Abstract
Background
In many clinical trials designed to assess the efficacy of anticancer treatments, overall survival (OS) is often used as a primary endpoint despite its several points of weakness.
Methods
This study evaluated the role of progression-free survival (PFS) in the first three lines of treatment as a potential surrogate endpoint of OS in patients with metastatic colorectal cancer (MCRC). One hundred and twenty patients with MCRC were enrolled in this study. The median PFS of the first-, second-, and third-lines of treatment and the OS were evaluated. The correlation between the time to progression and the OS was analyzed. The median PFS of the three lines of treatment were 8.5, 5, and 3 months, respectively.
Results
The median OS was 32.4 months. A modest correlation was found between the PFS to the first-line treatment with Folfox–avastin and OS. Similar data were obtained with the second-line treatment. However, no correlation was found between the PFS and OS during the third-line treatment. The regression analysis revealed that PFS is predictive of OS.
Conclusion
In brief, the PFS of the first- and second-lines of treatment could be a good candidate as a surrogate endpoint of OS in patients with MCRC.
Keywords: colorectal neoplasm, overall survival, progression-free survival, surrogate endpoint liver metastates, avastin, egorafenib
Introduction
Metastatic colorectal cancer (MCRC) is characterized by a very variable natural history. At the time of diagnosis, only 20–30% of patients have the disease confined to the liver. In the last 5 years, the role of chemotherapy in the treatment of MCRC has considerably increased. Standard regimens used to treat patients with MCRC are based on chemotherapy drugs such as fluoropyrimidines, oxaliplatin, and irinotecan (which can be used in combination and sequentially) and monoclonal antibodies targeting vascular endothelial growth factor (VEGF) such as bevacizumab.1 On the other hand, in patients with RAS (both KRAS and NRAS) wild-type tumors, monoclonal antibodies targeting epidermal growth factor receptor (EGFR) such as cetuximab and panitumumab are used as single agents or in combination with chemotherapy.2 However, regimens related to the first- or second-line treatment have a paucity of effectiveness in the course of treatment. Only a minority of patients with MCRC can benefit from all agents.3 In general, the combination of chemotherapy with targeted drugs, such as anti-VEGF and anti-EGFR monoclonal antibodies, gives better results.4 In fact, VEGF is one of the most important factors that regulate tumor angiogenesis which plays an important role in tumor progression, invasion, and metastasis to distant organs.5
These therapeutic regimens showed a statistically significant impact on overall survival (OS). To assess the efficacy of this anticancer treatment in clinical trials, the often used surrogate endpoint has been OS, but studies using the OS as the surrogate endpoint need a large number of patients and an extremely long time of follow-up which can delay the evaluation of treatment efficacy. The term surrogate endpoint was defined by The Biomarker Definitions Working Group as:
a biomarker that is intended to substitute for a clinical end point, and is expected to predict clinical benefit or harm (or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence.6
In their meta-analysis, Cremolini et al7 confirmed the role of progression-free survival (PFS) as a reliable surrogate for OS, thus justifying the surrogacy of PFS as a primary endpoint in first-line studies in MCRC. Furthermore, the PFS has also been tested in second- and further-lines of treatment of several targeted agents producing significant results.
A meta-analysis by Shi et al describes an existent but reduced relationship between PFS and OS in a large set of first-line MCRC trials. This meta-analysis suggests that a significant OS benefit from a single line of treatment will be increasingly challenging for a long post-progression survival times with a greater chance for heterogeneous patient treatment. For this reason, PFS remains an appropriate endpoint for first-line trials in MCRC.8
In our retrospective study, the purpose was to evaluate if PFS of the three lines of treatment can be a primary endpoint of OS in patients with MCRC. Consequently, this surrogate endpoint will allow us to reach a shorter time with a small number of patients to make new options more rapidly available.
Materials and methods
Study design
This retrospective study analyzed 120 patients diagnosed with pathologically confirmed MCRC, between January 2013 and March 2017 at the Medical Oncology Unit of the University of Palermo.
