Abstract
The perirhinal cortex (PRC) supports associative memory and perception, and PRC dysfunction impairs animals’ abilities to associate stimulus features across sensory modalities. PRC damage also leads to deficits in discriminating between stimuli that share features. While PRC-dependent stimulus discrimination has been shown to be impaired with advanced age, data regarding the abilities of older adults and other animals to form PRC-dependent associations have been equivocal. Moreover, the extent to which similar neural computations within the PRC support associative memory versus discrimination abilities have not been directly examined. In the current study, young and aged rats were cross-characterized on two PRC-dependent crossmodal object recognition (CMOR) tasks to test associative memory, and a LEGO® object discrimination task. In the CMOR tasks, rats were familiarized with an object with access to tactile input and then tested for recognition with visual input only. The relative exploration time of novel versus familiar objects indicated that aged rats showed preference for the novel over familiar object with and without an epoch of multimodal pre-exposure to the familiar object prior to the testing session. Furthermore, crossmodal recognition performance between young and aged rats was not significantly different. In contrast, for the LEGO object discrimination task, aged rats were impaired relative to young. Notably, aged rats who performed poorly on the LEGO object discrimination task had better performance on the CMOR tasks. The dissociation of discrimination and association abilities with age suggests that these behaviors rely on distinct neural computations within PRC-medial temporal lobe circuit.
Keywords: aging, medial temporal lobe, memory, recognition
Introduction
The perirhinal cortex (PRC) receives polymodal input from sensory association cortices (Burwell & Amaral, 1998a; Suzuki & Amaral, 1994), and is strongly reciprocally connected with the hippocampus and other medial temporal lobe regions (Agster & Burwell, 2013; Burwell & Amaral, 1998b; Lavenex, Suzuki, & Amaral, 2004). Through this circuitry, the PRC is poised to support the integration of sensory information within and across modalities (Holdstock, Hocking, Notley, Devlin, & Price, 2009; Jacklin, Cloke, Potvin, Garrett, & Winters, 2016; Murray & Bussey, 1999; Winters & Reid, 2010) that is incorporated into memories for specific environments or episodes that are critical for adaptive behavior. In support of this idea, lesions of the PRC impair rats’ abilities to perform a tactile-to-visual crossmodal object recognition task (Winters & Reid, 2010), even if the object is pre-sampled in both modalities prior to the task (Jacklin et al., 2016). The role of PRC in integrating stimulus features can be broadly conceptualized by suggesting that it plays a role in supporting the formation of associations between different stimuli or a stimulus and a location. For example, PRC lesions impair formation of object-place associations (Barker & Warburton, 2015; Hernandez, Reasor, et al., 2017; Jo & Lee, 2010a, 2010b), as well as object-in-context recognition (Heimer-McGinn, Poeta, Aghi, Udawatta, & Burwell, 2017).
In addition to associative memory, the PRC plays an integral role in high-level perceptual functions of the medial temporal lobe, such as object discrimination when the stimuli to be discriminated share a high degree of feature overlap, also referred to as ‘perceptual ambiguity’ (Ahn & Lee, 2015; Barense, Gaffan, & Graham, 2007; Bartko, Winters, Cowell, Saksida, & Bussey, 2007a, 2007b; Devlin & Price, 2007; Norman & Eacott, 2004). This aspect of PRC function is reported to be particularly vulnerable to the effects of advancing age, with older adults (Reagh et al., 2015; Ryan et al., 2012; Stark, Yassa, Lacy, & Stark, 2013; Toner, Pirogovsky, Kirwan, & Gilbert, 2009), monkeys (Burke et al., 2011), and rats (Burke et al., 2011; Johnson et al., 2017) showing selective deficits in discriminating between objects that share features relative to objects that are more distinct. Importantly, age-associated deficits in discrimination have also been observed for similar odorants (Yoder et al., 2017), suggesting that age-related impairments in this cognitive function are not confined to the visual modality.
In contrast to data that consistently show age-related impairments in discriminating between stimulus features, there are disparate reports of the impact of aging on the ability to associate stimulus features within and across sensory modalities. Some studies suggest that older adults are impaired on tasks assessing associative memory (Castel & Craik, 2003; Cohn, Emrich, & Moscovitch, 2008; Naveh-Benjamin, 2000; Old & Naveh-Benjamin, 2008). In contrast, more recent studies have suggested that associative memory is preserved with age due to the observation that older adults’ item memory benefits from visual integration within a context similar to young subjects (Memel & Ryan, 2017). Moreover, it is possible that deficits in associative memory can in part be accounted for by reduced memory for items, and that when this is controlled for, associative memory in older adults is similar to young subjects (Oedekoven, Jansen, Keidel, Kircher, & Leube, 2015). Thus, it remains unclear the extent to which normal aging is accompanied by reduced abilities to form and maintain associations.
Although it is evident that the PRC is critical for both discrimination and association, the extent to which the same underlying neural computations support these cognitive functions is not yet known. It remains to be determined if advancing age similarly affects discrimination and association abilities. In the current study, young and aged rats were cross-characterized on PRC-dependent crossmodal object recognition tasks, which tests the ability to bind together stimulus features experienced in different sensory modalities, and on a LEGO® object discrimination task, which tests the ability to differentiate between similar stimuli within a single sensory modality to obtain a reward. Performance across these tasks in old animals can be used to infer the extent to which discrimination and association rely on the same neural computations. Specifically, if performance on both tasks is similarly impaired in the aged rats, then overlapping mechanisms likely support these two processes. Conversely, if association and discrimination performance are not positively correlated, then distinct computations within the PRC and its associated circuits may be required for each task.
Methods
Subjects
A total of 30 young (4-6 months old) and 31 aged (22-26 months old) male Fischer 344 × Brown Norway F1 hybrid male rats (NIA colony, Taconic Farms) were used for this study. Each rat was behaviorally characterized on the crossmodal object recognition (CMOR; Winters & Reid, 2010) task, the spontaneous object recognition task with visual only or tactile only stimuli (SOR; Ennaceur & Delacour, 1988), and the LEGO object discrimination task (S.A. Johnson et al., 2017); see descriptions below. Figure 1A shows the timeline for all experimental procedures. Rats were individually housed and maintained on a reversed 12-hr light-dark cycle. All behavioral experiments were performed during the dark phase of the cycle. During recognition tasks, rats were given access to standard chow (Teklad LM-485, Harlan Labs) and water ad libitum. During the LEGO object discrimination task, rats were food-restricted on moist chow to between 80-85% of their free-feeding weight, with access to water ad libitum. All rats were handled for the week prior to testing. Additionally, all rats were handled daily for testing and completed daily weighing during discrimination testing. All experimental procedures were performed in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees at the University of Florida.
Figure 1. Experimental design.
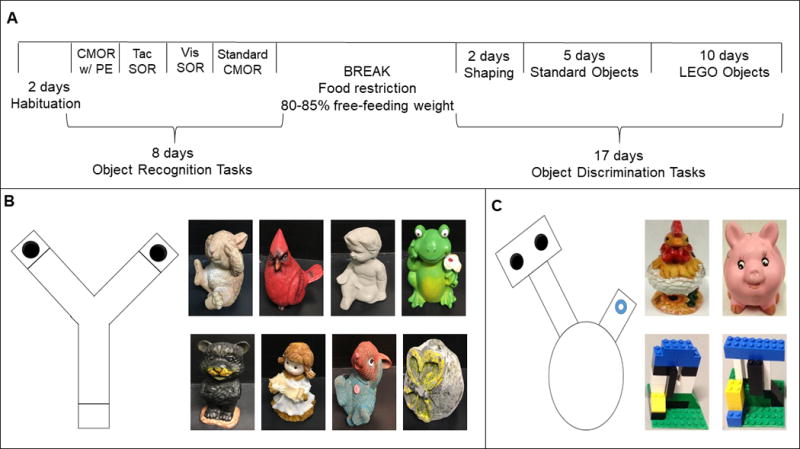
(A) Timeline of testing for all behavioral experiments. The order of object recognition conditions was pseudorandomized across animals. (B) Line drawing representing the testing apparatus for the object recognition task. The black circles indicate the location that objects were placed during all phases of recognition testing. Photographs show all objects that were used for recognition testing. Objects were made of plastic or ceramic. Triplicates of each object were obtained for testing to ensure that rodents could not use odorant cues to recognize or discriminate between objects and the novel versus familiar object within a task were counterbalanced across rats. (C) Line drawing representing the testing apparatus for the object discrimination task. The black circles indicate food wells where the food reward is placed for the rat to obtain. The blue ring in the right arm of the object discrimination apparatus represents the Froot Loop piece placed in the arm to be retrieved by the rat at the start of each trial. Photographs show the standard (top) and LEGO (bottom) objects used in the discrimination tasks.
