Abstract
Objectives
African American and Hispanic elderly are at elevated risk of both depression and cardiovascular disease, relative to non-Hispanic whites. Effective interventions are therefore needed to address depressive symptoms and to reduce these disparities. BRIGHTEN Heart was a behavioral randomized controlled trial to test the efficacy of a virtual team intervention in reducing depressive symptoms in minority elderly as measured by the 9-item Patient Health Questionnaire (PHQ9).
Study design
250 African American and Hispanic adults, age ≥60 years, with comorbid depression and overweight/obesity were randomized. Participants randomized to the Intervention condition received a social work evaluation, team-based electronic consultation, case management, and psychotherapy over a 12 month period. Control participants were enrolled in a membership program that provided health classes and other services to support chronic disease self-management. Blinded research assistants completed assessments at baseline, and 6 and 12 months postrandomization.
Results
The study population was characterized by low socioeconomic status, with 81.4% having a household income of less than $20,000. Although median depression scores were in the mild range, 25% of participants had scores showing moderate to severe depression at baseline. 75% of participants had four or more chronic conditions. Significant demographic and clinical differences were observed between the African American and Hispanic populations.
Conclusions
BRIGHTEN Heart was designed to rigorously test the efficacy of a multi-level intervention to reduce comorbid depressive symptoms and cardiovascular risk in minority elderly. Investigators successfully recruited a cohort well suited to testing the study hypothesis.
Keywords: Primary care, Older adults, Depression, Cardiometabolic syndrome, Multidisciplinary team intervention
1. Introduction
Multiple meta-analyses have indicated a strong bi-directional link between depression and cardiac disease [1–5,87]. Depression has been identified as one of four primary predictive pathways to ischemic heart disease among urban minorities, along with physical inactivity, increased waist circumference, and smoking [6]. Depression is one of the strongest predictors of non-adherence with medication regimens [7] and thus reduces the effectiveness of medications to control blood pressure, glucose, and lipids. Self-neglect is associated with depression [8], leading to decreased levels of physical activity [9], poor dietary habits [10], and increases in visceral fat [11].
Low socioeconomic status (SES) places minority older adults at significantly greater risk of depression and anxiety [12,13]. Chronic financial burden, crime and violence exposure, and housing instability further increase the risk of depression risk for this group [14–17]. Lack of access to appropriate mental health care exacerbates this situation. Older African Americans get treatment less frequently than non-Hispanic whites [13,18,19]. Spanish-speaking older adults have even less access to mental health care in the United States, particularly psychotherapy [20]. Thus, these lower socioeconomic ethnic minority groups experience more depression, anxiety, and stress and have limited opportunity for adequate treatment.
African Americans and Hispanics also have higher rates of most major cardiovascular disease risk factors compared to non-Hispanic whites [21–23]. While improvements in care over the past 20 years have led many populations to achieve improvements in cardiovascular mortality rates and risk factors (other than obesity and diabetes), the relative gap between whites and minority groups has remained constant or in some cases widened [24–30]. Upstream social factors, traumatic life events, and comorbid depression may explain why these improvements have not led to improved outcomes in African Americans and Hispanics. Therefore, interventions must target both depression and cardiovascular risk in order to be effective [31,32]. Programs that afford multi-level treatment of mental and physical health needs, with appreciation of cultural and environmental obstacles to care are critical.
The body of empirical evidence supporting the efficacy of interdisciplinary teams in treating older adults with multiple chronic conditions has been growing substantially in recent years [33–36]. Integrated care has been linked with increased access to mental health care [37–39], decreased length of stay in acute hospitals [40], decreased pain [41], improved physical functioning following stroke [42], improved treatment adherence [43,44], increased patient satisfaction [40,41], decreased mortality [45], and decreased health care expenditures [39,46].
The current paper describes the design of the BRIGHTEN Heart randomized control trial (RCT) and summarizes baseline characteristics of its African American and Hispanic participants. The primary aim of the BRIGHTEN Heart RCT was to test the efficacy of a multidisciplinary virtual team intervention based in urban safety-net primary care clinics in reducing depression symptoms in low-income older African Americans and Hispanics relative to a control group. Reduction in prevalence of cardiometabolic syndrome as compared to a control population was a secondary outcome. Medication adherence will be used as a mediator of clinical improvement. Pre-specified covariates that might moderate the impact of the intervention include race/ethnicity, gender, and age. In addition, because of the high prevalence of traumatic stress in the urban population, analyses will test whether such stressors moderated the results of the intervention.
2. Research design and methods
2.1. Study design
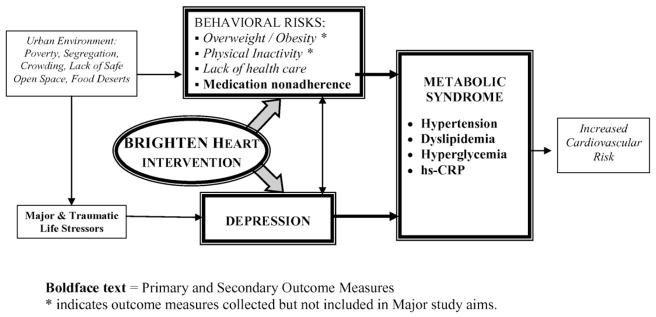
BRIGHTEN Heart was a randomized controlled trial designed to test the effects of a 12-month multidisciplinary team intervention delivered in primary care settings on decreasing depression symptoms and cardiometabolic risk factors in minority older adults relative to a control group. Participants were randomized to either the BRIGHTEN Heart intervention arm plus membership in the Rush Generations program, or Rush Generations program membership with usual clinic care. An overview of the study model is presented in Fig. 1. Institutional Review Board approval was obtained from all study sites. The study was monitored by the Data and Safety Monitoring Board of the Rush Center for Urban Health Equity.
Fig. 1.
Model of BRIGHTEN Heart, a multi-level intervention that targets both depression and health behaviors in older adults with comorbid depression and metabolic syndrome. Boldface text = Primary and Secondary Outcome Measures. * indicates outcome measures collected but not included in Major study aims.