This study was approved by the ethics committee of the Azienda Ospedaliera Universitaria Policlinico “P. Giaccone” at Palermo, and all patients provided written consent to review their medical records. Clinical data were collected from patients who received all three lines of chemotherapy treatment as follows: first-line treatment with Folfox–avastin, second-line treatment with Folfiri–avastin,9 and third-line treatment with regorafenib.10
All patients enrolled in this study had to meet the following inclusion criteria: 1) histologically or cytologically confirmed diagnosis of MCRC according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1); 2) performance status of 0–2 according to the Eastern Cooperative Oncology Group; 3) clinical or radiological evidence of metastatic disease (number of lesions ≥1) and with an adequate liver, renal, and blood functionality; and 4) patients who had RAS (both KRAS and NRAS) mutation. Patients were excluded if they were 1) hypersensitive to chemotherapeutic drugs administered in the three lines of treatment and their excipients or other formulation components, 2) diagnosed with other malignancies (with the exception of properly treated basal cell carcinoma of the skin), and 3) suffering from serious comorbidities not adequately controlled by other ongoing therapies (eg, liver disease, diabetes, infections, and heart disease).
Evaluation of the response and survival
Computerized tomography and magnetic resonance imaging were used to assess the response to treatment every 2 months. Tumor response was defined according to RECIST. PFS was defined as the period from the date of beginning treatment until the date of disease progression or death from any cause, while OS was defined as the period from the date of beginning treatment until the date of last follow-up, death, or final follow-up day of evaluation.
Statistical analysis
The median PFS of the first, second, third, and other lines of treatment were evaluated for the 120 patients included in the study. The normality of distribution was checked by using univariate indices of kurtosis and asymmetry with an acceptance threshold equal to 1. No variable violated normality indices. In order to provide a sociodemographic representation of the study group of patients and explore how to distribute the examined variables, descriptive statistical analyses were carried out. PFS and OS curves were estimated by using the Kaplan–Meier method. On the other hand, inferential statistical analyses were performed to detect any significant relationships among the considered variables. In particular, to analyze the correlation between PFS and OS, we used the linear correlation index of Bravais–Pearson (r) with a 95% confidence interval (CI). The relationship between the two variables was analyzed by linear regression (the OS represents the dependent variable Y and the PFS is the independent variable X). The values of the coefficient of determination R2 (which represents the proportion of variation between the observed values of Y explained by the linear regression of Y on X) and the slope values (increase of Y for any increase of X units) were considered. Data were represented by scatter plot and regression line. Given the sample size, parametric statistics and a threshold of P<0.05 value were used to evaluate the significance of the obtained results. Data processing was performed using the software Statistical Package for Social Science version 22.0.
Results
Treatment exposure and follow-up
In Table 1, we describe the demographic and clinical–pathological characteristics of the 120 patients with MCRC included in this study. However, it is important to note that at the time of diagnosis, the age of patients was between 35 and 80 years with a mean age of 58 years. About 87 patients had received the resection of the primary or one/two metastatic, 46 patients had mucinous histology, 26 patients had received surgery and thermoablation for liver metastases, six patients had received surgical lung metastases, and most of the patients did not have severe comorbidity; patients with severe comorbidities were excluded from the sample.
Table 1.
Baseline demographic and clinical characteristics (N=120)
| Characteristics | Patients |
|---|---|
| Age at diagnosis (range), years | 58 (35–80) |
| Sex | |
| Male | 68 |
| Female | 52 |
| Primary site | |
| Colon | 87 |
| Rectal | 33 |
| Size of metastasis | |
| 0.5–4 cm | 85 |
| 4.5–5.5 cm | 20 |
| >6 cm | 15 |
| Metastatic site | |
| Liver | 68 |
| Lung | 39 |
| Peritoneum | 16 |
Tolerability
In the group of patients treated with chemotherapeutic drugs administered in the third-line of treatment, the treatment-related toxicity was not relevant and side effects were evaluated after each course of therapy and reported in line with the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 4.0. Only 20% of patients reported these relevant adverse events such as hand–foot skin reaction, diarrhea, and fatigue.
