Abstract
Background
Multiple lines of evidence indicate that aspirin has an antineoplastic effect in the large bowel. Randomized clinical trials have been conducted to evaluate the effectiveness of aspirin for reducing the risk of colorectal adenomas. A meta-analysis of these trials will provide more precise estimates of the aspirin effect, both overall and in subgroups.
Methods
We combined data from all randomized double-blind placebo-controlled trials that evaluated aspirin for the prevention of colorectal adenomas. We used random-effects meta-analysis to estimate risk ratios and 95% confidence intervals (CIs) for the effect of aspirin on the occurrence of adenomas and of advanced lesions (ie, tubulovillous adenomas, villous adenomas, adenomas ≥1 cm in diameter, adenomas with high-grade dysplasia, or invasive cancer). All statistical tests were two-sided.
Results
We identified four clinical trials with 2967 randomly assigned participants. Each trial evaluated aspirin for the secondary prevention of colorectal adenomas. Doses of aspirin tested ranged from 81 to 325 mg/d. The average age of participants at baseline was 58 years, and 60% were male. Median follow-up was 33 months. A total of 2698 participants underwent colonoscopic follow-up and were included in the analysis of adenoma occurrence and advanced-lesion occurrence after randomization. Among these participants, adenomas were found in 424 (37%) of the 1156 participants allocated to placebo and in 507 (33%) of the 1542 participants allocated to any dose of aspirin. Advanced lesions were found in 12% of participants in the placebo group and in 9% of participants allocated to any dose of aspirin. The pooled risk ratio of any adenoma for any dose of aspirin vs placebo was 0.83 (95% CI = 0.72 to 0.96). This corresponded to an absolute risk reduction of 6.7% (95% CI = 3.2% to 10.2%). For any advanced lesion, the pooled risk ratio was 0.72 (95% CI = 0.57 to 0.90). We found no statistically significant effect modification for any of the baseline factors studied.
Conclusion
Aspirin is effective for the prevention of colorectal adenomas in individuals with a history of these lesions.
CONTEXT AND CAVEATS
Prior knowledge
Multiple lines of evidence including clinical trial data indicate that aspirin has an antineoplastic effect in the large bowel and reduces the risk of colorectal adenomas. However, a quantitative summary of efficacy is missing.
Study design
A meta-analysis of the association between aspirin use and the occurrence of adenomas and of advanced lesions using participant data from four randomized double-blind trials that evaluated higher-dose and/or lower-dose aspirin vs placebo for the secondary prevention of colorectal adenomas.
Contribution
Among 2698 participants who underwent colonoscopic follow-up after randomization, adenomas were found in 37% of those allocated to placebo and in 33% of those allocated to any dose of aspirin (advanced lesions were found in 12% and 9%, respectively).
Implications
This meta-analysis of clinical trial data indicates that aspirin reduces the risk of recurrence of colorectal adenomas.
Limitations
Dose–response patterns were not interpretable because only two studies investigated lower-dose aspirin. An analysis of cardiovascular and bleeding events was limited by the small numbers of events observed in any one trial. One trial was excluded from the analysis of adverse events.
From the Editors
Over the past 30 years, compelling data have emerged suggesting that nonsteroidal anti-inflammatory drugs (NSAIDs), particularly aspirin, can suppress carcinogenesis in the large bowel. For example, in numerous epidemiological studies, the use of aspirin or other NSAIDs has been associated with a reduced risk of colorectal cancer ( 1 ), and pooled results from two clinical trials have shown a protective effect in the primary prevention of sporadic colorectal cancer ( 2 ). In trials conducted among patients with familial adenomatous polyposis ( 3–7 ), treatment with other NSAIDS (sulindac or cyclooxygenase-2 inhibitors) caused existing adenomas to regress and suppressed the emergence of new lesions.
In case–control and cohort studies ( 2 , 8 , 9 ), aspirin use was associated with a reduced risk of colorectal cancer, especially after 10 years of use. Clinical trial data ( 2 ) also suggest that aspirin must be taken for many years before a protective effect on colorectal cancer emerges. The long duration of use required to prevent invasive cancer may reflect the time required for cancer to develop from precursor lesions.
Colorectal adenomas—the precursors to most colorectal cancers—would be expected to reflect the chemopreventive effects of aspirin sooner than invasive cancers because these lesions occur much earlier in the carcinogenic pathways. To date, results of four randomized clinical trials that formally assessed the effect of aspirin on the risk of adenomas have been published ( 10–13 ). Participants with a history of colorectal adenomas or cancer were recruited in each of the trials and were followed up for subsequent new adenomas. Data from each of these trials suggest that aspirin reduces the risk of subsequent new adenomas, but, to our knowledge, a quantitative summary of efficacy has not been reported. In addition, it is not clear what the dose–response patterns are or if particular groups are resistant (or more sensitive) to the chemopreventive effect of aspirin.
To better characterize the apparent chemopreventive effect of aspirin in the large bowel, we performed a meta-analysis of all available randomized clinical trials that investigated whether aspirin reduces the risk of colorectal adenomas.
Methods
Trial Inclusion Criteria
We identified candidate studies by conducting computerized searches of the Medline and Web of Science databases using the terms “aspirin,” “acetylsalicylic acid,” “salicylates,” “adenoma,” and “randomized clinical trial.” We also consulted with colleagues to identify unpublished studies that may exist. We included all placebo-controlled randomized trials of aspirin (acetylsalicylic acid) in any dose as a chemopreventive agent for sporadic large-bowel adenomas. To be included in this meta-analysis, a trial must have satisfied the following inclusion criteria: 1) the study was placebo controlled and double blinded; 2) individuals with familial adenomatous polyposis were excluded; 3) participants were treated for at least 1 year; 4) for each participant, colorectal polyp status at baseline was assessed with complete colonoscopy and no polyps were knowingly left in the bowel at that time; and 5) colorectal polyp occurrence after randomization was assessed by colonoscopic follow-up. In addition, each trial had to have obtained written informed consent from each participant and must have had appropriate institutional review board (IRB) approval.