2.2. Recruitment
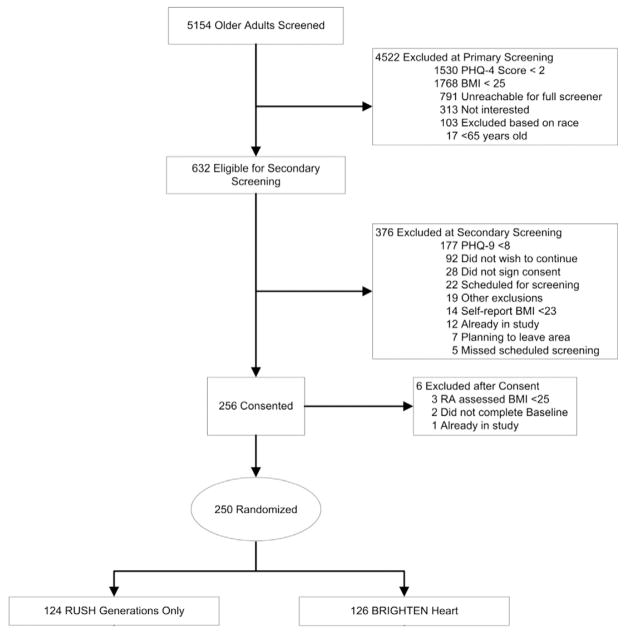
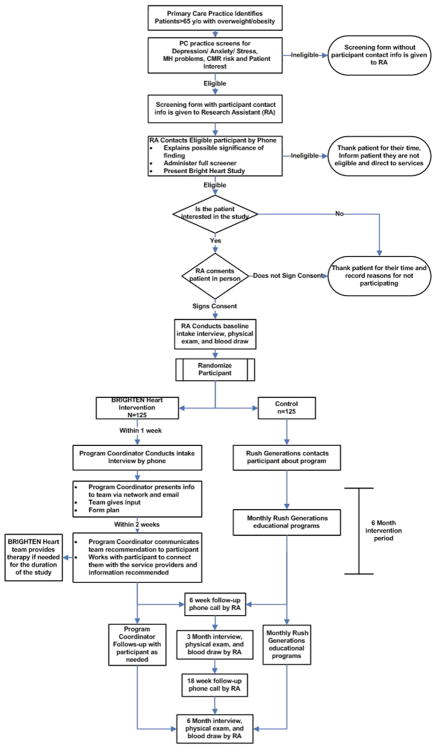
The CONSORT Diagram, showing study recruitment, is in Fig. 2. Participants were recruited from six clinics and Federally Qualified Health Centers in Chicago. Initially, those over the age of 65 were recruited. However, due to slow enrollment and a large sample of those aged 60–65 in our prior pilot, we decreased the age criteria to age 60 after the first 30 participants had been enrolled. Clinic nurses or medical assistants were asked to screen all patients age 60 or over using the Patient Health Questionnaire-4 [47] (PHQ-4) at the time they presented for a clinic visit (Fig. 3). Nurses then asked patients who had scores ≥2 on the PHQ2 if they wished to be contacted by the study. If interested, the patient provided their contact information and self-identified race or ethnicity on the screening form and the nurses indicated if the patient had a BMI >25. Research Assistants (RAs) then called those patients who expressed interest and had passed the initial screening to verify eligibility criteria and schedule in-person assessments. Participants were randomized by primary care clinic, ensuring balanced participation within each clinic. Participating clinics were chosen in part to achieve relatively equal numbers of African Americans and Hispanics in the study population.
Fig. 2.
CONSORT diagram.
Fig. 3.
Study protocol and workflow.
2.3. Eligibility criteria
Individuals were eligible to participate in the study if they met the following inclusion criteria: age 60 or older, overweight or obese as documented by BMI >25.0 (initial screen for cardiometabolic syndrome), self-identified as either Hispanic or African American, and had symptoms of depression as documented by a PHQ-9 [48,50, 51] (score of ≥8).
The 9-item PHQ-9 is the most widely used screening measure of depression that can also be used to estimate probable depression diagnosis [51]. The PHQ-9 has also been found to be valid for older adults [52], with an ethnically diverse population [53], and has a validated Spanish-language version [54,55]. Many studies use a minimum inclusion PHQ-9 score of 10 out of 27 total points. However, because older adults experience functional deficits, increased use of medical utilization, and higher mortality with subsyndromal levels of depression [56,57], investigators chose a lower score of 8 for inclusion in this study.
Participants were excluded if they had plans to leave the area during the next six months. RAs assessed potential participants’ decisional capacity by having them repeat back a description of the study and the key elements of the consent in their own terms [49]. Individuals unable to do so were excluded from randomization, as were persons with evidence of delusions or active psychosis. Individuals who voiced active suicidal ideation at the time of a consent visit were immediately put in contact with the study PI, a clinical psychologist, per safety protocol, and were not randomized. Other exclusion criteria included being currently in active psychotherapy, not having regular access to a telephone in their home, or current enrollment in another intervention trial.
2.4. Outcome assessment
All study outcome measures were administered by blinded RAs at baseline, six months, and 12-month follow-up. Additionally, the PHQ-9 was administered every 6 weeks by RAs via phone. Because assessment includes suicide risk, a safety protocol for all study staff was established that includes immediate contact of co-primary investigator (EE-T) or her licensed mental health provider designate while the participant is still on the phone or in the room for risk assessment. The licensed clinician assesses risk and directs the research assistant to call 911, enlist family or clinic support to obtain the appropriate level of service, and/or provide suicide hotline, as appropriate.
Secondary measures in this trial included cardiometabolic risk factors, stress symptoms, and medication adherence as shown in Table 1. RAs measured participant height, weight, waist circumference, and blood pressure. Dried Blood spots (DBS) were obtained via fingerstick, using the DBS kit supplied by the Rush University Proteomics Core laboratory. The collection kit contained a DBS collection filter paper (Whatman 903TM Protein Saver Card) for five spots of 50–80 μL blood samples. Blood was collected onto all 5 spots, allowed to dry at room temperature for 2 h and then stored at −20 °C. Assays were performed by a standard procedure used by ZRT laboratory, a CLIA certified diagnostic laboratory at Beaverton, Oregon, USA.
Table 1.
Variables measured at baseline, 6-month and 12-month follow-up.
| Primary outcome measures | Details |
|---|---|
| Depression symptoms: Patient Health Questionnaire (PHQ-9) | 10-item self-administered depression screening questionnaire that assesses the presence and severity of depressive symptoms over two-week period [48] |
| Secondary outcomes measures | |
| Hemoglobin A1c | Hemoglobin A1C levels reflect level of glycemic control in diabetes patients. Samples collected via finger stick and processed through Rush core laboratory |
| Blood pressure | After sitting for 10 min, participants’ blood pressure was taken three times at two-minute intervals using the Omron HEM-907XL automated sphygmomanometer (Omron Healthcare, Kyoto Japan). Second and third readings were averaged to obtain final score. Mean Arterial Pressure was calculated using the standard formula: DBP + 1/3 (SBP – DBP). |
| Lipids | Total Cholesterol, LDL-C, HDL-C, and triglycerides were measured after an 8 h fast. Samples were collected via finger stick and processed through RUSH core laboratory |
| hsCRP (C-reactive protein) | Elevations in C-reactive protein is one of the diagnostic criteria used for the Metabolic Syndrome. Samples were processed through the through RUSH core laboratory. |
| Body Mass Index | Weight measured using a balance beam digital scale (Health O Meter), reported to 1/10 kg (5 min). Height measured using wall mounted stadiometer. BMI calculated by dividing weight (kilograms) by height (meters squared). |
| Medications | Patients brought medication list to assessment visits and regimen was recorded by study RAs. Medications taken for depression, diabetes, hypertension, and lipid control are categorized. |
| Waist circumference | Nearest cm at narrowest part of torso, above iliac crest, below rib cage (4 min). |
| Mediating factors | |
| Stress: Past Stressor Events Form (PSE) | 10-item questionnaire measuring stressful events occurring within the past year [78,79] |
| Traumatic Stress Checklist Form | 11-item interviewer-administered questionnaire evaluating exposure to traumatic stress events during the course of one’s lifetime. The Lifetime Trauma Exposure from the Composite International Diagnostic Interview PTSD Module [79] |
| Posttraumatic stress disorder: PTSD Checklist-Civilian Short Form | 6-item questionnaire used to screen for symptoms of posttraumatic stress disorder [72] |
| Medication adherence: Morisky Medical Adherence Scale (MMAS) | 4-item questionnaire that assesses subjective impression of medication adherence behaviors [80] |
| Participant variables | |
| Participant demographic information | Self-reported demographic questionnaire which included questions about income, education, employment and insurance |
| Anxiety: Beck Anxiety Inventory (BAI) | 21-item questionnaire designed to screen for symptoms of anxiety as distinguished from symptoms of depression [81,82] |
|
Quality of Life: EQ-5D |
18-item questionnaire assessing health status based on five dimensions including: mobility, self-care, usual activities, pain/discomfort and anxiety/depression [83] |
| Short Form Health Survey (SF-12) | 12-item questionnaire used to assess functional health and well-being which includes physical and mental component summary scales [84,85] |
| Acculturation: Marin-Marin Acculturation Scale (Spanish speakers only) | 12-item questionnaire measuring acculturation with subscales for language use, media use, and preference for social relations [86] |
The Morisky Medication Adherence Scale was chosen as a general measure of medication adherence because (a) participants took a wide range of medications that would be untenable to measure; and (b) utilizing pill cap or other devices may negatively impact adherence by changing participants’ routines. Stress symptoms were measured with the Past Stressor Events Form (PSE), a validated 10-item questionnaire measuring stressful events occurring within the past year [78]. Prior history of major and traumatic life stress, which has been proposed as a possible moderator of treatment effect, was assessed using the Traumatic Stress Checklist Form [79], an 11-item interviewer-administered questionnaire adapted from the Composite International Diagnostic Interview PTSD module, and the PTSD Checklist-Civilian Short Form, a 6-item questionnaire used to screen for symptoms of posttraumatic stress disorder [72].
