Abstract
Task-specific dystonia is a movement disorder characterized by the development of a painless loss of dexterity specific to a particular motor skill. This disorder is prevalent among writers, musicians, dancers and athletes. No current treatment is predictably effective and the disorder generally ends the careers of affected individuals. There are a number of limitations with traditional dystonic disease models for task-specific dystonia. We therefore review emerging evidence that the disorder has its origins within normal compensatory mechanisms of a healthy motor system in which the representation and reproduction of motor skill is disrupted. We describe how risk factors for task-specific dystonia can be stratified and translated into mechanisms of dysfunctional motor control. The proposed model aims to define new directions for experimental research and stimulate therapeutic advances for this highly disabling disorder.
We enjoy marvelling at a musician in full flow during a performance, or the grace of a tennis player during a game. Although such activities appear effortless to the casual observer, these individuals have honed their motor ability through years of rigorous practice. We reward their expertise by filling concert halls and sports stadiums and consider such exquisite movement control one of the pinnacles of human development. However, in a proportion of individuals, this repetitive practice of motor skill comes at a price — the development of a painless loss of co-ordination specific to their skill, termed task-specific dystonia. The motor impairment only manifests during a single task yet its wider impact is considerable as some of the individuals affected define our arts and sports communities.
Task-specific dystonia is currently considered a subtype of dystonia1. However, a longstanding debate continues as to whether the dystonias represent a single disease entity with shared pathophysiology or whether each dystonia subtype is distinct2. Task-specific dystonia is unique in a number of ways1. Primarily, the isolated and highly task-specific nature of this condition ties the problem directly to the control of a specific motor task. In the affected body region other fine motor tasks are initially unaffected3. Another distinctive feature is the range of environmental risk factors highlighted in this framework which are repeatedly linked to symptomatology by patients and epidemiological studies4,5. In the past many studies have suggested that traditional neurophysiological markers of dystonia such as abnormal sensorimotor plasticity and impaired inhibition are also implicated in task-specific dystonia6. However general changes in plasticity, inhibition or somatosensory representation are unable to explain why only an individual task is affected (such abnormalities have also been documented in circuits sub-serving unaffected body regions7–9). Such neurophysiological markers are also highly variable in health and abnormalities do not reliably or specifically identify patients with task-specific dystonia10,11. These observations point to the need to search for addition ways to understand the disorder.
In this perspective article, we review emerging evidence that task-specific dystonia has its origins within normal compensatory mechanisms of a healthy motor system in which the representation and reproduction of motor skill is disrupted. We review mechanisms of skill learning in health and then describe how risk factors for task-specific dystonia can be stratified and translated into mechanisms of dysfunctional motor control. Finally we discuss the translational implications this motor control framework yields for the prevention and treatment of task-specific dystonia.
Motor skill learning in health
A broad definition of motor skill learning is any neuronal change that enables an organism to accomplish a motor task faster and more precisely than before12. Increasing expertise is characterized by optimization of speed and accuracy13, high consistency and reliability in achieving the movement goal (effectiveness), as well as fluent and economical movement execution and automaticity (efficiency)14.
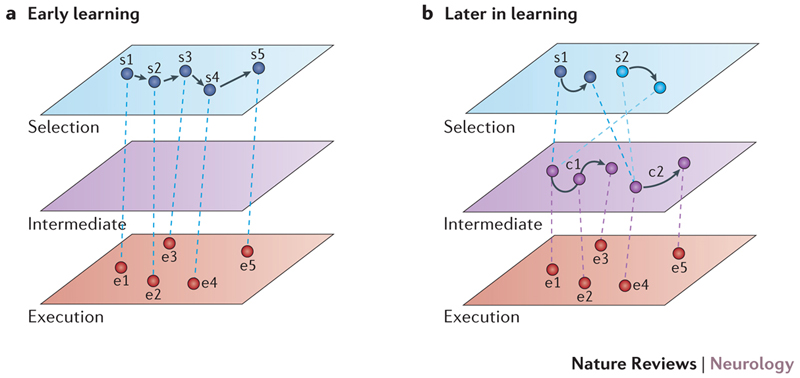
Studies of motor skill learning suggest that a hierarchical organization of neuronal networks encodes the different skill components required for expert performance12 (FIG. 1). The main broad division proposed within the motor hierarchy is between action selection and execution12,15. At the top is the selection level which links the task goals to motor control circuits. It takes into account the whole repertoire of movements and weighs up their motor costs and rewards before selecting the most appropriate response. At the bottom of the hierarchy is the execution level which involves neuronal populations co-ordinating the muscles to contract.
Figure 1. Motor hierarchy in skill learning.
a | In early learning, explicit or cognitive processing of task instructions occurs at the selection level. At the execution level, the most appropriate set of motor elements is mapped to task requirements12. b | Later in learning, task performance is largely automatic. Skill elements become encoded within the dynamic neural network as an intermediate level, and the flow of motor elements requires little explicit or cognitive control. Motor chunking refers to the linking of elemental execution representations within the intermediate level. For example, a specific motor goal at the selection level (s1) might initiate two motor chunks at the intermediate level (c1, c2) that lead to distinct motor sequences (e1–e3 in the case of c1 and e4–e5 in the case of c2). A second motor goal (s2) has a different order requirement but is built from the same components; initiating the two chunks in reverse order (c2 then c1) still produces the required motor behaviour (e4–e5 from c2 and e1–e3 from c1). These intermediate-level representations link elementary units, enabling them to be activated in a fluent manner without the need for direct mapping from the selection level. Permission obtained from Elsevier © Diedrichsen, J. & Kornysheva, K. Trends Cogn. Sci. 19, 227–233 (2015).
At the action execution level, experiments suggest that the primary motor cortex encodes small movement fragments or ‘motor synergies’ within stable neuronal networks16,17. Gestures as complex as grasping or licking can be reproducibly evoked via simple electrical stimulation of primary motor cortex (FIG. 2a18). Furthermore the range of motor synergies appears to depend on experience; skilled musicians have a motor synergy repertoire tailored to their trained instrument19. As such, representations at the execution level can be considered building blocks for the motor system.
Figure 2. Evidence for a motor hierarchy.
