Abstract
Introduction
The DCDC2 gene is involved in neuronal migration. Heterotopias have been found within the white matter of DCDC2-knockdown rats. A deletion in DCDC2/intron 2 (DCDC2d), which encompasses a regulatory region named ‘regulatory element associated with dyslexia 1’ (READ1), increases the risk for dyslexia. We hypothesized that DCDC2d can be associated to alterations of the white matter structure in general and in dyslexic brains.
Methods
Based on a full-factorial analysis of covariance (ANCOVA) model, we investigated voxel-based diffusion tensor imaging (VB-DTI) data of four groups of subjects: dyslexia with/without DCDC2d, and normal readers with/without DCDC2d. We also tested DCDC2d effects upon correlation patterns between fractional anisotropy (FA) and reading scores.
Results
We found that FA was reduced in the left arcuate fasciculus and splenium of the corpus callosum in subjects with versus without DCDC2d, irrespective of dyslexia. Subjects with dyslexia and DCDC2d showed reduced FA, mainly in the left hemisphere and in the corpus callosum; their counterpart without DCDC2d showed similar FA alterations. Noteworthy, a conjunction analysis in impaired readers revealed common regions with lower FA mainly in the left hemisphere. When we compared subjects with dyslexia with versus without DCDC2d, we found lower FA in the inferior longitudinal fasciculus and genu of the corpus callosum, bilaterally. Normal readers with versus without DCDC2d had FA increases and decreases in both the right and left hemisphere.
Discussion
The major contribution of our study was to provide evidence relating genes, brain and behaviour. Overall, our findings support the hypothesis that DCDC2d is associated with altered FA. In normal readers, DCDC2-related anatomical patterns may mark some developmental cognitive vulnerability to learning disabilities. In subjects with dyslexia, DCDC2d accounted for both common – mainly located in the left hemisphere – and unique – a more severe and extended pattern – alterations of white matter fibre tracts.
Keywords: Diffusion tensor imaging, DCDC2, READ1, Developmental dyslexia, Neuronal migration
1. Introduction
A disturbance in the genetically driven developmental mechanisms of early neuronal migration is at the basis of several neurodevelopment disorders, including developmental dyslexia (hereafter: dyslexia; Diaz & Gleeson, 2009). Dyslexia is an aetiologically heterogeneous condition, typically diagnosed in the first school years, characterized by an impaired reading acquisition in spite of adequate neurological and sensorial conditions, educational opportunities, and normal intelligence. Following earlier descriptions of high familial aggregation of the disorder, substantial heritability has been reported, with estimates across dyslexia and related quantitative traits (such as reading and spelling) ranging from .18 to .72 (Plomin & Kovas, 2005). A multifactorial threshold model of inheritance, whereby multiple genetic and environmental factors contribute to phenotypic variation, has been found as the most plausible mode of familial transmission of the disorder (Plomin & Kovas, 2005).
The DCDC2 gene has been recognized as one of the leading risk genes in dyslexia (Brkanac et al., 2007; Cope et al., 2012; Deffenbacher et al., 2004; Harold et al., 2006; Marino et al., 2012; Meng et al., 2005; Newbury et al., 2011; Powers et al., 2013; Schumacher et al., 2006; Wilcke et al., 2009; Zhong et al., 2013), and in reading abilities in the normal range (Lind et al., 2010; Scerri et al., 2011), even though negative results have been also reported (Becker et al., 2014; Ludwig et al., 2008; Parracchini et al., 2011). Data show that the DCDC2 gene is involved in neuronal migration and is most highly expressed in the entorhinal cortex, inferior and medial temporal cortex, hypothalamus, amygdala and hippocampus (Meng et al., 2005). The embryonic knockdown of the DCDC2 function in rodent neocortical progenitor cells results in postnatal small and scattered heterotopias within the white matter (Burbridge et al., 2008). The specific function of the Dcdc2 protein in neuronal migration has yet to be elucidated, but analyses of its protein structure provide some clues. It was found that Dcdc2 exhibits the same functional features displayed by the Dclk and Dcx proteins, which have been found to have a role in the axonal growth across the corpus callosum, and in neuronal migration within the cerebral cortex (Coquelle et al., 2006; Deuel et al., 2006; Koizumi, Tanaka, & Gleeson, 2006). A highly polymorphic, short-tandem repeat (named BV677278) located in the intron 2 of the DCDC2 gene was reported (Meng et al., 2005), for which a role as a regulatory region has been suggested (Meng et al., 2011). Recently, Powers et al. (2013) identified the BV677278-binding protein as the transcription factor ETV6, confirmed BV677278 as a regulatory element, and proposed a new name for BV677278, i.e., regulatory element associated with dyslexia 1 (READ1). As such, READ1 could substantially influence the function of the DCDC2 gene in neuronal migration. Noteworthy, a rare DCDC2 variant, i.e., a DCDC2/intron 2 deletion embedding READ1 (DCDC2d), was found to increase the risk of dyslexia by independent studies (Brkanac et al., 2007; Cope et al., 2012; Harold et al., 2006; Marino et al., 2012; Wilcke et al., 2009) although negative findings have also been reported (Ludwig et al., 2008; Powers et al., 2013). Interestingly, in healthy adult humans DCDC2d has been found associated with altered grey matter volumes in specific cortical regions (Meda et al., 2008), several of which correspond to those found altered by post-mortem studies of dyslexia (Galaburda, Sherman, Rosen, Aboitiz, & Geschwind, 1985). Furthermore, in adult healthy humans allelic variation in the DCDC2 gene has been associated with individual differences in fibre tracts – as those connecting the left medial temporal gyrus with the angular and supramarginal gyri, the superior longitudinal fasciculus as well as the corpus callosum (Darki, Peyrard-Janvid, Matsson, Kere, & Lingberg, 2012) – which are commonly found altered in neuroimaging studies of reading and dyslexia (Vandermosten, Boets, Wouters, & Ghesquiere, 2012; Wandell & Yeatman, 2013; Fig. 1 and Table 1).
Fig. 1.
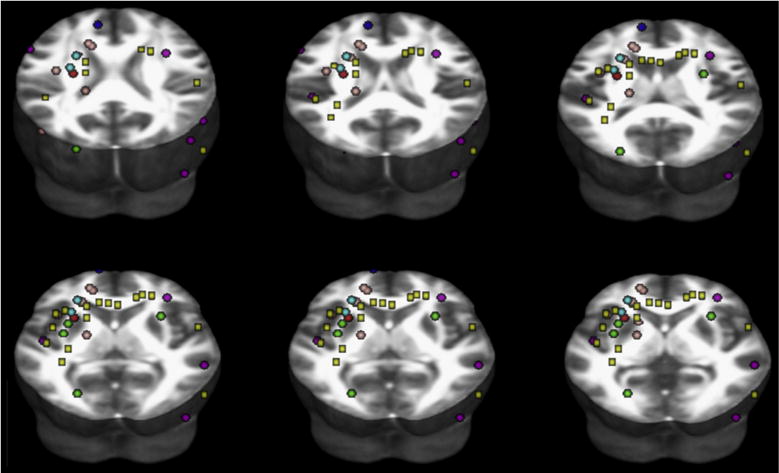
Map of coordinates in Montreal Neurological Institute space of significant findings of FA reductions in subjects with dyslexia compared to normal readers, as reviewed across DTI studies. We performed an online search in the database PUBMED from January 1996 to February 2011, using the keywords “diffusion tensor” and “developmental dyslexia”. We then checked the references lists for DTI additional studies. We included studies that reported (1) a minimum of six directions (2) anisotropy-based statistical comparisons between subjects with dyslexia and normal readers (3) stereotactic coordinates of FA whole-brain results (4) thresholds for significance, i.e., corrected for multiple comparisons or uncorrected with spatial extent thresholds. We also included one study that employed Tract-Based Spatial Statistics (Richards et al., 2008), and one that applied a priori defined anatomical regions of interest (Niogi & McCandliss, 2006). Six studies were included (Table 1). From each cluster of significant differences, we selected the millimetre-space coordinates of the voxel where the difference between subjects with dyslexia and normal readers was maximum. Coordinates reported in Talairach space were converted to Montreal Neurological Institute space. Spherical regions of interest (2 mm radius; see colours in Table 1) were then created and centred at the peak coordinates reported for each study. Yellow box regions of interest were instead created and centred at the coordinates reported in Table 4A (FA differences between DYS+ and NR− in our study). Finally, all regions of interest were re-referenced to the FMRIB58_FA 3D template in Montreal Neurological Institute space provided with the FSL package (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The placement of the regions of interest projected to different axial planes overlaid to the FMRIB58_FA 3D template is shown (www.fmrib.ox.ac.uk/fsl).
Table 1.
