Abstract
Release of neurotransmitter from synaptic vesicles requires the Ca+2/phospholipid binding protein, synaptotagmin 1. There is considerable evidence that cooperation between the tandem C2 domains of synaptotagmin is a requirement of regulated exocytosis; however, high-resolution structural evidence for this interaction has been lacking. The 2.7Å crystal structure of the cytosolic domains of human synaptotagmin 1 in the absence of Ca+2 reveals a novel closed conformation of the protein. The shared interface between C2A and C2B is stabilized by a network of interactions between residues on the C-terminal α-helix of the C2B domain and residues on Loops 1, 2 and 3 of the Ca+2 binding region of C2A. These interactions alter the overall shape of the Ca+2 binding pocket of C2A, but not that of C2B. Thus, synaptotagmin 1 C2A-C2B may utilize a novel regulatory mechanism whereby one C2 domain could regulate the other until an appropriate triggering event decouples them.
TOC image
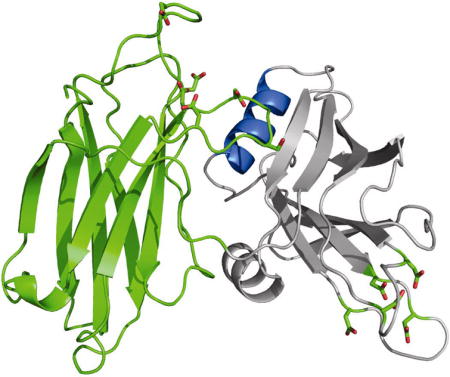
Ca+2-dependent release of neurotransmitter into the synaptic space is one of the fundamental tenets of modern neurobiology. This aspect of neuronal function has been studied for many years, yet the molecular details are only now being elucidated. The process begins with a neurotransmitter-filled synaptic vesicle progressing from a docked position to a primed, fusion-competent position next to the target membrane. Once the vesicle membrane and the target membrane fuse, neurotransmitter is released into the synaptic space. The merger of these two distinct bilayers, and the concomitant formation of a lipidic fusion pore, is mediated by Ca+2 and a number of proteins specific for the task. These include the protein complex formed between vesicle-localized R-SNAREs such as synaptobrevin and the Q-SNAREs such as SNAP-25 and syntaxin. This protein complex acts either as a staging platform for other constituents required for vesicle fusion (1), or it may directly participate in the actual fusion event itself (2). Regardless of the specific mechanism, the fusion of vesicle membranes with presynaptic membranes requires a calcium-ion sensor or trigger. The C2A-C2B domains of synaptotagmin 1 (Syt 1) possess the Ca+2-dependent phospholipid binding activity (3) and the SNARE binding activity predicted for such a trigger (4).
Syt 1 is a vesicle-localized transmembrane protein with two tandem C2 domains (C2A and C2B) at the C-terminus of the protein. There are two possible explanations for the tandem C2 domain architecture of Syt 1. Either the two domains are joined by a flexible linker and are completely independent, or there is some degree of cooperativity between domains that is essential to the overall function of the protein. These two scenarios are not mutually exclusive, as evidence exists for both. NMR analysis of Syt 1 C2A-C2B concluded that there were no detectable interaction between the domains (5). Also, the crystal structure of Syt 3, a homolog of Syt 1, displays no evidence of inter-domain interaction (6) (Figure 1B). On the other hand, the C2 domains of Syt 1 are known to function synergistically when compared to isolated C2 domains (7). Subsequent experiments concluded that a close physical coupling of the tandem domains is critical for function (8).
Figure 1.
