countries than the Philippines. However, it remains unclear whether the low GMTs observed with O139 in the current study may be due to the low immunogenicity of this antigen or the limited sensitivity of the assay used. Variation among laboratories in terms of the vibriocidal activity of sera infected with V cholerae O139 has been previously noted.18,19 A potential explanation may lie in the different complement dilutions used for the vibriocidal assay; indeed, a highly diluted complement, as used in the V. cholorae O1 assay, may not be sufficient to mediate killing of O139, which has a capsule.18 Strain-specific differences in the degree of expression of capsule may also impact the capacity of the assay strain to detect anti-O139 antibody.19 In the current study, the O139 strain used in the vibriocidal assay, CIRS 134-SR, is almost devoid of capsule and therefore, considered to have increased sensitivity in the assay compared with other previously used strains.20 Alternatively, limited circulation of O139 could be a factor leading to reduced immune response against this serotype21; indeed, to date O139 has not been isolated in the National Reference Laboratory for Enteropathogens in the Philippines. Baseline GMTs increased with age against all serotypes assessed, though only marginally so with O139. This observation was expected as cholera is endemic in the Philippines.3 Thus, the increasing GMTs with age reflect an increasing cumulative exposure to wild-type cholera over time.
We used a vibriocidal antibody assay to detect serum vibriocidal antibodies to strains of V. cholera O1 El Tor Inaba, El Tor Ogawa and V. cholerae O139. Although intestinal secretory IgA is probably the best predictor of protection, it is not a practical indicator to measure in the context of a large clinical trial.22 Currently, serum vibriocidal antibody is the most useful and most widely studied surrogate marker of intestinal immune response when the antigen is given orally. Detection of serum vibriocidal antibodies is thus considered the ‘gold standard’ in determining immune responses to V. cholerae infection.23 In a recent study, responses targeting V. cholerae LPS, including vibriocidal responses that correlate with protection against cholera, were shown to predominantly target the O-specific polysaccharide (OSP) component of the LPS.24 As the OSP defines serogroup specificity, this polysaccharide most likely contributes significantly to the observed immune responses to V. cholerae LPS. OSP ELISA may be a useful tool for examining immune responses specific to the sugar component of LPS, in the absence of contaminating V. cholerae proteins, for future immunological studies.
To date, there is no recognized vibriocidal antibody titer threshold for seroprotection. However, the 2-dose regimen of the OCV has been shown to confer 67% cumulative protective efficacy against confirmed cholera at 2 y, with only a marginal decrease in efficacy to 66% and 65% at 3 and 5 y follow up, respectively.5,6,8 Significant herd immunity conferred by the OCV to non-vaccinated individuals has also been demonstrated.25 In a recently published study, it was observed that a single-dose regimen of the OCV at 6 mo follow up was efficacious in older children (≥ 5 y of age) and in adults in a setting with a high level of cholera endemicity.26 The protective efficacy was 63%, 56%, and 16% against all cholera episodes among persons vaccinated at the age of 5–14 y, ≥ 15 y, and 1–4 y, respectively. The findings of the single-dose efficacy study correlate with the current and previous immunogenicity studies,4,12-14 which show robust immune responses after a single dose in those aged ≥ 5 y in a cholera-endemic population. However, further follow-up of the single-dose efficacy study will be required to ascertain the duration of protection and to confirm long-term efficacy of a single dose of this vaccine in older children and adults.
In conclusion, the 2-dose regimen of the killed bivalent whole cell OCV has a good safety profile and is well-tolerated across all age groups in the Philippines. The OCV elicited a robust immune response even after a single-dose, similar to that observed in previous studies undertaken in cholera-endemic populations. OCV would be a suitable additional tool to control cholera in this country.
Methods
Study design
This was a phase IV, single-arm, descriptive, open-label study undertaken at 2 centers in the Philippines from 29 Apr 2014 to 17 Jul 2014. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice. The study was approved by each study site's institutional review board. Written informed consent was obtained from all participants aged ≥ 18 y or their parents/legal representative if <18 y before study entry; participants aged ≥ 12 y also signed an assent or informed consent form depending on the study site's institutional review board regulations.
Individuals aged 1 y and older were eligible for inclusion. Toddlers aged less than 2 y had to be born at full term (≥ 37 weeks gestation) and/or with a birth weight ≥ 2.5 kg. Women of childbearing potential had to use an effective method of contraception, or be sexually abstinent for at least 4 weeks before the first vaccination until at least 4 weeks after the last vaccination.
Exclusion criteria included: pregnancy; breast feeding; previous vaccination against cholera in the preceding 5 years; history of clinically, serologically, or microbiologically confirmed cholera infection; high risk for cholera infection; antibiotics use within 1 week before the start of the study; known or suspected congenital or acquired immunodeficiency; and abdominal pain or cramps, loss of appetite, nausea, or vomiting within 24 hours before enrollment.