Summary of the findings from DTI studies that were published on dyslexia and that were included in Fig. 1.
| Study | Method | Sample
|
Mean age
|
Coordinates of white matter FA reductions poor readers versus normal readers
|
Colour in Fig. 1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Poor readers | Normal readers | Poor readers | Normal readers | x | y | z | |||
| Klingberg et al., 2000 a | VB analysis | 6 | 11 | 31.5 ± 5.3 | 23.1 ± 1.4 | −26 | −22 | 29 | RED |
| Niogi & McCandliss, 2006 | Region of interest | 8 | 7 | 8.31 ± 1.19 | 7.75 ± .66 | −28 | −11 | 26 | CYAN |
| −24 | −12 | 36 | |||||||
| Steinbrink et al., 2008 b | VB analysis | 8 | 8 | 20.1 ± 3.9 | 23.7 ± 4.3 | −21 | 27 | 6 | GREEN |
| −30 | 0 | 9 | |||||||
| −33 | −7 | 6 | |||||||
| −24 | −87 | 15 | |||||||
| 30 | 0 | 15 | |||||||
| Rimrodt, Peterson, Denckla, Kaufmann, & Cutting, 2010 b | VB analysis | 7 | 9 | 11 ± 3 | 11 ± 2 | −54 | 4 | 30 | VIOLET |
| −46 | −32 | 18 | |||||||
| 58 | 6 | 34 | |||||||
| −44 | 0 | −10 | |||||||
| −50 | −16 | −28 | |||||||
| 34 | −32 | 52 | |||||||
| 46 | −70 | −16 | |||||||
| 18 | 20 | −8 | |||||||
| 2 | −4 | −22 | |||||||
| 58 | −48 | 10 | |||||||
| Richards et al., 2008 b | Tract-Based | 14 | 7 | 30–45 (age range) | −14 | −1 | 36 | PINK | |
| Spatial Statistics | −17 | 11 | 2 | ||||||
| −16 | −4 | 40 | |||||||
| −18 | −47 | 33 | |||||||
| −46 | −28 | −12 | |||||||
| −37 | −23 | 32 | |||||||
| −21 | 9 | 20 | |||||||
| Keller & Just, 2009 b | VB analysis | 33 | 17 | 10 ± 1.1 | 9.8 ± 1 | −10 | 20 | 38 | BLUE |
Talairach coordinates.
Montreal Neurological Institute coordinates.
Neuroimaging studies have consistently revealed that dyslexia is linked to alterations of a left-hemispheric network, including the inferior frontal, temporo-parietal and occipitotemporal cortical regions (Brambati et al., 2004, 2006; Silani et al., 2005). The first two regions constitute a dorsal phonological route, whereas the occipito-temporal region hosts a ventral orthographical route. Furthermore, some studies suggest a role of the corpus callosum that drives the left lateralization of the reading network (Linkersdorfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Richlan, Kronbichler, & Wimmer, 2013; Vandermosten et al., 2012; Wandell & Yeatman, 2013). The recent computational methods that allow the study of brain structural properties via magnetic resonance imaging (MRI), such as voxel-based diffusion tensor imaging (VB-DTI) techniques, have greatly extended our knowledge of the morphology of dyslexia and consistently reported altered concentrations of white matter. Overall, DTI data on dyslexia converge in finding white matter abnormalities in multiple fibre bundles, i.e., superior longitudinal, arcuate, inferior longitudinal and inferior fronto-occipital fasciculi (mainly in the left hemisphere), and the whole corpus callosum (Vandermosten et al., 2012; Wandell & Yeatman, 2013; see Fig. 1 and Table 1 for a descriptive survey of the related literature), as anatomic correlates of the disorder.
Given the above evidence, we hypothesized that DCDC2d could: (1) be associated with disorganization of the white matter structure in general; (2) be associated with disorganization of the white matter structure in the dyslexic brain; (3) influence the correlation between reading performance and white matter structure.
To address these hypotheses we measured the fractional anisotropy (FA) – a parameter linked to axon packing and myelination (Beaulieu, 2002) – of four groups, namely, subjects with dyslexia with/without DCDC2d (hereafter: DYS+ and DYS−, respectively), and normal readers with/without DCDC2d (hereafter: NR+ and NR−, respectively). A 3 T MRI scanner was employed together with VB-DTI analyses. Furthermore, we tested DCDC2d effects upon the correlation patterns between FA and average reading. This was the first study to investigate subjects with dyslexia with an identified element of genetic susceptibility (DCDC2d) at a neuroanatomical level by means of FA analyses.
2. Material and methods
2.1. Ethics
The protocol was approved by the Scientific Review Board and the Ethical Committee of the “Eugenio Medea” and “San Raffaele” Scientific Institutes.
2.2. Subjects
Subjects with dyslexia were recruited from a sample of an ongoing genetic study cohort, which has been genotyped for DCDC2d gene for genetic association tests (n = 303; Marino et al., 2012). Inclusion criteria at the time of recruitment for the genetic study were: (1) either accuracy or speed z-scores ≤−2.0 standard deviations (SDs) on timed text-reading tests; or: (2) either accuracy or speed z-scores ≤−2.0 SDs on timed reading of single unrelated words or pronounceable nonwords lists (Cornoldi & Colpo, 1995, 1998; Sartori, Job, & Tressoldi, 1995); and: (3) full-scale IQ ≥ 85 (Wechsler Intelligence Scale for Children, Revised, 1981); and (4) absence of neurological or sensorial disorders. Thirty-seven subjects had DCDC2d (all heterozygous), and 266 subjects did not (allelic frequency of DCDC2d = .06). Subjects were asked to participate in the present study if they were 16–21 years old, were right-handed, had no metal dental braces, no head piercings, no upper back/head tattoos. Twenty-one subjects (11 DYS+ and 10 DYS−) met these inclusion criteria and gave their informed consent to participate in the present study.
Normal readers were recruited via two different ascertainment schemes: (1) subjects were contacted by word of mouth among high school and university students, 16–21 years old, with no metal dental braces, no head piercings, no upper back/head tattoos; informed written consent to participate in the present study and in the mouthwash-sample collection to obtain DNA was obtained from 25 subjects; genotyping for DCDC2d yielded four subjects with DCDC2d (all heterozygous) and 21 without DCDC2d (allelic frequency of DCDC2d = .08); (2) subjects were recruited from a genetic study of emotional and behavioural problems in a general population sample of adolescents (Frigerio et al., 2006; Nobile et al., 2007). Genotyping for DCDC2d of 631 subjects of this general population sample yielded 51 subjects with DCDC2d (two homozygous and 49 heterozygous) and 580 without DCDC2d (allelic frequency of DCDC2d = .08). We were interested in 32 subjects who were: (1) 16–21 years old, and (2) carriers of DCDC2d; 14 gave an informed written consent to participate in the present study.
All subjects were administered (1) reading – word reading, non-word reading – and spelling tasks – writing under dictation sentences containing homophones – from the “Batteria per la Valutazione della Dislessia e Disortografia Evolutiva” (Battery for the Assessment of Developmental Reading and Spelling Disabilities; Sartori et al., 1995); speed in total seconds and the number of errors were recorded, and z-scores were obtained based on grade norms from the general population (from second grade to last grade of high school; Sartori et al., 1995); (2) short-term memory tasks – letter forward/backward spans, number forward/backward spans – from ‘TEMA, Test di Memoria e Apprendimento’, which is the Italian translation of ‘TOMAL, Test of Memory and Learning’ (Reynolds & Bigler, 1994); scores were computed based on the number of accurate letters/numbers recalled in the correct order for each string, and z-scores were obtained based on grade norms from the general population (Reynolds & Bigler, 1994); (3) the Cattell’s Culture Free Test (Cattell & Cattell, 1981), which yielded a measure of non-verbal IQ; (4) phonemic awareness, ad-hoc made tasks, which included syllable displacement, spoonerism, phonemic blending; both the speed and the number of accurate answers were recorded; z-scores were obtained based on reference norms that were collected from 96 healthy subjects of comparable age and educational level as the sample subjects; (5) the Adult Dyslexia Checklist (Vinegrad, 1994). Hand preference was tested using the Briggs and Nebes Inventory (Briggs & Nebes, 1975). Socioeconomic status was based on parental occupation which was scored according to the Hollingshead nine-points scale, whereby a score ranging from 10 to 90 was assigned to each parental job, and the higher of the two scores was used when both parents were employed (Hollingshead, 1975). Subjects’ level of education was self-reported as the highest completed grade of high school or year of college at the time of assessment and was analyzed as a continuous variable (hereafter: education).
Normal readers had to meet the following criteria to be included in the study: (1) IQ ≥ 85; (2) word and non-word reading >−.5 SD; (3) at least two among letter forward/backward spans and number forward/backward spans >−.5 SD; (4) an Adult Dyslexia Checklist score ≤9, and (5) no neurological, psychiatric or sensorial disorders. To be included, all subjects had to have right hand dominance (≥+9 points based on the Briggs and Nebes Inventory) and no history of hand switching during their lifetime.
Overall, by the adoption of the above criteria we ended with 11 DYS+, 10 DYS−, 10 NR+, and 16 NR− to form the four groups for this study.
2.3. MRI acquisitions
Magnetic resonance images were collected with a 3 T Philips Achieva scanner (Best, The Netherlands). High-resolution anatomical scans were acquired using a T1-weighed 3D turbo field echo pulse sequence with the following parameters: repetition time = 8.06 msec, echo time = 4 msec, voxel size = .90 × .90 × 1 mm, number of slices = 150, matrix size = 245 × 256. Diffusion tensor images were acquired with echo planar imaging DTI pulse sequences and the following acquisition parameters: repetition time = 9775 msec, echo time = 58 msec, sense reduction factor = 2, voxel size = 1.835 × 1.835 × 2.3 mm, b = 1000 sec/mm2, 35 non-collinear directions of the diffusion gradients.