Apparatus and Testing Objects
Crossmodal object recognition tasks
The tasks were conducted in a Y-shaped apparatus as described in previous studies (Figure 1B; Forwood, Winters, & Bussey, 2005; Winters, Forwood, Cowell, Saksida, & Bussey, 2004; Winters & Reid, 2010). The apparatus was constructed from high white-painted wooden walls (41.91 cm tall) with a white base to facilitate automated tracking of the F344×BN rats. The start arm (41.91 cm × 16.51 cm × 17.78 cm) was separated from the two test arms by a plastic guillotine door (45.72 cm × 12.7 cm) to contain the rat before the start of testing. The two choice arms were equal in length. Both guillotine doors could be removed or inserted to prevent the rat from reaching the end of the arm. Objects made of plastic and ceramic were used as testing stimuli. Preliminary testing of exposure of rats from the same strain to testing objects did not reveal any significant object preferences. During CMOR testing, all objects were counterbalanced across rats. Objects were placed in the center of the end of the choice arms (41.91 cm × 16.51 cm × 6.35 cm) during both the sample and test phase of each condition. Between testing sessions, objects were cleaned with 70% ethanol. During testing, a white noise machine was used to minimize distraction based on extraneous noise.
During the sample phase of the CMOR and the Tactile-only SOR tasks, room lights (32 Watt bulbs) were turned off and a red lamp (25 Watt bulb) was positioned above the apparatus to deprive rats of visual input while enabling video recording for offline analysis. During the test phase of the CMOR and the Visual-only SOR tasks, room lights were turned on and the transparent plexiglas panels were inserted into the choice arms to prevent rats from touching or whisking against the objects.
Object exploration was measured using in-house video scoring software (Collector, Burke/Maurer Laboratories, Gainesville, FL). Scorers determined duration (seconds) of active exploration of the right and left objects separately. Active exploration was qualified by the rats’ proximity (~2 cm) to the object and explorative behaviors (nose facing object without rearing on or climbing on object).
LEGO object discrimination task
The LEGO object discrimination task was conducted in a black two-arm maze (Figure 1C; Hernandez et al., 2015). A start platform, 48.3 cm in diameter, gave off the two arms, each 84 cm long with a rectangular choice platform (31.75 cm × 24.13cm) at the end. The right arm was blocked halfway with a plastic barrier to restrict access to the choice platform, and was used as the ‘start’ zone. The choice platform in the left arm contained two food wells 2.5 cm in diameter and 1 cm deep, spaced 12.8 cm apart. During testing, a white noise machine was kept on to minimize extraneous noise. Objects made of plastic, ceramic, or constructed from LEGO blocks to share visible features (Fig 1C) were used as testing stimuli. During testing, objects were placed over the food wells to conceal a hidden food reward. Between testing sessions, objects were cleaned with 70% ethanol. During all phases of discrimination testing, the room lights were turned off and a lamp with a red bulb (25 Watt) and another lamp with a white bulb (100 Watt) were placed in opposite corners of the testing room facing the walls to provide indirect illumination of the maze and object stimuli.
General Testing Procedures
Crossmodal object recognition tasks
Two days prior to recognition testing, rats were introduced to the Y-shaped apparatus for a 17-minute period. For the first five minutes, the room lights were off and the red overhead lamp was turned on. The rat spent one minute in the start arm blocked in by the guillotine door before being released into the maze. The rat was given five minutes to freely explore before it was returned to its home cage for another five minutes. At the beginning of this resting period, the room lights were turned on, and the red light was turned off. The two transparent plexiglas dividers were secured at the end of the choice arms. Again, the rat was placed in the start arm for one minute before being released into the choice arms to freely explore for five more minutes. If necessary, rats were physically reset to the center of the two choice arms to encourage exploration. After this habituation phase, all rats completed 4 different spontaneous object recognition tasks: standard crossmodal object recognition (CMOR) without pre-exposure, CMOR with multimodal pre-exposure, tactile spontaneous object recognition, and visual spontaneous object recognition. Each task was given on different testing days with a day off between tasks, and the order of tasks was counterbalanced across animals with a Latin Square design so that the different possible orders of recognition tests had similar numbers of rats. This procedure normalized any potential order effects across object recognition tasks.
Rats were exposed to a unique set of objects for each of the four recognition tasks. Prior to testing, rats were assigned objects to be familiarized with during the sample phase and an object that would remain novel for testing. Two copies of the object to be initially familiarized with were presented at the end of each choice arm during the sample phase. One identical copy of the familiar object and a novel object were then presented during the test phase. Which object was novel, and the side in which the novel object was placed, were counterbalanced across animals.
Standard crossmodal object recognition without pre-exposure
Rats first completed the tactile-only sample phase, in which the room lights were turned off and the red lamp was turned on so that only tactile features were available to the animal. The designated familiar objects were placed in the two choice arms of the maze. The rat was placed in the start arm for one minute before it was allowed to enter the choice arms and freely explore for 3 minutes. If the rat was inactive for >1 min, it was gently moved to the center of the maze to encourage activity. After the sample phase, the rat was returned to the home-cage for 5 minutes, which remained in the testing room covered with a dark drape to minimize exposure to distractors. During the visual-only test phase, the room lights were then turned on and the red lamp was turned off. The transparent plexiglas panels were placed in front of the familiar and novel objects to restrict access to tactile information but allowing access to visual information. After spending 1 minute in the start arm, the guillotine door was raised and the rat was given 3 minutes to freely explore both choice arms.
Crossmodal object recognition with multimodal pre-exposure
The pre-exposure variant of the CMOR task was identical to the task described above, except that 24 hours prior to testing, rats were given a 2-minute pre-exposure to sample the familiar object stimuli in both visual and tactile modalities. During pre-exposure, both the room lights and red lamp were turned on and plexiglas panels were removed from the maze. Rats were placed in the start arm of the maze for one minute. Then, the guillotine door was raised and the rat was given two minutes to explore the pair of identical objects, placed in both arms of the Y-maze. If the rat failed to explore the objects during this initial pre-exposure phase, the rat was given an additional 2 minutes to explore the objects. If exploration did not occur during the entire 4 minutes, the animal was excluded.
Standard Object Recognition (SOR) Tasks
Tactile Standard Object Recognition
During both phases of testing, the room lights were turned off and the red lamp was turned on. The designated familiar objects were placed in the two choice arms of the maze. During the sample phase, the rat was placed in the start arm for one minute before being allowed to enter the maze and freely explore for three minutes. If the rat was inactive for >1 min, it was moved to the center of the maze. After the sample phase, the rat was returned to the home-cage for 5 minutes. During the test phase, one identical copy of the familiar objects and one novel object were placed in the choice arms and rats were given another three minutes to explore.
Visual Standard Object Recognition
During both phases of testing, the room lights were turned on and the red lamp was turned off. Plexiglas panels were placed in the end of each choice arm in front of the objects. During the sample phase, the designated familiar objects were placed in the two choice arms of the maze. The rat was placed in the start arm for one minute before being allowed to enter the maze and freely explore for three minutes. If the rat was inactive for a period of time, it was moved to the center of the maze. After the sample phase, the rat was returned to the home-cage for 5 minutes, which remained in the testing room and covered with a black sheet. During the test phase, an identical copy of the familiar object and one novel object were placed behind the transparent plexiglas panels in the choice arms and rats were given three minutes to explore.
LEGO® Block Object Discrimination Task
Shaping
Shaping, training, and testing procedures were carried out in a truncated version of those previously described (Johnson et al., 2017; Maurer et al., 2017). After all recognition testing was completed, rats were placed on a restricted feeding protocol and began shaping for the object discrimination task. During the shaping phase, rats were trained over five days to retrieve a food reward (Froot Loops, Kellogg’s Company, Battlecreek, MI) from one of the two food wells on the choice platform. At the beginning of each shaping session, the rat was placed in the start arm and given a piece of Froot Loop cereal. The rat was then guided to turn and exit the start arm and traverse the arm to the choice platform. The rat was then required to locate the food well with the piece of Froot Loop cereal. Once the rat had obtained this food reward, it would return to the start arm and receive a second piece of Froot Loop cereal. The side of the rewarded food well (left vs. right) was pseudorandomly varied across shaping trials. Rats completed two days of shaping regardless of accuracy.
Standard Object Discrimination
To provide procedural training for the object discrimination task, all animals first learned to discriminate between a pair of standard ‘junk’ objects similar to those used in CMOR and SOR tasks (plastic or ceramic figurines; Figure 1D) before moving on to the final phase of LEGO object discrimination. During this phase, one of the objects of the pair was assigned as the target object, always covering the food well containing the food reward, while the alternate object served as a ‘lure’ object. The same pair of objects was used to train all rats in the study, however the object serving as the target was counterbalanced across rats. Trials were identical to those during the shaping phase, except that upon reaching the choice platform, rats were taught to displace the target object to obtain the concealed food reward. In the case of an incorrect response, if the rat chose to displace the lure object, the food reward and both objects were quickly removed from the choice platform and the rat was required to return to the start location to initiate a new trial. In the case of correct responses, in which the rat selected the target object, the rat was allowed to consume the food reward before returning to the start arm and receiving a second food reward to initiate the next trial. Rats were trained for 5 sessions of 32 trials/day before moving on to the LEGO object discrimination phase.