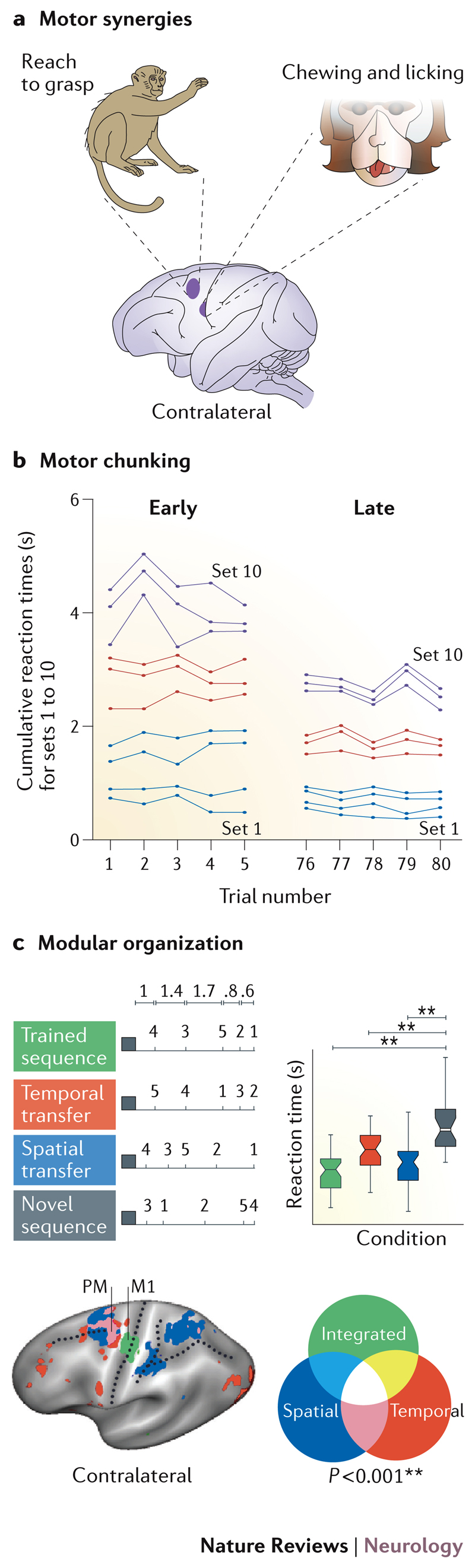
a | Motor synergies are fragments of movement sequences encoded within the motor cortex. Complex gestures such as grasping or licking can be reproducibly evoked by electrical stimulation of the shaded regions of primary motor and premotor cortices in monkeys b | The results of an experiment in which humans learned to do 10 sets of two button presses. Each point on the graph represents one set, and the spacing between two adjacent lines corresponds to the time interval between the onsets of each set. During early learning (trials 1–5) the total time taken for the sequence to be executed was longer and dispersion of the 10 sets through time was approximately even. During late learning (trials 76–80) the total time taken for the entire sequence has decreased and motor chunking is evident — the different sets are grouped into three chunks (set 1–4, set 5–7, set 8–10). c) Evidence for a modular representation of rhythm or ‘temporal chunking’. The timing and finger order of four different sequences are shown: trained (green); temporal transfer (red); spatial transfer (blue); and novel (grey). Behavioural benefits (reaction time decreases) of new sequences retaining either the trained temporal or spatial features are seen in comparison to entirely novel sequences. Multivariate analysis of functional MRI data reveals independent representations (red, blue) of these spatial and temporal features, some of which occur in overlapping (pink) regions of the premotor (PM) cortex. The primary motor cortex (M1), by contrast, contains integrated (that is, non-separable) representations of the two sequence features (green). Part a adapted with permission from Elsevier © Graziano, M. S. Trends Cogn. Sci. 20, 121–132 (2016)18. Part b adapted with permission from Springer © Sakai, K. Exp. Brain Res. 152, 229-242 (2003)22. Part c adapted with permission from Elsevier © Diedrichsen, J. & Kornysheva, K. Trends Cogn. Sci. 19, 227–233 (2015).
In the early stages of skill learning, most task requirements are thought to be explicitly processed at the action selection level, and then directly mapped to the most appropriate execution elements (FIG. 1a)12. However, this is an effortful and time-consuming process, as multiple alternatives within the movement repertoire need to be considered. Therefore, later in learning intermediate level skill representations are thought to be formed, binding together elementary execution components (such as motor synergies) within a dynamic control network (FIG. 1b). Subsequently, the selection level may only need to trigger a corresponding intermediate network which reduces load at the selection level and involves less explicit processing of task requirements.
One experimental line of evidence that supports the existence of intermediate representations is the concept of ‘motor chunking’ — the grouping of elementary components of a sequential action into one representational unit20. With learning, as the completion of motor sequences becomes faster and more accurate, sequence execution starts to show idiosyncratic temporal groupings or chunks21. Novel sequences are performed faster if previously established chunks of trained motor sequences are preserved, than if the chunks are regrouped22. As such, these chunks are thought to be linked at the intermediate level, and the transfer or flexibility of motor skill learning across different actions can be explained by the reuse of existing chunks in new motor sequences (FIG. 2b)23. Chunk-specific neuronal activity has been shown experimentally in (pre-)supplementary motor, lateral premotor cortical areas and the striatum23–27.
Besides the sequential order of individual movements, complex skill reproduction requires the integration of other features such as the temporal profile or rhythm of the sequence. Interestingly, such sequence characteristics may be encoded separately. Advantages emerge when previously trained spatial or temporal features of movement sequences are transferred to new spatio-temporal combinations separately suggesting that these movement signatures are represented independently within the brain (FIG 2c)28–30. Accordingly, chunk-specific activity in striatal medium spiny neurons in rodents is not modulated by changes in the speed or timing of the trained motor action, suggesting that these neurons specify the order ofmovements in a chunk, but not its full spatiotemporal implementation 31. At the same time motor sequence learning often leads to a separable representation of temporal features, which can be understood as a more abstract form of chunking – a temporal grouping of sequence elements which is transferable across different movements and effectors30,32,33.
Thus, within this hierarchical model of motor skill learning, intermediate-level representations are thought to provide an architecture that enables the flexible modification and recombination of acquired movement chunks and timing, maximizing both the plasticity and efficiency of the motor skill network involving primarily cortical and striatal areas. This function contrasts with spatiotemporally intricate but stereotyped reflex movements, such as swallowing controlled at the brainstem level, which have a limited capacity for modification. The cortico-spinal and cortico-striatal pathways may thus be biologically predisposed for flexible control of skilled movements34.
This literature exploring motor skill learning in health can offer important insights into disorders of skill reproduction such as task-specific dystonia.
Risk factors for task-specific dystonia
Both genetic and environmental factors are thought to be important in the aetiology of task-specific dystonia. Genetic influences are suggested by the male preponderance and positive family history of movement disorders in a significant proportion of patients and the ARSG gene (encoding arylsulfatase G) has been identified as a possible susceptibility locus35–37. Until better characterised, the mechanism by which genetic and epigenetic factors could influence the risk profile of an individual remains wide (e.g. gating of synaptic plasticity, determination of personality traits, musical ability).
Environmental risk factors associated with task-specific dystonia are diverse and there is great heterogeneity between patients38,39. Importantly many of these risk factors suggest potential mechanisms by which motor control may malfunction and offer directions for therapeutic intervention. A pragmatic manner to assess for such risk factors, which can also be used to guide rehabilitation strategy, is to identify factors associated with each of the essential components required for the performance of a given skill (task, tool, periphery and central nervous system (FIG. 3)). The periphery describes the characteristics of the body region that performs the task with the tool. The central nervous system includes the network that encodes skill performance modulated by the individual’s psychological state. As all components are required to work in concert to maintain task performance, a change in one component prompts a change or shift in other components (FIG. 3). A further dynamic element is that the risk factor profile of an individual can also change over time and it is often useful to qualify risk factors as predisposing, triggering and/or maintaining influences.
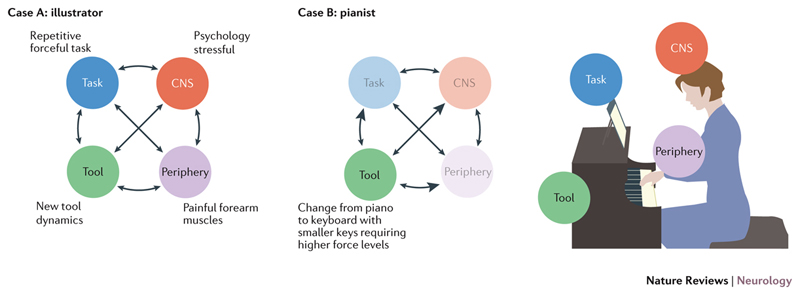
Figure 3. Components required for skill performance and the dynamic interactions between risk factors.