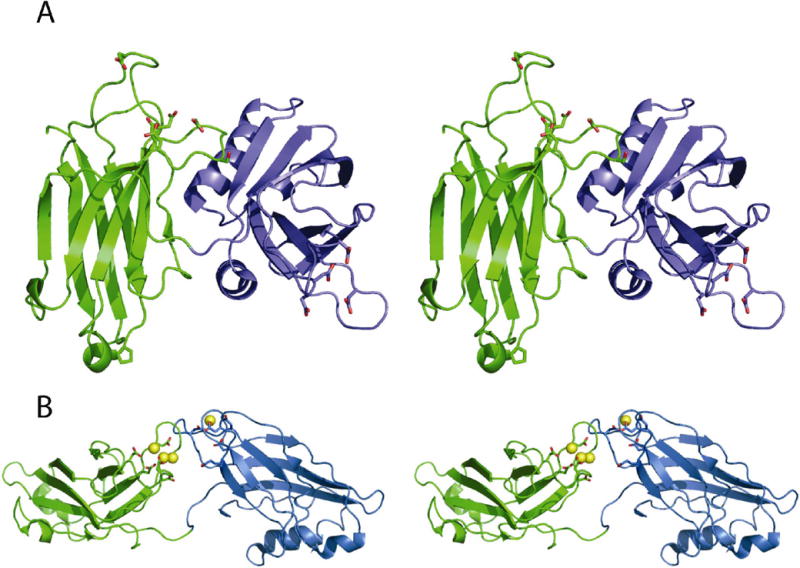
The open and closed conformations of synaptotagmin. (A) Stereoview of Syt1 C2A-C2B in the closed configuration. The C2A domain is colored as green ribbons. The C2B domain is colored as blue ribbons. The six known Ca+2-binding residues are shown as sticks. (B) Stereo diagram of synaptotagmin 3 C2A-C2B in the open configuration (pdb code: 1DQV). The C2A domain in Syt3 is rendered as green ribbons. The C2B domain in Syt3 is rendered as blue ribbons. Yellow spheres represent magnesium ions.
Each C2 domain of synaptotagmin 1 is formed from 8 β-strands arranged around a Greek key topology, known as the C2 key (9). The curvature of the domains is governed by a series of tandem β-bulges, some of which are unique to the C2 domain. Both the C2A and C2B domains of Syt 1 have extended loops at one end of the domain (Loops 1, 2, and 3) that have been specialized for Ca+2/phospholipid binding (10). Three ions can bind in a cup-like depression formed from these loops, but only Loops 1 and 3 contribute calcium-ion binding residues. Loop 2 contributes to the overall shape of the Ca+2 binding pocket, but does not directly participate in the coordination of cations. Hydrophobic residues at the apices of Loop 1 and Loop 3 embed the domain in the target membrane (11, 12). In the isoforms of Syt that bind Ca+2, five conserved acidic residues on Loops 1 and 3 coordinate the binding of Ca+2. Both X-ray studies and NMR analysis of the cation binding properties of the Syt 1 C2A domain confirm that the C2A domain can accommodate 3 calcium ions (10, 13), while the C2B domain binds two (14, 15). However, the isolated C2 domains show no gross structural change when comparing the Ca+2-free and Ca+2-bound states (16); therefore, any structural change associated with full-length synaptotagmin function must rely on relative domain motions and not intra-domain changes.
Experimental Procedures
Protein expression and purification
Human synaptotagmin 1 C2A-C2B was obtained via PCR from a human hippocampal Quick-Clone cDNA library (Clontech). An 858 nucleotide fragment comprising Syt 1 C2A-C2B residues 141-422 was directly cloned into PCR2.1 (Invitrogen), excised using BamH1 and Xho1 and subsequently subcloned into pGEX4T-1 (GE-Healthcare). DNA sequence analysis confirmed insertion in the correct reading frame with respect to the GST fusion partner. The cytosolic fragment of human synaptotagmin 1, containing only the cytosolic C2A and C2B, was over-expressed in E. coli as a GST-fusion protein (17) and purified.
Data collection Diffraction
Data were collected at the Stanford Synchrotron Radiation Laboratory (SSRL) on beamline 11-1 at a wavelength of 0.97Å (Table 1). The data sets were collected at 100 K using an ADSC image plate detector. Data were integrated, reduced, and scaled using HKL2000 (18). The crystals were indexed in the orthorhombic space group P212121; data statistics are summarized in Table 1.
Table 1.