Survival analysis
The median PFS of the first three lines of treatment were 8.5 months (range 4–23 months), 5 months (range 4–7.5 months), and 3 months (range 2–5.5 months), respectively. Among the 120 patients enrolled, the interim analysis of survival (last follow-up in March 2017) showed a median OS of 32.4 months (range 12–38.5). The Bravais–Pearson index allowed us to show a significant correlation between the PFS of the first-line treatment with Folfox–avastin and OS, with a value of r (95% CI) of 0.64 (0.25–0.81) and a P-value of 0.0021. Similar data were obtained in the second-line treatment with Folfiri–avastin, with an r value (95% CI) of 0.60 (0.20–0.80) and a P-value of 0.0054. While in the third-line with regorafenib, no correlation between the PFS and OS was observed; the r value (95% CI) was 0.33 (−0.05 to 0.68) and P=0.05 (Table 2). The regression analysis between PFS of the first-line of treatment with Folfox–avastin and OS showed an R2 value of 0.50 with a slope of 1.47 (P=0.0021) (Figure 1). The linear regression between PFS of the second-line of treatment with Folfiri–avastin and OS showed an R2 value of 0.16 with a slope of 1.98 (P=0.080) (Figure 2). The regression analysis between PFS of the third-line of treatment has not led to a statistically significant value (Figure 3). However, modest association between the PFS and OS was confirmed with regression analysis in the first two lines of treatment (Figures 1 and 2).
Table 2.
Pearson’s correlation between progression-free survival (PFS) and overall survival (OS) in the three lines of treatment (N=120)
| Treatment line | r value (95% CI) | P-value |
|---|---|---|
| PFS first-line/OS | 0.64 (0.25 to 0.81) | P=0.0021 |
| PFS second-line/OS | 0.60 (0.20 to 0.80) | P=0.0054 |
| PFS third-line/OS | 0.33 (-0.05 to 0.68) | P=0.05 |
Figure 1.
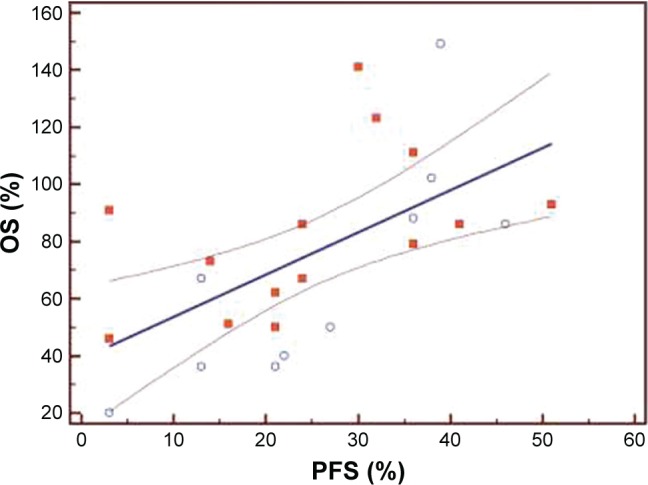
Regression analysis between progression-free survival (PFS) and overall survival (OS) of patients in the first-line of treatment (N=120).
Figure 2.
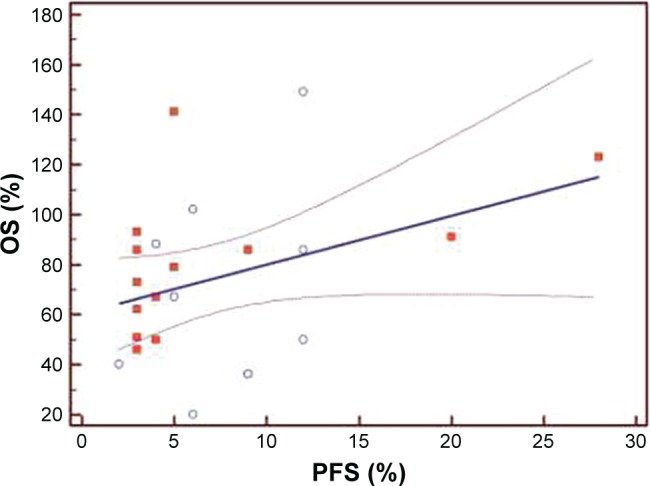
Regression analysis between progression-free survival (PFS) and overall survival (OS) of patients in the second-line of treatment (N=120).