Risk factors can be comprehensively identified by considering all the components that interact in the performance of a given skill (central nervous system, periphery, task and tool). For example, case A exemplifies an illustrator that had taken a prestigious but demanding new job in animation. The patient was required to use a tablet and stylus rather than the usual paint brush (tool) and the work required 1000s of dots demanding forceful demarcation with the stylus (task). The patient also worked for many hours until a painful forearm overuse injury occurred (periphery) and was highly stressed attempting to make imposed deadlines (psychology). Here multiple risk factors seemed to interact in the development of task-specific dystonia. Others patients present with fewer risk factors. The pianist in case B was a classical pianist that took a job playing in a musical. This required a subtle change in instrument such that the pianist was using a smaller keyboard with keys that were less responsive than a piano. In this case the change in tool was the dominant risk factor with repercussions for task parameters and sensorimotor control (indicated by the arrows linking skill components).
Task
The highest relative prevalence of task-specific dystonia is found in musicians (1 in 100 for musicians’ dystonia, versus 1 in 15,000 for writers’ dystonia40,41) and the specific influences of task can be readily exemplified in this group. For example, dystonia in musicians preferentially involves the hand demanding the highest spatiotemporal acuity (right hand in keyboard players, left hand in players of bowed instruments)4,38,41. In bow-arm dystonia although the effectors involved in movements are much larger than those involved in hand movements, the spatiotemporal demands involved in producing a pure note are similarly high. Greater neuronal organization might therefore be needed to achieve the desired skill, as such task requirements are a large departure from the evolutionary designed role of large muscle groups. Task-specific dystonia is more frequently observed in classical musicians, in part owing to the requirement for classical musicians to execute performances according to the invariant temporal and spatial parameters predicated by their sheet music42. Motor impairments are less frequently seen in jazz musicians, as a certain flexibility of note and tempo is intrinsic to this music form43. Task-specific dystonia also typically affects the performance of highly rehearsed skills, tasks that have been performed repetitively for many hours38. Professional musicians typically accumulate 10,000 hours of practice prior to symptom onset44. Thus, high-risk groups are characterised by exceptionally high task accuracy requirements, a high cost associated with any deviation from predefined parameters, and highly rehearsed motor skills.
Tool
Changes in the presentation of patients with task-specific dystonia over the centuries highlight the importance of specific tools in the pathogenesis of task-specific dystonia5,45,46. In the 19th century, the change from feather quills to steel nibs in scriveners and clerks was the cause of a dramatic increase in prevalence of occupational motor problems in the British Civil Service47. The new nibs altered the dynamics of the tool (and corresponding task kinematics) and writers did not have to stop periodically to sharpen steel nibs, which offered a brief rest from what would otherwise be a continuous task (predisposing to muscle fatigue)47,48. More than 10% of telegraph operators communicating in Morse code developed motor problems49. Here, the requirement for stereotyped and individuated finger movements seemed to be particularly problematic50. Crucially, when Morse keys were replaced by keyboards, most operators experienced relief from their symptoms49. By contrast, computer-related dystonia is infrequently described in the literature, perhaps owing to the use of ergonomic keyboard designs51. In musicians, the incidence of task-specific dystonia increases as the string instrument size decreases, suggesting that tools requiring a higher spatial resolution confer an increased risk of task-specific dystonia 52. Clearly, tools largely confer risk as a result of the specific task requirements. However, occasionally the risks conferred by task and tool are independent (for example the higher incidence of task-specific dystonia in classical rather than jazz pianists) and a tool is not an essential component for the development of task-specific dystonia, as exemplified by the occurrence of task-specific singing impairment53.
Periphery
Fatigue, overuse and injury are important risk factors for task-specific dystonia38,39,47. For example, facial injuries can precipitate motor impairments affecting the embouchure in wind and brass instrument players43,54. In some individuals, injury might be caused by excessive practice or performance. However, injury of the body part in a context removed from the task also increases the risk of task-specific dystonia4. Anatomical limitations of the body region required for the task are another important consideration in the assessment of task-specific dystonia. Some individuals are born with a musculoskeletal system that favours skilled performance (such as an optimal range of motion) whereas others have biomechanical constraints that predispose them to develop motor dysfunction39.
Central nervous system
All individuals are likely to have a ceiling capacity of their nervous system for encoding the different elements (such as timing) of a movement. Determinants of this capacity are likely to include a combination of nature (inherent talent or capacity for neural plasticity and processing) and nurture (exposure and training). Furthermore, exposure might have to occur within a window during which the conditions most favour skill learning. Musicians who start practising after the age of 10 have an increased risk of developing task-specific dystonia 42. This is after the most sensitive periods of neural development have occurred in which training is thought to have its greatest effects on brain structure and behaviour55.
Finally, multiple cognitive and emotional processes can influence motor control. Compared to unaffected musicians, those with task-specific dystonia are six times more likely to exhibit elevated anxiety, perfectionism and evidence of stress and such characteristics seem to predate the onset of dystonia56,57,58. There is a clear presence in some patients of performance-related stress in the run up to development of task-specific dystonia. In one case series in which dystonia was evident only when writing a single letter or number, all cases were characterised by the need to repetitively write the letter or number under stressful situations59.
Implications for motor control
Interestingly, such risk factors for task-specific dystonia can be translated into mechanisms by which the motor control system becomes vulnerable to malfunction. Here we discuss a number of uniting themes by which dysfunctional neural representations of motor skill may arise.
Neural correlates of skill expertise
Particularly in professional musicians and athletes, the limitations of the neural networks supporting their skill expertise are likely to be an important contributory factor in the development of task-specific dystonia (FIG. 4)60. Experimental data suggest that the repetitive practice of long sequences of movements can lead to the formation of progressively longer motor chunks over time25,61,62, leading to performance gains that are increasingly contextual and tied to the individual task or body region25,63–67. Poor transfer of these performance gains to other tasks seems to be accentuated if a narrow training repertoire is applied, in contrast to more varied training approaches68. Practice predating the development of task-specific dystonia is often particularly extensive and stereotyped (for example, some musicians frequently practice each section of music in the same manner with the same rhythm and same fingering, over and over again). In such highly rehearsed tasks, intermediate-level representations that previously conferred flexibility for related tasks (such as those involving the same chord transitions or rhythms), could become redundant as highly stereotyped sequences begin to dominate the movement repertoire. The original transferrable chunk structure could disappear, as the concatenation into long execution bound synergies effectively replaces such intermediate elements (FIG. 4a). Such an architecture within the motor hierarchy could reliably encode an extreme optimization of performance parameters that pushes variability towards zero (FIG. 4b), but retains little capacity for flexibility and generalization to other contexts.