Adverse events tracked include all-cause (including mental health) hospitalizations, emergency department visits, and death. Medical adverse events are adjudicated by a cardiologist and psychiatric events by a psychiatrist, both within the academic medical center but not directly affiliated with the study.
2.5. Retention plan
Several techniques were implemented to retain participants once randomized to study conditions. All study participants were asked for at least two alternate contacts with telephone numbers at enrollment. In addition, a blinded RA called participants every 6 weeks to administer the PHQ-9 to maintain contact. Finally, the study protocol included a series of stepped incentives to encourage not only enrollment but full participation for the 12 months of study follow-up. Randomized participants received $20 for completing the baseline measures, $30 for follow-up at 6 months, and $50 for completion of 12-month follow-up.
2.6. The BRIGHTEN Heart intervention
The development of the BRIGHTEN intervention model is described elsewhere [58]; a brief description is provided below. The expansion of the BRIGHTEN model, originally developed to treat depression in older adults, to address cardiometabolic syndrome is based on epidemiologic studies linking depression, physical activity, increased waist circumference, and smoking to increased cardiac risk among urban minorities. As shown in Fig. 1, depression is at the center of these risk factors.
For many older adults and minority populations the diagnosis and treatment of depression are stigmatized, and may be rejected [59,60]. In addition, the failure of trials such as ENRICHD has demonstrated that depression treatment alone is not successful in reducing myocardial infarction rates among persons with known coronary artery disease [61]. BRIGHTEN Heart is designed to reduce the risk of heart disease for at risk older African Americans and Hispanics using a multilevel intervention consisting of (1) a team-based electronic consultation, (2) case management, and (3) evidence-based psychotherapy. The following paragraphs describe each element of the intervention.
Assessment and electronic team consultation. Once randomized to the BRIGHTEN Heart intervention, the bilingual Program Coordinator (geriatric social worker) conducted a semi-structured assessment of mental health and cardiovascular risk. Results of that assessment were shared with the interdisciplinary “virtual” team when the Program Coordinator sent an email notifying members to log on to the secure website to read the evaluation. This secure website was a new development for this project. In the original BRIGHTEN process [58], team members communicated via encrypted email within the hospital fire wall. Since teams now included primary care physicians outside of the hospital fire wall, additional security was required to maintain confidentiality of participant information. Team members included: the participant’s primary care provider (PCP); a geropsychologist; a psychiatrist; a social worker; a chaplain; a dietitian; a pharmacist; and an occupational therapist. Each team member logged on to the secure website to provide recommendations for each participant’s overall health, and specifically regarding depression and cardio-metabolic syndrome. Recommendations were shared with the participant by the Program Coordinator, and together they develop a patient-centered Action Plan based on participant priorities. Adherence to treatment is measured by participant completion of the Action Plan.
Care management. The Program Coordinator connected participants to all agreed upon services, and provided ongoing care management as needed, including monthly phone follow-up calls. Care management included facilitating referrals to health care providers accepting the participant’s insurance, or lack thereof, and coordination of these services with the participant’s PCP; transportation services; SHIP (Senior Health Insurance program) counseling; organizations providing free or low-cost eye glasses, hearing aids, and dentures; support groups; legal services; and home health assistance.
Psychotherapy. Psychotherapy was recommended for all intervention participants to manage depression and/or health behavior; those who accepted this recommendation participated in either cognitive behavioral (CBT) [62,63] or interpersonal psychotherapy (IPT) [64,65] by supervised BRIGHTEN Heart psychology and social work fellows. Selection of CBT or IPT was made based on participant primary concerns about individual coping or relationship issues, and number of sessions is determined by clinical judgment of patient need relative to symptom alleviation and relapse prevention. Services were provided in the participant’s primary care clinic, and in their preferred language (English or Spanish). Fellows and Program Coordinator received one hour of individual and one hour of group supervision weekly by the unblinded co-Principal Investigator (EE-T) and a co-Investigator (RG). Supervisors listened to a random sample of session recordings to check for fidelity to theoretical base. Supervision sessions also addressed participants’ individual and cultural tailoring needs.
2.6.1. Additional services
The patient-centered model of BRIGHTEN Heart facilitates referrals to providers and services beyond BRIGHTEN Heart core staff (Social work and Psychology fellows) based on virtual team recommendations [58]. Participation in these services is measured by participant self-report, as tracking providers city-wide was not tenable, nor sustainable in a treatment model. Examples of additional services included, some patients received antidepressant medications from their primary care provider or psychiatrist. While study staff communicated with these providers about participant response to these medications, the project protocol did not dictate medication changes. Use of antidepressant medication will be analyzed as a potential mediator. BRIGHTEN Heart services were designed to complement existing clinic and community services, not control or replace those services.
2.6.1.1. Intervention fidelity
In addition to direct weekly supervision of study interventionists, as described above, each step of the BRIGHTEN process (team consultation, care management, psychotherapy, and referrals for services) was documented in the intervention database and monitored by the Rush Data Management Center. Monthly control reports ensured that the intervention was delivered as intended.
2.7. Control group
A suitable control condition for such a trial provides access to health education, social support, and supportive services, but without the directed team intervention addressing depressive symptoms and cardiovascular disease prevention. To achieve this end, individuals randomized to the control condition were given membership in the Rush Generations program. Rush Generations is a free membership program organized by the academic health center for older adults, people who care for older adults, and anyone with an interest in healthy aging. The focus of the program is to provide chronic disease prevention and management through a wide range of health and aging-related programs and services, including a twice yearly health fair, hospital-based and community-based programming, civic engagement opportunities, and individual and family consultations with social work staff. A lecture series offered twice monthly by experts in the fields of health and aging provides practical information and resources on topics such as cognitive health, nutrition & exercise, diabetes, and Medicare & prescription drugs that participants can integrate into their daily lives for improved health and well-being. Participants randomized to this condition were encouraged, but not required, to attend programming. Rush Generations staff were asked to record BRIGHTEN Heart participants’ program attendance and provide attendance data to BRIGHTEN Heart staff.