2.4. VB-DTI analysis
After correction for Eddy currents, tensor was calculated from DTI and FA maps were created using Brainvisa Software (www.brainvisa.info). T2 (b = 0) images were first spatially normalised to the echo planar imaging template and used for the calculation of normalisation parameters. It must be acknowledged that a VB-DTI approach may be sensitive to problems concerning the precise overlap between the same regions in different brains, which may lead to inaccurate registrations, especially in regions close to the edges of very different FA values such as ventricles. In order to limit the effects of miss-registration, co-registered FA maps were carefully inspected and successively masked in order to include only white matter voxels before normalisation parameters were applied on these maps to create FA templates. Original tensor maps were then normalised to these new templates. Maps were smoothed (6 × 6 × 6 mm3) and were then analyzed with SPM5 software on a voxel-by-voxel basis. Specifically, tensor maps were entered in a full-factorial analysis of variance (ANOVA) model including the four groups (DYS+, DYS−, NR+ and NR−) as a between-group factor (independent measurements, equal variance not assumed). The critical threshold for identifying significant FA differences was set at p < .05 false discovery rate (FDR) corrected at the voxel level (k = 100 voxels). The anatomical localization of statistically significant clusters was investigated in SPM5 using probabilistic maps (Bürgel et al., 2006; Hua et al., 2008) and the Anatomy toolbox (Eickhoff et al., 2005) and white matter atlases included in FSL (www.fmrib.ox.ac.uk/fsl).
2.5. VB-DTI correlation analysis
A composite of all dyslexia-related traits was derived for each subject with dyslexia by averaging neuropsychological z-scores (hereafter: average reading). By using SPM5, we tested the DCDC2d effects upon the correlation patterns between FA and average reading. First, we tested the a priori hypothesis that the correlation patterns between FA and average reading differed in DYS+ versus DYS− in fibre tracts which have been shown to be highly relevant for dyslexia (see Fig. 1). Second, we performed a multiple regression analysis for correlation of FA with average reading including the four groups, i.e., DYS+, DYS−, NR+ and NR−. To identify regions that were strongly engaged in reading, we restricted the analyses to fibre tracts that showed FA increase with average reading in a progressive mode in NR−, chosen as a reference point, using an inclusive mask procedure. For the identified fibre tracts with an a priori hypothesis, the critical threshold was set at p < .0042 uncorrected at the voxel level (Bonferroni correction for 12 between-group post-hoc tests) for each tract.
3. Results
3.1. Demographic assessment, genotypes and neuropsychological characteristics of participants
Table 2a summarizes descriptives and related statistics for the socio-demographic measures and Adult Dyslexia Checklist score in the four groups. At the Bonferroni-corrected level of significance, there were significant group differences in education, IQ and Adult Dyslexia Checklist scores. All measures displayed acceptable distribution as tested by the Shapir-o–Wilk test of normality, except for education (p = .008 and p = .004, respectively, in NR− and NR+) and socio-economic status (p < .001 in NR−). A test of homogeneity of variance for these data showed no significant differences across groups. We found a strong correlation between age and education (r = .737, p < .001); the correlations were weak or non-significant for the remaining comparisons (Table S1, Supplemental Material). Table 2b shows post-hoc groups’ comparisons for the socio-demographic measures and Adult Dyslexia Checklist score in the four groups. At the Bonferroni-corrected level of significance, significant differences were found in subjects with dyslexia versus normal readers for age, education, socio-economic status and IQ. The Adult Dyslexia Checklist was significantly different in subjects with dyslexia versus normal readers, as expected, but, importantly, it was not significantly different in DYS+ versus DYS−. No socio-demographic measures differed significantly in DYS+ versus DYS−, and in NR+ versus NR−. Genotypes and neuropsychological scores are reported for each subject DYS+ and DYS− in Tables S2 and S3 (Supplemental Material). Descriptives and related statistics for neuropsychological tests are presented in Table 3. Group mean differences were tested by non-parametric Kruskal–Wallis ANOVA, given that most distributions deviated significantly from normality. Table S4 (Supplemental Materials) shows post-hoc groups’ comparisons for the neuropsychological variables in the four groups. At the Bonferroni-corrected level of significance, post-hoc analyses revealed significantly worse performances in all tasks for individuals with dyslexia (either with or without DCDC2d) compared to normal readers (either with or without DCDC2d). Performance on all neuropsychological tests was not significantly different in DYS+ versus DYS−, and in NR+ versus NR− (Table S4, Supplemental Materials). Average reading did not differ in DYS+ versus DYS− (Mann–Whitney U = 54.00, Z = −.07, p = .94) and in NR+ versus NR− (Mann–Whitney U = 95.00, Z = .79, p = .45). Box-and-whisker plots revealed no significant statistical outliers in the four groups for averaging reading.
Table 2a.
Means, SDs, distribution scores and related statistics of socio-demographic measures and Adult Dyslexia Checklist score in subjects with dyslexia and normal readers. Bonferroni-corrected level of significance p = .008 (.05 divided by six comparisons).
| Total sample n = 47 | DYS+ n = 11 | DYS− n = 10 | NR+ n = 10 | NR− n = 16 | ANOVA p-value | Test of homogeneity of variance p-value | |
|---|---|---|---|---|---|---|---|
| Sex (males) | 26 | 5 | 6 | 5 | 10 | .809 | n.a. |
| Age | |||||||
| Mean (SD) | 18.02 (2.24) | 17.55 (2.38) | 16.40 (.97) | 19.10 (1.91) | 18.69 (2.38) | .019 | .024 |
| Skewness | .30 | −.04 | .81 | −.77 | .32 | ||
| Kurtosis | −.86 | −1.48 | .69 | 1.71 | −1.2 | ||
| Shapiro–Wilk test of normality p-value | .021 | .448 | .015 | .528 | .040 | ||
| IQ | |||||||
| Mean (SD) | 108.94 (16.14) | 104.00 (16.14) | 93.80 (5.49) | 117.80 (13.46) | 116.25 (14.78) | <.001 | .043 |
| Skewness | .54 | 1.35 | −.12 | .88 | −.06 | ||
| Kurtosis | −.79 | .98 | −1.09 | .44 | −1.17 | ||
| Shapiro–Wilk test of normality p-value | .009 | .028 | .511 | .403 | .20 | ||
| Education | |||||||
| Mean (SD) | 11.49 (1.68) | 10.18 (1.78) | 10.80 (1.23) | 12.80 (1.55) | 12.00 (1.10) | .001 | .229 |
| Skewness | −.40 | .45 | .47 | −1.61 | −1.39 | ||
| Kurtosis | −.65 | −.89 | −.54 | 4.54 | 2.52 | ||
| Shapiro–Wilk test of normality p-value | .004 | .184 | .389 | .008 | .004 | ||
| Socio-economic status | |||||||
| Mean (SD) | 63.40 (22.77) | 48.18 (24.01) | 64.00 (16.47) | 61.00 (26.01) | 75.00 (17.89) | .029 | .667 |
| Skewness | −.65 | −.16 | −.81 | −.80 | −.56 | ||
| Kurtosis | −.06 | .12 | 1.24 | .13 | −1.70 | ||
| Shapiro–Wilk test of normality p-value | .001 | .337 | .045 | .294 | <.001 | ||
| Adult Dyslexia Checklist | |||||||
| Mean (SD) | 6.49 (5.88) | 13.91 (3.99) | 9.40 (2.46) | 2.50 (2.73) | 1.56 (1.86) | <.001 | .011 |
| Skewness | .54 | −1.05 | .26 | .82 | 1.31 | ||
| Kurtosis | −1.03 | −.49 | −1.07 | −.69 | 1.05 | ||
| Shapiro–Wilk test of normality p-value | <.001 | .003 | .502 | .101 | .004 | ||
Table 2b.
Post-hoc groups’ comparisons for the sociodemographic measures and Adult Dyslexia Checklist score in the four study groups.
| Mann–Whitney U | Monte Carlo significancea | ||
|---|---|---|---|
| DYS+ versus DYS− |
Age | 40.0 | .15 |
| IQ | 34.0 | .07 | |
| Adult Dyslexia | 20.5 | .01 | |
| Checklist | |||
| Education | 41.5 | .18 | |
| Socio-economic status | 31.5 | .04 | |
| DYS+ versus NR− |
Age | 65.5 | .14 |
| IQ | 47.5 | .02 | |
| Adult Dyslexia | .0 | <.001 | |
| Checklist | |||
| Education | 36.0 | .004 | |
| Socio-economic status | 32.0 | .002 | |
| DYS+ versus NR+ |
Age | 35.5 | .09 |
| IQ | 23.5 | .01 | |
| Adult Dyslexia | 1.0 | <.001 | |
| Checklist | |||
| Education | 15.5 | .002 | |
| Socio-economic status | 36.0 | .10 | |
| DYS− versus NR− |
Age | 31.5 | .01 |
| IQ | 11.5 | <.001 | |
| Adult Dyslexia | .5 | <.001 | |
| Checklist | |||
| Education | 36.5 | .02 | |
| Socio-economic status | 52.0 | .13 | |
| DYS− versus NR+ |
Age | 11.5 | .001 |
| IQ | .5 | <.001 | |
| Adult Dyslexia | 2.5 | <.001 | |
| Checklist | |||
| Education | 14.5 | .003 | |
| Socio-economic status | 48.5 | .46 | |
| NR− versus NR+ |
Age | 65.5 | .23 |
| IQ | 77.0 | .44 | |
| Adult Dyslexia | 52.5 | .24 | |
| Checklist | |||
| Education | 42.5 | .02 | |
| Socio-economic status | 55.0 | .09 |
Bonferroni-corrected level of significance: p = .008, i.e., .05 divided by six comparisons.
Table 3.