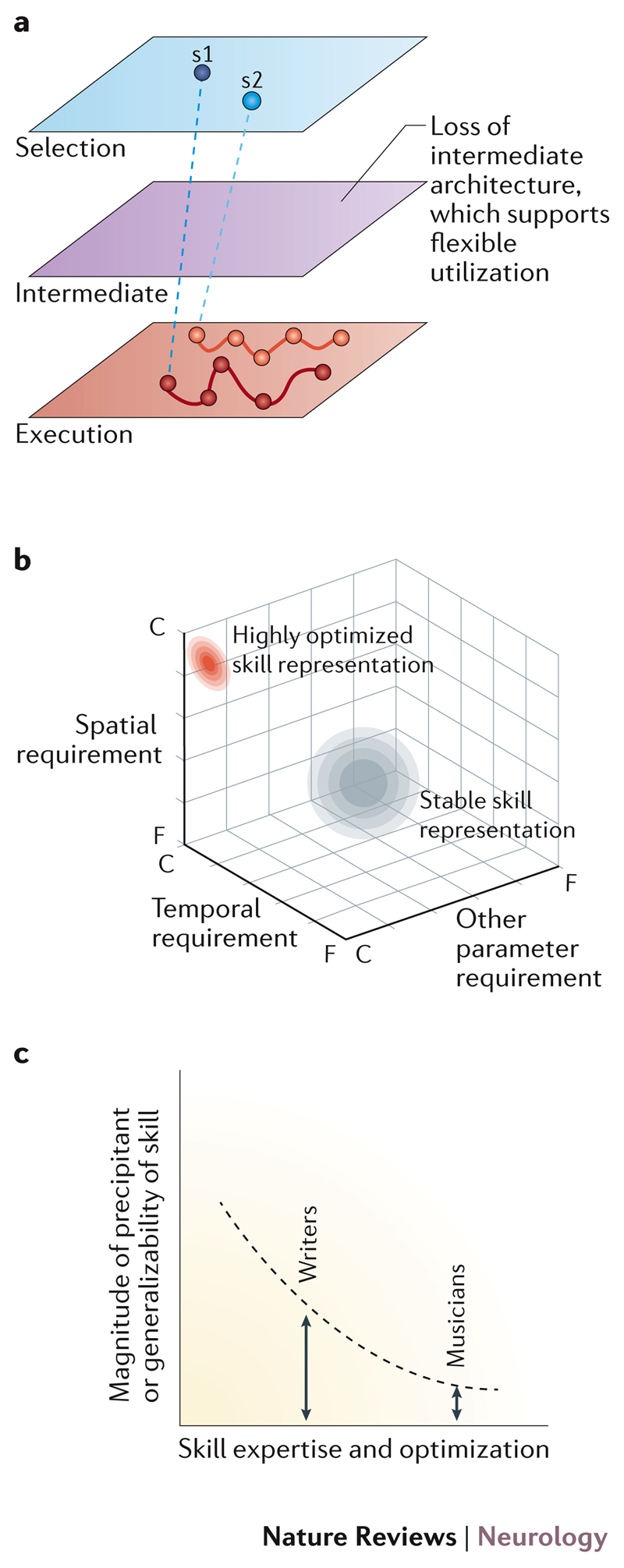
Figure 4. Vulnerabilities of highly skilled representations.
a | The neural architecture of overlearned skills is not well defined, but here the highly trained skill is represented as a rigid synergy-like execution pattern, which has poor flexibility due to limited use of motor chunking at the intermediate level. b | Stable everyday tasks (grey region) require a moderate range of spatial and temporal accuracy, and are encoded at approximately the midpoint between floor (F) and ceiling (C) values. By contrast, performers strive to improve time and accuracy functions within movement goals that are tightly prescribed (requiring minimal motor variability and error rates that approach zero). Thus, highly optimised skill representations (red region) require encoding of spatial and temporal accuracy at very close to ceiling values. The cost of such optimization is largely unknown. A high degree of optimizationis likely to limit the flexibility to respond to new task requirements (narrow diameter of skill representation) and other parameters may not be optimized (shown close to floor). c | With increasing skill expertise, the magnitude of the precipitant of task-specific dystonia decreases. In part, this association may be due to the reduced generalizability of highly optimized skill representations.
Capacity versus requirement
Many triggers for task-specific dystonia can be helpfully conceptualized as an unresolvable mismatch between the capacity of the motor system and the task requirements39. Capacity in this sense is defined by the limits of the neural control network and the periphery (for example, the range of feasible movements at a particular joint)39. Requirement is the exact movement trajectory, timing, force and accuracy required of the body in order to achieve the desired movement goals, as largely defined by the task and tool. Some mismatches between capacity and requirement are biomechanical in nature. For example, a task with a high force requirement will limit the capacity to make individuated finger movements with greater unintentional and undesired movements of neighbouring fingers69–71. Changes in capacity due to fatigue or injury of the body can also result in an effector system that responds more variably to a given motor command. Alternatively, a change in task requirements might result from external factors, such as changes in the size of a tool or an attempt by the performer to change their instrumental technique. If the neural representation of a skill can accommodate this change in the task requirement by adjusting and scaling its motor commands to maintain performance, an effective neural compensation has been found39. If, however, the new task requirement cannot be accommodated by the existing representation, the performer is pushed outside the overlearned boundaries of the skill. The inability to transfer the highly optimized skill across different parameter requirements will cause performance to break down because no effective motor compensation is available39. As we have alreadly described in relation to professional performers affected by task-specific dystonia, skill representations that are highly optimized are likely to be particularly narrow in their ability to cope with a change in task requirements. This could help to explain the high prevalence of task-specific dystonia in these individuals and why the triggering factors are often subtle (FIG. 4c)72–74.
Ill-equipped corrective mechanisms
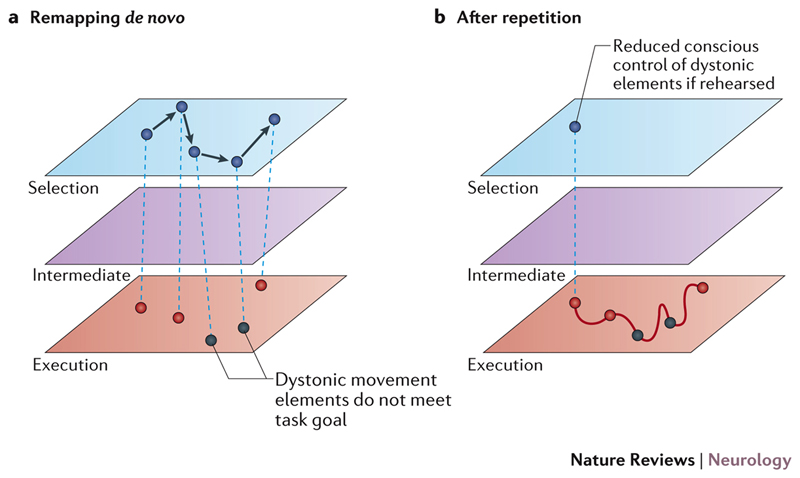
Once a critical mismatch between capacity and requirement has occurred, novel motor control strategies alien to the existing neural representation of skill must be employed to maintain task performance. However, de novo motor control solutions are unlikely to be able to match or maintain the level of skill performance that was formerly encoded by a hierarchy of neuronal elements optimized over many years of practice. The skills that are usually affected in task-specific dystonia are characterized by automaticity with little conscious control of movement 75. By contrast, during de novo learning, task requirements are explicitly mapped to basic execution elements22, a time-consuming process that conflicts with the demand for rapid task reproduction within a millisecond timescale. Access to subcomponents of more-abstract movement elements, which previously underpinned some features of expert task performance, is limited. Thus, once task performance has broken down, alternative motor control options are ill-equipped to immediately reinstate motor performance using new elements. Inappropriate and dysfunctional movements are likely to be produced, which are unable to match required task performance levels, marking the onset of task-specific dystonia (FIG. 5a).
Figure 5. The development of task-specific dystonia.
a | If the existing hierarchical representation can no longer accommodate task requirements, novel motor control options must be sought. Solutions are likely to require mechanisms comparable to early learning states, in which task requirements are explicitly mapped to basic execution elements. Such mechanisms are ill-equipped to immediately reinstate previous levels of skill performance, which were encoded by a hierarchy of neuronal elements optimised over many years of practice. Movements that are either inappropriate or non-physiological might start to be produced, which can be classed as dystonic as they no longer attain task goals. b | If dystonic movements are rehearsed they are likely to become encoded in a manner similar to any other learned sequence of movements, with a shift of motor control towards increased automaticity and reduced explicit cognitive monitoring of movement sequences. In this situation dystonic movement sequences become increasingly difficult to correct.