2.8. Data management and analysis plan
Power calculations were based on published data from the IMPACT intervention trial [66], in which a reduction of 30% (4.1 points) in PHQ-9 depression score was defined as clinically meaningful [67]. We allowed for the possibility that the control group may improve by up to 10% (1.4 points) by virtue of being in the study. If the control group improved by 10% and the intervention group improved by 30%, the difference between the two treatment arms would be 2.7 points on the PHQ-9, corresponding to an effect size of 0.44. With 125 participants per group and an attrition rate of 20%, we will have >85% power to detect a difference of 2.7 points between the two treatment arms. We will be able to detect effect sizes for secondary outcomes ranging from 0.40 to 0.46 with at least 80% power at alpha = 0.05.
To calculate each participant’s change score, we will subtract the baseline PHQ-9 score from the 6-month score. To assess whether depressive symptoms change in the intervention group, we will use a one sample t-test or a Wilcoxon Signed Rank test, if the distribution of the change in PHQ-9 is not well approximated by a normal distribution. To assess whether depressive symptoms change more in the intervention than in the control group, we will use a two-sample t-test or the Mann–Whitney test, should a non-parametric test be warranted. Data from all participants enrolled in the study will be used. If the intervention group change from baseline is significantly larger than zero, we will conclude that the intervention is effective. If the intervention group change from baseline is significantly larger than the control group change, we will conclude that the intervention is more effective than the control treatment. Linear models will be used to test whether the treatment effect on the change in depressive symptom from baseline to 6 months is independent of pre-specified covariates of age, gender, and ethnicity.
To investigate the effect of treatment on depressive symptoms over time (baseline, 6 months, 12 months), we will fit a linear mixed-effects model, regressing PHQ-9 at each actual time point on time since baseline. Although all participants are scheduled to be measured at three exact time points, in practice, there is fluctuation. Using a mixed-effects model allows us to incorporate the actual time on study. We will include random effects for starting level and rate of change. Such partitioning of the variability in the data allows for more precise estimates of random variation and more precise tests of the hypothesis. The basic model will be repeated, adjusting for age at baseline, gender, and ethnicity.
We will repeat analyses described above for other measures, including stress and cardiometabolic risk factors. To test for a difference in medication adherence, as measured by the Morisky scale, between baseline and 6 months in the intervention group a chi-square test or equivalent logistic regression will be used. To test for a difference in the change in medication adherence from baseline to 6 months between the treatment and control group, we will use logistic regression. The outcome variable will be medication adherence at 6 months, and the predictors will be baseline adherence and treatment group. If the treatment parameter is significant then we will conclude that the intervention arm significantly changed medication adherence.
3. Findings/baseline characteristics
3.1. Recruitment
Study recruitment started in January 2011 and was completed by December 2014. A CONSORT diagram of recruitment is presented in Fig. 2. Nursing staff at participating primary care clinics screened 5154 patients and 632 met initial eligibility criteria. RAs contacted eligible patients to administer final study criteria. 256 consented to the study and 250 were randomized, giving an eligible-to-enrolled ratio of 2.47. Participants were randomized to either the BRIGHTEN Heart program (n = 126) or the control group (n = 124).
3.2. Baseline characteristics
Demographic characteristics of the total sample are displayed in Table 2. At baseline, the mean age for participants was 67 years. The majority of the participants were female (80.4%) and 30.8% of the study participants reported that they were living with a spouse or life partner. The population was of lower socioeconomic and educational status, with 78.8% of the study sample having an average annual income of less than $20,000, 56.0% with less than a high school degree, and 25% were uninsured. The Hispanic sample had a low level of acculturation, with 90% reporting Spanish as their preferred language. There were significant differences in demographic, physical, and psychosocial characteristics between the African American and Hispanic participants. Overall, Hispanic participants were of significantly lower SES than their African American counterparts including years of education, annual income and insurance status. Both African American and Hispanic groups were taking a median of 5 medications, which is relatively low given the high number of chronic conditions. Though more African American participants had four or more chronic conditions than Hispanics, 75.4% of the total sample had four or more chronic conditions, pointing to the extent of health complexity across this sample.
Table 2.
Participant characteristics.
| Variable | Total sample (N = 250) | African American (N = 125) | Hispanic (N = 125) | p-valuea b |
|---|---|---|---|---|
| Female, n (%) | 201 (80.4) | 102 (81.6) | 99 (79.2) | 0.75025 |
| Age, median (q1, q3) | 67 (64, 71) | 68 (65, 73) | 66 (63, 70) | 0.011 |
| Age, categorized, n (%) | 0.0076 | |||
| 60–64 | 71 (28.4) | 28 (22.4) | 43 (34.4) | |
| 65–74 | 141 (56.4) | 70 (56.0) | 71 (56.8) | |
| 75–88 | 38 (15.2) | 27 (21.6) | 11 (8.8) | |
| Education, number of years, median (q1, q3) | 9 (5, 12) | 12 (11, 14) | 5 (2, 7) | <0.0001 |
| Education, categorized, n (%) | <0.0001 | |||
| Less than high school | 140 (56.0) | 34 (27.2) | 106 (84.8) | |
| High school diploma or equivalent | 36 (14.4) | 29 (23.2) | 7 (5.6) | |
| Vocational school, some college, associate degree | 50 (20.0) | 45 (36.0) | 5 (4.0) | |
| College or graduate degree | 20 (8.0) | 15 (12.0) | 5 (4.0) | |
| Other | 4 (1.6) | 2 (1.6) | 2 (1.6) | |
| Relationship status, n (%) | <0.0001 | |||
| Single | 50 (20.0) | 40 (32.0) | 10 (8.0) | |
| Living with spouse/life partner | 77 (30.8) | 18 (14.4) | 59 (47.2) | |
| Divorced/living separately from spouse/life partner | 71 (28.4) | 37 (29.6) | 34 (27.2) | |
| Widowed | 52 (20.8) | 30 (24.0) | 22 (17.6) | |
| Average annual income b, n (%) | 0.0049 | |||
| <10,000 k | 98 (39.2) | 44 (35.2) | 54 (43.2) | |
| 10,000–19,999 k | 99 (39.6) | 58 (46.4) | 41 (32.8) | |
| >20,000 k | 45 (18.0) | 23 (18.4) | 22 (17.6) | |
| Unknown/no answer given | 8 (3.2) | 0 | 8 (6.4) | |
| Insurance c, n (%) | <0.0001 | |||
| Medicare only | 73 (29.9) | 50 (40.0) | 23 (19.3) | |
| Medicaid only | 20 (8.2) | 10 (8.4) | 10 (8.0) | |
| Medicare plus private | 14 (5.7) | 8 (6.4) | 6 (5.0) | |
| Medicare plus Medicaid | 46 (18.9) | 35 (28.0) | 11 (9.2) | |
| No insurance | 63 (25.8) | 14 (11.2) | 49 (41.2) | |
| Other/private | 28 (11.5) | 8 (6.4) | 20 (16.8) | |
| Employment, n (%) | <0.0001 | |||
| Full time care-giver | 5 (2.0) | 1 (0.8) | 4 (3.2) | |
| Part time care-giver | 19 (7.6) | 9 (7.2) | 10 (8.0) | |
| Retired | 134 (53.6) | 94 (75.2) | 40 (32.0) | |
| Unemployed/disabled | 79 (31.6) | 21 (16.8) | 58 (46.4) | |
| Refused/do not know | 13 (5.2) | 0 (0) | 13(10.4) | |
| Psychosocial factors, median (q1, q3) | ||||
| BAI d | 13 (5, 22) | 6 (3, 11) | 21 (14, 27) | <0.001 |
| hsCRP (C-reactive protein) | 3.2 (1.6, 7.2) | 3.9 (2.0, 10.0) | 2.6 (1.3, 6.4) | 0.0138 |
| Traumatic stress checklist e | 3 (1, 4) | 3 (1, 5) | 2 (1, 4) | 0.0057 |
| PSE f | 2.5 (1, 4) | 2 (2, 3) | 3 (1, 4) | 0.02520 |
| PTSD g | 11 (8, 15) | 10 (7, 12) | 12 (9, 16.5) | <0.0001 |
| SF-12 h | ||||
| SF-12 PCS | 25 (24.8, 25.2) | 25 (24.8, 25.2) | 25 (24.8, 25.2) | 0.439 |
| SF-12 MCS | 18.7 (18.3, 19.1) | 19.1 (18.8, 19.4) | 18.5 (18.1, 18.7) | <0.0001 |
| Patient complexity factors | ||||
| Total number of medications, median (q1, q3) | 5 (3, 7) | 5 (3, 7) | 5 (3, 6) | 0.6306 |
| Number of chronic conditions including depression symptoms i, n (%) | 0.0019 | |||
| 1 (depression only) | 4 (1.6) | 3 (2.4) | 1 (0.8) | |
| 1–2 (excluding depression) | 57 (22.8) | 17 (13.6) | 40 (32.0) | |
| 3–4 (excluding depression) | 127 (50.8) | 67 (53.6) | 60 (48.0) | |
| 5+ (excluding depression) | 62 (24.8) | 38 (30.4) | 24 (19.2) | |
Wilcoxon used for comparing non-normal data, Chi-square used for categorical data, t-tests used for normally distributed continuous data, Fisher’s exact test used for categorical data with cell counts <5.