Data Collection
Rather than combine published (or provided) summary statistics from each study, we obtained participant-level data from each identified trial, a preferred approach to meta-analysis ( 14 ). We sought the following data elements: randomized aspirin or placebo treatment group; age at study entry (in years at last birthday); sex; race; baseline body mass index (BMI); number of lifetime adenomas before randomization; family history of colorectal cancer; baseline smoking status; duration of randomized treatment; pill-taking compliance; occurrence and timing of death during the treatment period; occurrence and timing of myocardial infarctions, strokes, major bleeding, or new cancer diagnoses during the treatment period; and timing and outcome of each colonoscopic follow-up examination during the treatment period, including the type, size, and location of each adenoma found and whether high-grade dysplasia was present. These data, if available, were sent in a de-identified data file to Dartmouth Medical School for analysis as a combined dataset. This study was determined to be exempt from IRB review by the Dartmouth College Human Subjects Review Committee.
Statistical Analysis
The primary endpoint was the occurrence of any colorectal adenoma after randomization. Secondary endpoints were the occurrence of advanced lesions, defined as tubulovillous adenomas (25%–75% villous features), villous adenomas (≥75% villous features), large adenomas (≥1 cm in diameter), adenomas with high-grade dysplasia, or invasive cancer, and the occurrence of adverse events. The statistical analysis of the combined datasets followed standard random-effects meta-analysis methods ( 15–17 ) in a two-stage approach. In the first stage, each clinical trial was analyzed separately to obtain trial-specific estimates of the relative risk of adenoma and advanced lesions for the aspirin group vs the placebo group. The trial-specific risk ratios were then combined using standard methods for random-effects meta-analysis ( 15 ). A similar method was used to combine trial-specific absolute risk reductions. All P values were derived from two-sided tests, and we considered a P value less than .05 to be statistically significant. Between-study heterogeneity was assessed using the Q statistic and the I 2 statistic. An I 2 value of greater than 50%, or a P value less than .05 for the Q statistic, was taken to indicate heterogeneity ( 18 , 19 ). Within each clinical trial, the analysis population was defined as all randomly assigned participants who had undergone at least one follow-up colonoscopy. This approach represents a modified intention-to-treat analysis.
We used forest plots to summarize overall results for the effect of aspirin on the risks of any adenoma and any advanced lesion. Subgroup analyses were performed to evaluate the consistency of the aspirin treatment effect within specific subgroups of patients defined according to their baseline characteristics. These analyses were performed using the same methods as described above, but they focused on data for the subgroup of interest. Wald tests were used to assess the interaction between aspirin and subgroup. Six subgroup factors were defined in the analysis protocol: sex; age (≤54, 55–63, or ≥64 years, based on tertiles of the data); BMI [<25, 25–29.9, or ≥30.0 kg/m 2 , based on cut points established by the National Heart, Lung and Blood Institute ( 20 )]; family history of colorectal cancer, defined as a first-degree relative diagnosed with the disease (present or absent); number of lifetime adenomas (1 or ≥2, to divide the sample into two groups of similar size); and the presence of advanced lesions at the examination that was required to determine study eligibility (present or absent). We also performed analyses that considered colonoscopic examinations that occurred within four follow-up intervals following randomization: 0 to <12, 12 to <24, 24 to <38, and ≥38 months. These intervals were chosen to compare effects from year to year after randomization. However, because most of the clinical trials specified a 3-year follow-up interval, we set the final cut point at 38 months rather than 36 months. This cut point ensured that follow-up examinations that occurred near the planned examination time fell within only one of the analysis time intervals (ie, the interval from 24 to <38 months).
Results
Trials Identified
We identified four clinical trials that satisfied the inclusion criteria: the Aspirin/Folate Polyp Prevention Study (AFPPS) ( 10 ), the Colorectal Adenoma Prevention Study [Cancer and Leukemia Group B (CALGB) 9270] ( 11 ), the United Kingdom Colorectal Adenoma Prevention (ukCAP) Study ( 12 ), and the Association pour la Prevention par l’Aspirine du Cancer Colorectal (APACC) Study ( 13 ). Table 1 summarizes the trial designs. Three of the four trials (AFPPS, ukCAP, and APACC) recruited participants with a recent history of sporadic colorectal adenoma and excluded individuals with a history of invasive large-bowel cancer, and one trial (CALGB 9270) specifically recruited patients who had been treated for colorectal cancer. Other eligibility criteria for the trials were similar—each trial excluded individuals with inflammatory bowel disease, those with a clinical need for aspirin treatment, and those who could not take aspirin.
Table 1.
Summary of trial designs *
| Trial (reference) | No. of subjects randomly assigned | Calendar years of recruitment | Study population | Recruitment site | Treatment groups |
| AFPPS (10) | 1121 | 1994–1998 | Recent history of sporadic colorectal adenomas | United States and Canada | Placebo vs 81 mg/d aspirin vs 325 mg/d aspirin, with or without folic acid |
| CALGB 9270 (11) | 635 | 1993–2000 | Previous colorectal cancer | United States | Placebo vs 325 mg/d aspirin |
| ukCAP (12) | 939 | 1997–2001 | Recent history of sporadic colorectal adenomas | United Kingdom and Denmark | Placebo vs 300 mg/d aspirin |
| APACC (13) | 272 | 1996–2000 | Recent history of sporadic colorectal adenomas | France | Placebo vs 160 mg/d aspirin vs 300 mg/d aspirin |
AFPPS = Aspirin/Folate Polyp Prevention Study; CALGB = Cancer and Leukemia Group B; ukCAP = United Kingdom Colorectal Adenoma Prevention; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal.
All four studies used an adenoma endpoint. Two trials (AFPPS and APACC) compared lower-dose aspirin (defined as 81 or 160 mg/d) and higher-dose aspirin (defined as 300 or 325 mg/d) with placebo. The remaining trials (CALGB 9270 and ukCAP) compared higher-dose aspirin with placebo.
Each of the trials had a defined endoscopy follow-up schedule. AFPPS and ukCAP intended to examine participants 3 years after the baseline examination, and APACC examined participants at 1 and 4 years after the baseline examination. CALGB 9270 examined participants with early-stage disease at 4 years after the baseline examination and all other patients at 3 years after the baseline examination. This trial was stopped early because of the efficacy of the intervention. AFPPS and APACC each had central pathology review of lesions removed from the large bowel during follow-up examinations. CALGB 9270 and ukCAP relied solely on local pathologists to determine lesion characteristics.