Participants were asked to attend 4 study visits (visit 1–4), at d 0, 14 (+ 2 d), 28 (+ 2 d) and 44 (+ 7 d). Telephone contacts were scheduled for d 8 ( ± 2 d) and 22 ( ± 2 d).
Vaccines
Participants received 2 doses of OCV 14 d apart, with a 2-day window (Day 0 and Day 14 [+2 d]). The vaccine was provided as a 1.5 mL buffered solution containing thiomersal 0.02% (w/v) and 2100 ELISA units (EU) of formalin- or heat-killed whole-cell bacterial lipopolysaccharide (LPS) from 5 strains of V. cholerae O1 and O139: 600 EU of LPS from V. cholerae O1 Inaba El Tor strain Phil 6973 formalin-killed; 300 EU of LPS from V. cholerae O1 Ogawa classical strain Cairo 50 heat-killed; 300 EU of LPS from V. cholerae O1 Ogawa classical strain Cairo 50 formalin-killed; 300 EU of LPS from V. cholerae O1 Inaba classical strain Cairo 48 heat-killed; 600 EU of LPS from V. cholerae O139 strain 4260B formalin-killed.
The vaccine was presented as a white suspension, which had to be vigorously shaken to obtain a homogeneous turbid white suspension. The vaccine suspension was poured into the recipients' mouth, followed by a drink of water if needed.
Reactogenicity and safety
Participants were kept under observation for 30 minutes after each vaccination to assess the occurrence of any immediate adverse events or reactions. Participants or their parents/legally acceptable representatives were provided with safety diary cards and digital thermometers to record for 7 d (i.e., D0 to D7) axillary temperature, intensity of solicited systemic reactions (diarrhea, fever, nausea, vomiting, abdominal pain, itching, rash, weakness, cough, vertigo, and dryness of mouth), and action undertaken to treat each event. All reactions, except for fever, were recorded on a 4-point severity scale (grade 1, 2, 3 or 4) in the diary card; grade 1 indicated minimal symptoms and grade 4, severe symptoms requiring a visit to the emergency department or hospitalization. Fever was graded on a 3-point scale during statistical analysis as follows: grade 1, for temperatures ≥ 38.0°C to ≤ 38.4°C; grade 2, ≥38.5°C to ≤ 38.9°C; and grade 3, ≥ 39.0°C.
In addition, any unsolicited non-serious adverse event was recorded during the 14 d after the first vaccination dose and during the 30 d after the second dose. These were graded on a 3-point scale as follows: grade 1, no interference with activity; grade 2, some interference with activity; and grade 3, prevents daily activity.
Serious adverse events (SAE) were collected and assessed throughout the trial, from inclusion until 30 d after the last vaccination. The study investigators assessed the causal relationship between each unsolicited adverse event or SAE and the OCV as either “not related” or “related.”
Immunogenicity assessments
Blood samples were collected before the first dose and the second dose, and 14 d after the second dose (study visits 1, 2 and 3). The blood samples were left to clot at room temperature for up to 2 hours, centrifuged to separate the serum, and the serum stored at −20°C or lower before analysis.
Assessment of serum vibriocidal antibodies to specific strains of V. cholerae O1 El Tor Inaba, El Tor Ogawa and V. cholerae O139 was undertaken using the microtiter technique at a central laboratory, the International Vaccine Institute (IVI) in Seoul, Korea.27,28 Two-fold serial dilutions of pre- and post-immunization serum samples were tested side-by-side in duplicate, with the mean of the 2 determinations taken as the final titer. Titers were adjusted in relation to a reference serum specimen included in each test to compensate for variations between analyses on different occasions. The assay was repeated if a ≥ 2-fold difference was noted between the results of the duplicate tests. Seroconversion after dose 1 or dose 2 was defined as 4-fold or greater rises in serum vibriocidal antibody titers from baseline.
Statistical analyses
No hypothesis was tested. All analyses were descriptive, and presented by age strata where possible. The number of participants enrolled in the study was determined arbitrarily.
The geometric mean titers (GMTs) and the percentages of participants who seroconverted and their 95% CIs were calculated using the normal approximation for quantitative data and the exact binomial method (Clopper-Pearson method) for proportions, respectively.29 For GMTs, it was assumed that log10 transformation of the titers followed a normal distribution. First, the mean and 95% interval were calculated on log10 (titers) using the usual calculation for normal distribution; then antilog transformations were applied to the results of the calculations.