Data collection and refinement statistics
| Syt1 C2AB | |
|---|---|
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (A) | 82.3, 86.3, 147.2 |
| α,β,γ (°) | 90,90,90 |
| Resolution (A) | 50-2.7 (2.85-2.7)* |
| Rsym or Rmerge (%) | 8.6 (44)* |
| I/Iσ | 13 (2.8)* |
| Completeness (%) | 99.5 (99.9)* |
| Redundancy | 4.0 (4.0)* |
| Refinement | |
| Resolution (Å) | 46-2.7 |
| No. reflections | 29304 |
| Rwork / Rfree | 25.3/23.0 |
| No. atoms | |
| Protein | 4514 |
| Ligand/ion | 8 |
| Water | 43 |
| B-factors (Å2) | Chains A+B |
| Protein | 47 |
| Ligand/ion | 40.5 |
| Water | 33 |
| R.m.s. deviations | |
| Bond lengths (A) | 0.007 |
| Bond angles (°) | 1.3 |
| Ramachandran Map‡ | |
| Most favored regions (%) | 89.2 |
| Additional allowed region (%) | 10.8 |
| Generously allowed region (%) | 0 |
| Disallowed region (%) | 0 |
All data collected from a single frozen crystal.
Values in parentheses are for highest-resolution shell.
For chains (A+B) in the ASU
Structure Determination and Refinement
Crystals were grown in the absence of Ca+2 as described elsewhere (17). The structure of synaptotagmin 1 C2A-C2B was solved via molecular replacement using Phaser (19) using diffraction data collected from one crystal to 2.7 Å (Table 1). Initial model coordinates were obtained using the C2A domain (1BYN) and the C2B domain (1TJX). Model building was done with Coot (20). Refinement of the model was carried out with CNS with a random subset of all data set aside for calculation of the Rfree (10%). Manual adjustments to the models were carried out with Coot. After refinement of the protein was complete, solvent molecules were assessed followed by manual adjustments. Chloride ions were assigned based on chemical environment, refined B-factor vs. the other water molecules and available coordination potential. The structure of C2A-C2B was verified by examining a simulated-annealed omit map generated with CNS v1.2 (Figure S1). There were no residues in disallowed regions of the Ramachandran map (Table 1). All figures were rendered with Pymol (21).
Results and Discussion
To examine whether the C2 domains of Syt 1 interact with each other prior to a Ca+2-dependent switch, we solved the 2.7 Å crystal structure (Table 1) of the complete cytosolic portion of Syt1 C2AB (Figure 1A). The protein was purified in the presence of EDTA, and no additional sources of divalent cations were added at the crystallization stage. We show that the two C2 domains of Syt 1 are capable of forming specific inter-domain interactions between amino acids on the H-A helix of C2B and residues on Loops 1, 2 and 3 of C2A. These interactions distort Loop 3 of C2A relative to the conformation of the same loop in the isolated C2A domain. The two Syt1 C2AB molecules in the asymmetric unit show the same interactions; further, there are no crystal contacts that would artificially distort Loop 3 of C2A. Therefore, this orientation likely represents the inter-domain interactions that are present in Syt1 prior to the Ca+2 flux.
Overall structure of Syt1 C2A-C2B
As this is the first atomic structure of Syt 1 C2A-C2B, we have defined the extent of the C2 domains, as well as the linker, based on their structural contributions to the overall fold of the protein. The residue numbering used throughout will correspond to the well-described rat Syt1 protein sequence, unless otherwise noted. The human Syt1 primary sequence is essentially identical to the rat/mouse sequence with the exception of one additional amino acid at the extreme N-terminus. We define a residue as part of the C2 domain “motif” if it interacts with other amino acids in the fold either by contributing a hydrogen bond to peptide backbone residues or by packing next to other secondary structural elements within the C2 domain. By this definition, the C2A domain extends from residue Leu-142 to Gln-263. The 9-residue linker extends from Ser-264 to Lys-272. The C2B domain of Syt 1 extends from Leu-273 to Lys-423. The two C2 domains of human Syt 1 share ~33% primary sequence identity; however, C2B possesses additional residues that correspond to two α-helices near its C-terminus (H-A and H-B) (14, 15). Primary sequence alignment of all the known human isoforms of synaptotagmin show that while the H-A helix is a common feature of most C2B domains, the H-B helix is present in only a sub-set of the paralogs (Figure 4).
Figure 4.