Follow-up for adverse events varied considerably across trials. AFPPS attempted to follow up all participants through the dates of the expected year 3 examinations, whereas in APACC and ukCAP, adverse event data were ascertained only for participants who were on treatment or who had study examinations. Adverse event data for CALGB 9270 were considered unreliable by the study investigators because of inconsistencies in the computerized database, and thus the data regarding bleeding, cardiovascular events, and cancer diagnoses from this trial were not included in this analysis. We included only the deaths that occurred during the treatment period of all studies, including CALGB 9270, which was terminated early due to efficacy.
Primary analyses reports have been published for AFPPS ( 10 ), CALGB 9270 ( 11 ), and ukCAP ( 12 ). APACC has reported year 1 results only ( 13 ).
Characteristics of the Study Participants
Table 2 describes the baseline characteristics of all 2967 randomly assigned participants by treatment group. Of these, 1289 participants were allocated to placebo and 1678 to aspirin. A total of 450 participants were randomly assigned to lower-dose aspirin and 1228 to higher-dose aspirin. The mean age of all participants was approximately 58 years, and approximately 60% were male. Smoking status was not available from CALGB 9270, nor was information regarding family history of colorectal cancer. Data on race were not available from APACC. Consequently, data regarding some characteristics were missing for substantial proportions of the participants. Most participants (71%) were non-Hispanic white or had a BMI of 25 kg/m 2 or higher. Most (54%) of the participants whose adenoma history was known had only one lifetime adenoma. Among those with information available regarding family history of colorectal cancer, 31% had a first-degree relative who had been diagnosed with colorectal cancer. The high proportions of males, those with unknown number of lifetime adenomas, and those with a family history of colorectal cancer in the lower-dose aspirin group reflect the characteristics of participants in the two studies that offered two aspirin doses, not imbalances in randomization.
Table 2.
Baseline characteristics of participants in all trials
| Characteristic | Placebo | Aspirin in any dose | Aspirin, 81 or 160 mg/d | Aspirin, 300 or 325 mg/d |
| No. of participants | 1289 | 1678 | 450 | 1228 |
| Age, y | ||||
| Mean (SD) | 58.5 (9.9) | 58.2 (9.7) | 57.6 (9.8) | 58.4 (9.6) |
| ≤54, No. (%) | 459 (36) | 602 (36) | 169 (38) | 433 (35) |
| 55–63, No. (%) | 380 (29) | 546 (33) | 153 (34) | 393 (32) |
| ≥64, No. (%) | 450 (35) | 530 (32) | 128 (28) | 402 (33) |
| Sex, No. (%) | ||||
| Male | 755 (59) | 1014 (60) | 295 (66) | 719 (59) |
| Female | 534 (41) | 664 (40) | 155 (34) | 509 (41) |
| Body mass index, kg/m 2 | ||||
| Mean (SD) | 27.2 (4.5) | 27.3 (4.6) | 27.1 (4.3) | 27.3 (4.7) |
| <25.0, No. (%) | 366 (28) | 461 (27) | 143 (32) | 318 (26) |
| 25.0–29.9, No. (%) | 451 (35) | 647 (39) | 215 (48) | 432 (35) |
| ≥30, No. (%) | 232 (18) | 311 (19) | 90 (20) | 221 (18) |
| Unknown, No. (%) | 240 (19) | 259 (15) | 2 (0) | 257 (21) |
| Race or ethnic group, No. (%) | ||||
| Non-Hispanic white | 880 (68) | 1228 (73) | 329 (73) | 899 (73) |
| Non-Hispanic black | 55 (4) | 59 (4) | 22 (5) | 37 (3) |
| Hispanic | 43 (3) | 43 (3) | 16 (4) | 27 (2) |
| Other | 16 (1) | 28 (2) | 10 (2) | 18 (1) |
| Unknown | 295 (23) | 320 (19) | 73 (16) | 247 (20) |
| Tobacco smoking status, No. (%) | ||||
| Never smoked | 328 (25) | 492 (28) | 200 (44) | 292 (24) |
| Former smoker | 306 (24) | 476 (28) | 179 (40) | 297 (24) |
| Current smoker | 173 (13) | 209 (12) | 69 (15) | 140 (11) |
| Unknown | 482 (37) | 501 (30) | 2 (0) | 499 (41) |
| Family history of colorectal cancer, No. (%) | ||||
| No known history | 505 (39) | 673 (40) | 231 (51) | 442 (36) |
| First-degree relative | 205 (16) | 342 (20) | 131 (29) | 211 (17) |
| Other relative | 28 (2) | 35 (2) | 24 (5) | 11 (1) |
| Unknown | 551 (43) | 628 (37) | 64 (14) | 564 (46) |
| No. of lifetime adenomas at baseline, No. (%) | ||||
| 1 | 525 (41) | 729 (43) | 214 (48) | 515 (42) |
| ≥2 | 444 (34) | 630 (38) | 235 (52) | 395 (32) |
| Unknown | 320 (25) | 319 (19) | 1 (0) | 318 (26) |
All of the trials required participants to avoid taking aspirin and NSAIDs during the treatment period of the trial. Therefore, very few participants were regular users of these drugs before randomization. Rates of regular use (defined as use at least 3 d/wk) before randomization did not exceed 5% in any of the studies, and treatment groups were not statistically significantly unbalanced in this regard.
Compliance and Follow-up
Compliance with the study treatments and procedures was generally good. Overall pill-taking compliance (expressed as the percentage of expected number of study pills taken) tended to be slightly higher in the any-dose aspirin group than in the placebo group ( Table 3 ). Among the four studies, mean pill-taking compliance ranged from approximately 69% to approximately 92%. At least 81% of participants in each of the studies and at least 90% of the participants in three studies (AFPPS, ukCAP, and APACC) had at least one follow-up examination ( Supplementary Table 1 , available online). (The early termination of CALGB 9270 contributed to the lower percentage of participants with colonoscopic follow-up in that study.) In total, 2698 participants underwent colonoscopic follow-up. The remaining 269 participants were either lost to follow-up or died and were excluded from the analysis of adenoma and advanced-lesion occurrence. The median follow-up of all participants was approximately 33 months. APACC included a 4-year examination and thus had the longest median follow-up (47.2 months vs 32.2, 31.3, and 37.5 months for AFPPS, CALGB 9270, and ukCAP, respectively). Follow-up duration and rate of colonoscopic follow-up were well balanced across the treatment groups ( Supplementary Table 1 , available online).