Encoding of dystonic movement
If stereotyped dystonic movements are repeatedly practiced they will become encoded in a similar manner to any other sequence of movements. Conscious control of the dystonic movement elements declines, causing frustration for individuals with undiagnosed task-specific dystonia as they attempt to implement strategies to address their movement difficulties (FIG. 5b). This formulation might partially explain one of the most puzzling features of task-specific dystonia: why normal task performance, which was previously achievable, can no longer be easily reinstated. Skill representations that are activated for a particular context or performance goal could become corrupted, with dystonic movements incorporated into their architecture60.
Psychology of motor control
Finally, the influence that misdirected cognitive influences can have on skill performance is worth emphasizing 76. An attentional focus on the mechanics of movement rather than on the external consequences or goals of movement has consistently been shown to worsen skill performance14. Deterioration in function can be shown experimentally for writing (when attention is focused on hand movements rather than output of the tool)52 as well as musical performance (when attention is focused on finger movements rather than the sound)77. Personality traits seen in musicians with task-specific dystonia such as anxiety and perfectionism are linked to a highly attentive manner of motor control, a situation that has repercussions for both development of the disorder and how we treat it14. The negative effects of self-focus are commonly discussed within the sports science literature (for example in relation to ‘the yips’ in golfers)76 but might be equally relevant in forms of motor impairment that share phenomenology in musicians and writers (such as motor block or choking under pressure 59,76)(Box 1). Aside from personality traits, other triggering factors such as injury, pain and explicit attempts to alter technique or performance will also naturally focus attention on the body region, to the detriment of motor control14. Misplaced attention can impede the normally automatic reproduction of highly skilled tasks. For a subset of patients with task-specific dystonia, this is an important mechanism through which performance can deteriorate.
Box 1. Features of task-specific dystonia.
Task-specificity
The task specificity of task-specific dystonia varies. Some individuals have a deficit only for writing particular letters or playing stereotyped musical phrases38,59. The corresponding deficit must, therefore, be encoded within the specific representation required for that particular motor context. In other individuals, difficulty with other tasks also develops over time (although a gradient in severity usually persists, such that the presenting task remains the most severely affected)96,97. In these patients the dystonic movement is likely to be encoded within a representation that the brain starts to use in multiple contexts (for example, if one of the motor synergies involved with a particular piano sequence is similar to that required when typing). In a minority of patients, task-specific dystonia also starts to affect the contralateral hand, which could either reflect modification of a new representation by persistent risk factors, or recruitment of existing dysfunctional representations that are required for performing a given task regardless of which hand is used (such as effector-independent representations that control sequence learning98).
An apparently task-selective deficit can be the presenting feature of other dystonia syndromes and movement disorders as a lesser pathological insult to the motor system is required to reveal a deficit in a skilled compared to an unskilled action42,99,100. However in contrast to task-specific dystonia a generalized motor impairment then becomes apparent 101.
Sensory tricks/maneuvers
Once task-specific dystonia is established, distorting sensory feedback from the affected body part (for example, by putting on a plastic or latex glove to play the instrument) can lead to short-term performance improvements in a minority of patients102,103. From a motor control perspective, the model of the skilled movement needs to be updated to produce an altered prediction of the sensory feedback to achieve baseline motor production104–106. In the un-adapted state, shortly after putting on the glove, the increased mismatch between feedback prediction and actual sensory feedback may be helpful in disrupting the over-learnt dysfunctional motor synergies through error-driven plasticity 107. However, in the adapted state, we would expect this positive effect to cease: the corrupted motor model has undergone adaptation but is structurally unchanged, and the short-term benefits would likely be lost. However, the period of heightened plasticity at the beginning of adaptation might present a unique time window for retraining, in the rare patients who respond with objective improvements to sensory distortion102.
Subtypes
Task-specific motor impairment can be divided into subtypes such as overuse injury, choking under pressure, dynamic stereotype and dystonia. Each subtype is likely to have differences of emphasis in both causative mechanisms and how they are encoded centrally43,47. For example, it has been suggested that dynamic stereotypes might represent an early modifiable form of motor impairment that can develop into dystonia if sufficient maintaining risk factors persist43.
Translational implications
Prevention
Defining task-specific dystonia as a modifiable disorder of motor control has the important implication that a proportion of cases of task-specific dystonia might be preventable. Many occupational forms of task-specific dystonia are characterized by mismatches between natural capability and the tool or task requirements. Improving the ergonomics of tools and limiting task parameters that stress the motor system might be beneficial41. However, professional musicians and athletes cannot modify their tool or task requirement to any great extent. As such, prevention strategies that focus on the control system, maximizing the ‘resilience’ of relevant representations in the brain and nurturing a healthy psychology profile, could reduce the risk of task-specific dystonia. Practice plans that emphasize flexibility of motor performance should be encouraged and ‘healthy’ practice routines defined by musicians have a reassuring resonance within the framework we outline42. For example, pianists at the Moscow Conservatory are encouraged to practice on pianos with different weights, and the famous cellist Rostropovich recommended practising different versions of difficult sections and experimenting with rubato and altered emphasis (which subtly change movement parameters) so that ‘the brain is relieved of the pressure of performing an action in a single rigid way’78. Such practice techniques could feasibly consolidate intermediate level connections and reduce the development of rigid effector representation, facilitating flexibility and resilience when any changes in task parameters are required.
Retraining dysfunctional movements
Once a motor problem has developed, careful assessment of potential risk factors should reveal the mechanism profile specific to that individual. This is important owing to the great heterogeneity within this group of patients. For example, although psychological factors are likely to be influential in a subset of patients with task-specific dystonia, a considerable proportion do not exhibit any signs of anxiety, perfectionism or stress58. The identification of mismatches between task requirement and capacity is already a widely established tool used to pragmatically guide selection of treatment strategies within rehabilitation disciplines (termed the ‘person–environment-occupation model’)79. Techniques from the sports science literature can also be integrated into treatment plans as the role of attention in task-specific dystonia becomes increasingly appreciated. For example, focusing attention away from the mechanics of movement and onto the goals of movement can help to prevent anxiety-related blocks in performance43,80.
Unfortunately cure of task-specific dystonia remains difficult to achieve reliably. Retraining therapies for patients with task-specific dystonia have shown encouraging results but often include techniques based on traditional models of dystonic pathophysiology, which might not be optimal81. Overall, our framework predicts that multifaceted interventions tailored to specific individuals’ risk profiles will represent the best overall treatment approach for task-specific dystonia82. Centres in which practitioners have access to combined therapeutic approaches report better outcomes for patients with task-specific dystonia than do centres lacking such resources83.
Role of traditional dystonia treatments
Conventional dystonia treatments rarely offer adequate relief in task-specific dystonia. Oral medications (such as trihexyphenidyl) have been tried with inconsistent responses and their use is often limited by adverse effects83–85. An initial benefit from botulinum toxin injections in specialist settings is often seen, but marked variability in responses to this treatment, and difficulty in avoiding disabling weakness, mean that only a subset continue this therapy long term83,86,87. It is likely that botulinum toxin injections are able to treat the end point of task-specific dystonia — the inappropriate muscle contractions associated with performing a given task — but cannot address underlying mechanisms. Studies of non-invasive brain stimulation (such as transcranial magnetic stimulation), which aim to either disrupt or augment the defining physiology in a favourable manner, are increasingly attempted in patients with task-specific dystonia9,88. Theoretically, the most attractive design pairs stimulation with concurrent task-relevant behavioural training (thereby activating the corresponding neuronal network subserving that skill) 89. Finally, deep brain stimulation and thalamotomy have also been trialled in non case-controlled series90–95. Randomised controlled trials are needed to offer validation of these more invasive approaches.