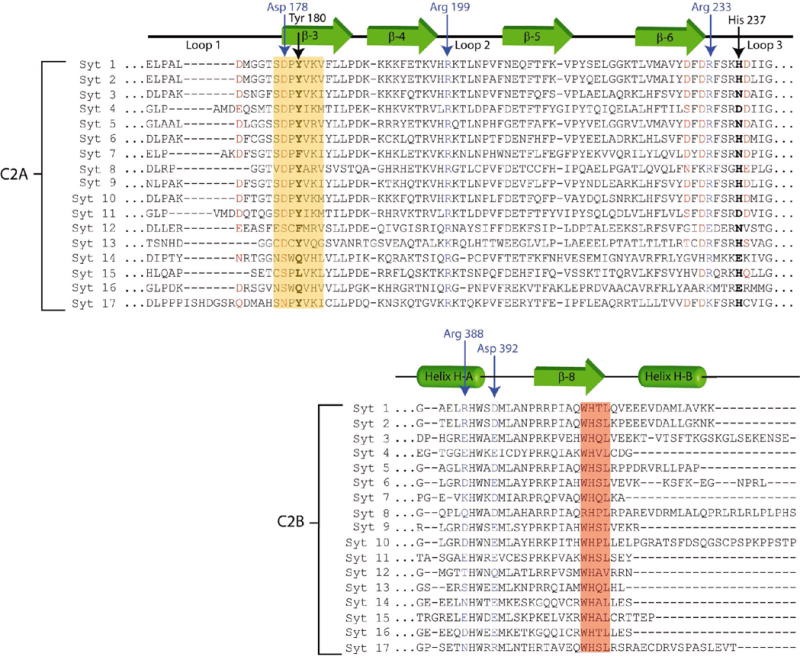
ClustalW alignment of the reported human synaptotagmin paralogs. The green arrows correspond to β-sheet structures in the C2 domains. The green tubes represent α-helical regions in the C2B domain. The top half of the alignment shows residues Glu-169 to Gly-242 in human Syt 1 C2A; the bottom half only shows residues Gly-385 to Lys-422 in C2B. Residues highlighted in red correspond to acidic residues in Syt1 C2A known to coordinated Ca+2. Red residues in Syt 2-17 share homologous positions, and in most cases, contribute to Ca+2 binding in those isoforms. The residues highlighted in the yellow box are those in β-strand 3 implicated in modulated Loop 3 structure of Syt1. The other residues homologous to the “SDPYVKV” motif within the yellow box may play similar roles in other paralogs. Residues in blue were observed forming salt bridges between C2A and C2B in Syt1. Residues boxed in red comprise the conserved “WHxL” motif.
Variable loop conformations of Syt1 C2A-C2B
Syt 1 crystallizes as a dimer in the asymmetric unit. While we do not ascribe physiological meaning to the dimer itself, it does allow us to compare the loop structures between the two molecules of the asymmetric unit and the isolated C2 domains, within the limits of our resolution range and refinement protocol. In this Syt 1 C2A-C2B crystal structure, the tertiary fold of both C2B domains is similar. Further, the fold of the C2B domains in our crystal is similar to that of the isolated C2B domain (14, 15). The RMSD between the isolated C2B structure and the C2B domain in the context of the tandem C2 domains is 0.58 Å over all Cα residues. We conclude that no part of C2B undergoes significant structural change in the context of C2A-C2B.
When comparing the two C2A domains present in the crystallographic asymmetric unit versus the isolated Syt 1 C2A domain, there are significant differences in loops 1 and 3 (Figure 2B). These differences are partly due to the hinge-like behavior of the loops in the unliganded state of the C2A domains in this crystal. Because of this hinge motion, Asp-172, one of the Ca+2-binding residues on Loop 1, was traced near the surface of the domain in one molecule, while the other conformation was directed in towards the Ca+2 binding pocket (Figure 2A). The flexible nature of this loop was not observed for C2B, as both loops 1 and 3 appear to be well ordered in this crystal form.
Figure 2.