Table 3.
Overall pill-taking compliance *
| Study | Placebo | Aspirin in any dose | Aspirin, 81 or 160 mg/d | Aspirin, 300 or 325 mg/d |
| AFPPS | ||||
| Mean percentage of study pills taken (SD) | 90.3 (20.5) | 91.7 (18.8) | 91.9 (18.8) | 91.6 (18.7) |
| Missing/unknown, No. (%) | 18 (5) | 24 (3) | 13 (3) | 11 (3) |
| CALGB 9270 | ||||
| Mean percentage of study pills taken (SD) | 74.9 (28.5) | 79.4 (26.8) | NA | 79.4 (26.8) |
| Missing/unknown, No. (%) | 22 (7) | 21 (7) | NA | 21 (7) |
| ukCAP | ||||
| Mean percentage of study pills taken (SD) | 80.9 (31.6) | 77.1 (35.2) | NA | 77.1 (35.2) |
| Missing/unknown, No. (%) | 53 (11) | 48 (10) | NA | 48 (10) |
| APACC | ||||
| Mean percentage of study pills taken (SD) | 68.5 (36.3) | 75.7 (32.9) | 76.8 (32.8) | 74.6 (33.3) |
| Missing/unknown, No. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| All trials | ||||
| Mean percentage of study pills taken (SD) | 80.8 (29.4) | 84.1 (27.8) | 89.4 (22.4) | 82.1 (29.3) |
| Missing/unknown, No. (%) | 93 (7) | 93 (6) | 13 (3) | 80 (7) |
AFPPS = Aspirin/Folate Polyp Prevention Study; CALGB = Cancer and Leukemia Group B; NA = not applicable; ukCAP = United Kingdom Colorectal Adenoma Prevention; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal.
Adenoma and Advanced-Lesion Occurrence
The analyses of adenoma occurrence and advanced-lesion occurrence after randomization were restricted to the 2698 participants for whom colonoscopic follow-up information was available. Overall, approximately 35% of participants with colonoscopic follow-up were diagnosed with one or more recurrent adenomas, and the proportions varied considerably across studies, ranging from approximately 22% to 52% of participants. The lowest proportion was seen in CALGB 9270 (22%), which possibly reflects the fact that these participants did not have an entire colorectal mucosa left at risk after undergoing a colon or rectum resection for their cancer.
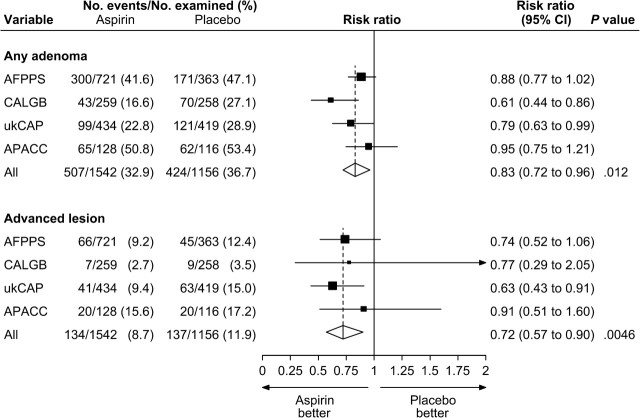
Figure 1 summarizes the random-effects meta-analysis comparing aspirin in any dose to placebo. Among participants with colonoscopic follow-up, adenomas were found in 424 (37%) of the 1156 participants allocated to placebo and in 507 (33%) of the 1542 participants allocated to any dose of aspirin ( Figure 1 ). We observed a statistically significant 17% relative reduction in the risk of any adenoma for aspirin in any dose vs placebo (pooled risk ratio [RR] = 0.83; 95% confidence interval [CI] = 0.72 to 0.96). This corresponded to a statistically significant 6.7% (95% CI = 3.2% to 10.2%) absolute risk reduction. Among participants with colonoscopic follow-up, advanced lesions were found in 137 (12%) participants in the placebo group and in 134 (9%) participants allocated to any dose of aspirin ( Figure 1 ), which corresponded to a statistically significant relative risk reduction of 28% for aspirin in any dose (RR = 0.72; 95% CI = 0.57 to 0.90).
Figure 1.
Random-effects risk ratio forest plot comparing any aspirin vs placebo. Trial-specific risk ratios are shown as black squares , with the size of the square being inversely proportional to the trial-specific risk ratio variance. Horizontal lines represent 95% confidence intervals for the trial-specific risk ratios. Pooled risk ratios are shown as diamonds . The middle of each diamond corresponds to the risk ratio, and the width of each diamond represents the 95% confidence interval. The vertical dashed lines provide a visual comparison of the pooled risk ratio with the corresponding trial-specific risk ratios. Tests for heterogeneity are as follows. For any adenoma, Q = 5.13 ( P = 0.16) and I 2 = 41.5. For advanced lesion, Q = 1.27 ( P = .74) and I 2 = 0.0. AFPPS = Aspirin/Folate Polyp Prevention Study; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal; CALGB = Cancer and Leukemia Group B; CI = confidence interval; ukCAP = United Kingdom Colorectal Adenoma Prevention.