Descriptive analyses of neuropsychological variables in the four study groups.
| Neuropsychological task |
DYS+
|
DYS−
|
NR−
|
NR+
|
Non-parametric One-way ANOVAa |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Skewness | Kurtosis | Mean | SD | Skewness | Kurtosis | Mean | SD | Skewness | Kurtosis | Mean | SD | Skewness | Kurtosis | X2 | df | p | |
| Word reading, error | −2.00 | 2.40 | −1.04 | .02 | −2.50 | 2.60 | −.63 | −1.14 | .46 | .48 | −2.38 | 5.31 | .25 | .71 | −1.36 | .11 | 26.54 | 3 | <.001 |
| Word reading, speed | −3.54 | 2.65 | −.98 | .71 | −2.69 | 1.98 | −.38 | −.64 | .66 | .51 | −.21 | −.53 | 1.00 | .63 | .16 | −.66 | 33.78 | 3 | <.001 |
| Non-word reading, error | −.79 | 1.21 | −.63 | −1.23 | −1.52 | 1.32 | −.62 | 1.31 | .88 | .36 | −.25 | −1.36 | .94 | .40 | −.86 | .91 | 29.82 | 3 | <.001 |
| Non-word reading, speed | −4.63 | 4.46 | −1.30 | 1.37 | −2.95 | 2.53 | −1.41 | 2.20 | .69 | .73 | .70 | .54 | 1.23 | 1.07 | .73 | −.55 | 32.41 | 3 | <.001 |
| Sentences containing homophones | −3.85 | 4.93 | −.46 | −1.75 | −2.50 | 3.05 | −1.59 | 1.85 | −.18 | 1.31 | −2.29 | 6.35 | .10 | .52 | .48 | −2.28 | 10.74 | 3 | .013 |
| Letter forward span | −1.42 | .78 | .99 | 1.51 | −1.47 | 1.33 | −.17 | −1.35 | .54 | .61 | −.42 | −.59 | .54 | .76 | −.67 | .15 | 27.15 | 3 | <.001 |
| Letter backward span | −1.12 | .95 | −.03 | −1.05 | −.73 | 1.35 | 1.23 | .71 | .44 | .83 | −.26 | −.08 | 1.21 | 1.31 | −1.09 | .72 | 19.27 | 3 | <.001 |
| Number forward span | −1.45 | .83 | .84 | .17 | −1.55 | .46 | .69 | −.22 | .29 | .61 | −.47 | .34 | −.10 | .94 | .94 | 2.64 | 28.78 | 3 | <.001 |
| Number backward span | −.94 | .53 | −1.97 | 4.79 | −.90 | .63 | −.41 | −.53 | .50 | 1.05 | .80 | −.52 | .60 | .97 | .44 | −.64 | 26.20 | 3 | <.001 |
| Syllable displacement, accuracy | −1.13 | 1.57 | −.51 | −.87 | −1.34 | 1.63 | −.22 | .45 | .34 | .73 | −1.26 | 1.05 | .59 | .34 | −.08 | 2.17 | 15.06 | 3 | .002 |
| Syllable displacement, speed | −5.04 | 4.63 | −.94 | −.63 | −5.24 | 3.13 | −1.41 | 1.59 | .24 | 1.12 | −.97 | 1.24 | .48 | 1.00 | −.32 | −1.17 | 30.57 | 3 | <.001 |
| Spoonerism, accuracy | −1.27 | 1.30 | −.23 | −.85 | −2.66 | 1.03 | .03 | −1.24 | .55 | .47 | −.26 | −1.35 | .11 | .82 | .04 | −1.45 | 29.28 | 3 | <.001 |
| Spoonerism, speed | −3.02 | 1.80 | −.71 | 1.03 | −4.21 | 2.33 | −.55 | 2.16 | .23 | .96 | −.70 | .14 | .08 | 1.32 | −.60 | .20 | 29.78 | 3 | <.001 |
| Phonemic blending, accuracy | −1.23 | 1.07 | .25 | −.43 | −1.59 | 1.48 | .15 | −1.36 | −.04 | 1.00 | −1.14 | .79 | .02 | .78 | −.50 | .90 | 13.64 | 3 | .003 |
| Phonemic blending, speed | −3.34 | 4.87 | −2.27 | 5.19 | −1.81 | 3.22 | −1.07 | .13 | .11 | 1.00 | −.96 | .22 | .28 | 1.67 | −1.39 | .91 | 13.42 | 3 | .004 |
Bonferroni-corrected level of significance: p = .003, i.e., .05 divided by 15 comparisons.
3.2. VB-DTI analysis
Significant differences in several white matter tracts between groups emerged, and were optimally localized based on the post-mortem white matter fibre tract atlas (Bürgel et al., 2006) and the DTI-derived atlas (Catani & Thiebaut de Schotten, 2008) based on virtual in vivo dissections of diffusion tensor datasets.
To control for confounders, age, socio-economic status and IQ were entered as covariates in subsequent analyses of imaging data. Although education was significantly different between subjects with dyslexia and normal readers (Table 2b), it was not used as an additional covariate to avoid multi-collinearity issues, since it showed a strong correlation with age.
First, we performed an F-contrast testing for significant differences among the four groups (at p < .001 uncorrected at the voxel level). The ANCOVA revealed significant differences in the left temporal segment of the arcuate fasciculus (x = −48, y = −38, z = 2; cluster = 22 voxels) and in the splenium of the corpus callosum (x = −24, y = −42, z = 4; cluster = 29 voxels). Plots of parameter estimates for each group revealed that these differences were related to a main effect of DCDC2d, i.e., lower FA in the two above mentioned regions for groups with DCDC2d compared to groups without DCDC2d (Fig. 2).
Fig. 2.
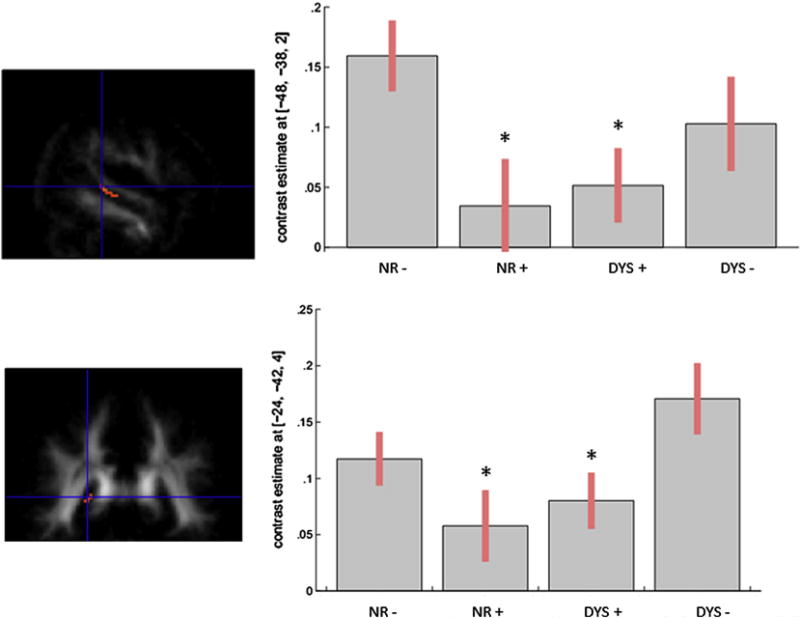
Main effect of DCDC2d for FA values in the arcuate fasciculus (upper part: x = −48, y = −38, z = 2) and in the splenium of the corpus callosum (lower part: x = −24, y = −42, z = 4) in the four groups. Plots of parameter estimates of FA values are represented for each tract for the four groups.
In post-hoc analyses, DYS+ versus NR− showed FA decreases in multiple anatomical fibre tracts. The altered fibres belonged to the genu and body of the corpus callosum and to the parietal segment of the superior longitudinal fasciculus bilaterally and, in the left hemisphere, to the arcuate fasciculus (dorsal and ventral segments), to the inferior longitudinal fasciculus (occipital–temporal and occipital segments) extending posterior to the optical radiations, as well as to the inferior cerebellar pedunculus. Noteworthy, the reverse comparison of FA did not show any above-threshold results (Fig. 3 left panel; Table 4A). Subjects with DYS− versus NR− showed significant FA decreases in the left hemisphere, namely in the inferior longitudinal fasciculus (in the occipital segment extending anterior to the temporal portion), in the dorsal segment of the arcuate fasciculus and in the splenium of the corpus callosum. Lower FA emerged also in the left inferior cerebellar pedunculus and in the medium cerebellar peduncles bilaterally, as well as in the genu and body of the corpus callosum in the right hemisphere. The reverse comparison revealed no significant differences (Fig. 3 right panel; Table 4B). DYS+ versus DYS− showed FA decreases in the left hemisphere, i.e., in the inferior longitudinal fasciculus extending to the acoustic radiation, and in the right hemisphere in the inferior longitudinal fasciculus (anterior temporal portion) and extensively in the genu of the corpus callosum. No regions with significant lower FA values were detected in DYS− versus DYS+ (Table 4C). When NR+ were compared to NR−, FA reductions were found in the genu of the corpus callosum bilaterally and in the body of the corpus callosum in the right hemisphere (Table 4D). In addition, we found FA increases in the left hemisphere, in the inferior longitudinal fasciculus (in proximity of the acoustic radiations), in the dorsal segment of the arcuate fasciculus, and in the inferior fronto-occipital fasciculus (temporal segment), and in the right hemisphere, in the inferior fronto-occipital fasciculus, and in the body and splenium of corpus callosum (Table 4E). Finally, to determine commonalities, i.e., common regions with lower FA in subjects with dyslexia, we employed a conjunction analysis (under the null hypothesis) for the contrasts ‘lower FA in DYS+ versus NR−’ and ‘lower FA in DYS− versus NR−’. The statistical parametric mapping (SPM) conjunction map (with threshold set at p < .001 uncorrected; k > 10) revealed common differences mainly located in the left hemisphere, i.e., the occipital and occipito-temporal segments of the inferior longitudinal fasciculus, both the dorsal and ventral segments of the arcuate fasciculus and the splenium of corpus callosum. In the right hemisphere, lower common FA values were in the genu and body of the corpus callosum and, bilaterally in the parietal segment of the superior longitudinal fasciculus (Table 4F).