Total family income before taxes.
n = 6 Hispanic participants did not know their insurance status.
Beck Anxiety Inventory, response format: 0–3 (not at all to severely-it bothered me a lot), score range: 0–63. Higher score indicates greater anxiety severity.
Traumatic Stress Checklist, response format: yes, no, don’t know, refuse, higher score indicated experiencing more stressful events over a lifetime.
Past Stressor Events Form, response format: yes, no, refuse, higher score indicates experiencing more stressful events over past year.
PCL-Civilian Short Form, response format: range 1–5 (not at all to extremely), score range: 0–30. Higher scores indicate more severe symptoms of PTSD.
Short Form Health Survey, includes physical and mental component summary scales. Higher scores indicate worse health status.
Chronic conditions include heart attack or myocardial infarction, hypertension, diabetes, cancer, stroke, kidney problems, currently on dialysis, arthritis, lung disease, liver disease, asthma, sleep apnea, neurological disease, obesity (BMI ≥30).
3.3. Primary and secondary outcome measures
Median baseline depression scores were in the mild range (PHQ = 11/27), with a smaller but significant number in the moderate to severe range (24.8% scored ≥15/27). Hispanics reported significantly higher levels of depression symptoms (12/27) than African Americans (11/27; p < 0.0287), with twice as many Hispanics demonstrating severe depression as indicated by PHQ-9 of 15 or greater (33.6% of Hispanics vs 16% of African Americans). The use of anti-depressant medications at baseline was lower among African Americans than Hispanics (13.6%, vs 28% of Hispanics).
The cardiometabolic syndrome has been used to describe a cluster of metabolic risk factors associated with insulin resistance and the development of atherosclerotic cardiovascular disease. Several professional societies have generated criteria for the syndrome; BRIGHTEN Heart has used the widely accepted NHLBI/American Heart Association criteria [68]. According to this definition, cardiometabolic syndrome is diagnosed when three of the following five criteria are met: elevated waist circumference (≥102 cm in men or ≥88 cm in women), elevated blood pressure (≥130/85 mmHg or taking prescribed antihypertensive medications), reduced HDL-Cholesterol (<40 mg/dL in men or <50 mg/dL in women, or on lipid lowering medications), elevated triglycerides (≥150 mg/dL or on drug treatment), or elevated fasting blood glucose(≥100 mg/dL or on glucose lowering medications). For this study, Hemoglobin A1c ≥ 5.7 was used as a surrogate for elevated fasting blood glucose.
Sufficient data was available from 225 of the 250 randomized participants (90%) to determine whether or not they met criteria for the diagnosis of the syndrome; data missing at baseline was most often due to technical difficulties in collecting and processing laboratory samples. Of those 225 participants, 190 (84.4%) met at least three criteria, and thus fulfill the diagnosis of the cardiometabolic syndrome.
While there was no significant difference in BMI between African American and Hispanic participants, there were differences in other cardiometabolic criteria. As seen in Table 3, Hispanics were significantly more likely to be on glucose-lowering medications than were African Americans (46.4% vs 29.6%, p = 0.00899). Hispanics were also more likely to have baseline dyslipidemias. Although African Americans were more likely to take anti-hypertensive medication than Hispanics at baseline, this difference was not statistically significant, nor were there differences in baseline blood pressure measures.
Table 3.
Study outcomes.
| Variable | Total sample (N = 250) | African American (N = 125) | Hispanic (N = 125) | p-value a |
|---|---|---|---|---|
| Depression symptoms | ||||
| PHQ-9 b, median (q1, q3) | 11 (9, 14) | 11 (9, 13) | 12 (9, 15) | <0.0287 |
| PHQ-9, categorized n (%) | 0.004 | |||
| 8–9 | 86 (34.4) | 47 (37.6) | 39 (31.2) | |
| 10–14 | 102 (40.8) | 58 (46.4) | 44 (35.2) | |
| 15–19 | 47 (18.8) | 18 (14.4) | 29 (23.2) | |
| > = 20 | 15 (6) | 2 (1.6) | 13 (10.4) | |
| Cardiometabolic syndrome, n (%) c | 190 (84.4) | 96 (87.3) | 94 (81.7) | 0.2745 |
| Overweight/obesity d | 192 (77.4) | 99 (79.2) | 93 (75.6) | 0.5451 |
| Glucose intolerance e | 185 (80.8) | 95 (84.8) | 90 (76.9) | 0.1355 |
| Low HDL cholesterol f | 89 (38.4) | 35 (29.7) | 54 (47.4) | 0.0069 |
| Elevated triglycerides g | 155 (70.5) | 70 (66.0) | 85 (74.6) | 0.1849 |
| Elevated blood pressure h | 222 (89.9) | 117 (95.9) | 105 (84.0) | 0.0026 |
| Body Mass Index (BMI), median (q1, q3) | 31.6 (27.8, 36) | 32 (28.1, 36.3) | 31.3 (27.5, 35.2) | 0.2069 |
| BMI ≥30, n (%) | 151 (60.4) | 76 (60.8) | 75 (60.0) | 1.0 |
| Waist circumference (cm), median (q1,q3) | 105 (98, 115) | 106 (99, 115) | 104 (97, 114) | 0.1835 |
| Blood Pressure (BP) | ||||
| Systolic BP (mm Hg), median (q1, q3) | 138 (126.5, 153) | 138 (127.5, 155) | 138.5 (126, 150) | 0.7396 |
| Diastolic BP (mm Hg), median (q1, q3) | 78.5 (69, 85) | 79 (68.5, 86.5) | 77 (69, 84) | 0.3976 |
| Lipids | ||||
| Total cholesterol (mg/dL), median (q1, q3) | 189 (163, 223) | 198.5 (168, 228) | 181.5 (160, 209.5) | 0.0100 |
| HDL cholesterol (mg/dL), median (q1, q3) | 49 (40, 61) | 54 (45, 65) | 45 (38, 56) | 0.0001 |
| Triglycerides (mg/dL), median (q1, q3) | 153 (111, 228) | 133 (96, 201) | 171.5 (128.5, 240.5) | 0.0014 |
| HBA1c, median (q1, q3) | 6.4 (5.7, 7.6) | 6.3 (5.7, 7.5) | 6.6 (5.7, 7.9) | 0.502 |
| Medications (%) | ||||
| Glucose lowering medications | 95 (38) | 37 (29.6) | 58 (46.4) | 0.00899 |
| Lipid lowering medications | 87 (34.8) | 37 (29.6) | 50 (40) | 0.11084 |
| Blood pressure lowering medications | 178 (71.2) | 95 (76) | 83 (66.4) | 0.12414 |
| Anti-depressant medications | 52 (20.8) | 17 (13.6) | 35 (28.0) | 0.0077 |
| Adherence | ||||
| Low adherence i (%) | 176 (70.4) | 98 (78.4) | 78 (62.4) | 0.00824 |
Wilcoxon used for comparing non-normal data, Chi-square used for categorical data, t-tests used for normally distributed continuous data, Fisher’s exact test used for categorical data with cell counts <5.