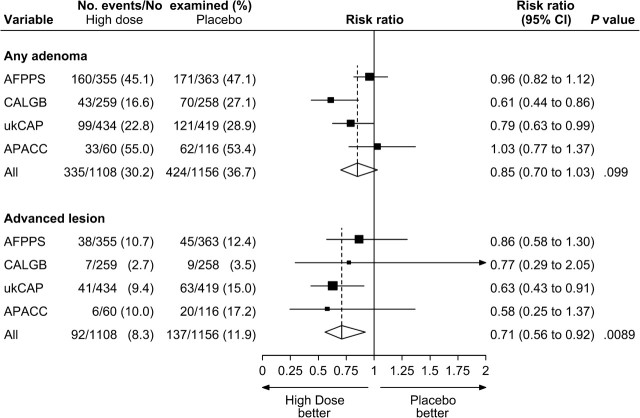
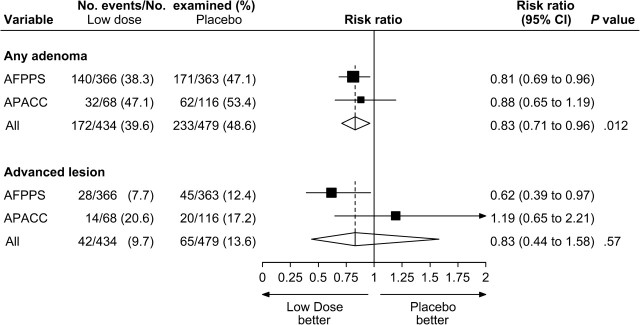
Figures 2 and 3 summarize the pooled comparisons of higher-dose aspirin vs placebo and lower-dose aspirin vs placebo, respectively. Among the 1108 participants who were randomly assigned to higher-dose aspirin and who underwent colonoscopic follow-up, adenomas were found in 335 (30%) and advanced lesions were found in 92 (8%). Among the 434 participants who were randomly assigned to lower-dose aspirin and who underwent colonoscopic follow-up, adenomas were found in 172 (40%) and advanced lesions were found in 42 (10%). For high-dose aspirin vs placebo, we observed a non–statistically significant relative risk reduction of 15% for any adenoma (RR = 0.85; 95% CI = 0.70 to 1.03); however, the absolute risk reduction of 5.7% was statistically significant (95% CI = 1.5% to 9.9%). We observed a statistically significant relative risk reduction of 29% for advanced lesions (RR = 0.71; 95% CI = 0.56 to 0.92) ( Figure 2 ). For low-dose aspirin vs placebo, we observed a statistically significant relative risk reduction of 17% for any adenoma (RR = 0.83; 95% CI = 0.71 to 0.96), which corresponded to a statistically significant absolute risk reduction of 8.4% (95% CI = 1.9% to 14.8%). We also observed a non–statistically significant relative risk reduction of 17% for advanced lesions (RR = 0.83; 95% CI = 0.44 to 1.58) ( Figure 3 ).
Figure 2.
Random-effects risk ratio forest plot comparing higher-dose aspirin (300 or 325 mg/d) vs placebo. Trial-specific risk ratios are shown as black squares , with the size of the square being inversely proportional to the trial-specific risk ratio variance. Horizontal lines represent 95% confidence intervals for the trial-specific risk ratios. Pooled risk ratios are shown as diamonds . The middle of each diamond corresponds to the risk ratio, and the width of each diamond represents the 95% confidence interval. The vertical dashed lines provide a visual comparison of the pooled risk ratio with the corresponding trial-specific risk ratios. Tests for heterogeneity are as follows. For any adenoma, Q = 7.51 ( P = .057) and I 2 = 60.1. For advanced lesion, Q = 1.87 ( P = .60) and I 2 =0.0. AFPPS = Aspirin/Folate Polyp Prevention Study; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal; CALGB = Cancer and Leukemia Group B; CI = confidence interval; ukCAP = United Kingdom Colorectal Adenoma Prevention.
Figure 3.
Random-effects risk ratio forest plot comparing lower-dose aspirin (81 or 160 mg/d) vs placebo. Trial-specific risk ratios are shown as black squares , with the size of the square being inversely proportional to the trial-specific risk ratio variance. Horizontal lines represent 95% confidence intervals for the trial-specific risk ratios. Pooled risk ratios are shown as diamonds . The middle of each diamond corresponds to the risk ratio, and the width of each diamond represents the 95% confidence interval. The vertical dashed lines provide a visual comparison of the pooled risk ratio with the corresponding trial-specific risk ratios. Tests for heterogeneity are as follows. For any adenoma, Q = 0.21 ( P = .65) and I2 = 0.0. For advanced lesion, Q = 2.89 ( P = .089) and I2 = 65.4. AFPPS = Aspirin/Folate Polyp Prevention Study; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal; CI = confidence interval.
We compared higher-dose aspirin vs lower-dose aspirin using the two trials that provided relevant data (AFPPS and APACC). This analysis involved 849 participants (415 allocated to higher-dose aspirin and 434 allocated to lower-dose aspirin). The pooled risk ratio comparing higher-dose aspirin vs lower-dose aspirin was 1.18 (95% CI = 1.01 to 1.37), with an absolute risk reduction of 7.0% (95% CI = 0.4% to 13.6%) in favor of lower-dose aspirin. For any advanced lesion, the pooled risk ratio was 0.89 (95% CI = 0.32 to 2.47).
There was evidence of between-study heterogeneity for the following comparisons based on relative risk: higher-dose aspirin vs placebo for any adenoma ( Q = 7.51, P = .057; I2 = 60.1%), lower-dose aspirin vs placebo for advanced lesions ( Q = 2.89, P = .089; I2 = 65.4%), and higher-dose aspirin vs lower-dose aspirin for advanced lesions ( Q = 4.25, P = .04; I2 = 76.5). For the first two of these three comparisons, heterogeneity was established solely based on an I2 statistic greater than 50%. No statistically significant heterogeneity was observed for comparisons based on absolute risk reductions.
Subgroup Analyses
Results of the random-effects meta-analysis for all adenomas according to subgroups of participants revealed no statistically significant effect modification for any of the baseline factors studied ( Figure 4 ). However, the estimated effect of aspirin in any dose on advanced lesions was substantially greater among those with a family history of colorectal cancer (RR = 0.53; 95% CI = 0.33 to 0.83) than among those without (RR = 0.92; 95% CI = 0.67 to 1.28; Pinteraction = .05).
Figure 4.