Conclusions
In this Perspectives article, we have presented task-specific dystonia in the context of motor skill learning in health, suggesting that they are two sides of the same coin. Our framework integrates established risk factors for task-specific dystonia with known mechanisms of motor skill learning, and describes how they might interact to disrupt the neural representation of motor skills. We hope that this perspective will help to define new directions for research and to promote much-needed therapeutic advances.
Acknowledgements
M.J.E.’s research is partially funded by a National Institute for Health Research (NIHR) grant relating to a study for which he is the principal investigator.
Glossary terms
- Automaticity
Mode of motor control in which movements operate with very little conscious knowledge of the actions required to perform them.
- Chunking
Collection of elementary units which have been inter-associated and stored in memory which act as a coherent, integrated group when retrieved.
- Dystonia
Movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements, postures, or both.
- Individuation
Degree to which one can move a single finger without unintended movements of the other fingers of the same hand.
- Representation
Activity in neural substrates containing information on the external or internal state of the system including motor output.
- Motor hierarchy
Organisation of the motor system in a series of layers with each level having specific roles in the motor control of movement generation
- Motor synergies
Elemental action units which are characterized by coordinated group of weighted muscle activations in space and time.
Biographies
Author biographies
Anna Sadnicka is a Chadburn Clinical Lecturer at St George’s University of London, UK. She is fascinated by the neural control of movement and how this is disturbed in movement disorders. She recently completed a PhD as a Guarantors of Brain Clinical Research Fellow at University College London, UK, in which she used neurophysiology, robotics and neuroimaging to probe the pathophysiology of dystonia.
Katja Kornysheva is a Lecturer (Associate Professor) at Bangor University, Wales, UK. Her research investigates how the nervous system enables us to learn and store the temporal and spatial dynamics of movement. She develops new methods to uncover neural markers of skilled performance and plasticity at the systems level in humans and at the single-cell level in mice.
John Rothwell is a Professor of Human Neurophysiology at University College London, UK. His laboratory specializes in devising new neurophysiological techniques to study the human motor system in intact awake volunteers, and to investigate the pathophysiology of movement disorders.
Mark Edwards is a Professor of Neurology at St George’s University of London, UK. His laboratory focuses on the science of motor control and its application to disorders of movement. He has an international profile in the investigation and treatment of patients with movement disorders and has a special interest in task-specific motor disorders.
Footnotes
Author contributions A.S. K.K. J.C.R. M.J.E.
A.S. and K.K. researched data for the article, wrote the manuscript, contributed substantially to discussions of its content, and undertook review and editing of the manuscript before submission. J.C.R. and M.J.E. also contributed substantially to discussions of the article content and to review or editing of the first draft.
Competing interests
A.S. and K.K. declare that they have no competing interests. J.C.R. declares that he has received speaker travel costs from the Movement Disorders Society. M.J.E. declares that he receives royalties from publication of the Oxford Specialist Handbook Of Parkinson's Disease and Other Movement Disorders (Oxford University Press, 2008) and that he has received honoraria for speaking from UCB pharmaceuticals.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Subject ontology terms
Health sciences / Neurology / Neurological disorders / Dystonia
[URI /692/617/375/1444]
Health sciences / Neurology / Neurological disorders / Movement disorders
[URI /692/617/375/346]
Health sciences / Medical research / Experimental models of disease
[URI /692/308/1426]
References
- 1.Albanese A, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese A. How Many Dystonias? Clinical Evidence. Front Neurol. 2017;8:18. doi: 10.3389/fneur.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann A, Grossbach M, Baur V, Hermsdorfer J, Altenmuller E. Musician's dystonia is highly task specific: no strong evidence for everyday fine motor deficits in patients. Med Probl Perform Art. 2015;30:38–46. doi: 10.21091/mppa.2015.1006. [DOI] [PubMed] [Google Scholar]
- 4.Altenmuller E, Jabusch HC. Focal hand dystonia in musicians: phenomenology, etiology, and psychological trigger factors. J Hand Ther. 2009;22:144–154. doi: 10.1016/j.jht.2008.11.007. quiz 155. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Ruiz PJ. Task-specific dystonias: historical review--a new look at the classics. J Neurol. 2013;260:750–753. doi: 10.1007/s00415-012-6696-y. [DOI] [PubMed] [Google Scholar]
- 6.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Movement disorders : official journal of the Movement Disorder Society. 2013;28:958–967. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quartarone A, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- 9.Pirio Richardson S, et al. Research Priorities in Limb and Task-Specific Dystonias. Front Neurol. 2017;8:170. doi: 10.3389/fneur.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. A reflection on plasticity research in writing dystonia. Mov Disord. 2014;29:980–987. doi: 10.1002/mds.25908. [DOI] [PubMed] [Google Scholar]
- 11.Kassavetis P. Reassessing the role of motor surround inhibition in dystonia. 2016 doi: 10.1016/j.jns.2018.04.015. under review. [DOI] [PubMed] [Google Scholar]
- 12.Diedrichsen J, Kornysheva K. Motor skill learning between selection and execution. Trends Cogn Sci. 2015;19:227–233. doi: 10.1016/j.tics.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telgen S, Parvin D, Diedrichsen J. Mirror reversal and visual rotation are learned and consolidated via separate mechanisms: recalibrating or learning de novo? J Neurosci. 2014;34:13768–13779. doi: 10.1523/JNEUROSCI.5306-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wulf G. Attentional focus and motorlearning: a review of 15 years. International Review of Sport and Exercise Psychology. 2013;6:77–104. [Google Scholar]
- 15.Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72:469–476. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overduin SA, d'Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. 2012;76:1071–1077. doi: 10.1016/j.neuron.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churchland MM, et al. Neural population dynamics during reaching. Nature. 2012;487:51–56. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graziano MS. Ethological Action Maps: A Paradigm Shift for the Motor Cortex. Trends Cogn Sci. 2016;20:121–132. doi: 10.1016/j.tics.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Gentner R, et al. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010;20:1869–1874. doi: 10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Gobet F, et al. Chunking mechanisms in human learning. Trends Cogn Sci. 2001;5:236–243. doi: 10.1016/s1364-6613(00)01662-4. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum DA, Kenny SB, Derr MA. Hierarchical control of rapid movement sequences. J Exp Psychol Hum Percept Perform. 1983;9:86–102. doi: 10.1037//0096-1523.9.1.86. [DOI] [PubMed] [Google Scholar]
- 22.Sakai K, Kitaguchi K, Hikosaka O. Chunking during human visuomotor sequence learning. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2003;152:229–242. doi: 10.1007/s00221-003-1548-8. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014;17:423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 25.Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron. 2012;74:936–946. doi: 10.1016/j.neuron.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- 27.Penhune VB, Steele CJ. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res. 2012;226:579–591. doi: 10.1016/j.bbr.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Ullen F, Bengtsson SL. Independent processing of the temporal and ordinal structure of movement sequences. J Neurophysiol. 2003;90:3725–3735. doi: 10.1152/jn.00458.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kornysheva K, Sierk A, Diedrichsen J. Interaction of temporal and ordinal representations in movement sequences. J Neurophysiol. 2013;109:1416–1424. doi: 10.1152/jn.00509.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornysheva K, Diedrichsen J. Human premotor areas parse sequences into their spatial and temporal features. Elife. 2014;3:e03043. doi: 10.7554/eLife.03043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F. Effector-independent voluntary timing: behavioural and neuroimaging evidence. Eur J Neurosci. 2005;22:3255–3265. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- 32.Kornysheva K. Encoding Temporal Features of Skilled Movements-What, Whether and How? Adv Exp Med Biol. 2016;957:35–54. doi: 10.1007/978-3-319-47313-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konoike N, et al. Temporal and Motor Representation of Rhythm in Fronto-Parietal Cortical Areas: An fMRI Study. PLoS One. 2015;10:e0130120. doi: 10.1371/journal.pone.0130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt A, et al. Etiology of musician's dystonia: familial or environmental? Neurology. 2009;72:1248–1254. doi: 10.1212/01.wnl.0000345670.63363.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohmann K, et al. Genome-wide association study in musician's dystonia: a risk variant at the arylsulfatase G locus? Mov Disord. 2014;29:921–927. doi: 10.1002/mds.25791. [DOI] [PubMed] [Google Scholar]