Patient Health Questionnaire-9, 9 items, range 0–3 (Not at all to nearly every day). Higher scores indicate greater depression severity.
Cardiometabolic syndrome missing data (n = 25)
Waist ≥102 cm (men) or ≥88 cm (women), missing data (n = 2)
Hemoglobin A1c ≥5.7 or on Glucose-lowering medications, missing data (n = 21)
<1.0 mmol/L (40 mg/dL) (men); <1.3 mmol/L (50 mg/dL) (women), missing data (n = 18)
≥1.7 mmol/L (150 mg/dL) or on lipid lowering medicines, missing data (n = 30)
≥130/85 mmHg or on blood pressure lowering medicines, missing data (n = 3)
Morisky Medication Adherence Scale, response format: range yes–no. Low adherence = MMAS score ≥3
Health-related quality of life was far lower than published older adult averages on the SF-12; our total sample scored 15–20 points lower for physical functioning, and 34–35 points lower for mental functioning on the SF-12 [69,70]. Within our sample, physical functioning was similar between ethnic groups (median = 25), though African Americans reported significantly higher mental functioning (median = 19.1) than Hispanics (median = 18.5). The latter is consistent with reported differences in depression. In contrast, our sample scored similarly (median = 0.80) to other American older adults on the EQ5-D [71] and no differences were noted between African Americans and Hispanics.
3.4. Mediator variables
Medication adherence was generally poor, with 70.4% reporting low adherence. African Americans (78.4%) reported a significantly higher rate of poor medication adherence than Hispanics (62.4%; p = 0.00824). Participants reported a median of three traumatic events in their lifetime, 2.5 in the last year; African Americans endorsed significantly more lifetime traumatic stress events, while Hispanics scored higher for stressful events in the past year (PSE). A cut off score of ≥14 on the PCL-C short form is used to identify traumatized patients in general medical settings [72]. Median Post-Traumatic Stress Disorder (PTSD) symptoms were 11/30, with Hispanics reporting significantly higher symptom levels (median = 12/30) than African Americans (10/30, p < 0.0001).
4. Discussion
Depression is a significant issue among older adults, and the burden of depression is greater among African American and Hispanic older adults. The impact of depression is not limited to mental health, but also physical well-being, with increased risk of cardiovascular disease. The significance of the BRIGHTEN Heart trial lies in its engagement of a challenging population, along with implementation and rigorous evaluation of an intervention to reduce both symptoms of depression and cardiometabolic risk in this health disparity population. In order to achieve this aim, several challenges had to be addressed.
The first of these challenges was participant engagement. Study inclusion criteria presented four challenges to recruitment: race/ethnicity, depression, age, and poverty. Barriers to the participation of African Americans and Hispanics in clinical trials have been well documented [73–75], and include trust, lower health literacy, limited English proficiency, and costs associated with study participation. As the primary enrollment criterion, all of our participants have symptoms of depression, and many have other behavioral risk factors such as anxiety and traumatic stress. Depression among older adults is characterized by loss of interest, fatigue, and diminished ability to concentrate or make decisions [76], all of which are barriers to giving consent and participating in research. The successful recruitment of participants for BRIGHTEN Heart should encourage mental health researchers to make greater efforts to engage minority elderly in intervention trials.
In addition to depression, the older adults recruited for BRIGHTEN Heart have significant comorbid illness. The primary comorbidity that BRIGHTEN Heart targets is metabolic syndrome. Given that the syndrome is rarely listed in problem lists in the patient medical record, and few patients are aware that they have the condition, it was necessary to find a surrogate measure to identify study participants. In this depressed minority older adult population, the use of BMI over 25 as an enrollment criterion was highly effective, as it provided a population in which 84.4% met formal criteria for metabolic syndrome.
Also noteworthy is that over 75% of study participants had three or more major chronic illnesses in addition to depression. While this pattern of multiple chronic conditions is commonly seen in clinical practice with older adults [77], comorbidities are often a criterion for exclusion from research study participation. The BRIGHTEN Heart study population is therefore highly representative of depressed older adults in “real world” settings, suggesting that findings from the trial will have external validity.
A strength of the study design is its methodological rigor which includes key components such as independent randomization, an intervention supervised by a single clinician leader (EE), and outcomes assessments by blinded RAs. These features ensure that assessments are free of bias and any observed differences between the intervention and control conditions can be reasonably attributed to the BRIGHTEN Heart intervention.
Baseline findings do, however, suggest an important analytic challenge, as well as an opportunity. Although inclusion criteria and enrollment settings were the same for all participants, significant differences are observed in the demographics, mental health, and physical health status between African Americans and Hispanics in the trial. Socioeconomic and health differences between the African American and Hispanic populations may require that implementation of the BRIGHTEN Heart intervention be adapted to meet the distinct needs of each group. Intergroup differences will also need to be addressed in data analyses; equal numbers of African Americans and Hispanics in the study cohort will facilitate comparisons of outcomes between the two groups.
5. Conclusion
The BRIGHTEN Heart trial has been designed to rigorously test a multi-level intervention for vulnerable minority older adults with depression and elevated cardiovascular risk. The study has successfully enrolled this difficult-to-reach target population. We are now well-positioned to determine the efficacy of the BRIGHTEN Heart virtual team-based intervention, based in a primary care environment, in improving both mental health and physical well-being in older adults. If found to be efficacious, the BRIGHTEN Heart intervention could lead to more effective approaches to address depression and cardiovascular risk in vulnerable communities affected by health disparities.
Acknowledgments
This research was part of the Rush Center for Urban Health Equity, which was funded by the National Institutes of Health (NIH) through the National Institute for Heart Lung and Blood (NHLBI), grant number 1P50HL105189-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Lindsey Mitchell, David Mata, Serena Silvestry, and Syed Quadri provided assistance with data collection.