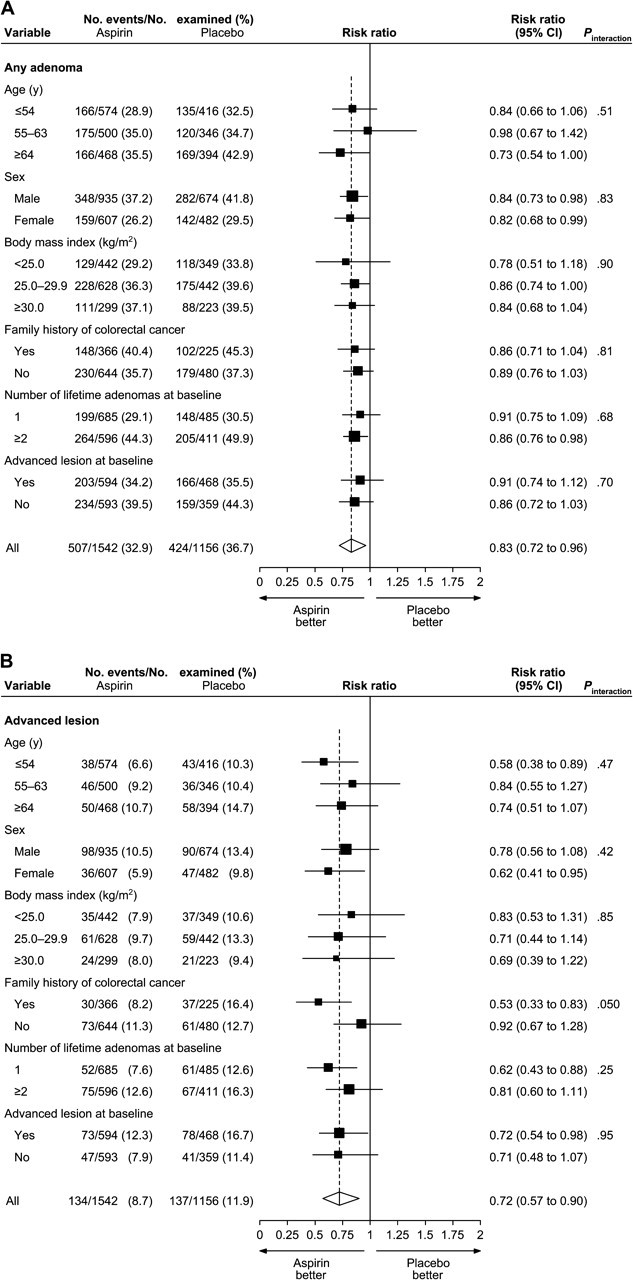
Random-effects risk ratio forest plot comparing any aspirin vs placebo by subgroups. A) Any adenoma. B) Advanced lesion. Subgroup-specific risk ratios are shown as black squares , with the size of the square being inversely proportional to the subgroup-specific risk ratio variance. Horizontal lines represent 95% confidence intervals for the subgroup-specific risk ratios. Overall pooled risk ratios are shown as diamonds . The middle of each diamond corresponds to the risk ratio, and the width of each diamond represents the 95% confidence interval. The vertical dashed line provides a visual comparison of the pooled risk ratio with the subgroup-specific risk ratios. P values (two-sided) are based on Wald tests for interaction. Among those with follow-up data, body mass index was missing for 173 participants in the aspirin groups and 142 participants in the placebo group; family history of colorectal cancer was missing for 532 participants in the aspirin groups and 451 participants in the placebo group, including all participants in Cancer and Leukemia Group B (CALGB 9270); number of lifetime adenomas at baseline was missing for 261 participants in the aspirin groups and 260 participants in the placebo group, including all participants in CALGB 9270; advanced-lesion status at baseline was missing for 96 participants in the aspirin groups and 71 participants in the placebo group, with participants in CALGB 9270 being excluded from this subgroup analysis. CI = confidence interval.
Analyses by Time of Examination
Random-effects meta-analysis for any-dose aspirin vs placebo by time since randomization revealed that greatest reduction in risk for all adenomas occurred during the first year of follow-up (RR = 0.62; 95% CI = 0.48 to 0.81) and that aspirin had no effect on risk beyond 38 months (RR = 0.99; 95% CI = 0.78 to 1.26) ( Figure 5 ). Most of the follow-up examinations occurred 24–38 months after randomization; for this time period, the pooled risk ratio was 0.84 (95% CI = 0.74 to 0.96). For advanced lesions, the most pronounced effects were also in the first year of follow-up, when the pooled risk ratio comparing aspirin in any dose vs placebo was 0.47 (95% CI = 0.24 to 0.90); the corresponding pooled risk ratios for the subsequent time intervals were 0.91 (95% CI = 0.42 to 2.00) for 12–24 months, 0.72 (95% CI = 0.52 to 1.00) for 23–38 months, and 0.71 (95% CI = 0.37 to 1.35) for 38 months or longer.
Figure 5.
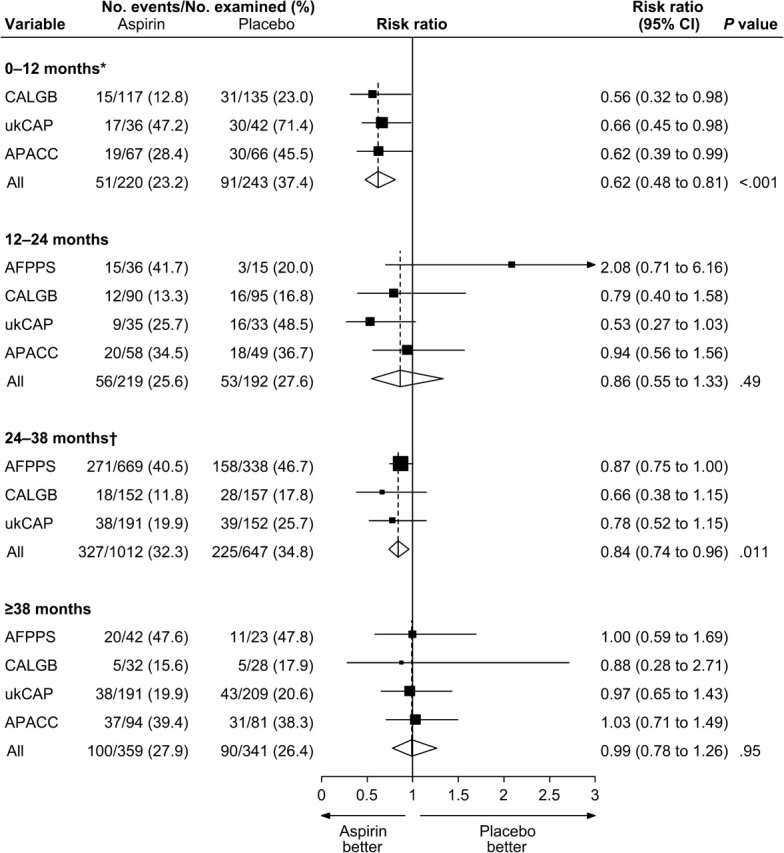
Random-effects risk ratio forest plot comparing any aspirin vs placebo in terms of adenoma risk by time interval after randomization. Trial-specific risk ratios are shown as black squares , with the size of the square being inversely proportional to the trial-specific risk ratio variance. Horizontal lines represent 95% confidence intervals for the trial-specific risk ratios. Pooled risk ratios are shown as diamonds . The middle of each diamond corresponds to the risk ratio, and the width of each diamond represents the 95% confidence interval. The vertical dashed lines provide a visual comparison of the pooled risk ratios with the corresponding trial-specific risk ratios. *The AFPPS trial had no examinations during the 0- to 12-month interval. †The APACC trial had only two examinations during the 24- to 38-month interval. AFPPS = Aspirin/Folate Polyp Prevention Study; CALGB = Cancer and Leukemia Group B; APACC = Association pour la Prevention par l’Aspirine du Cancer Colorectal; CI = confidence interval; ukCAP = United Kingdom Colorectal Adenoma Prevention.