LEGO® Block Object Discrimination
Procedures for the LEGO object discrimination phase were identical to those for the standard object discrimination phase, except that objects were constructed from LEGO blocks to share approximately 63% of visible features (Figure 1D; Johnson et al. 2017). Rats completed discrimination training sessions of 32 trials/day for 10 consecutive days.
Statistical Analyses
Rats were excluded from final analyses if they did not explore the stimulus objects during sample or test phases of the task. Additionally, rats were excluded if they displayed neophobic behavior, which was considered complete lack of exploration of the novel object during the test phase. A number of animals were excluded due to lack of exploration. This is likely due to many of the rats being overweight from ad libitum feeding, which can reduce exploratory behavior. The current student chose not to food restrict during recognition testing, as the aged rats are over conditioned relative to young, and therefore take longer to reach comparable levels of motivation to explore for food (Hernandez, Hernandez, et al., 2017; Johnson et al., 2016; S. A. Johnson et al., 2017). Such an interaction between restriction and age that can confound the detection of age-related differences. Moreover, over-restriction can also interfere with rats innate tendency to explore objects (Carter, Leeuwenburgh, Daniels, & Foster, 2009). A chi-squared test for association revealed that only in the standard CMOR task was age significantly associated with task exclusion (χ[1] = 4.68, p = 0.03), with more aged rats being excluded. Importantly, when all rats were included on this task, results of the final analyses were similar. Furthermore, there was not a significant association between age and task exclusion for the CMOR task with pre-exposure (χ[1] = 0.04, p = 0.84), tactile-only standard object recognition (χ[1] = 2.39, p = 0.12), or visual-only standard object recognition (χ[1] = 0.51, p = 0.47), meaning that rules for exclusion of these tasks did not preferentially exclude aged over young rats.
Data are presented as mean values ± standard error of the mean (SEM). Statistical analyses were completed with IBM Statistical Package for the Social Sciences (SPSS) version 24 for Windows. Independent samples t-tests were used to determine age-related differences in behavioral performance. Paired-samples t-tests were employed to determine within-group differences in behavioral performance. To determine if there were interactions between age and mean exploration time of the familiar and novel objects, repeated-measures ANOVAs were used with age as a between-subjects variable. Post hoc analyses were used where applicable, most commonly simple contrasts with Bonferroni corrections applied. To determine if discrimination ratio (time spent exploring the novel object relative to time spent exploring the familiar object; Burke et al., 2010; 2011), significantly differed from chance performance (equal time spent exploring each object, discrimination ratio = 0) and that rats displayed preference for novelty (discrimination ratio > 0), a Bonferroni-corrected one-sample t-test was used. Furthermore, Principal Components Analysis was performed on all testing condition variables to determine if any particular components explained the variance in the original variables across age groups. Eigenvalues above 1.0 were considered meaningful. Within components, loadings above 0.5 in either direction were considered meaningful contributions. A K-means cluster analysis was used to determine homogenous groups of good and poor performers of young and aged rats on the LEGO object discrimination task to facilitate an analysis of preference for novelty on selected association tasks, based on the results of the Principal Components Analyses. Across tests, significance was considered for p-values less than 0.05.
Results
Crossmodal Object Recognition Tasks
Standard crossmodal object recognition without multimodal pre-exposure
To assess the ability of young and aged rats to recognize a familiar object that was learned in one sensory modality and re-experienced in a different modality, rats were familiarized with an identical object pair under a red light and then tested under lighted conditions in which the familiar and novel test objects were placed behind a plexiglas screen. Access to tactile information during familiarization but visual input during test requires animals to generalize object features across distinct sensory inputs. Eighteen (18) aged rats and 25 young rats showed adequate exploration during both epochs, and were included in the final analysis. Table 1 shows the mean exploration times during the familiarization phases of all recognition tasks for young and aged rats. For the standard CMOR task without pre-exposure, the exploration times of young rats (mean = 8.94 seconds +/− 0.84) were not significantly different from exploration times of aged rats (mean = 7.39 seconds +/− 1.10) (Table 1). This suggests that potential age-related differences in object sampling during the familiarization phase did not confound recognition in the test phase.
Table 1.
Mean exploration times during familiarization phase in young and aged rats
| Standard CMOR Task Sampling | Pre-exposure CMOR Task | CMOR with pre-exposure Sampling | Tactile-only SOR Task Sampling | Visual-only SOR Task Sampling | |
|---|---|---|---|---|---|
| Young | 8.94 (0.84) | 11.14 (1.40) | 7.35 (0.12) | 10.80 (0.89) | 8.55 (1.83) |
| Aged | 7.39 (1.10) | 7.41 (1.32) | 5.98 (0.98) | 10.69 (1.51) | 7.26 (1.69) |
| T-Test–Young v. Aged | t(41) = −1.14, p = 0.26 | t(41) = −1.93, p = 0.06 | t(41) = −0.89, p = 0.38 | t(39) = −0.07, p = 0.95 | t(38) = −0.52, p = 0.61 |
Note. CMOR = Crossmodal object recognition SOR = Spontaneous Object recognition
During the test phase, repeated-measures ANOVA indicated a significant main effect (novel versus familiar), such that the time spent exploring the novel object was significantly greater than the time spent exploring the familiar object (Figure 2A; F[1,41] = 6.73, p < 0.05). However, the main effect of age on total exploration time during the test phase was not significant (F[1,41] = 0.57, p = 0.46). While the interaction effect between object type (novel versus familiar) and age also did not reach statistical significance (F[1,41] = 1.18, p = 0.28), there was a trend for the young rats to explore the familiar object more than the aged rats (t[41] = −1.76, p = 0.09). This trend between age groups was not observed in the exploration times of the novel object (t[41] = −0.10, p = 0.93). Within age groups, time spent exploring the novel object relative to the familiar object was significantly greater in the aged rats (t[17] = 2.39, p < 0.05), but not the young (t[24] = 1.17, p = 0.25). These data suggest that the aged rats were able to use an experience in one modality to recognize an object in a different modality.
Figure 2. Standard Crossmodal Object Recognition (CMOR) without multimodal pre-exposure performance.
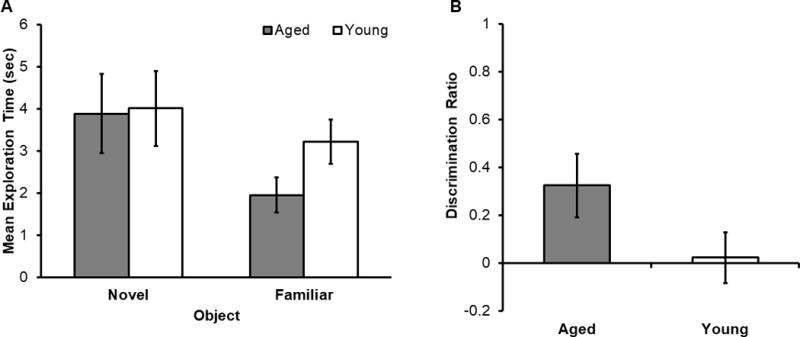
(A) Mean exploration time (seconds) for the novel and familiar objects in young (white) and aged (grey) rats. The main effect of object (novel versus familiar) was significant with more time spent exploring the novel object (F[1,41] = 6.73, p < 0.05). However, the main effect of age on total exploration time was not significant (F[1,41] = 0.57, p = 0.46). While the interaction effect between object type (novel versus familiar) and age also did not reach statistical significance (F[1,41] = 1.18, p = 0.28), there was a trend for the young rats to explore the familiar object more than the aged rats (t[41] = −1.76, p = 0.09). (B) Mean discrimination ratio for young (white) and aged (grey) rats. The aged rats (T[17] = 2.39, p < 0.05), but not young (T[24] = 1.17, p = 0.25), showed a discrimination ratio that was significantly different from 0.
Raw exploration times were used to calculate discrimination ratios for young and aged rats, which summarize an animal’s tendency to explore the novel object over the familiar one. There was a trend for the discrimination ratio of young rats to be lower when compared to the aged rats (Figure 2B; t[41] = 1.80, p = 0.08). Moreover, when the discrimination ratio was compared to 0 with one-sample t-tests for each age group separately, the aged rats (t[17] = 2.47, p < 0.05), but not young (t[24] = 0.23, p = 0.82), showed a discrimination ratio that was significantly different from 0. These data further support the notion that the aged rats were able to use experience with an object in the tactile modality to recognize the same object in the visual modality during crossmodal object recognition at short delays, and may even show a preferential exploration of the novel object when it is exposed in a distinct modality relative to young animals.