Fig. 3.
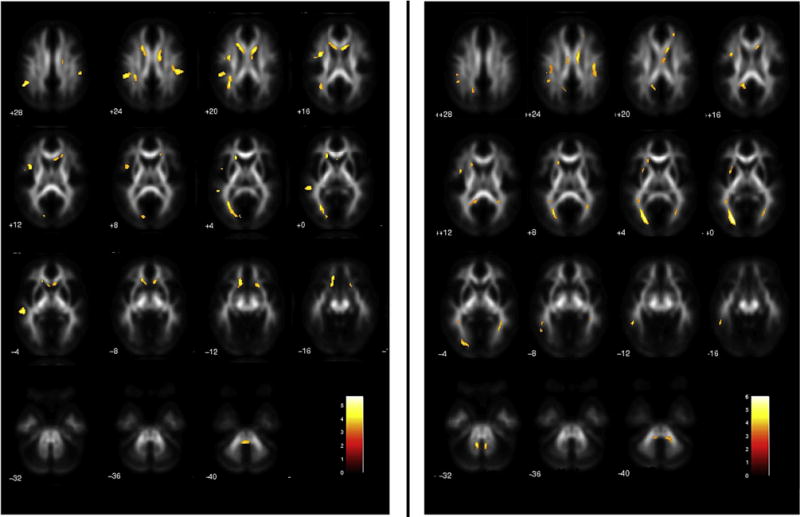
Statistical parametric maps of decreased FA in DYS + versus NR− (left panel), and in DYS − versus NR− (right panel). Threshold: p < .05 FDR corrected at the voxel level (k > 100 voxels).
Table 4.
Regions with decreased or increased FA resulting from groups’ contrasts of interest as an output of the full-factorial ANCOVA model. Only significant differences thresholded at p = .05, FDR corrected at the voxel level and a cluster extent of 100 voxels are reported.
| Regions | Left/right | Cluster extent | Z | Coordinates
|
|||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A Decreased FA DYS+ versus NR− | |||||||
| Left hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | L | 192 | 4.66 | −48 | −50 | 32 | |
| Superior longitudinal fasciculus (parietal segment) | L | * | 4.29 | −48 | −38 | 22 | |
| Arcuate fasciculus (dorsal segment) | L | 145 | 4.28 | −34 | 0 | 18 | |
| Arcuate fasciculus (dorsal segment) | L | * | 3.46 | −44 | −4 | 18 | |
| Arcuate fasciculus (dorsal segment) | L | * | 3.43 | −34 | −10 | 22 | |
| Arcuate fasciculus (ventral segment) | L | 111 | 4.08 | −50 | −30 | −2 | |
| Arcuate fasciculus (ventral segment) | L | * | 4.01 | −52 | −36 | 0 | |
| Inferior longitudinal fasciculus (occipital–temporal segment) | L | 168 | 4.09 | −30 | −66 | 4 | |
| Inferior longitudinal fasciculus (occipital segment) | L | * | 3.72 | −26 | −76 | 2 | |
| Optic radiation | L | * | 3.4 | −12 | −88 | 8 | |
| Corpus callosum (genu) | L | 262 | 3.96 | −16 | 24 | 0 | |
| Corpus callosum (genu) | L | * | 3.93 | −14 | 22 | −14 | |
| Corpus callosum (body) | L | 164 | 3.81 | −16 | 20 | 20 | |
| Corpus callosum (body) | L | * | 3.7 | −10 | 14 | 18 | |
| Corpus callosum (body) | L | * | 3.42 | −12 | 4 | 24 | |
| Corpus callosum (splenium) | L | 123 | 3.99 | −34 | −52 | 20 | |
| Corpus callosum (splenium) | L | * | 3.8 | −30 | −40 | 22 | |
| Inferior cerebellar pedunculus | L | 244 | 4.01 | −4 | −40 | −46 | |
| Right hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | R | 100 | 4.14 | 52 | −30 | 26 | |
| Corpus callosum (genu) | R | 225 | 4.04 | 12 | 10 | 20 | |
| Corpus callosum (genu) | R | * | 3.31 | 20 | 24 | 12 | |
| Corpus callosum (genu) | R | * | 3.86 | 20 | 20 | −14 | |
| Corpus callosum (body) | R | * | 3.74 | 14 | −4 | 24 | |
| B Decreased FA DYS− versus NR− | |||||||
| Left hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | L | 101 | 3.59 | −36 | −26 | 26 | |
| Superior longitudinal fasciculus (parietal segment) | L | * | 3.76 | −46 | −48 | 32 | |
| Arcuate fasciculus (dorsal segment) | L | 102 | 3.65 | −18 | 24 | 4 | |
| Arcuate fasciculus (dorsal segment) | L | * | 3.55 | −20 | 18 | 10 | |
| Arcuate fasciculus (dorsal segment) | L | * | 3.52 | −28 | 6 | 2 | |
| Arcuate fasciculus (dorsal segment) | L | * | 3.29 | −26 | 14 | 0 | |
| Inferior longitudinal fasciculus (occipital segment) | L | 283 | 5.04 | −26 | −78 | 0 | |
| Inferior longitudinal fasciculus (temporo-occipital segment) | L | * | 4.65 | −30 | −70 | 2 | |
| Inferior longitudinal fasciculus (temporo-occipital segment) | L | * | 4.5 | −30 | −68 | 2 | |
| Inferior longitudinal fasciculus (temporal segment) | L | * | 3.2 | −30 | −62 | 8 | |
| Corpus callosum (splenium) | L | 101 | 3.76 | −12 | −52 | 16 | |
| Corpus callosum (splenium) | L | * | 3.56 | −12 | −58 | 22 | |
| Corpus callosum (splenium) | L | * | 3.46 | −18 | −60 | 28 | |
| Cerebellar pedunculus medius | L | 141 | 4.05 | −8 | −50 | −32 | |
| Cerebellar pedunculus inferior | L | * | 3.87 | −6 | −40 | −46 | |
| Right hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | R | ||||||
| Corpus callosum (genu) | R | 124 | 3.99 | 12 | 8 | 22 | |
| Corpus callosum (body) | R | * | 4.06 | 12 | 0 | 24 | |
| Cerebellar pedunculus mediusa | R | * | 3.85 | 8 | −40 | −50 | |
| C Decreased FA in DYS+ versus DYS− | |||||||
| Left hemisphere | |||||||
| Inferior longitudinal fasciculus (acoustic radiations) | L | 148 | 4.9 | −36 | −24 | 12 | |
| Right hemisphere | |||||||
| Inferior longitudinal fasciculus (anterior temporal portion) | R | 573 | 5.64 | 52 | −22 | −8 | |
| Inferior longitudinal fasciculus (anterior temporal portion) | R | * | 4.67 | 50 | −14 | −14 | |
| Inferior longitudinal fasciculus (anterior temporal portion) | R | * | 4.29 | 52 | −6 | −20 | |
| Corpus callosum (genu) | R | 877 | 4.86 | 6 | 40 | 6 | |
| Corpus callosum (genu) | R | * | 4.64 | 2 | 34 | 26 | |
| Corpus callosum (genu) | R | * | 4.12 | 4 | 36 | 14 | |
| Corpus callosum (genu) | R | 159 | 4.53 | 20 | 26 | 2 | |
| Corpus callosum (genu) | R | * | 3.66 | 10 | 16 | 0 | |
| Corpus callosum (genu) | R | * | 3.3 | 16 | 0 | 20 | |
| D Decreased FA in NR+ versus NR− | |||||||
| Left hemisphere | |||||||
| Corpus callosum (genu) | L | 190 | 6.2 | −18 | 18 | 8 | |
| Right hemisphere | |||||||
| Corpus callosum (genu) | R | 156 | 3.81 | 14 | 22 | 12 | |
| Corpus callosum (body) | R | * | 4.19 | 12 | −4 | 22 | |
| E Increased FA in NR+ versus NR− | |||||||
| Left hemisphere | |||||||
| Arcuate fasciculus (dorsal segment) | L | 162 | 4.77 | −28 | 26 | 2 | |
| Inferior occipito-frontal fasciculus | L | * | 3.37 | −32 | 6 | −12 | |
| Inferior longitudinal fasciculus (acoustic radiations) | L | 303 | 5.5 | −44 | −30 | 8 | |
| Inferior longitudinal fasciculus (acoustic radiations) | L | * | 4.82 | −36 | −34 | 12 | |
| Right hemisphere | |||||||
| Inferior fronto-occipital fasciculus | R | 506 | 5.46 | 44 | −78 | −12 | |
| Inferior fronto-occipital fasciculus | R | * | 4.92 | 42 | −76 | −2 | |
| Inferior fronto-occipital fasciculus | R | * | 3.92 | 34 | −80 | −20 | |
| Corpus callosum (body) | R | 455 | 3.7 | 6 | 24 | 18 | |
| Corpus callosum (splenium) | R | * | 4.45 | 6 | −20 | 32 | |
| Corpus callosum (splenium) | R | * | 3.82 | 8 | −32 | 22 | |
| F Common regions with decreased FA in both groups of subjects with dyslexia | |||||||
| Left hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | L | 13 | 3.72 | −46 | −48 | 32 | |
| Arcuate fasciculus (dorsal segment) | L | 33 | 3.94 | −38 | 6 | 14 | |
| Arcuate fasciculus (ventral segment) | L | 23 | 3.89 | −50 | −48 | −12 | |
| Inferior longitudinal fasciculus (occipital–temporal segment) | L | 96 | 4.59 | −30 | −66 | 4 | |
| Inferior longitudinal fasciculus (occipital segment) | L | * | 4.09 | −26 | −76 | 2 | |
| Corpus callosum (splenium) | L | * | 3.4 | −30 | −62 | 6 | |
| Right hemisphere | |||||||
| Superior longitudinal fasciculus (parietal segment) | R | 18 | 3.42 | 42 | −26 | 24 | |
| Corpus callosum (genu) | R | 68 | 3.9 | 12 | 6 | 22 | |
| Corpus callosum (body) | R | * | 3.54 | 12 | −4 | 24 | |
Multiple peaks within the cluster are shown in subsequent lines. The SPM conjunction map was thresholded at p < .001 uncorrected with a minimum cluster extent of 10 voxels.