Two main analysis sets were used: the Full Analysis Set (FAS) and the Safety Analysis Set (SAS). The FAS was defined as those participants who had received at least one dose of the vaccine. The SAS for any one dose was defined as the subset of participants who had received at least one dose; post-dose 1 and post-dose 2, the SASs were defined as those participants who received the corresponding dose, respectively.
Supplementary Material
Disclosure of potential conflicts of interest
MRZC received a research grant from Sanofi Pasteur. MLAMG received personal fees from Sanofi Pasteur during the conduct of the study, and personal fees and non-financial support from Sanofi Pasteur outside the submitted work (including honoraria for lectures, participation in speakers' bureaus and travel grants to attend medical conferences). MSD was an employee of the vaccine manufacturer (Shantha Biotechnics Private Limited) at the time of the study conduct and is currently an employee of Sanofi Pasteur. NAD'C, VJM, BNP are employees of Shantha Biotechnics Private Limited, and YT and ED employees of Sanofi Pasteur.
Acknowledgments
The authors thank all the volunteers who participated in the study. Authors also thank Anvar Rasuli, Karina Abalos, Marie-Claude Bonnet and Martin Dupuy for their valuable contribution to the study. Editorial assistance with the preparation of the manuscript was provided by Juliette Gray of inScience Communications, Springer Healthcare, funded by Sanofi Pasteur.
Funding
Funding for this study was provided by Sanofi Pasteur.
References
- [1].Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832. PMID:26043000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deen JL, von Seidlein L, Sur D, Agtini M, Lucas ME, Lopez AL, Kim DR, Ali M, Clemens JD. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl Trop Dis. 2008;2:e173. doi: 10.1371/journal.pntd.0000173. PMID:18299707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lopez AL, Macasaet LY, Ylade M, Tayag EA, Ali M. Epidemiology of cholera in the Philippines. PLoS Negl Trop Dis. 2015;9:e3440. doi: 10.1371/journal.pntd.0003440. PMID:25569505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kanungo S, Paisley A, Lopez AL, Bhattacharya M, Manna B, Kim DR, Han SH, Attridge S, Carbis R, Rao R, et al.. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine. 2009;27:6887-93. doi: 10.1016/j.vaccine.2009.09.008. PMID:19761838 [DOI] [PubMed] [Google Scholar]
- [5].Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, et al.. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. PMID:22028938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, et al.. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1694-702. doi: 10.1016/S0140-6736(09)61297-6. PMID:19819004 [DOI] [PubMed] [Google Scholar]
- [7].Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, Seidlein LV, Carbis R, Han SH, Shin SH, et al.. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One. 2008;3:e2323. doi: 10.1371/journal.pone.0002323. PMID:18523643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, et al.. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13:1050-6. doi: 10.1016/S1473-3099(13)70273-1. PMID:24140390 [DOI] [PubMed] [Google Scholar]
- [9].Charles RC, Hilaire IJ, Mayo-Smith LM, Teng JE, Jerome JG, Franke MF, Saha A, Yu Y, Kováč P, Calderwood SB, et al.. Immunogenicity of a killed bivalent (O1 and O139) whole cell oral cholera vaccine, Shanchol, in Haiti. PLoS Negl Trop Dis. 2014;8:e2828. doi: 10.1371/journal.pntd.0002828. PMID:24786645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Severe K, Rouzier V, Anglade SB, Bertil C, Joseph P, Deroncelay A, Mabou MM, Wright PF, Guillaume FD, Pape JW. Effectiveness of Oral Cholera Vaccine in Haiti: 37-Month Follow-Up. Am J Trop Med Hyg. 2016;94:1136-42. doi: 10.4269/ajtmh.15-0700. PMID:26928838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Health Organization Oral cholera vaccine stockpile for cholera emergency response. Available from: http://www.who.int/cholera/vaccines/Briefing_OCV_stockpile.pdf (Accessed 16May2015) 2013
- [12].Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, et al.. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine. 2011;29:8285-92. doi: 10.1016/j.vaccine.2011.08.108. PMID:21907255 [DOI] [PubMed] [Google Scholar]