Crossmodal object recognition with multimodal pre-exposure
Previous studies have shown that novelty preference on the CMOR task can be facilitated by giving rats a brief pre-exposure to objects in which both tactile and visual information is available 24 hours prior to the testing session (Reid, Jacklin, & Winters, 2012; James M Reid, Jacklin, & Winters, 2014). After excluding animals due to poor overall object sampling, 21 aged rats and 22 young rats were included in the final analysis of the CMOR task with multimodal pre-exposure. Analysis of raw exploration times during the 3-min pre-exposure phase revealed that the mean exploration time of young rats (mean = 11.14 seconds +/− 1.4) trended toward greater exploration time than aged rats (mean = 7.41 seconds +/− 1.32; t[41] = −1.93, p = 0.06) (Table 1). During the familiarization phase, the mean exploration time of young rats (mean = 7.35 seconds +/−0.12) was not significantly different from mean exploration time of aged rats (Table 1; mean = 5.98 seconds +/− 0.98).
During the test phase, repeated-measures ANOVA indicated that the time rats spent exploring the novel object was significantly greater than the time spent exploring the familiar object (Figure 3A; F[1,41] = 11.50, p = 0.02), despite the exploration time differences between age groups during pre-exposure. The main effect of age did not reach statistical significance (F[1,41] = 0.20, p = 0.66). Moreover, there was no significant interaction effect between object (novel versus familiar) and age on exploration time (F[1,41] = 0.15, p = 0.70). Likewise, there was no significant difference in the time spent by aged and young rats exploring the novel (t[41] = 0.47, p = 0.64) or familiar object (t[41] = 0.22, p = 0.83). These data indicate that aged and young rats were similarly able to discriminate between the novel and familiar object on the CMOR task with multimodal pre-exposure. Consistent with these results, the mean discrimination ratio of young rats was not significantly different from that of the aged rats (Figure 3B; t[41] = 1.24, p = 0.22). Importantly, the mean discrimination ratio of all rats together was significantly different from 0 (t[42] = 4.52, p < 0.001; one-sample t-test), further indicating preference for the novel object in both young and aged rats when multimodal pre-exposure was provided.
Figure 3. Crossmodal Object Recognition (CMOR) with multimodal pre-exposure performance.
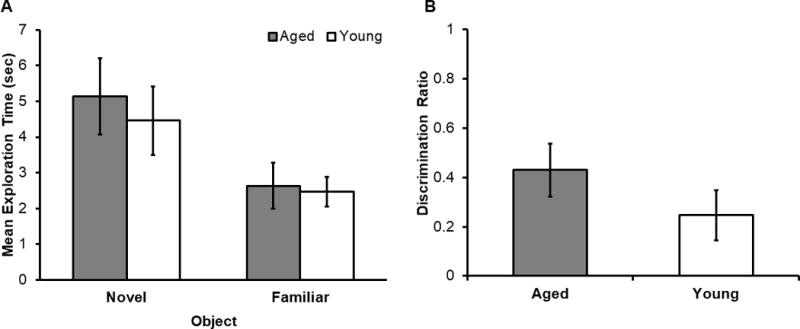
(A) Mean exploration time (seconds) for the novel and familiar objects in young (white) and aged (grey) rats. Significantly more time was spent exploring the novel object relative to the familiar object (F[1,41] = 11.50, p = 0.02). However, there was not a significant main effect of age (F[1,41] = 0.20, p = 0.66), nor was the interaction between exploration time and age significant (F[1,41] = 0.15, p = 0.70). (B) Mean discrimination ratio in young (white) and aged (grey) rats. The discrimination ratio of young and aged rats did not significantly differ between the age groups (t[41] = 1.24, p = 0.22), but were significantly different from 0 (t[42] = 4.52, p < 0.001).
Comparison across CMOR Tasks
A repeated-measures ANOVA on the discrimination ratio variable was used to assess a potential difference between performance on the two different CMOR tasks in young and aged rats. The ANOVA indicated that the difference between the discrimination ratios of the CMOR tasks with and without multimodal pre-exposure was not significant (F[1,29] = 2.45, p = 0.13). The main effect of age, however, approached significance (F[1,29] = 3.55, p = 0.07), with aged rats having higher discrimination ratios. The interaction effect between CMOR task (with and without pre-exposure) and age on discrimination ratio was also not significant (F[1,29] = 0.91, p = 0.35). Interestingly, Bonferroni-corrected post-hoc analyses of the between-subjects effect of age revealed a nearly significant difference between CMOR task performance for young rats (F[1,29] = 3.79, p = 0.06), but not for aged rats (F[1,29] = 0.16, p = 0.69). This suggests that, in the young rats, but not the aged, recognition performance may have benefitted from the multimodal pre-exposure epoch. Alternatively, it is conceivable that the young rats identified a familiar object experienced in a new modality as novel, which is consistent with their slightly elevated exploration times of the familiar object.
Standard Object Recognition Task Performance
To control for potential sensory deficits that could confound CMOR performance, all animals completed two versions of the SOR task: 1) tactile-only SOR task, in which both familiarization and test phases were conducted under red light with no access to visual information, 2) visual-only SOR task, in which lights were on, but the objects were placed behind transparent plexiglas screens for both the familiarization and test phases. After animals with low object sampling during the familiarization phase were removed, 17 aged rats and 23 young rats were included in the analysis of performance on the tactile-only SOR task. Additionally, 18 aged rats and 21 young rats were included for the visual-only SOR task.
Tactile standard object recognition
During the familiarization phase, the exploration times of young rats (mean = 10.80 seconds +/− 0.89) were not significantly different from the exploration times of aged rats (Table 1; mean = 10.69 seconds +/− 1.51). Moreover, during the test phase, repeated-measures ANOVA indicated that the time spent exploring the novel object was significantly greater than the time spent exploring the familiar object (Figure 4A; F[1,39] = 11.80, p < 0.01). The main effect of age on total exploration time during the test phase, however, was not significant (F[1,39] = 0.06, p = 0.81). The interaction effect between object type (novel versus familiar) and age also did not reach statistical significance (F[1,39] = 0.05, p = 0.82). Consistent with the interpretation that young and aged rats had a similar preference for the novel over the familiar object in the tactile-only condition, there was not a statistically significant difference in the time aged and young rats spent exploring the novel object (Figure 4B; F[39] = −0.05, p = 0.96) or the familiar object (t[39] = −0.33, p = 0.75). Finally, the mean discrimination ratio of all rats was significantly different from 0 (t[40] = 5.90, p < 0.001; one-sample), and discrimination ratios of young and aged rats did not significantly differ (t[39] = 0.54, p = 0.59).
Figure 4. Comparison of Visual and Tactile Standard Object Recognition (SOR) Performance across Age Groups.
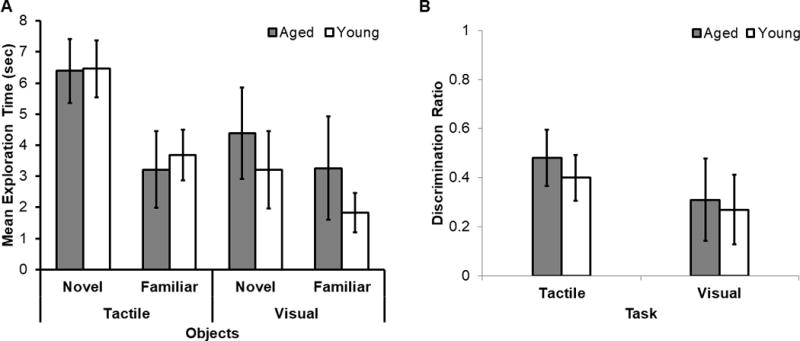
(A) Mean exploration time (seconds) for the novel and familiar objects in young (white) and aged (grey) rats for the tactile- and visual-only SOR tasks. For the tactile-only SOR task, rats spent significantly more time exploring the novel compared to the familiar objects (F[1,39] = 11.80, p < 0.01). This did not vary as a function of age group (F[1,39] = 0.06, p = 0.81). For the Visual-only SOR task, there were not significant effects of exploration time of novel versus familiar objects (F[1,38] = 0.94, p = 0.34), age (F[1,38] = 1.05, p = 0.31), or interaction between exploration time and age (F[1,38] = 0.01, p = 0.92). (B) Mean discrimination ratios for the tactile and visual-only SOR tasks in young (white) and aged (gray) rats. Discrimination ratios of young and aged rats did not significantly differ in the tactile-only SOR task (t[39] = 0.54, p = 0.59) or in the visual-only SOR task (t[38] = 0.18, p = 0.86). The discrimination ratio was significantly different from 0, however, for both the tactile-only SOR task (t[40] = 5.90, p < 0.001) and the visual-only SOR task (t[39] = 2.69, p = 0.01).