Drs. Emery-Tiburcio and Rothschild are Co-Principal Investigators for the trial and oversaw all aspects of the study design and implementation and manuscript preparation. Ms. Golden is an Investigator involved in implementation of both control and intervention conditions. Dr. Mack is a study interventionist and participated in study implementation and manuscript preparation. Dr. Li is the primary study biostatistician; she and Ms. Avery supported study implementation, recruitment, and fidelity; with Ms. Wang, they prepared and analyzed all data in this report. Dr. Powell is the Principal Investigator of the Rush Center for Urban Health Equity and provided critical guidance to the design and implementation of the trial.
References
- 1.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis1. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 2.Barth J, Sehumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 3.Van Melle JF, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 4.Wulsin LR, Singal SB. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–10. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 5.Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatr. 2006;51(12):730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 6.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMatteo M, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 8.Dyer CB, Pavlik VN, Murphy KP, Hyman DJ. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205–208. doi: 10.1111/j.1532-5415.2000.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 9.Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Kritchevsky SB, Tylavsky F, Harris TB, Ayonayon HN, Newman AB. Factors associated with impaired appetite in well-functioning community-dwelling older adults. J Nutr Elder. 2007;26(1–2):27–43. doi: 10.1300/J052v26n01_02. [DOI] [PubMed] [Google Scholar]
- 11.Vogelzangs N, Kritchevsky SB, Beekman ATF, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65(12):1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster A, Bosma H, Kempen G, Penninx B, Beekman A, Deeg D. The role of psychosocial factors, physical health status, and behavioral factors. J Psychosom Res. 2006;61(5):619–627. doi: 10.1016/j.jpsychores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Brenes GA, Knudson M, McCall WV, Williamson JD, Miller ME, Stanley MA. Age and racial differences in the presentation and treatment of generalized anxiety disorder in primary care. J Anxiety Disord. 2008;22(7):1128–1136. doi: 10.1016/j.janxdis.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arean P, Alvidrez J. The prevalence of psychiatric disorders and subsyndromal mental illness in low-income, medically ill elderly. Int J Psychiatry Med. 2001;31(1):9–24. doi: 10.2190/YGL6-N54R-08RW-1BF4. [DOI] [PubMed] [Google Scholar]
- 15.Krause NBE. Financial strain, economic values, and somatic symptoms in later life. Psychol Aging. 1992;7(1):4–14. doi: 10.1037//0882-7974.7.1.4. [DOI] [PubMed] [Google Scholar]
- 16.Rothermund K, Brandtstädter J. Depression in later life: cross-sequential patterns and possible determinants. Psychol Aging. 2003;18(1):80–90. doi: 10.1037/0882-7974.18.1.80. [DOI] [PubMed] [Google Scholar]
- 17.Mills T, Alea N, Cheong J. Differences in the indicators of depressive symptoms among a community sample of African–American and Caucasian older adults. Community Ment Health J. 2004;40(4):309–331. doi: 10.1023/b:comh.0000035227.57576.46. [DOI] [PubMed] [Google Scholar]
- 18.Skaer TL, Sclar DA, Robison LM, Galin RS. Trends in the rate of depressive illness and use of antidepressant pharmacotherapy by ethnicity/race: an assessment of office-based visits in the United States, 1992–1997. Clin Ther. 2000;22(12):1575–1589. doi: 10.1016/s0149-2918(00)83055-6. [DOI] [PubMed] [Google Scholar]
- 19.Young AS, Klap R, Sherbourne DC, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz JC, Patel SR, Balan IC, Bell MA, Blanco C. Yellow horse brave heart, M. Toward an adaptation of interpersonal psychotherapy for hispanic patients with DSM-IV major depressive disorder. J Clin Psychiatry. 2009;70(2):214–222. doi: 10.4088/jcp.08m04100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Writing Group Members. Lloyd-Jones D, Adams R, et al. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 22.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race, and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rep. 2009;13:1–8. [PubMed] [Google Scholar]
- 23.Mensah G, Mokdad A, Ford E, Greenlund K, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 24.Keppel K, Bilheimer L, Gurley L. Improving population health and reducing health care disparities. Health Aff. 2007;26(5):1281–1292. doi: 10.1377/hlthaff.26.5.1281. [DOI] [PubMed] [Google Scholar]
- 25.Keppel K, Garcia T, Hallquist S, Ryskulova A, Agress L. Comparing racial and ethnic populations based on healthy people 2010 objectives. Healthy People Stat Notes. 2008;26(1–16) [PubMed] [Google Scholar]
- 26.Keppel KG, Pearcy JN, Wagener DK. Trends in racial and ethnic-specific rates for the health status indicators: United States, 1990–98. Healthy People 2000 Stat Notes. 2002;23:1–16. [PubMed] [Google Scholar]
- 27.Keppel KG, Pearcy JN, Weissman JS. Trends in racial disparities in care. N Engl J Med. 2005;353(19):2081–2085. [PubMed] [Google Scholar]
- 28.Margellos H, Silva A, Whitman S. Comparison of health status indicators in Chicago: are black–white disparities worsening? Am J Public Health. 2004;94(1):116–121. doi: 10.2105/ajph.94.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitman S. Racial disparities in health: taking it personally. Public Health Rep. 2001;116(5):387–389. doi: 10.1093/phr/116.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva A, Whitman S, Margellos H, Ansell D. Evaluating Chicago’s success in reaching the healthy people 2000 goal of reducing health disparities. Public Health Rep. 2001;116(5):484–494. doi: 10.1016/S0033-3549(04)50076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan G, Everson SA, Lynch JW. The Contribution of Social and Behavioral Research to an Understanding of the Distribution of Disease: A Multilevel Approach. In: Smedley BD, Syme SL, editors. Promoting Health: Intervention Strategies from Social and Behavioral Research. National Academies Press; Washington D.C: 2000. [PubMed] [Google Scholar]
- 32.Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff. 2008;27(2):339–349. doi: 10.1377/hlthaff.27.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skultety K, Zeiss A. The treatment of depression in older adults in the primary care setting: an evidence based review. Health Psychol. 2006;25:665–674. doi: 10.1037/0278-6133.25.6.665. [DOI] [PubMed] [Google Scholar]
- 34.Bruce ML, Ten Have TR, Reynolds CF, III, Katz II, Schulberg HC, Mulsant BH. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 35.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ. Effectiveness of collaborative care for older adults with Alzheimer’s disease in primary care: a randomized controlled trial. JAME. 2006;295(2148–57) doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 36.Ciechanowski P, Wagner E, Schmaling K, Schwartz S, Williams B, Diehr P. Community-integrated home-based depression treatment in older adults: a randomized control trial. J Am Med Assoc. 2004;291:1569–1577. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]
- 37.Hedrick S, Chaney E, Felker B, Liu C, Hasenberg N, Heagerty P. Effectiveness of collaborative care depression treatment in veterans’ affairs primary care. J Intern Med. 2003;18(9–16) doi: 10.1046/j.1525-1497.2003.11109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartels SJ, Coakley EH, Zubritsky C, et al. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161(8):1455–1462. doi: 10.1176/appi.ajp.161.8.1455. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Hedrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54(5):698–704. doi: 10.1176/appi.ps.54.5.698. [DOI] [PubMed] [Google Scholar]