This peak is part of the cluster with maxima at −8 −40 −50 located in the left hemisphere.
To further investigate potential confounds effects due to age differences across groups, we performed pairwise comparisons of FA values (at p < .05 FDR corrected at the voxel level; k = 100 voxels), using age-matched subgroups and including the covariates that remained significant for each comparison. Two sets of subgroups were extracted and submitted to two-sample t-test analyses: (1) 11 DYS+ versus 11 age-matched NR− (p-values for age = .709, socio-economic status = .009, IQ = .13 by Student’s T-tests; socio-economic status was used as covariate in subsequent analyses of FA values); (2) 10 DYS− versus 10 age-matched NR− (age = .15, socio-economic status = .009, IQ = .002 by Student’s T-tests; socio-economic status and IQ were used as covariates in subsequent analyses of FA values). When DYS+ were compared to NR−, we found FA decreases in the genu and body of the corpus callosum, in the parietal segment of the superior longitudinal fasciculus and in the arcuate fasciculus (dorsal and ventral segments; see Fig. S1a, Supplemental Material). By applying a more lenient threshold (at p < .005 uncorrected; k > 10), FA decrease emerged also in the inferior longitudinal fasciculus (occipital–temporal and occipital segments; Fig. S1b, Supplemental Material). When DYS− were compared to NR−, findings completely overlapped those observed in the analogous post-hoc analyses of the ANCOVA (Fig. S2, Supplemental Material).
To exclude collinearity between age and socio-economic status (both linear and non-linear effects) and FA values across the whole brain, we performed correlation analyses in DYS+ and DYS−. A significant positive correlation (at p < .001 uncorrected at the voxel level; k > 50 voxels) between age (linear) and FA values was found in the left inferior longitudinal fasciculus, temporal segment (x = −46, y = −8, z = −16; Z = 4.05) and the right arcuate fasciculus, ventral segment (x = 58, y = −48, z = −24; Z = 3.49). Noteworthy, these correlations were outside the fibre tracts where significant differences in FA values were found in VB-DTI analyses in DYS+ and DYS− when compared to NR−, i.e., inferior longitudinal fasciculus, occipital–temporal and occipital segment, and left arcuate fasciculus, ventral segment (Fig. 3; Table 4A and B). No other significant correlations were found.
3.3. VB-DTI correlation analysis
In the first set of analyses, we focused on fibre tracts relevant for dyslexia (Fig. 1). We found significant differences in the correlation patterns between FA and average reading in DYS− versus DYS+, in the following left-hemispheric fibre tracts: arcuate fasciculus (ventral segment: x = −38, y = −26, z = −8; cluster = 22 voxels), inferior longitudinal fasciculus (temporal segment: x = −46, y = −6, z = −10; cluster = 40 voxels), arcuate fasciculus (dorsal segment: x = −22, y = 0, z = 12; cluster = 70 voxels), superior longitudinal fasciculus/arcuate fasciculus (dorsal segment: x = −14, y = −8, z = 32; cluster = 135 voxels) and inferior frontal–occipital fasciculus (x = −30, y = 6, z = −8; cluster = 43 voxels; Fig. 4). To explore the different patterns of correlation in the two groups, plots of parameter estimates for local maxima for each cluster were computed. As displayed in Fig. 4, the correlation between FA and average reading in DYS− was positive in all fibre tracts, meaning that a better average reading paralleled higher FA and therefore better organized fibre bundles, and it was significantly stronger in each site compared to the correlation found in DYS+.
Fig. 4.
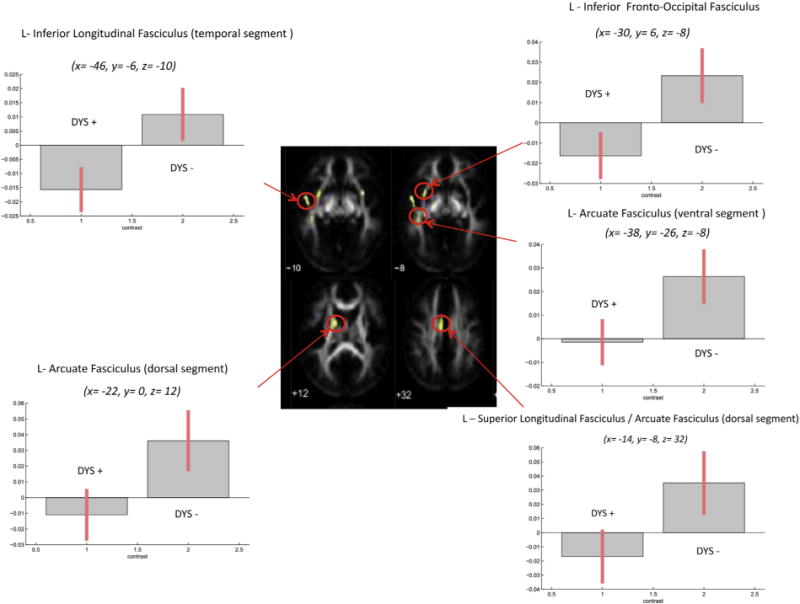
Fibre tracts exhibiting significant positive correlations of FA and average reading in DYS − versus DYS +. Plots of parameter estimates are represented for each tract for the two groups. Bars indicate correlation coefficients, which are measures of the strength of the relationship of FA and average reading.
In the second set of analyses, fibre tracts exhibiting significant correlations between FA and average reading in NR− (identified by inclusive masking procedure, see Section 2.5) included the splenium of the corpus callosum (x = −12, y = −50, z = 12; cluster = 97 voxels) and the optic radiations (x = −26, y = −80, z = 0; cluster = 12 voxels) in the left hemisphere (Fig. 5). Inclusive masking analyses revealed significant differences in the correlation patterns between FA values and average reading in DYS− versus DYS+ in both the splenium of the corpus callosum and optic radiations; correlations in DYS− were positive and stronger compared to correlations in DYS+. Furthermore, significant differences in the correlation patterns emerged in NR− versus NR+ in the splenium of the corpus callosum but not in the optic radiations, and in NR− versus both DYS+ and DYS− in both the splenium of the corpus callosum and optic radiations.
Fig. 5.
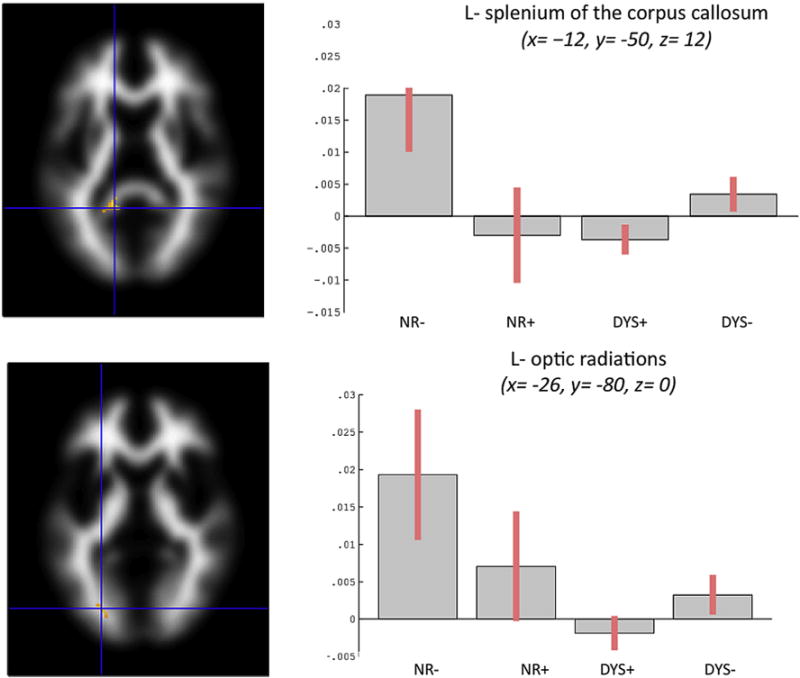
Fibre tracts exhibiting significant positive correlations of FA and average reading in NR− (identified by inclusive masking procedure). Bars indicate correlation coefficients, which are measures of the strength of the relationship of FA and average reading. Significant differences in correlation patterns were found in DYS − versus DYS + in both the splenium of the corpus callosum and optic radiations. Furthermore, significant differences emerged in NR− versus NR+ in the splenium of the corpus callosum but not in the optic radiations, and in NR− versus both DYS + and DYS − in both the splenium of the corpus callosum and optic radiations.