- 37.Nibbeling E, et al. Accumulation of rare variants in the arylsulfatase G (ARSG) gene in task-specific dystonia. J Neurol. 2015;262:1340–1343. doi: 10.1007/s00415-015-7718-3. [DOI] [PubMed] [Google Scholar]
- 38.Altenmuller E, Jabusch HC. Focal dystonia in musicians: phenomenology, pathophysiology and triggering factors. Eur J Neurol. 2010;17(Suppl 1):31–36. doi: 10.1111/j.1468-1331.2010.03048.x. [DOI] [PubMed] [Google Scholar]
- 39.Leijnse JN, Hallett M, Sonneveld GJ. A multifactorial conceptual model of peripheral neuromusculoskeletal predisposing factors in task-specific focal hand dystonia in musicians: etiologic and therapeutic implications. Biol Cybern. 2015;109:109–123. doi: 10.1007/s00422-014-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutt JG, Muenter MD, Melton LJ, 3rd, Aronson A, Kurland LT. Epidemiology of dystonia in Rochester, Minnesota. Advances in neurology. 1988;50:361–365. [PubMed] [Google Scholar]
- 41.Altenmuller E, Jabusch HC. Focal dystonia in musicians: phenomenology, pathophysiology, triggering factors, and treatment. Med Probl Perform Art. 2010;25:3–9. [PubMed] [Google Scholar]
- 42.Altenmuller E, Ioannou CI, Lee A. Apollo's curse: neurological causes of motor impairments in musicians. Prog Brain Res. 2015;217:89–106. doi: 10.1016/bs.pbr.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Altenmuller E, Ioannou CI, Raab M, Lobinger B. Apollo's curse: causes and cures of motor failures in musicians: a proposal for a new classification. Adv Exp Med Biol. 2014;826:161–178. doi: 10.1007/978-1-4939-1338-1_11. [DOI] [PubMed] [Google Scholar]
- 44.Ericsson KA, Krampe RT, Heizmann S. Can we create gifted people? Ciba Found Symp. 1993;178:222–231. doi: 10.1002/9780470514498.ch14. discussion 232-249. [DOI] [PubMed] [Google Scholar]
- 45.Ramazzini B. The diseases of writers and amanuenses. A treatise on the disease of workmen. [“De mobis artificum diatriba” 1705]. Bell Andrew., translator. 1700 [Google Scholar]
- 46.Solly S. Scrivener’s palsy or the paralysis of writers. Lancet. 1864 Lecture I, 1864; 2: 709–11 Lecture II, 1865;1:84–6; Lecture III, 1865;1:113–5. [Google Scholar]
- 47.Pritchard MH. Writer's cramp: is focal dystonia the best explanation? JRSM Short Rep. 2013;4:1–7. doi: 10.1177/2042533313480071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce JM. A note on scrivener's palsy. Journal of neurology, neurosurgery, and psychiatry. 2005;76:513. doi: 10.1136/jnnp.2004.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson D. An Australian study of telegraphists' cramp. Br J Ind Med. 1971;28:280–285. doi: 10.1136/oem.28.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zabaleta ME, et al. Assessment of former and newly developed HBV assays in a Third World setting. Journal of medical virology. 1992;38:240–245. doi: 10.1002/jmv.1890380403. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K, et al. Computer mouse-related dystonia: a novel presentation of task-specific dystonia. Journal of neurology. 2012;259:2221–2222. doi: 10.1007/s00415-012-6519-1. [DOI] [PubMed] [Google Scholar]
- 52.Altenmuller E, Baur V, Hofmann A, Lim VK, Jabusch HC. Musician's cramp as manifestation of maladaptive brain plasticity: arguments from instrumental differences. Ann N Y Acad Sci. 2012;1252:259–265. doi: 10.1111/j.1749-6632.2012.06456.x. [DOI] [PubMed] [Google Scholar]
- 53.Halstead LA, McBroom DM, Bonilha HS. Task-specific singing dystonia: vocal instability that technique cannot fix. J Voice. 2015;29:71–78. doi: 10.1016/j.jvoice.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Torres-Russotto D, Perlmutter JS. Task-specific dystonias: a review. Ann N Y Acad Sci. 2008;1142:179–199. doi: 10.1196/annals.1444.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci. 2013;33:1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jabusch HC, Muller SV, Altenmuller E. Anxiety in musicians with focal dystonia and those with chronic pain. Mov Disord. 2004;19:1169–1175. doi: 10.1002/mds.20110. [DOI] [PubMed] [Google Scholar]
- 57.Enders L, et al. Musician's dystonia and comorbid anxiety: two sides of one coin? Mov Disord. 2011;26:539–542. doi: 10.1002/mds.23607. [DOI] [PubMed] [Google Scholar]
- 58.Ioannou CI, Altenmuller E. Psychological characteristics in musicians dystonia: a new diagnostic classification. Neuropsychologia. 2014;61:80–88. doi: 10.1016/j.neuropsychologia.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Shamim EA, et al. Extreme task specificity in writer's cramp. Mov Disord. 2011;26:2107–2109. doi: 10.1002/mds.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frucht SJ. Focal Task-specific Dystonia-From Early Descriptions to a New, Modern Formulation. Tremor Other Hyperkinet Mov (N Y) 2014;4:230. doi: 10.7916/D8VD6WHP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramkumar P, et al. Chunking as the result of an efficiency computation trade-off. Nat Commun. 2016;7:12176. doi: 10.1038/ncomms12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acuna DE, et al. Multifaceted aspects of chunking enable robust algorithms. Journal of neurophysiology. 2014;112:1849–1856. doi: 10.1152/jn.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ingram JN, Howard IS, Flanagan JR, Wolpert DM. Multiple grasp-specific representations of tool dynamics mediate skillful manipulation. Curr Biol. 2010;20:618–623. doi: 10.1016/j.cub.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogawa T, Kawashima N, Ogata T, Nakazawa K. Limited transfer of newly acquired movement patterns across walking and running in humans. PLoS One. 2012;7:e46349. doi: 10.1371/journal.pone.0046349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houldin A, Chua R, Carpenter MG, Lam T. Limited interlimb transfer of locomotor adaptations to a velocity-dependent force field during unipedal walking. J Neurophysiol. 2012;108:943–952. doi: 10.1152/jn.00670.2011. [DOI] [PubMed] [Google Scholar]
- 66.Wu YH, Truglio TS, Zatsiorsky VM, Latash ML. Learning to combine high variability with high precision: lack of transfer to a different task. J Mot Behav. 2015;47:153–165. doi: 10.1080/00222895.2014.961892. [DOI] [PubMed] [Google Scholar]
- 67.Wiestler T, Diedrichsen J. Skill learning strengthens cortical representations of motor sequences. Elife. 2013;2:e00801. doi: 10.7554/eLife.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boutin A, et al. Practice makes transfer of motor skills imperfect. Psychol Res. 2012;76:611–625. doi: 10.1007/s00426-011-0355-2. [DOI] [PubMed] [Google Scholar]
- 69.Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]
- 70.van Duinen H, Gandevia SC. Constraints for control of the human hand. The Journal of physiology. 2011;589:5583–5593. doi: 10.1113/jphysiol.2011.217810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ejaz N, Hamada M, Diedrichsen J. Hand use predicts the structure of representations in sensorimotor cortex. Nature neuroscience. 2015;18:1034–1040. doi: 10.1038/nn.4038. [DOI] [PubMed] [Google Scholar]
- 72.Altenmuller E, Muller D. A model of task-specific focal dystonia. Neural Netw. 2013;48:25–31. doi: 10.1016/j.neunet.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Potter P. Task specific focal hand dystonia: understanding the enigma and current concepts. Work. 2012;41:61–68. doi: 10.3233/WOR-2012-1261. [DOI] [PubMed] [Google Scholar]
- 74.Frucht SJ. Focal task-specific dystonia in musicians. Adv Neurol. 2004;94:225–230. [PubMed] [Google Scholar]
- 75.Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards MJ, Rothwell JC. Losing focus: How paying attention can be bad for movement. Movement disorders : official journal of the Movement Disorder Society. 2011;26:1969–1970. doi: 10.1002/mds.23920. [DOI] [PubMed] [Google Scholar]
- 77.Porter JM, Nolan RP, Ostrowski EJ, Wulf G. Directing attention externally enhances agility performance: a qualitative and quantitative analysis of the efficacy of using verbal instructions to focus attention. Front Psychol. 2010;1:216. doi: 10.3389/fpsyg.2010.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demenga, T. (2014).