Visual standard object recognition
During the familiarization phase, the exploration times of young rats (mean = 8.55 seconds +/− 1.83) were not significantly different from the exploration times of aged rats (Table 1; mean = 7.26 seconds +/− 1.69). During the test phase, repeated-measures ANOVA indicated that the time spent exploring the novel object was not significantly greater than the time spent exploring the familiar object (Figure 4A; F[1,38] = 0.94, p = 0.34). The main effect of age on total exploration time during the test phase was also not significant (F[1,38] = 1.05, p = 0.31). The interaction effect between object type (novel versus familiar) and age also did not reach statistical significance (F[1,38] = 0.01, p = 0.92). While the total exploration times for the novel versus familiar objects did not significantly differ, the mean discrimination ratio of all rats was significantly different from 0 (Figure 4B; t[39] = 2.69, p = 0.01; one-sample t-test). The discrimination ratio did not significantly differ between the young and aged rats (t[38] = 0.18, p = 0.86). These data suggest that in the visual-only condition, with no tactile input, both young and aged rats discriminated between novel and familiar objects.
LEGO® Object Discrimination Task Performance
Twenty-one (21) aged and 22 young rats that participated in the CMOR task were tested for two days on a standard object discrimination task (Figure 1C, top panels) to acquire the procedural aspects of displacing 3-dimensional objects to retrieve a food reward. Rats then completed 10 consecutive days of the LEGO object discrimination task (Figure 1C, bottom panels). To study only the rats that met inclusionary criteria for both CMOR tasks, 11 aged and 13 young rats were included in the final analyses of the LEGO object discrimination task.
Standard object discrimination
Performance on the standard object discrimination task was represented by mean percent correct responses out of the 32 trials completed each day. A repeated-measures ANOVA with the within-subjects factor of test day (day 1 versus day 2) and between-subjects factor of age revealed that there was not a significant difference between performance of rats on day 1 versus day 2 of standard object discrimination training (Figure 5; F[1,41] = 0.16, p = 0.69). Additionally, the main effect of age (F[1,41] = 0.07, p = 0.79) and the interaction of day of testing and age were not significant (F[1,41] = 0.36, p = 0.55). This suggests aged and young rats did not differ in their rate of procedural learning of the discrimination task.
Figure 5. Standard Object Discrimination Performance by Age Group.
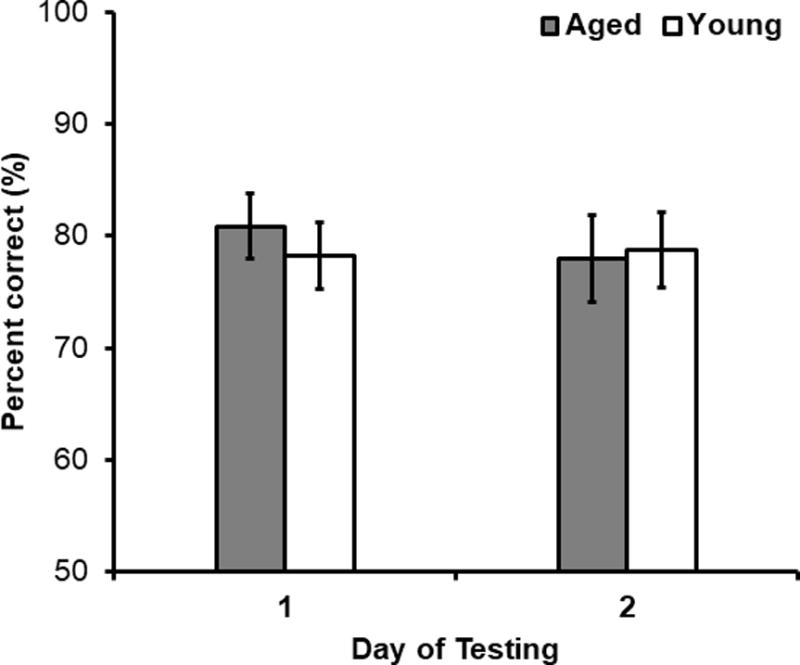
Standard object discrimination percent correct (%) as a function of test day in young (white) and aged (grey) rats. Performance did not significantly differ between test day (F[1,41] = 0.16, p = 0.69), or age group (F[1,41] = 0.07, p = 0.79). Moreover, the interaction effect between testing day and rat age was not significant (F[1,41] = 0.36, p = 0.55).
LEGO object discrimination
First, a repeated-measures ANOVA with the within-subjects factor of test day and between-subjects factor of age was used to compare performance across days of testing for young and aged rats that were included in the CMOR tasks. Due to the fixed number of trials and testing days completed, each rat performed the same number of problems and had identical exposure to the objects. Performance on the LEGO object discrimination task was taken as mean percent correct responses out of the 32 trials completed each day. During the first several days of testing, rats were performing at or below chance and were still acquiring the procedural aspects of the task. Thus, analyses of test performance began on day 3, which is the first day that average performance significantly differed from chance (T[21] = 2.92, p < 0.01). The ANOVA on test days 3-10 revealed a significant overall effect of testing day on task accuracy (Figure 6A; F[7,1] = 11.48, p < 0.001). The main effect of age was not significant (F[1,21] = 2.62, p = 0.12), however, the interaction between testing day and age was significant (F[7,1] = 2.90, p = 0.007). Thus, post hoc tests were assessed to compare mean percent correct scores on all days between age groups. The mean percent accuracy of young rats was significantly better than aged rats on day 10 (Figure 6B; F[1,21] = 9.88, p = 0.005), reflecting the higher discrimination task accuracy of young rats over test days compared to aged rats.
Figure 6. LEGO Object Discrimination Performance by Age Group.
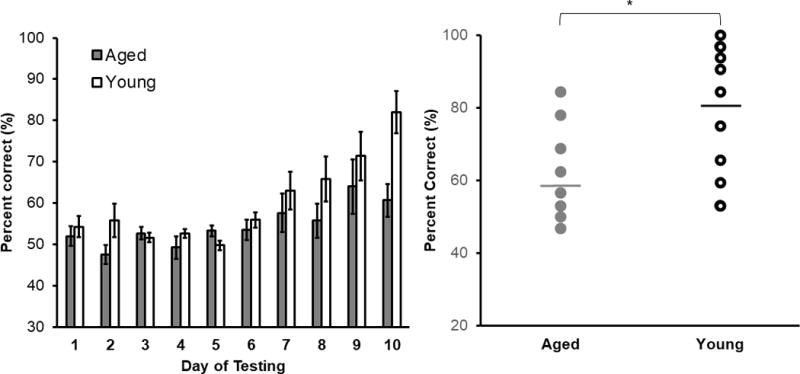
(A) LEGO object discrimination percent correct (%) as a function of test day in young (white) and aged (grey) rats. The main effect of testing day on LEGO object discrimination task performance was significant (F[7,1] = 11.48, p < 0.001). The main effect of age (F[1,21] = 2.62, p = 0.12). Notably, the interaction of testing day and age was significant (F[7,1] = 2.90, p = 0.007) when collapsed across test days 3 through 10. Performance was significantly different between young and aged rats on day 10 (F[1,21] = 9.88, p = 0.005). (B) Individual performance on LEGO object discrimination on Day 10 in young and aged rats. Horizontal lines indicated group means.
Relationship Between Crossmodal Object Recognition and Discrimination Performance
To determine the patterns of association among performances on the five different behavioral tasks, discrimination ratio measures of recognition performance and percent correct on Day 10 of LEGO object discrimination testing were subject to principal component analysis (PCA). PCAs were performed separately for each age group to determine whether tasks clustered differently for young and aged rats. For young rats, a PCA with a varimax rotation was performed due to the occurrence of split loadings. This analysis revealed no problematic redundancies for any task variables (problematic redundancy > 0.7 across multiple components). According to total variance explained, a model using the top two components (Eigenvalues > 1.0), explained 70.50% of the variance in the performance indicators, with the first component accounting for 38.56% of the variance. Item communalities were moderate to high (ranging from 0.66 to 0.91) for all items except Visual-only SOR, which was low (0.43).
In the young rats PCA indicated that, the standard CMOR task without multimodal pre-exposure and the LEGO object discrimination task both positively loaded onto the first component (0.91, 0.87), while the Tactile-only SOR contributed to a negative loading (−0.58). This suggests that young rats with better CMOR performance with no stimulus pre-exposure were also better at discriminating between similar stimuli, but worse at recognition in the tactile modality. CMOR with multimodal pre-exposure, tactile-only SOR, and visual-only SOR all positively loaded onto the second component (0.86, 0.57, 0.66). These data indicated that for young rats, in which an association between the visual and tactile features of the object were established during pre-exposure, recognition performance reflected more preference for the novel object in both the tactile and visual modalities.