Figure 3.
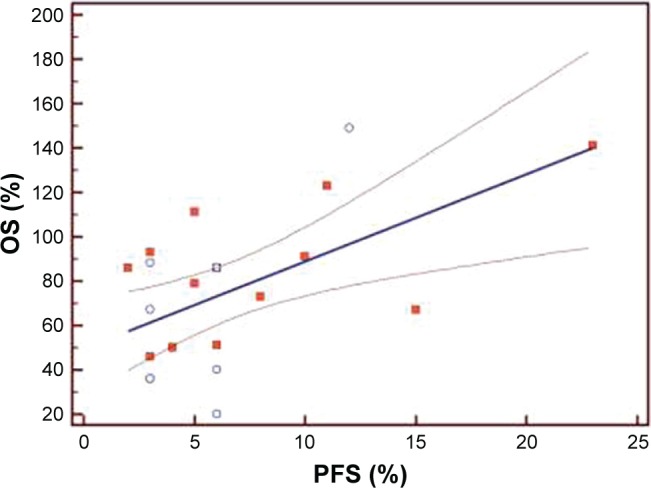
Regression analysis between progression-free survival (PFS) and overall survival (OS) of patients in the third-line of treatment (N=120).
Discussion
Despite the common practice that OS still represents the primary endpoint for the most part of Phase III randomized trials, its use has several points of weakness, including a large number of patients in the studies, an extremely long time of follow-up, and the confounding role of subsequent lines of treatment. Therefore, the oncology community is investigating the potential role of new surrogate endpoints available for clinical use in order to promote a faster evaluation of the effectiveness of new therapies,11,12 whereby very few have already shown a certain clinical validity. However, the definition of a surrogate endpoint is still a matter of debate. There is a general consensus on two points: first, a good correlation between the surrogate endpoint and the gold standard; second, the surrogate endpoint must be able to show the effect of treatment on the primary endpoint. A number of studies have evaluated the potential of new surrogate endpoints in different cancer settings13–15 and showed that PFS seems to be a good surrogate endpoint of OS in the evaluation of the treatment of MCRC.16,17 An analysis of the data collected from 11 randomized trials tested the validity of a number of potential surrogate endpoints of OS (tumor response, disease control, PFS, and time to progression) in the evaluation of the treatment of MCRC.18 Hellmann et al19 have proposed the use of the pathological response as a surrogate endpoint of survival in the evaluation of neoadjuvant treatment of non–small cell lung cancer.19 Other very significant data have emerged from studies on surrogate endpoint of survival in the treatment of glioblastoma,20 renal cell carcinoma,21 and ovarian cancer.22 Despite the small sample of patients included in our study, we have shown that the PFS of the first-line treatment with Folfox–avastin may be a good surrogate endpoint of OS in patients with MCRC. Even if a strong correlation requires R2 values to be >0.90, it is clear that the first-line of treatment affects 40% of survival, or 60% considering only the data of deceased patients. The slope values indicate that an improvement of 1 month in PFS corresponds to about 2 months of OS benefit.23–25 The PFS of the third-line treatment with regorafenib showed a rather low level of correlation with the OS, accounting for only 16% survival. However, the PFS of the second-line of treatment with Folfori–avastin has moderate levels of correlation. In brief, our study found that the PFS of the first- and second-lines of treatment could be a good candidate as a surrogate endpoint of OS in patients with MCRC. Our study allows us to conclude that PFS can be a variable surrogate endpoint in the first- and second-lines of treatment, in accordance with other clinical trials. The negative correlation obtained in the analysis of the third-line of treatment cannot be explained by a real lack of relationship between the variables examined, but rather by a shortage of studies dealing with the efficacy of PFS in targeted therapy.