- 79.Mallinson T, Hammel J. Measurement of participation: intersecting person, task, and environment. Arch Phys Med Rehabil. 2010;91:S29–33. doi: 10.1016/j.apmr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 80.Toledo SD, et al. Sports and performing arts medicine. 5. Issues relating to musicians. Arch Phys Med Rehabil. 2004;85:S72–74. doi: 10.1053/j.apmr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 81.McKenzie AL, et al. Differences in physical characteristics and response to rehabilitation for patients with hand dystonia: musicians' cramp compared to writers' cramp. J Hand Ther. 2009;22:172–181. doi: 10.1016/j.jht.2008.12.006. quiz 182. [DOI] [PubMed] [Google Scholar]
- 82.Butler K, et al. Embracing the clincial heterogeneity of task-specific dystonia and the implications for therapy: a feasibiltiy study. (under review) [Google Scholar]
- 83.van Vugt FT, Boullet L, Jabusch HC, Altenmuller E. Musician's dystonia in pianists: long-term evaluation of retraining and other therapies. Parkinsonism Relat Disord. 2014;20:8–12. doi: 10.1016/j.parkreldis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Jabusch HC, Zschucke D, Schmidt A, Schuele S, Altenmuller E. Focal dystonia in musicians: treatment strategies and long-term outcome in 144 patients. Mov Disord. 2005;20:1623–1626. doi: 10.1002/mds.20631. [DOI] [PubMed] [Google Scholar]
- 85.Termsarasab P, Thammongkolchai T, Frucht SJ. Medical treatment of dystonia. J Clin Mov Disord. 2016;3:19. doi: 10.1186/s40734-016-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kruisdijk JJ, Koelman JH, Ongerboer de Visser BW, de Haan RJ, Speelman JD. Botulinum toxin for writer's cramp: a randomised, placebo-controlled trial and 1-year follow-up. J Neurol Neurosurg Psychiatry. 2007;78:264–270. doi: 10.1136/jnnp.2005.083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lungu C, Karp BI, Alter K, Zolbrod R, Hallett M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750–753. doi: 10.1002/mds.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho HJ, Hallett M. Non-Invasive Brain Stimulation for Treatment of Focal Hand Dystonia: Update and Future Direction. J Mov Disord. 2016;9:55–62. doi: 10.14802/jmd.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kimberley TJ, Schmidt RL, Chen M, Dykstra DD, Buetefisch CM. Mixed effectiveness of rTMS and retraining in the treatment of focal hand dystonia. Front Hum Neurosci. 2015;9:385. doi: 10.3389/fnhum.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taira T, Hori T. Stereotactic ventrooralis thalamotomy for task-specific focal hand dystonia (writer's cramp) Stereotact Funct Neurosurg. 2003;80:88–91. doi: 10.1159/000075165. [DOI] [PubMed] [Google Scholar]
- 91.Horisawa S, et al. Stereotactic Thalamotomy for Hairdresser's Dystonia: A Case Series. Stereotact Funct Neurosurg. 2016;94:201–206. doi: 10.1159/000446612. [DOI] [PubMed] [Google Scholar]
- 92.Horisawa S, et al. Gamma Knife Ventro-Oral Thalamotomy for Musician's Dystonia. Mov Disord. 2017;32:89–90. doi: 10.1002/mds.26726. [DOI] [PubMed] [Google Scholar]
- 93.Horisawa S, Goto S, Nakajima T, Kawamata T, Taira T. Bilateral Stereotactic Thalamotomy for Bilateral Musician's Hand Dystonia. World Neurosurg. 2016;92:585 e521–585. doi: 10.1016/j.wneu.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 94.Horisawa S, Taira T, Goto S, Ochiai T, Nakajima T. Long-term improvement of musician's dystonia after stereotactic ventro-oral thalamotomy. Ann Neurol. 2013;74:648–654. doi: 10.1002/ana.23877. [DOI] [PubMed] [Google Scholar]
- 95.Fukaya C, et al. Thalamic deep brain stimulation for writer's cramp. J Neurosurg. 2007;107:977–982. doi: 10.3171/JNS-07/11/0977. [DOI] [PubMed] [Google Scholar]
- 96.Sheehy MP, Marsden CD. Writers' cramp-a focal dystonia. Brain. 1982;105(Pt 3):461–480. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- 97.Rosset-Llobet J, Candia V, Fabregas S, Ray W, Pascual-Leone A. Secondary motor disturbances in 101 patients with musician's dystonia. J Neurol Neurosurg Psychiatry. 2007;78:949–953. doi: 10.1136/jnnp.2006.107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiestler T, Waters-Metenier S, Diedrichsen J. Effector-independent motor sequence representations exist in extrinsic and intrinsic reference frames. J Neurosci. 2014;34:5054–5064. doi: 10.1523/JNEUROSCI.5363-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritz K, et al. Screening for dystonia genes DYT1, 11 and 16 in patients with writer's cramp. Mov Disord. 2009;24:1390–1392. doi: 10.1002/mds.22632. [DOI] [PubMed] [Google Scholar]
- 100.Chung SJ, Lee JH, Lee MC, Yoo HW, Kim GH. Focal hand dystonia in a patient with PANK2 mutation. Mov Disord. 2008;23:466–468. doi: 10.1002/mds.21880. [DOI] [PubMed] [Google Scholar]
- 101.Sadnicka A, et al. Task-specific dystonia: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2016;87:968–974. doi: 10.1136/jnnp-2015-311298. [DOI] [PubMed] [Google Scholar]
- 102.Paulig J, Jabusch HC, Grossbach M, Boullet L, Altenmuller E. Sensory trick phenomenon improves motor control in pianists with dystonia: prognostic value of glove-effect. Front Psychol. 2014;5:1012. doi: 10.3389/fpsyg.2014.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng FP, Grossbach M, Altenmuller EO. Altered sensory feedbacks in pianist's dystonia: the altered auditory feedback paradigm and the glove effect. Front Hum Neurosci. 2013;7:868. doi: 10.3389/fnhum.2013.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 105.Wolpert DM, Miall RC. Forward Models for Physiological Motor Control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 106.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schonewille M, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]