For aged rats, the PCA revealed no problematic redundancies of any task variables (problematic redundancy > 0.7 across multiple components). According to total variance explained, a model using the top two components (Eigen values > 1.0), explained a total of 82.98% of the variance in the performance indicators, with the first component accounting for 48.13% of the variance. Item communalities were moderate to high (ranging from 0.77 to 0.99) for all items, lending further support to the consideration of two components.
According to the PCA, the LEGO object discrimination task and the tactile-only SOR task positively loaded onto the first component (0.84 and 0.70, respectively), while the standard CMOR task and the CMOR task with multimodal pre-exposure did not (−0.80 and −0.75, respectively). This suggests that while tactile recognition is associated with better discrimination performance, the aged rats that performed below the mean on the LEGO object discrimination task, showed a greater preference for the novel over the familiar object when the familiar object was encountered in a distinct modality. This indicates that poor discriminators were better able to associate visual and tactile features in order to recognize a previously experienced object. Performance on the visual-only SOR task loaded strongly onto the second component (0.99), while tactile-only recognition had a negative loading (−0.51). These data suggest that rats with better recognition performance in the tactile modality had worse performance in the visual modality.
Together, the PCA results indicated that day 10 LEGO object discrimination performance in aged rats was negatively related to performance on CMOR tasks. Conversely, performance of young rats on the LEGO object discrimination task was associated with better recognition on the standard CMOR task. To further examine these relationships, we compared performance on the CMOR tasks between rats that were good or poor performers on LEGO object discrimination. To do this, a K-Means cluster analysis was used to determine whether the discrimination data on day 10 (Figure 6) significantly fit a bimodal distribution. This analysis revealed a significant bimodal distribution for aged rats (F[17] = 50.71 p < 0.001). For young rats, the K-Means Cluster Analysis also revealed a significant bimodal distribution of performance on Day 10, with 14 rats performing at or around a mean of 90.95% and 8 rats performing at or around a mean of 53.5% (F[20] = 112.11, p < 0.001). Because of the bimodal distribution of performance of both aged and young rats in the LEGO object discrimination task, both age groups were separated into subgroups of “Good Performers” and “Poor Performers” based on whether each rat performed above or below their respective group mean on the final day of LEGO object discrimination testing (Day 10). To determine whether rats’ abilities to generalize across modalities without multimodal pre-exposure related to object discrimination performance, one-sample t-tests comparing the CMOR discrimination ratio to 0 were calculated for each of the four different discrimination groups (Aged Good and Poor Performers, and Young Good and Poor Performers). Only the discrimination ratio of Aged Poor Performers on the standard CMOR task was significantly different from 0 (t[8] = 2.60, p = 0.03) (Figure 7A). The discrimination ratios of Aged Good, Young Good, and Young Poor Performers were not significantly different from 0 (t[4] = −0.59, p = 0.59; t[9] = 1.63, p = 0.14; t[8] = −0.60, p = 0.57; respectively). These data suggest that aged rats with worse discrimination performance were better able to associate object features across sensory modalities.
Figure 7. CMOR performance within LEGO object discrimination performance subgroups.
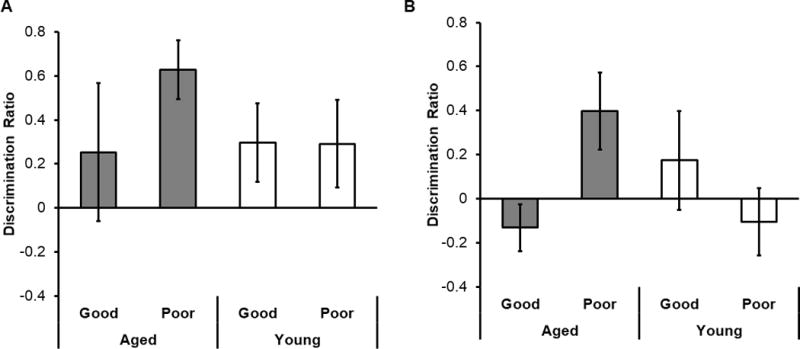
(A) Mean discrimination ratio for the standard CMOR task without multimodal pre-exposure in young and aged rats separated into good and poor performing subgroups based on mean LEGO object discrimination percent accuracy. Only the Aged Poor Performers showed a significant preference for novelty on the standard CMOR task (t[8] = 2.60, p = 0.03). (B) Same as in A, but for the CMOR with multimodal pre-exposure task. Again, only the Aged Poor Performers showed significant preference for novelty (t[6] = 3.99, p = 0.007).
To determine whether rats’ abilities to associate across modalities when they were pre-exposed to test objects related to LEGO object discrimination performance, the discrimination ratios on the CMOR task with multimodal pre-exposure was compared to 0 with one-sample t-tests for all 4 LEGO object discrimination groups separately. Similar to the results described above, only the discrimination ratio of Aged Poor Performers on the CMOR with multimodal pre-exposure task was significantly different from 0 (t[6] = 3.99, p = 0.007) (Figure 7B). Discrimination ratios of Aged Good, Young Good, and Young Poor Performers were not significantly different from 0 (t[5] = 1.23, p = 0.27; t[8] = 1.66, p = 0.14; t[6] = 1.46, p = 0.20; respectively). Together, these data suggest aged rats with the best associative abilities perform worse during perceptually similar discrimination testing. Notably, this inverse relationship between PRC-dependent behaviors is not observed in young animals.
Discussion
The current study aimed to investigate the relationship between two distinct perirhinal (PRC)-cortical dependent abilities in the context of cognitive aging. To assess this question, aged and young rats were cross-characterized on PRC-dependent crossmodal recognition (CMOR) tasks and a LEGO object discrimination task. On the CMOR tasks, with and without pre-exposure to test objects, aged rats explored the novel object more than the familiar object. Thus, old animals were able to associate object features across visual and tactile modalities (Figure 2A, 3A). This observation is consistent with data from older adults showing that binding of individual visual features for objects does not decline in advanced age (Hoefeijzers, González Hernández, Magnolia Rios, & Parra, 2017; Isella, Molteni, Mapelli, & Ferrarese, 2015; Parra, Abrahams, Logie, & Sala, 2009; Read, Rogers, & Wilson, 2016).
One aspect of the current data is the high exclusion rate of both aged and young rats due to insufficient exploration of sample or pre-exposure objects. While food and weight restriction are not commonly reported in standard object recognition procedures, this practice is frequently used to increase exploratory behavior. Brief caloric restriction procedures in the Fischer 344 × Brown Norway F1 hybrid rats used here, however, are difficult in aging studies due to the differential restriction responses of young and aged rats. Specifically, aged rats of this strain are over conditioned relative to young, and therefore take longer to reach a comparable level of motivation to explore for food (Hernandez, Hernandez, et al., 2017; S. A. Johnson et al., 2017). This can be normalized across age groups over long restriction protocols, as utilized in the object discrimination experiment reported here. Over-restriction, however, can result in reductions in exploratory behavior in spontaneous object recognition tasks (Carter et al., 2009). Thus, the CMOR and SOR procedures used here did not employ any restriction, which may have contributed to some animals showing less exploratory behavior. Nonetheless, there was a significant interaction between association and discrimination abilities in aged rats.
The observation that advanced age does not result in reduced ability to recognize an object in multiple modalities on a PRC-dependent CMOR task may appear somewhat surprising in the context of previously reported age-associated changes in PRC neuron activity (Burke, Hartzell, Lister, Hoang, & Barnes, 2012; S. N. Burke et al., 2014; Maurer, Burke, Diba, & Barnes, 2017), and reductions in the BOLD signal in the PRC of older adults (Lee et al., 2012). Moreover, age-associated changes in the biochemistry of PRC have also been widely reported (Liu, Chary, et al., 2008; Liu, Gupta, Jing, & Zhang, 2008; Liu, Jing, Collie, Chary, & Zhang, 2009; Liu, Jing, & Zhang, 2009; Liu, Smith, & Darlington, 2008; Moyer, Furtak, McGann, & Brown, 2011). While the current behavioral data may appear in conflict with the neurobiology, the aged rats in the current study performed worse, compared to young rats, at discriminating between objects that shared features (Figure, 6B; S. A. Johnson et al., 2017). The observed discrimination deficits with age are consistent with data from humans (Reagh et al., 2015; Stark, Stevenson, Wu, Rutledge, & Stark, 2015; Stark et al., 2013; Toner et al., 2009; Yassa et al., 2010; Yassa, Mattfeld, Stark, & Stark, 2011), monkeys (S. N. Burke et al., 2011), and rats (Burke, Wallace, Nematollahi, Uprety, & Barnes, 2010; S.A. Johnson et al., 2017). Together the current data, along with previous reports, indicate that advanced age dissociates associative versus discrimination functions of the PRC, with the latter being particularly vulnerable to normative aging processes.