Colorectal Cancer Occurrence
The AFPPS, ukCAP, and APACC trials reported incident colorectal cancer. CALGB 9270 was not included in this analysis because it studied a population with a history of colorectal cancer, whereas the remaining trials involved participants with no history of colorectal cancer. As would be expected in any population that was under endoscopic surveillance, the numbers of colorectal cancers observed were small. Across the combined AFPPS, ukCAP, and APACC study populations, the percentage of participants diagnosed with incident colorectal cancer was similar in the any-dose aspirin and placebo groups (0.54% vs 0.62%, respectively; P = .81).
Adverse Events
Serious adverse events after randomization were uncommon ( Table 4 ). Rates of death (ie, the percentages of participants who died) were similar in the any-dose aspirin and placebo groups (0.95% vs 0.85%, respectively; P = .85), as were the rates of myocardial infarction (0.48% vs 0.31%; P = .57). Of the 12 participants who had a stroke, all were randomly assigned to an aspirin group ( P = .002). Most of the stroke cases were apparently thrombotic events; only one—in a participant with a subarachnoid hemorrhage—was thought to be hemorrhagic. Rates of major bleeding were similar among participants allocated to any-dose aspirin and those allocated to placebo (2.50% and 2.79%, respectively; P = .64). Rates of new cancer diagnoses (ie, the percentages of participants who received a new diagnosis of any invasive cancer) were not statistically significantly different in the any-dose aspirin and placebo groups (2.62% vs 1.86%, respectively; P = .18), although the invasive cancer rate in the any-dose aspirin group was somewhat higher than that in the placebo group.
Table 4.
Adverse events in all trials combined
| Adverse event | No. of participants (%) |
P * | |||
| Placebo (n = 1289) | Aspirin in any dose (n = 1678) | Aspirin, 81 or 160 mg/d (n = 450) | Aspirin, 300 or 325 mg/d (n = 1228) | ||
| Death | 11 (0.85) | 16 (0.95) | 3 (0.67) | 13 (1.06) | .85 |
| Myocardial infarction | 4 (0.31) | 8 (0.48) | 2 (0.44) | 6 (0.49) | .57 |
| Stroke | 0 (0.00) | 12 (0.66) | 3 (0.67) | 9 (0.65) | .002 |
| Major bleeding | 36 (2.79) | 42 (2.50) | 11 (2.44) | 31 (2.52) | .64 |
| Invasive cancer | 24 (1.86) | 44 (2.62) | 18 (4.00) | 26 (2.12) | .18 |
| Colorectal cancer | 8 (0.62) | 9 (0.54) | 2 (0.44) | 7 (0.57) | .81 |
| Any event above | 71 (5.51) | 104 (6.20) | 29 (6.44) | 75 (6.12) | .48 |
P values (two-sided) are from Fisher's exact test comparing placebo vs aspirin in any dose.
Discussion
We obtained data from all randomized trials that studied aspirin for the chemoprevention of colorectal adenomas and used patient-level data to conduct a meta-analysis. In aggregate, nearly 3000 participants were included in four trials that were conducted in the late 1990s. Two trials studied 300 or 325 mg aspirin vs placebo; the other two trials included a lower dose of aspirin. Three trials recruited patients with recent adenomas, and one trial enrolled patients with colorectal cancer who had received curative treatment. Each of the trials had a 3- or 4-year intervention period. Compliance and follow-up in the trials were generally quite good.
Overall, we found a statistically significant 17% decrease in the relative risk of adenoma for aspirin in any dose vs placebo, which corresponded to a 6.7% absolute risk reduction. We also observed a 28% decrease in the relative risk of advanced lesions. All of the trials included in this meta-analysis involved a higher-dose aspirin arm (≥300 mg/d). In comparisons of higher-dose aspirin vs placebo, there was a 15% non–statistically significant decrease in the relative risk of any adenoma; however, the corresponding absolute risk reduction of 5.7% was statistically significant. We observed a statistically significant 29% reduction in the relative risk of advanced lesions for higher-dose aspirin vs placebo. In the two trials that evaluated lower doses of aspirin (doses of ≤160 mg/d) vs placebo, there was a statistically significant 17% decrease in the relative risk of any adenoma (absolute risk reduction = 8.4%) and a non–statistically significant 17% reduction in the relative risk of advanced lesions. These comparisons of the two dose levels with placebo suggest that there is no difference between higher- and lower-dose aspirin in the effect on all adenomas and that higher-dose aspirin provides a greater risk reduction than lower-dose aspirin for advanced lesions. However, a direct comparison of higher-dose vs lower-dose aspirin showed statistically significantly greater risk reduction for all adenomas with lower-dose aspirin. A similar comparison for advanced lesions yielded inconsistent and highly variable results. The difference between these findings and the comparisons with placebo is likely due to the limited or lack of efficacy of higher-dose aspirin in the two studies that also investigated the lower doses.
In general, the most reliable dose–response findings are those derived from studies that investigate multiple doses in a single population. The surprising lack of efficacy of the higher aspirin doses in the two multiple-dose trials, together with the unusual dose–response patterns (ie, greater efficacy with lower dose) that were observed in those trials, prevents secure conclusions regarding the relative efficacy of lower-dose vs higher-dose aspirin.
For any aspirin dose and for higher-dose aspirin, the effects observed were stronger for advanced lesions than for any adenomas. The same pattern has been observed in randomized trials of calcium supplementation ( 21 ) and difluoromethylornithine plus sulindac for the chemoprevention of adenomas ( 22 ). It is advantageous that aspirin is effective for preventing advanced lesions because these lesions tend to progress more rapidly to invasive cancer.