4. Discussion
The major contribution of our study was to provide clear, in vivo evidence of white matter disorganization related to the DCDC2-mediated genetic vulnerability. There is a relative paucity of studies relating genes, brain and behaviour in the developmental cognitive neurosciences, and in dyslexia specifically. Since genes are distal contributors whereas the brain is the proximal driver of human behaviour, we believe that data on the anatomical pathways from genes to behaviour are essential to the field.
Overall, we found FA alterations in all groups with DCDC2d included in this study, both normal readers and subjects with dyslexia. DCDC2d accounted per se and irrespective of dyslexia for lower FA in the left temporal segment of the arcuate fasciculus and in the splenium of the corpus callosum (Fig. 2). Moreover, NR+ compared to NR− showed several FA increases and some decreases in numerous white matter tracts bilaterally (Table 4D and E), and significantly weaker correlations between FA and average reading in the splenium of the corpus callosum (Fig. 5). Our findings are consistent with previously reported data using MRI in healthy subjects. Meda et al. (2008) found that DCDC2d carriers had increased grey matter volume in multiple brain regions of the left hemisphere compared to non-carriers. In a fMRI study, Cope et al. (2012) found significant associations between DCDC2/READ1 and brain activation in the left anterior inferior parietal lobe and in the right lateral occipital temporal gyrus during reading tasks, and a nominally significant association between DCDC2d and activation in the left anterior inferior parietal lobule. Darki et al. (2012) found that the DCDC2 gene was associated with the left temporo-parietal region. Taken together, DCDC2d-related anatomical patterns, irrespective of dyslexia, may mark some sub-threshold, developmental vulnerability to cognitive deficits. Future studies in this field, which should include the assessment of a wider range of cognitive processes, may be able to address this question.
DYS+ showed decreased FA when compared to NR−; this was mainly found in the left hemisphere, including the arcuate, the superior longitudinal and inferior longitudinal fasciculi. FA reductions were not confined to the left temporoparietal and frontal bundles; rather, they pertained extensively to the genu and body of the corpus callosum, supporting the idea that DCDC2d is associated to widespread white matter abnormalities in dyslexia (Fig. 3 left panel; Table 4A). Interestingly, these abnormalities (1) were found in fibre bundles that have been consistently shown to connect the crucial components of the reading network (Wandell & Yeatman, 2013), (2) were found altered in previous DTI studies of dyslexia (Table 1; Fig. 1), and (3) were similar to those found altered in DYS− (Table 4B). Not surprisingly, the conjunction analysis in the two groups of subjects with dyslexia revealed commonalities in the FA alterations that were mainly located in the left hemisphere, i.e., in the inferior longitudinal and arcuate fasciculi, and in the splenium of corpus callosum. Lower common FA values were also found in the right genu and body of the corpus callosum and in the superior longitudinal fasciculus, bilaterally (Table 4F). These findings show that DCDC2d contributes to a prevailing anatomical pattern that is common to dyslexia of unspecified origin. Consistently with what has been established in dyslexia from diverse cultural backgrounds (Paulesu et al., 2001), here we show for the first time that aetiologically different forms of dyslexia, with/without DCDC2d, share common white matter alterations, adding further evidence to the biological unity hypothesis of dyslexia (Paulesu et al., 2001; Ziegler, 2006). Inasmuch as white matter abnormalities were found in brain areas relevant for the integration of auditory-language processes, our findings in subjects with dyslexia and DCDC2d are consistent with the prevailing hypothesis that dyslexia results from a specific deficit of the auditory-phonological representation (Gabrieli, 2009; Goswami, 2011; Ramus, 2003; Vellutino, Fletcher, Snowling, & Scanlon, 2004). Nevertheless, abnormalities were also found in the optic radiations, in the inferior longitudinal fasciculus, in the corpus callosum and in the inferior cerebellar pedunculus, in line with other views that dyslexia might be the outcome of a multi-system impairment affecting multiple neurocognitive domains (Menghini et al., 2010; Pernet, Andersson, Paulesu, & Demonet, 2009).
When DYS+ were compared to DYS−, we found FA reductions in the same fibre bundles that had been found altered in the conjunction analysis, i.e., left inferior longitudinal fasciculus and right genu of the corpus callosum, and, additionally, in the right inferior longitudinal fasciculus (Table 4C). This finding suggests that DCDC2d in dyslexia can also account for some elements of uniqueness, which include a more severe and extended pattern of altered FA. Furthermore, DYS+ versus DYS− showed a significant weaker correlation between FA and average reading, suggesting that DCDC2d in dyslexia might also affect specific aspects of reading abilities conveyed by these fibre tracts (Fig. 4). Nevertheless, caution is warranted when dealing with single genetic variants and complex phenotypes. Multiple genes and multiple environmental factors are at the basis of dyslexia, and each factor is expected to typically account for 1–2% of the variance of the dyslexic phenotype at the most. When relating genes, brain and behaviour, complex interactive patterns are expected, rather than clear-cut simple one-to-one relationships.
In sum, our findings show brain’s white matter anatomical abnormalities in individuals with DCDC2d, regardless of the diagnosis of dyslexia. This is compatible with Galaburda, LoTurco, Ramus, Fitch, and Rosen’s (2006) hypothesis that some risk variants of genes implicated in neuronal migration influence brain development, and lead to neuroanatomical variations that belong to the ‘anatomical phenotype’ of dyslexia. Likewise, the finding that NR+ may present with anatomical alterations without developing dyslexia is in accordance with a multifactorial threshold model of the liability to reading disability. Just as dyslexia can be a common final outcome of multiple genetic and non-genetic elements of risk that vary widely across individuals, the DCDC2d-related anatomical patterns may contribute to dyslexia and/or other cognitive phenotypes depending on co-occurring risk factors.
Our results must be viewed in light of some limitations. First, the methodology adopted in the current study involving an orthogonal comparison of participants with/without DCDC2d and with/without dyslexia, given the rarity of DCDC2d, had an impact on the size of the groups, which was small, and may have indeed limited the power of the analyses. For the same reason, the matching of the experimental groups relative to age, socio-economic status and IQ was impractical to achieve, since it would have implied a further reduction of the sample size. Although we were aware that this approach could only partially solve the problem, to overcome the disparity between subjects with dyslexia and normal readers relative to age, socio-economic status and IQ, we opted to use the latter as regressors in the ANCOVA of imaging data. We additionally performed pairwise comparisons of FA values using age-matched subsamples. Given that findings from ANCOVA and pairwise comparisons were overlapping, and that age correlated with FA outside fibre tracts for which groups’ differences were found, we believe that the possibility that our findings were affected by age differences across groups is unlikely.
Second, most frequent comorbid disorder of dyslexia, Attention Deficit Hyperactivity Disorder (ADHD), was not assessed in this study. This disorder has been indeed associated to DCDC2 (Couto et al., 2009) and, we are aware of its possible comorbidity with dyslexia in our samples. Nevertheless, ADHD diagnosis in adults (such as in our sample) is very challenging and highly time-consuming, as criteria require evidence of symptom onset in preschool years, and attestation of impact on activities and school performance. Accuracy of retrospective self-reports is limited by the difficulty to recall, and clinical interviews, although being a reliable tool, are highly time-consuming (Mannuzza, Klein, Klein, Bessler, & Shrout, 2002). To ensure high levels of compliance of study subjects – as the highest possible sample size was the priority – the duration of the protocol was set between 60 and 90 min for each subject, which implied to limit the assessment to reading abilities and imaging acquisition, which were the main focus of the study.
Finally, subjects with dyslexia in our study might have had an alteration of white matter in the reading circuits as a consequence of lifetime poor reading. Supporting this possibility is the recent finding that effective remediation affected white matter in young subjects with dyslexia (Keller & Just, 2009; Meyler, Keller, Cherkassky, Gabrieli, & Just, 2008). While it is not possible to completely exclude that dysfunctional reading skills may cause alterations of white matter, when taking into account studies with adults (Vandermosten et al., 2012; Wandell & Yeatman, 2013; Fig. 1 and Table 1), children with dyslexia (Deutsch et al., 2005; Niogi & McCandliss, 2006), pre-readers with a family-history of dyslexia (Raschle, Zuk, & Gaab, 2012), and studies using reading-level matched, normal readers as controls (Hoeft et al., 2006), comparable imaging findings consistently emerge, suggesting instead a reverse causality.
While these findings need both replication in independent and larger samples and consideration of the potential limitations, our study can promote an initial understanding of the relationship between genetic and brain abnormalities in developmental disorders. Understanding the function of neuronal migration genes and their relationships with cognitive and neuroanatomical phenotypes are targets of utmost importance for future studies.
Supplementary Material
Acknowledgments
We thank all the young adults who took part in this study. We also want to express our gratitude towards A. Citterio for helping in neuropsychological data collection, G. Menozzi for technical assistance on databases and C. Saccuman and A. Iadanza for assistance in MRI scan acquisition.