In the third-line treatment, the survival time can be very short; hence, the correlation is not reliable as in the first-line treatment. Since, in metastatic colorectal disease, today the survival exceeds 36 months from diagnosis, the use of surrogate endpoints can be useful, even if the standard still remains for the OS. For this reason, in the near future, a multicenter clinical trial including a larger pool of patients is needed to support the use of PFS to third-line treatment with target agents as a surrogate endpoint of OS.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yang Q, Yin C, Liao F, et al. Bevacizumab plus chemotherapy as third- or later-line therapy in patients with heavily treated metastatic colorectal cancer. Onco Targets Ther. 2015;8:2407–2413. doi: 10.2147/OTT.S88679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 3.Costa T, Nuñez J, Felismino T, Boente L, Mello C. REOX: evaluation of the efficacy of retreatment with an oxaliplatin-containing regimen in metastatic colorectal cancer: a retrospective single-center study. Clin Colorectal Cancer. 2017;16(4):316–323. doi: 10.1016/j.clcc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Abrams TA, Meyer G, Schrag D, Meyerhardt JA, Moloney J, Fuchs CS. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106(2):djt371. doi: 10.1093/jnci/djt371. [DOI] [PubMed] [Google Scholar]
- 5.Saravanan S, Pari L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem Biol Interact. 2016;245:1–11. doi: 10.1016/j.cbi.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 7.Cremolini C, Antoniotti C, Pietrantonio F, et al. Surrogate endpoints in second-line trials of targeted agents in metastatic colorectal cancer: a literature-based systematic review and meta-analysis. Cancer Res Treat. 2016;49(3):834–845. doi: 10.4143/crt.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, de Gramont A, Grothey A, Zalcberg J, Chibaudel B, Schmoll HJ. Individual patient data analysis of progression-free survival versus overall survival as a first-line end point for metastatic colorectal cancer in modern randomized trials: findings from the analysis and research in cancers of the digestive system database. J Clin Oncol. 2015;33(1):22–28. doi: 10.1200/JCO.2014.56.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartwright TH, Yim YM, Yu E, Chung H, Halm M, Forsyth M. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. 2012;11(4):238–246. doi: 10.1016/j.clcc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Ohhara Y, Fukuda N, Takeuchi S, et al. Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol. 2016;15(9):642–655. doi: 10.4251/wjgo.v8.i9.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26(12):1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 12.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21(1):7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 13.Bikov KA, Mullins CD, Hung A, Seal B, Onukwugha E, Hanna N. Patterns of biologics use across treatment lines in elderly (age >65) Medicare patients with metastatic colon cancer. Oncologist. 2016;21(6):676–683. doi: 10.1634/theoncologist.2015-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 15.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–1398. doi: 10.1001/jamainternmed.2015.2829. [DOI] [PubMed] [Google Scholar]
- 16.Ciani O, Davis S, Tappenden P, et al. Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care. 2014;30(3):312–324. doi: 10.1017/S0266462314000300. [DOI] [PubMed] [Google Scholar]
- 17.Michiels S, Saad ED, Buyse M. Progression-free survival as a surrogate for overall survival in clinical trials of targeted therapy in advanced solid tumors. Drug. 2017;77(7):713–719. doi: 10.1007/s40265-017-0728-y. [DOI] [PubMed] [Google Scholar]
- 18.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2017;26(12):1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 19.Hellmann MD, Chaft JE, William WN, Jr, et al. University of Texas MD Anderson Lung Cancer Collaborative Group Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):42–50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han K, Ren M, Wick W, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5):696–706. doi: 10.1093/neuonc/not236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halabi S, Rini B, Escudier B, Stadler WM, Small EJ. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic renal cell carcinoma. Cancer. 2014;120(1):52–60. doi: 10.1002/cncr.28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose PG, Tian C, Bookman MA. Assessment of tumor response as a surrogate endpoint of survival in recurrent/platinum-resistant ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;117(2):324–329. doi: 10.1016/j.ygyno.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25(33):5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 24.Giessen C, Laubender RP, Ankerst DP, et al. Surrogate endpoints in second-line treatment for mCRC: a systematic literature-based analysis from 23 randomised trials. Acta Oncol. 2015;54(2):187–193. doi: 10.3109/0284186X.2014.938830. [DOI] [PubMed] [Google Scholar]
- 25.Petrelli F, Pietrantonio F, Cremolini C, et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer. 2015;51(7):800–807. doi: 10.1016/j.ejca.2015.02.011. [DOI] [PubMed] [Google Scholar]