As would be expected given the endoscopic surveillance of the study populations, there were only small numbers of colorectal cancers observed in the four adenoma trials. We observed no statistically significant effect of aspirin on colorectal cancer occurrence. However, this apparent lack of effect does not negate the chemopreventive potential of aspirin in the large bowel. The aggregate data were not powered to detect an effect on invasive colorectal cancer, and in any case, longer treatment and follow-up are known to be required for such an effect to emerge ( 2 , 8 ).
We evaluated whether baseline factors modified the effect of aspirin in any dose. Our random-effects meta-analysis for all adenomas detected no statistically significant effect modification for any of the baseline factors studied. For advanced lesions, the effect of aspirin was substantially greater among those with a family history of colorectal cancer, but this result did not achieve statistical significance. None of the other baseline factors studied modified the effect of aspirin on advanced lesions to a statistically significant extent.
Overall, the largest benefit of aspirin (in any dose) appeared during the first year after randomization (RR = 0.62). However, only one study (APACC) scheduled a protocol examination at 1 year; there were also substantial numbers of patients in CALGB 9270 who had early examinations. Thus, many of the examinations that occurred within 1 year from randomization were not follow-up examinations prescribed by protocol. Beyond 38 months after randomization, aspirin and placebo had nearly equivalent effects on the risk for all adenomas (RR = 0.99).
In the three studies that recruited adenoma patients (AFPPS, ukCAP, and APACC), participants who had an examination earlier than called for by the study protocol were likely a select (and probably atypical) group of patients who might have had clinical indications for the examination, such as bleeding. This selection of participants for early examination, together with the smaller numbers of participants in some of the follow-up intervals, complicates interpretation of the interval-specific risk ratios.
We saw no differences in the rates of adverse events comparing aspirin vs placebo in terms of mortality, myocardial infarction, major bleeding, and all-site invasive cancer. However, there was a statistically significantly higher rate of strokes (most of which were thought to be ischemic) among aspirin-treated participants than among those who received placebo. There is no ready explanation for these findings. Aspirin use does seem to increase risk of hemorrhagic stroke modestly ( 23–25 ). However, large randomized trials that focused on cardiovascular outcomes have reported that in patients without vascular disease (and who are roughly similar to participants in the adenoma trials), aspirin had no substantial effect on ischemic stroke risk in men ( 23 , 24 ), and may reduce this risk in women ( 23 ).
Our meta-analysis has a number of strengths. It included all known randomized clinical trials that have tested aspirin as a chemopreventive agent against colorectal adenomas. The trials were generally well conducted, with high compliance and generally high follow-up rates. The sample size in the pooled studies was substantial, providing good statistical power. Thus, this analysis is likely to have high validity.
However, this analysis also has substantial limitations. First, because only two studies (APACC and AFPPS) investigated lower-dose aspirin, our findings regarding dose–response patterns are not as convincing as our findings regarding overall adenoma occurrence and advanced-lesion occurrence. One of these two studies (APACC) was relatively small and had a substantial number of late dropouts, further hampering interpretation of the aspirin dose–response relationship. Second, our analysis of cardiovascular and bleeding events is quite limited. The entry criteria for the trials included the absence of a need for aspirin, which is a known cardioprotective drug. This criterion assured that the study population was at relatively low risk of cardiovascular events, and so the numbers of events observed in any one trial were modest. Furthermore, none of the trials focused on cardiovascular disease, and the clinical detail regarding individual cardiovascular events is incomplete. Third, our exclusion of CALGB 9270 from the analysis of adverse events because of concerns regarding the completeness of adverse event reporting further reduced the numbers of endpoints analyzed.
In summary, this meta-analysis of clinical trial data indicates that aspirin reduces the risk of recurrence of colorectal adenomas. This effect emerged rather quickly after the initiation of aspirin use, seemed more marked for advanced lesions than for adenomas overall, and was seen in essentially all subgroups examined. Our findings suggest that aspirin interferes with colorectal carcinogenesis relatively early in the progression from normal mucosa to adenoma or advanced lesion. The substantial size of the relative reduction in risk seen in our analysis (28% for advanced adenomas) and seen in clinical trials that evaluated the effect of aspirin on colorectal cancer risk (26% reduction) ( 2 ) indicates the potentially important health benefits of aspirin use. Of course, these benefits need to be considered in the context of all of the health effects of aspirin, positive and negative. As noted above, there is extensive research regarding the largely positive cardiovascular effects of aspirin ( 23–25 ). The possibility of cancer prevention can now be added to future considerations of risk and benefit.
Funding
This study was supported by a research grant from Bayer AG to J.A. Baron.
Supplementary Material
Footnotes
The authors gratefully acknowledge the dedicated work of those who contributed to the clinical trials included in this report, especially the study participants.
J. A. Baron is currently a paid consultant to Bayer AG (a manufacturer of aspirin and NSAIDs) and is a former paid consultant to Merck & Co Inc, which marketed Vioxx, another NSAID.
Research for AFPPS was supported in part by a grant from the National Cancer Institute (CA 59005) to the Polyp Prevention Study Group (John A. Baron, MD, Principal Investigator). The research for Cancer and Leukemia Group B (CALGB) 9270 was supported in part by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601).
The sponsor commented on initial drafts of the protocol for the meta-analysis. The sponsor had no role in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
References
- 1.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 2.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 3.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. New Engl J Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 4.Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101(3):635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 5.Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas JM. Effects of long-term sulindac therapy on colonic polyposis. Ann Internal Med. 1991;115(12):952–954. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihara K. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9(13):4756–4760. [PubMed] [Google Scholar]
- 7.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 8.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. New Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 11.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 12.Logan RFA, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 14.Oxman AD, Clarke MJ, Stewart LA. From science to practice. Meta-analyses using individual patient data are needed. JAMA. 1995;274(10):845–846. doi: 10.1001/jama.274.10.845. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(15):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. New York: Springer; 1998. [Google Scholar]
- 18.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575–1600. doi: 10.1002/sim.1188. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Panel on the Identification EvaluationTreatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 21.Wallace K, Baron JA, Cole BF, et al. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96(12):921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 22.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prevent Res. 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 24.Eidelman RS, Hebert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med. 2003;163(17):2006–2010. doi: 10.1001/archinte.163.17.2006. [DOI] [PubMed] [Google Scholar]
- 25.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280(22):1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.