- [13].Kanungo S, Desai SN, Nandy RK, Bhattacharya MK, Kim DR, Sinha A, Mahapatra T, Yang JS, Lopez AL, Manna B, et al.. Flexibility of oral cholera vaccine dosing-a randomized controlled trial measuring immune responses following alternative vaccination schedules in a cholera hyper-endemic zone. PLoS Negl Trop Dis. 2015;9:e0003574. doi: 10.1371/journal.pntd.0003574. PMID:25764513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Desai SN, Akalu Z, Teshome S, Teferi M, Yamuah L, Kim DR, Yang JS, Hussein J, Park JY, Jang MS, et al.. A randomized, Placebo-Controlled trial evaluating safety and immunogenicity of the killed, Bivalent, whole-cell oral cholera vaccine in Ethiopia. Am J Trop Med Hyg. 2015;93:527-33. doi: 10.4269/ajtmh.14-0683. PMID:26078323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Concha A, Giraldo A, Castaneda E, Martinez M, de la Hoz F, Rivas F, Depetris A, Svennerholm AM, Sack DA. Safety and immunogenicity of oral killed whole cell recombinant B subunit cholera vaccine in Barranquilla, Colombia. Bull Pan Am Health Organ. 1995;29:312-21. PMID:8605522 [PubMed] [Google Scholar]
- [16].Anh DD, Canh DG, Lopez AL, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH, et al.. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine. 2007;25:1149-55. doi: 10.1016/j.vaccine.2006.09.049. PMID:17055622 [DOI] [PubMed] [Google Scholar]
- [17].Iyer AS, Bouhenia M, Rumunu J, Abubakar A, Gruninger RJ, Pita J, Lino RL, Deng LL, Wamala JF, Ryan ET, et al.. Immune responses to an oral cholera vaccine in internally displaced persons in South Sudan. Sci Rep. 2016;6:35742. doi: 10.1038/srep35742. PMID:27775046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kossaczka Z, Shiloach J, Johnson V, Taylor DN, Finkelstein RA, Robbins JB, Szu SC. Vibrio cholerae O139 Conjugate Vaccines: synthesis and immunogenicity of V. cholerae O139 Capsular Polysaccharide conjugates with recombinant Diphtheria toxin mutant in mice. Infect Immunity. 2000;68:5037-43. doi: 10.1128/IAI.68.9.5037-5043.2000. PMID:10948122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Losonsky GA, Lim Y, Motamedi P, Comstock LE, Johnson JA, Morris JG, Tacket CO, Kaper JB, Levine MM. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin Diagn Lab Immunol. 1997;4:264-9. PMID:9144361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qadri F, Svennerholm AM, Shamsuzzaman S, Bhuiyan TR, Harris JB, Ghosh AN, Nair B, Weintraub A, Faruque SM, Ryan ET, et al.. Reduction in capsular content and enhanced bacterial susceptibility to serum killing of Vibrio cholerae O139 associated with the 2002 cholera epidemic in Bangladesh. Infect Immun. 2005;73:6577-83. doi: 10.1128/IAI.73.10.6577-6583.2005. PMID:16177333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].World Health Organization Cholera, 2012. Wkly Epidemiol Rec. 2013;88:321-34. PMID:23980290 [PubMed] [Google Scholar]
- [22].World Health Organization Guidelines for the production and control of inactivated Oral Cholera vaccines. WHO Technical Report Series No 924., Annex 3 World Health Organization: Geneva: 2004. www.who.int/biologicals/publications/trs/areas/vaccines/cholera/129-149.pdf. (accessed 27 March 2017) [Google Scholar]
- [23].World Health Organization The immunological basis for immunization series. Module 14: Cholera http://apps.who.int/iris/bitstream/10665/44367/1/9789241599740_eng.pdf. (accessed 27March2017) [Google Scholar]
- [24].Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, Afrin S, Akter A, Alam MM, Rahman A, et al.. Immune responses to O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 Ogawa in adult Bangladeshi recipients of an oral killed cholera vaccine and comparison to responses in patients with cholera. Am J Trop Med Hyg. 2014;90:873-81. doi: 10.4269/ajtmh.13-0498. PMID:24686738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44-9. doi: 10.1016/S0140-6736(05)66550-6. PMID:15993232 [DOI] [PubMed] [Google Scholar]
- [26].Qadri F, Wierzba TF, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Asaduzzaman M, Akter A, Khan A, et al.. Efficacy of a single-dose, inactivated oral cholera vaccine in Bangladesh. N Engl J Med. 2016;374:1723-32. doi: 10.1056/NEJMoa1510330. PMID:27144848 [DOI] [PubMed] [Google Scholar]
- [27].Attridge SR, Johansson C, Trach DD, Qadri F, Svennerholm AM. Sensitive microplate assay for detection of bactericidal antibodies to Vibrio cholerae O139. Clin Diagn Lab Immunol. 2002;9:383-7. PMID:11874883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang JS, Kim HJ, Yun C-H, Kang S-S, Im J, Kim H-S, Han SH. A semi-automated vibriocidal assay for improved measurement of cholera vaccine-induced immune responses. J Microbiol Methods. 2007;71:141-6. doi: 10.1016/j.mimet.2007.08.009. PMID:17888533 [DOI] [PubMed] [Google Scholar]
- [29].Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857-72. doi: 10.1002/(SICI)1097-0258(19980430)17:8%3c857::AID-SIM777%3e3.0.CO;2-E. PMID:9595616 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.