Abbreviations
- VB
voxel-based
- DTI
diffusion tensor Imaging
- FA
fractional anisotropy
- DCDC2d
DCDC2/intron 2 deletion
- READ1
regulatory element associated with dyslexia 1
- DYS+
subjects with dyslexia with DCDC2d
- DYS−
subjects with dyslexia without DCDC2d
- NR+
normal readers with DCDC2d
- NR−
normal readers without DCDC2d
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cortex.2014.04.016.
References
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Becker J, Czamara D, Scerri TS, Ramus F, Csépe V, Talcott JB, et al. Genetic analysis of dyslexia candidate genes in the European cross-linguistic NeuroDys cohort. European Journal of Human Genetics. 2014;22(5):675–680. doi: 10.1038/ejhg.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Danna M, Lanzi G, Stella G, et al. Neuropsychological deficits and neural dysfunction in familial dyslexia. Brain Research. 2006;1113(1):174–185. doi: 10.1016/j.brainres.2006.06.099. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63(4):742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11(3):230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Brkanac Z, Chapman NH, Matsushita MM, Chun L, Nielsen K, Cochrane E, et al. Evaluation of candidate genes for DYX1 and DYX2 in families with dyslexia. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2007;144B(4):556–560. doi: 10.1002/ajmg.b.30471. [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Wang Y, Volz AJ, Peschansky VJ, Lisann L, Galaburda AM, et al. Postnatal analysis of the effect of embryonic knockdown and overexpression of candidate dyslexia susceptibility gene homolog DCDC2 in the rat. Neuroscience. 2008;152(3):723–733. doi: 10.1016/j.neuroscience.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29(4):1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Culture Fair: una piccola batteria di test per la misura del fattore “g”. Firenze: Organizzazioni Speciali.; 1981. [Google Scholar]
- Cope N, Eicher JD, Meng H, Gibson CJ, Hager K, Lacadie C, et al. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. Neuroimage. 2012;63(1):148–156. doi: 10.1016/j.neuroimage.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle FM, Levy T, Bergmann S, Wolf SG, Bar-El D, Sapir T, et al. Common and divergent roles for members of the mouse DCX superfamily. Cell Cycle. 2006;5(9):976–983. doi: 10.4161/cc.5.9.2715. [DOI] [PubMed] [Google Scholar]
- Cornoldi C, Colpo G. Gruppo MT Nuove prove di lettura MT per la scuola media inferiore. Firenze: Organizzazioni Speciali; 1995. [Google Scholar]
- Cornoldi C, Colpo G. Prove di lettura MT per la scuola elementare – 2. Firenze: Organizzazioni Speciali; 1998. [Google Scholar]
- Couto JM, Gomez L, Wigg K, Ickowicz A, Pathare T, Malone M, et al. Association of attention-deficit/hyperactivity disorder with a candidate region for reading disabilities on chromosome 6p. Biological Psychiatry. 2009;66(4):368–375. doi: 10.1016/j.biopsych.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F, Peyrard-Janvid M, Matsson H, Kere J, Lingberg T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biological Psychiatry. 2012;72:671–676. doi: 10.1016/j.biopsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Deffenbacher KE, Kenyon JB, Hoover DM, Olson RK, Pennington BF, DeFries JC, et al. Refinement of the 6p21.3 quantitative trait locus influencing dyslexia: linkage and association analyses. Human Genetics. 2004;115(2):128–138. doi: 10.1007/s00439-004-1126-6. [DOI] [PubMed] [Google Scholar]
- Deuel TA, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49(1):41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Diaz AL, Gleeson JG. The molecular and genetic mechanisms of neocortex development. Clinics in Perinatology. 2009;36(3):503–512. doi: 10.1016/j.clp.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Frigerio A, Vanzin L, Pastore V, Nobile M, Giorda R, Marino C, et al. The Italian preadolescent mental health project (PrISMA): rationale and methods. International Journal of Methods in Psychiatric Research. 2006;15(1):22–35. doi: 10.1002/mpr.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325(5938):280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nature Neuroscience. 2006;9(10):1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends in Cognitive Sciences. 2011;15(1):3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Harold D, Paracchini S, Scerri T, Dennis M, Cope N, Hill G, et al. Further evidence that the KIAA0319 gene confers susceptibility to developmental dyslexia. Molecular Psychiatry. 2006;11(12):1085–1091. doi: 10.1038/sj.mp.4001904. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Mirtindale JL, Meyler A, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. Journal of Neuroscience. 2006;26(42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49(1):55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Lind PA, Luciano M, Wright MJ, Montgomery GW, Martin NG, Bates TC. Dyslexia and DCDC2: normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. European Journal of Human Genetics. 2010;6:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig KU, Schumacher J, Schulte-Korne G, König IR, Warnke A, Plume E, et al. Investigation of DCDC2 intron 2 deletion/compound short tandem repeat polymorphism in a large German dyslexia sample. Psychiatric Genetics. 2008;18(6):310–312. doi: 10.1097/YPG.0b013e3283063a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 2002;159(11):1882–1888. doi: 10.1176/appi.ajp.159.11.1882. [DOI] [PubMed] [Google Scholar]
- Marino C, Meng H, Mascheretti S, Rusconi M, Cope N, Giorda R, et al. DCDC2 genetic variants and susceptibility to developmental dyslexia. Psychiatric Genetics. 2012;22:25–30. doi: 10.1097/YPG.0b013e32834acdb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, et al. Polymorphism of DCDC2 reveals differences in cortical morphology of healthy individuals – a preliminary voxel based morphometry study. Brain Imaging and Behavior. 2008;2(1):21–26. doi: 10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Powers NR, Tang L, Cope NA, Zhang PX, Fuleihan R, et al. A dyslexia-associated variant in DCDC2 changes gene expression. Behavior Genetics. 2011;41(1):58–66. doi: 10.1007/s10519-010-9408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, et al. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Benassi M, Bolzani R, Facoetti A, Giovagnoli S, et al. Different underlying neurocognitive deficits in developmental dyslexia: a comparative study. Neuropsychologia. 2010;48(4):863–872. doi: 10.1016/j.neuropsychologia.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JD, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 2008;46(10):2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Parracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ, et al. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behavior Genetics. 2011;41(1):90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nobile M, Giorda R, Marino C, Carlet O, Pastore V, Vanzin L, et al. Socioeconomic status mediates the genetic contribution of the dopamine receptor D4 and serotonin transporter linked promoter region repeat polymorphisms to externalization in preadolescence. Development and Psychopathology. 2007;19(4):1147–1160. doi: 10.1017/S0954579407000594. [DOI] [PubMed] [Google Scholar]
- Parracchini S, Ang QW, Stanley FJ, Monaco AP, Pennell CE, Whitehouse AJ. Analysis of dyslexia candidate genes in the Raine cohort representing the general Australian population. Genes Brain and Behavior. 2011;10(2):158–165. doi: 10.1111/j.1601-183X.2010.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, et al. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Démonet JF. When all hypotheses are right: a multifocal account of dyslexia. Human Brain Mapping. 2009;30(7):2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131(4):592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Powers NR, Eicher JD, Butter F, Kong Y, Miller LL, Ring SM, et al. Alleles of a polymorphic ETV6 binding site in DCDC2 confer risk of reading and language impairment. American Journal of Human Genetics. 2013;93(1):19–28. doi: 10.1016/j.ajhg.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Current Opinion in Neurobiology. 2003;13(2):212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Bigler ED. Test TEMA – Memoria e apprendimento. Edizioni Erickson; 1994. [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, et al. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. American Journal of Neuroradiology. 2008;29(6):1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: a meta-analysis of voxelbased morphometry studies. Human Brain Mapping. 2013;34(11):3055–3065. doi: 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46(6):739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori G, Job R, Tressoldi PE. Batteria per la valutazione della dislessia e della disortografia evolutiva. Firenze: Organizzazioni Speciali; 1995. [Google Scholar]
- Scerri TS, Morris AP, Buckingham LL, Newbury DF, Miller LL, Monaco AP, et al. DCDC2, KIAA0319 and CMIP are associated with reading-related traits. Biological Psychiatry. 2011;70(3):237–245. doi: 10.1016/j.biopsych.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Anthoni H, Dahdouh F, König IR, Hillmer AM, Kluck N, et al. Strong genetic evidence of DCDC2 as a susceptibility gene for dyslexia. American Journal of Human Genetics. 2006;78(1):52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, et al. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, et al. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46(13):3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific learning disability (dyslexia) what have we learned in the past four decades. Journal of Child Psychology and Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Vinegrad M. A revised adult dyslexia checklist. Educare. 1994;48:21–23. [Google Scholar]
- Wandell BA, Yeatman JD. Biological development of reading circuits. Current Opinion in Neurobiology. 2013;23:1–8. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Examiner’s manual Wechsler intelligence scale for children Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wilcke A, Weissfuss J, Kirsten H, Wolfram G, Boltze J, Ahnert P. The role of gene DCDC2 in German dyslexics. Annals of Dyslexia. 2009;59(1):1–11. doi: 10.1007/s11881-008-0020-7. [DOI] [PubMed] [Google Scholar]
- Zhong R, Yang B, Tang H, Zou L, Song R, Zhu LQ, et al. Meta-analysis of the association between DCDC2 polymorphisms and risk of dyslexia. Molecular Neurobiology. 2013;47:435–442. doi: 10.1007/s12035-012-8381-7. [DOI] [PubMed] [Google Scholar]
- Ziegler JC. Do differences in brain activation challenge universal theories of dyslexia? Brain Language. 2006;98(3):341–343. doi: 10.1016/j.bandl.2005.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