In support of the dissociation between PRC-dependent association and discrimination, principal component analysis (PCA) revealed that for the aged rats only, poorer performance on the LEGO object discrimination task was associated with better recognition on both CMOR tasks. In contrast, young rats with higher discrimination ratios in the standard CMOR task tended to perform better at LEGO object discrimination. To further explore the relationship between crossmodal association and discrimination suggested by the PCA, young and aged rats were subdivided into groups of poor and good performers on the LEGO object discrimination task based on Day 10 percent correct, and CMOR performance was examined within these subgroups. Interestingly, aged rats that performed poorly on the LEGO object discrimination task showed a significant preference for novelty on both CMOR tasks, while aged rats that were good discriminators did not. This relationship did not exist in young rats. The dissociation of discrimination and association abilities strictly in aged rats suggests that association and discrimination rely on distinct PRC-dependent computations that are differentially impacted by age-related processes. Moreover, while the young PRC may be able to support both the ability to associate and discriminate, aged PRC circuits that maintain an ability to support stimulus discrimination may do so at the expense of computations critical for associative memory. One potential caution to be considered in the current data, however, is the possibility that a task order effect could have differentially impacted the young and aged rats, as object recognition testing always preceded object discrimination testing. While it is unlikely that PRC impairments developed over the 2-3 weeks between when recognition testing ended and discrimination testing began, this possibility will need to be explored further.
The variability in aging phenotypes observed in the current data may partially account for discrepant findings regarding associative memory deficits in older adults (e.g., Memel & Ryan, 2017; Naveh-Benjamin, 2000). Additionally, these findings support an emerging framework in which the PRC supports different levels of sensory processing via participation in two parallel networks that are reciprocally connected to the postrhinal cortex. One network, is involved in the formation of gist-like associations, and could be critical for CMOR task performance. The other network is critical for representations of specific sensory details that may be required for discrimination when stimuli shared features. Available data, along with the data reported here, indicate that the detail network may be particularly vulnerable in advanced age (Burke et al., in press).
The preservation of crossmodal association with age is supported by recent findings showing that visual integration, allowing for the unitization of objects with compatible scenes, improves the associative memory of older adults (Memel & Ryan, 2017). This is presumably due to an automatic process of object-context binding that occurs during encoding, and does not decline in older adults (Memel & Ryan, 2017). A similarly positive impact of semantic integration on associative memory has been previously found for verbal material in young (Bader, Mecklinger, Hoppstadter, & Meyer, 2010), and older adults (Bastin & Van der Linden, 2003);(Biss, Campbell, & Hasher, 2013; D’Angelo, Noly-Gandon, Kacollja, Barense, & Ryan, 2017; Zheng, Li, Xiao, Broster, & Jiang, 2015). It has been suggested that this is due to older adults’ predisposition to the ‘hyper-binding’ of randomly co-occurring stimuli in their environment, leading to association of information with short temporal distance (Campbell, Hasher, & Thomas, 2010).
It is important to note that there was a trend for the discrimination ratio of young rats to be lower when compared to aged rats on the standard CMOR task, which may suggest that young rats’ reduced exploration of the familiar object in a novel context was “adaptive” and that this recognition of novel object-context pair was absent in the aged rats, resulting in contradictory “better” performance on the CMOR task. An additional caveat of the current data is that exploration-based recognition memory paradigms are prone to the influence of other moderating variables that may impact exploration, such as anxiety, neophobia, fatigue, lack of appetite/motivation, and task experience. These factors are especially important to consider in an aging cohort in which age groups may respond differently to elements of the experimental design such as food restriction. Moreover, despite the Latin Square design used to vary recognition testing order, it is feasible that task performance may have been impacted by experience differentially in aged and young rats. Due to the varied impact of these factors across age groups, our interpretation of the data presented here favors a synthesis of the data obtained from the different behavioral paradigms and the significant negative relationship of task performance in aged rats between association and discrimination abilities. Specifically, our interpretation of aged rats’ behavior on the CMOR tasks is dependent on the relationship to their behavior on the discrimination task. Together, these data suggest that there is a dissociation between association and discrimination abilities within the PRC network.
The current data have implications for the clinical assessment of age-related disease and distinguishing between cognitive decline occurring in normal aging versus preclinical Alzheimer’s disease (AD). The ability to discriminate between stimuli that share features may ubiquitously decline in both aging and the early stages of Alzheimer’s disease (Bakker, Albert, Krauss, Speck, & Gallagher, 2015; Bakker et al., 2012; S.A. Johnson et al., 2017; Yoder et al., 2017). This lack of specificity for detecting impairments in normal aging versus disease could mitigate the utility of using discrimination-based tasks for the early detection of pathology. In contrast to discrimination, several studies have shown that the binding of different object features remains intact in healthy aging (Hoefeijzers et al., 2017; Isella et al., 2015; Parra et al., 2009; Read et al., 2016). In contrast, the binding of different stimulus features, which is critical for successful CMOR performance, is vulnerable in early stages of Alzheimer’s disease (Cecchini et al., 2017; Della Sala, Kozlova, Stamate, & Parra, 2016; Parra et al., 2017) and even shows declines in asymptotic carriers of familial Alzheimer’s disease (Parra et al., 2011). These data indicate that neuropsychological assessment to detect preclinical Alzheimer’s disease should include tests of feature binding, similar to the CMOR procedures used in the current paper. This approach may optimize the therapeutic window by enhancing our clinical detection of early pathological accumulation.
Acknowledgments
We thank Michael Burke for constructing the behavioral apparatus. This work was supported by the McKnight Brain Research Foundation, National Institute on Aging (R01AG049722), and a UF University Scholars Program Award to KTC.
References
- Agster KL, Burwell RD. Hippocampal and subicular efferents and afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Behav Brain Res. 2013;254:50–64. doi: 10.1016/j.bbr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JR, Lee I. Neural correlates of object-associated choice behavior in the perirhinal cortex of rats. J Neurosci. 2015;35(4):1692–1705. doi: 10.1523/JNEUROSCI.3160-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader R, Mecklinger A, Hoppstadter M, Meyer P. Recognition memory for one-trial-unitized word pairs: evidence from event-related potentials. Neuroimage. 2010;50(2):772–781. doi: 10.1016/j.neuroimage.2009.12.100. [DOI] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. doi:S0028-3932(07)00216-3 [pii] [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. Object-in-Place Associative Recognition Memory Depends on Glutamate Receptor Neurotransmission Within Two Defined Hippocampal-Cortical Circuits: A Critical Role for AMPA and NMDA Receptors in the Hippocampus, Perirhinal, and Prefrontal Cortices. Cereb Cortex. 2015;25(2):472–481. doi: 10.1093/cercor/bht245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007a;27(10):2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. doi:27/10/2548 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 2007b;14(12):821–832. doi: 10.1101/lm.749207. doi:14/12/821 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17(1):14–24. [PubMed] [Google Scholar]
- Biss RK, Campbell KL, Hasher L. Interference from previous distraction disrupts older adults’ memory. J Gerontol B Psychol Sci Soc Sci. 2013;68(4):558–561. doi: 10.1093/geronb/gbs074. [DOI] [PubMed] [Google Scholar]