- 40.Friedman DM, Berger DL. Improving team structure and communication: a key to hospital efficiency. Arch Surg. 2004;139(1194–8) doi: 10.1001/archsurg.139.11.1194. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg DB, Gittell JH, Lusenhop RW, Kautz CM, Wright J. Beyond our walls: impact of patient and provider coordination across the continuum on outcomes for surgical patients. Health Serv Res. 2007;42(1p1):7–24. doi: 10.1111/j.1475-6773.2006.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strasser DC, Falconer JA, Stevens AB, et al. Team training and stroke rehabilitation outcomes: a cluster randomized trial. Arch Phys Med Rehabil. 2008;89(1):10–15. doi: 10.1016/j.apmr.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 43.Roy-Byrne PB, Katon W, Cowley DS, Russo J. A randomized effectiveness trial of collaborative care for patients with panic disorder in primary care. Arch Gen Psychiatry. 2001;58(869–76) doi: 10.1001/archpsyc.58.9.869. [DOI] [PubMed] [Google Scholar]
- 44.Katon W, Russo J, Von Korff M, Lin E, Simon G, Bush T. Long-term effects of a collaborative care intervention in persistently depressed primary care patients. J Gen Intern Med. 2002;17:741–748. doi: 10.1046/j.1525-1497.2002.11051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birbeck G, Zingmond D, Cui X, Vickrey B. Multispecialty stroke services in California hospitals are associated with reduced mortality. Neurology. 2006;66(10):1527–1532. doi: 10.1212/01.wnl.0000203993.93763.b8. [DOI] [PubMed] [Google Scholar]
- 46.Gade G, Venohr I, Conner D, McGrady K, Beane J, Richarson RH. Impact of an in-patient palliative care team: a randomized controlled trial. J Palliat Med. 2008;11(2):180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 47.Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. 2009;50(6):613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 48.Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. J Am Med Assoc. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 49.Applebaum PS, Grisso T. MacCAT-CR: MacArthur competence assessment tool for clinical research. 2001:84. [Google Scholar]
- 50.Spitzer RL, Williams JBW, Kroenke K, Hornyak R, McMurray J. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD patient health questionnaire obstetrics-gynecology study. Obstet Gynecol. 2000;183(3):759–769. doi: 10.1067/mob.2000.106580. [DOI] [PubMed] [Google Scholar]
- 51.Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann. 2002;32(509–21) [Google Scholar]
- 52.Phelan E, Williams B, Meeker K, Bonn K, Frederick J, Logerfo J, Snowden M. A study of the diagnostic accuracy of the PHQ-9 in primary care elderly. BMC Fam Pract. 2010;11:63. doi: 10.1186/1471-2296-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang FY, Chung H, Kroenke K, Spitzer RL. Racial and ethnic differences in the relationship between depression severity and functional status. Psychiatr Serv. 2006;57(4):498–503. doi: 10.1176/ps.2006.57.4.498. [DOI] [PubMed] [Google Scholar]
- 54.Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679–686. doi: 10.1097/00006842-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Baca E, Saiz J, Agüera L, Caballero L, Fernández-Liria A, Ramos J, Gil A, Madrigal M, Porras A. Validation of the Spanish version of PRIME-MD: a procedure for diagnosing mental disorders in primary care. Actas Esp Psiquiatr. 1999;27(6):375–383. [PubMed] [Google Scholar]
- 56.Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry. 2008;16(5):406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- 57.Vahia IV, Meeks TW, Thompson WK, et al. Subthreshold depression and successful aging in older women. Am J Geriatr Psychiatry. 2010;18(3):212–220. doi: 10.1097/JGP.0b013e3181b7f10e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emery EE, Lapidos S, Eisenstein A, Ivan I, Golden R. The BRIGHTEN program: implementation and evaluation of a program to bridge resources of an interdisciplinary geriatric health team via electronic networking. The Gerontologist. 2012;52(6):857–865. doi: 10.1093/geront/gns034. [DOI] [PubMed] [Google Scholar]
- 59.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African–American, Hispanic, and white primary care patients. Med Care. 2003;41(4):479–489. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 60.Cooper LA, Brown C, Vu HT, et al. Primary care patients’ opinions regarding the importance of various aspects of care for depression. Gen Hosp Psychiatry. 2000;22(3):163–173. doi: 10.1016/s0163-8343(00)00073-6. [DOI] [PubMed] [Google Scholar]
- 61.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. J Am Med Assoc. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 62.Scogin F, McElreath L. Efficacy of psychosocial treatments for geriatric depression: a quantitative review. J Consult Clin Psychol. 1994;62(1):69–74. doi: 10.1037//0022-006x.62.1.69. [DOI] [PubMed] [Google Scholar]
- 63.Ayers C, Sorrell J, Thorp S, Wetherell J. Evidence-based psychological treatments for late-life anxiety. Psychol Aging. 2007;22(1):8–17. doi: 10.1037/0882-7974.22.1.8. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds CF, III, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression. A randomized controlled trial in patients older than 59 years. J Am Med Assoc. 1999;281(1):39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- 65.Sloane RB, Staples FR, Schneider LS. Interpersonal Psychotherapy Versus Nortriptyline for Depression in the Elderly. In: Burrows G, Norman TR, Dennerstein L, editors. Clinical and Pharmacological Studies in Psychiatric Disorders. John Libbey; London: 1985. [Google Scholar]
- 66.Loewe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with The Patient Health Questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Unützer J IMPACT Investigators, Center for Health Services Research. IMPACT Intervention Manual. Los Angeles, California: 1999. [Google Scholar]
- 68.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of The National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 69.Everard KM, Lach HW, Fisher EB, Baum MC. Relationship of activity and social support to the functional health of older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55(4):S208–S212. doi: 10.1093/geronb/55.4.s208. [DOI] [PubMed] [Google Scholar]
- 70.Pettit T, Livingston G, Manela M, Kitchen G, Katona C, Bowling A. Validation and normative data of health status measures in older people: The Islington study. Int J Geriatr Psychiatry. 2001;16(11):1061–1070. doi: 10.1002/gps.479. [DOI] [PubMed] [Google Scholar]
- 71.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43(11):1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 72.Lang AJ, Stein MB. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav Res Ther. 2005;43(5):585–594. doi: 10.1016/j.brat.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Sinclair S, Hayes-Reams P, Myers HF, Allen W, Hawes-Dawson J, Kington R. Recruiting African Americans for Health Studies: Lessons from the Drew-RAND Center on Health and Aging. In: Levkoff SE, editor. Recruitment and Retention in Minority Populations. Springer Publishing Company; New York: 2000. p. 39. [Google Scholar]
- 74.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blazer D. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 77.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS. Neighborhood disadvantage, stress, and drug use among adults. J Health Soc Behav. 2001;42(151–165) [PubMed] [Google Scholar]
- 79.Mills K, Teesson M, Darke S, Ross J. Reliability of self-reported trauma exposure among people with heroin dependence: a longitudinal investigation. J Trauma Stress. 2007;20(3):313–323. doi: 10.1002/jts.20219. [DOI] [PubMed] [Google Scholar]
- 80.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 82.Beck AT, Steer RA. Relationship between the Beck Anxiety Inventory and the Hamilton Anxiety Rating Scale with anxious outpatients. J Anxiety Disord. 1991;5(3):213–223. [Google Scholar]
- 83.EuroQol — a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 84.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 86.Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9(2):183–205. [Google Scholar]
- 87.Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med. 2012;42(4):683–693. doi: 10.1017/S0033291711001784. [DOI] [PubMed] [Google Scholar]