- Burke SN, Hartzell AL, Lister JP, Hoang LT, Barnes CA. Layer V perirhinal cortical ensemble activity during object exploration: a comparison between young and aged rats. Hippocampus. 2012;22(10):2080–2093. doi: 10.1002/hipo.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Advanced age dissociates dual functions of the perirhinal cortex. J Neurosci. 2014;34(2):467–480. doi: 10.1523/JNEUROSCI.2875-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-Associated Deficits in Pattern Separation Functions of the Perirhinal Cortex: A Cross-species Consensus. Behavioral Neuroscience. 2011;125(6):836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–573. doi: 10.1037/a0020893. doi:2010-20760-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-Binding: A Unique Age Effect. Psychol Sci. 2010;21(3):399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64(8):850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Craik FI. The effects of aging and divided attention on memory for item and associative information. Psychology and aging. 2003;18(4):873. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Cecchini MA, Yassuda MS, Bahia VS, de Souza LC, Guimarães HC, Caramelli P, Parra MA. Recalling feature bindings differentiates Alzheimer’s disease from frontotemporal dementia. J Neurol. 2017 doi: 10.1007/s00415-017-8614-9. [DOI] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychology and aging. 2008;23(1):93. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- D’Angelo MC, Noly-Gandon A, Kacollja A, Barense MD, Ryan JD. Breaking down unitization: Is the whole greater than the sum of its parts? Mem Cognit. 2017 doi: 10.3758/s13421-017-0736-x. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Kozlova I, Stamate A, Parra MA. A transcultural cognitive marker of Alzheimer’s Disease. Int J Geriatr Psychiatry. 2016 doi: 10.1002/gps.4610. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Price CJ. Perirhinal contributions to human visual perception. Curr Biol. 2007;17(17):1484–1488. doi: 10.1016/j.cub.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15(3):347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Heimer-McGinn VR, Poeta DL, Aghi K, Udawatta M, Burwell RD. Disconnection of the Perirhinal and Postrhinal Cortices Impairs Recognition of Objects in Context But Not Contextual Fear Conditioning. J Neurosci. 2017;37(18):4819–4829. doi: 10.1523/JNEUROSCI.0254-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Campos KT, Truckenbrod LM, Sakarya Y, McQuail JA, Burke SN. The Anti-Epileptic Ketogenic Diet Alters Hippocampal Transporter Levels and Reduces Adiposity in Aged Rats. J Gerontol A Biol Sci Med Sci. 2017 doi: 10.1093/gerona/glx193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Maurer AP, Reasor JE, Turner SM, Barthle SE, Johnson SA, Burke SN. Age-related impairments in object-place associations are not due to hippocampal dysfunction. Behav Neurosci. 2015;129(5):599–610. doi: 10.1037/bne0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Reasor JE, Truckenbrod LM, Lubke KN, Johnson SA, Bizon JL, Burke SN. Medial prefrontal-perirhinal cortical communication is necessary for flexible response selection. Neurobiol Learn Mem. 2017;137:36–47. doi: 10.1016/j.nlm.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefeijzers S, González Hernández A, Magnolia Rios A, Parra MA. Feature Binding of Common Everyday Items Is Not Affected by Age. Front Aging Neurosci. 2017;9:122. doi: 10.3389/fnagi.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Hocking J, Notley P, Devlin JT, Price CJ. Integrating visual and tactile information in the perirhinal cortex. Cereb Cortex. 2009;19(12):2993–3000. doi: 10.1093/cercor/bhp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Molteni F, Mapelli C, Ferrarese C. Short term memory for single surface features and bindings in ageing: A replication study. Brain Cogn. 2015;96:38–42. doi: 10.1016/j.bandc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Jacklin DL, Cloke JM, Potvin A, Garrett I, Winters BD. The Dynamic Multisensory Engram: Neural Circuitry Underlying Crossmodal Object Recognition in Rats Changes with the Nature of Object Experience. J Neurosci. 2016;36(4):1273–1289. doi: 10.1523/JNEUROSCI.3043-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee I. Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. J Neurosci. 2010a;30(29):9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee I. Perirhinal cortex is necessary for acquiring, but not for retrieving object-place paired association. Learn Mem. 2010b;17(2):97–103. doi: 10.1101/lm.1620410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Sacks PK, Turner SM, Gaynor LS, Ormerod BK, Maurer AP, Burke SN. Discrimination performance in aging is vulnerable to interference and dissociable from spatial memory. Learn Mem. 2016;23(7):339–348. doi: 10.1101/lm.042069.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Turner SM, Santacroce LA, Bizon JL, Maurer AP, Burke SN. Age-related impairments in discriminating perceptually similar objects parallel those observed in humans. Accepted, Hippocampus. 2017 doi: 10.1002/hipo.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Turner SM, Santacroce LA, Carty KN, Shafiq L, Bizon JL, Burke SN. Rodent age-related impairments in discriminating perceptually similar objects parallel those observed in humans. Hippocampus. 2017;27(7):759–776. doi: 10.1002/hipo.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. J Comp Neurol. 2004;472(3):371–394. doi: 10.1002/cne.20079. [DOI] [PubMed] [Google Scholar]
- Liu P, Chary S, Devaraj R, Jing Y, Darlington CL, Smith PF, Zhang H. Effects of aging on agmatine levels in memory-associated brain structures. Hippocampus. 2008;18(9):853–856. doi: 10.1002/hipo.20448. [DOI] [PubMed] [Google Scholar]
- Liu P, Gupta N, Jing Y, Zhang H. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience. 2008;155(3):789–796. doi: 10.1016/j.neuroscience.2008.06.033. doi:S0306-4522(08)00964-0 [pii] [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Collie ND, Chary S, Zhang H. Memory-related changes in L-citrulline and agmatine in the rat brain. Hippocampus. 2009;19(7):597–602. doi: 10.1002/hipo.20561. [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Zhang H. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. 2009;164(2):611–628. doi: 10.1016/j.neuroscience.2009.08.029. doi:S0306-4522(09)01387-6 [pii] [DOI] [PubMed] [Google Scholar]
- Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62(11):834–841. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Burke SN, Diba K, Barnes CA. Attenuated Activity Across Multiple Cell Types and Reduced Monosynaptic Connectivity in the Aged Perirhinal Cortex. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.0531-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memel M, Ryan L. Visual integration enhances associative memory equally for young and older adults without reducing hippocampal encoding activation. Neuropsychologia. 2017;100:195–206. doi: 10.1016/j.neuropsychologia.2017.04.031. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Furtak SC, McGann JP, Brown TH. Aging-related changes in calcium-binding proteins in rat perirhinal cortex. Neurobiol Aging. 2011;32(9):1693–1706. doi: 10.1016/j.neurobiolaging.2009.10.001. doi:S0197-4580(09)00328-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1170. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behav Brain Res. 2004;148(1–2):79–91. doi: 10.1016/s0166-4328(03)00176-1. doi:S0166432803001761 [pii] [DOI] [PubMed] [Google Scholar]
- Oedekoven CS, Jansen A, Keidel JL, Kircher T, Leube D. The influence of age and mild cognitive impairment on associative memory performance and underlying brain networks. Brain imaging and behavior. 2015;9(4):776–789. doi: 10.1007/s11682-014-9335-7. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. American Psychological Association; 2008. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Sala SD. Age and binding within-dimension features in visual short-term memory. Neurosci Lett. 2009;449(1):1–5. doi: 10.1016/j.neulet.2008.10.069. [DOI] [PubMed] [Google Scholar]
- Parra MA, Mikulan E, Trujillo N, Sala SD, Lopera F, Manes F, Ibanez A. Brain information sharing during visual short-term memory binding yields a memory biomarker for familial Alzheimer’s disease. Curr Alzheimer Res. 2017 doi: 10.2174/1567205014666170614163316. [DOI] [PubMed] [Google Scholar]
- Parra MA, Sala SD, Abrahams S, Logie RH, Méndez LG, Lopera F. Specific deficit of colour-colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia. 2011;49(7):1943–1952. doi: 10.1016/j.neuropsychologia.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Read CA, Rogers JM, Wilson PH. Working memory binding of visual object features in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23(3):263–281. doi: 10.1080/13825585.2015.1083937. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, Yassa MA. Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus. 2015 doi: 10.1002/hipo.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Jacklin DL, Winters BD. Crossmodal object recognition in rats with and without multimodal object pre-exposure: no effect of hippocampal lesions. Neurobiol Learn Mem. 2012;98(3):311–319. doi: 10.1016/j.nlm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Reid JM, Jacklin DL, Winters BD. Delineating prefrontal cortex region contributions to crossmodal object recognition in Rats. Cerebral Cortex. 2014;24(8):2108–2119. doi: 10.1093/cercor/bht061. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cardoza JA, Barense MD, Kawa KH, Wallentin-Flores J, Arnold WT, Alexander GE. Age-related impairment in a complex object discrimination task that engages perirhinal cortex. Hippocampus. 2012;22(10):1978–1989. doi: 10.1002/hipo.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Stevenson R, Wu C, Rutledge S, Stark CE. Stability of age-related deficits in the mnemonic similarity task across task variations. Behav Neurosci. 2015;129(3):257–268. doi: 10.1037/bne0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51(12):2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16(5):338–342. doi: 10.1101/lm.1315109. doi:16/5/338 [pii] [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24(26):5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Reid JM. A distributed cortical representation underlies crossmodal object recognition in rats. J Neurosci. 2010;30(18):6253–6261. doi: 10.1523/JNEUROSCI.6073-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. doi:1101567108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Gaynor LS, Burke SN, Setlow B, Smith DW, Bizon JL. Interaction between age and perceptual similarity in olfactory discrimination learning in F344 rats: relationships with spatial learning. Neurobiol Aging. 2017;53:122–137. doi: 10.1016/j.neurobiolaging.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Li J, Xiao F, Broster LS, Jiang Y. Electrophysiological evidence for the effects of unitization on associative recognition memory in older adults. Neurobiol Learn Mem. 2015;121:59–71. doi: 10.1016/j.nlm.2015.03.006. [DOI] [PubMed] [Google Scholar]