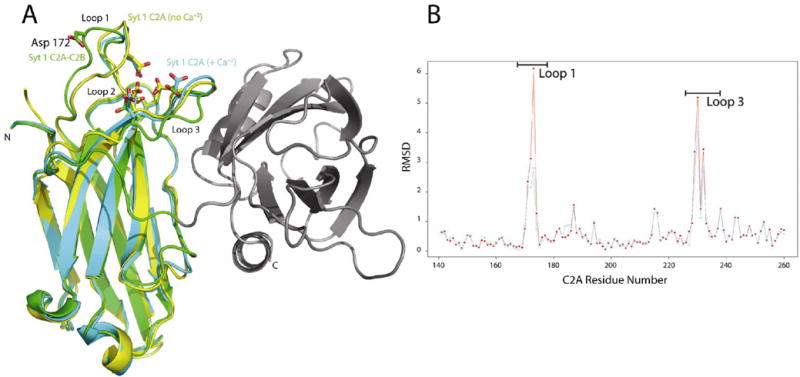
(A) Superposition of the isolated Syt1 C2A domain (1RSY, yellow); the Ca+2-saturated Syt1 C2A domain (1BYN, cyan); the C2A (in this crystal structure, green). The C2B domain is show in grey. The Ca+2 binding residues for the C2A domain are shown as sticks. The more distal position of Asp 172 in the C2AB structure is highlighted. The N- and C- termini of the synaptotagmin C2A-C2B protein are labeled as N and C. (B) RMSD plot over all Cα atoms between each of the C2A domains in the C2A-C2B crystal structure and the isolated C2A domain (1RSY). The red curve is the C2A domain of molecule A vs. 1RSY and the blue curve represents molecule B vs. 1RSY. The residues range corresponding to Loop 1 and 3 are shown by the bars above the major peaks. NCS restraints were used throughout the refinement with the exception of residue of Loop 1 and 3 in C2A, where considerable differences in the path of the loops were observed.
The structural variations observed in loop 3 of C2A is evident when comparing C2A-C2B to either the isolated Syt 1 C2A crystal structure (with no Ca+2) (9) or Syt 1 C2A with saturating Ca+2 (22) (Figure 2A). Regardless of the Ca+2 occupancy of the isolated C2A domain, the absolute position of Loop 3 resembles that of the C2B domains; consequently, the Ca+2-associating residues are oriented to bind calcium ion. However, in the C2A-C2B domain structure, this loop is restrained in an alternate conformation that is not consistent with the typical Ca+2 coordination geometry previously observed in the C2A domain. Typical calcium ion coordination by proteins utilizes a pentagonal bipyramidal array with an average 2.4 Å separation to oxygen atoms (23). This type of coordination is not possible while Loop 3 is restrained by C2B. Therefore, given this geometry, one would not expect C2A to bind Ca+2 in the presence of C2B, while in the closed conformation.
Modulation of Loop 3 in C2A
The stabilization of Loop 3 in this alternate position involves key residues in the “…SDPYVKV…” sequence in C2A. This motif is highly conserved across many C2 sequences, so the potential to modulate Loop 3 may be a common feature of the C2 domain (Figure 4). The Ca+2-binding conformation of Loop 3 in the isolated C2A domain is stabilized by a hydrogen bond between His-237 (H-bond acceptor) and the central Tyr in the sequence motif listed above (Tyr-180, the H-bond donor) (Figure 5B). However, in the human Syt 1 C2A-C2B structure, an alternate interaction of His-237 with Thr-406 in the C2B domain allows Loop 3 in C2A to collapse (Figure 5A). The importance of this Tyr on β-sheet 3 at this special position is supported by genetic evidence.
Figure 5.
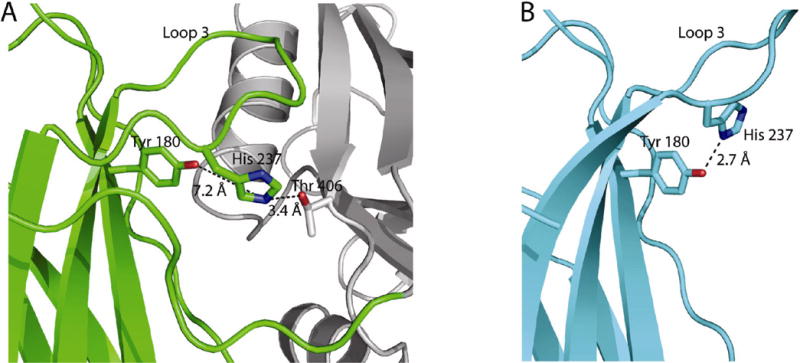
The rotameric position of His-237 depends on whether C2A is coupled to C2B. (A) the His-237 residue can pair with Thr-406 in C2B. C2B is rendered as grey helices and ribbons. (B) the hydrogen bonding partner of His 237 in the isolated C2A domain of rat synaptotagmin 1 (1RSY).
In the Drosophila Syt 1 C2B domain, the mutation of Tyr-364 to Asn (known as the AD3 mutation) abolishes Ca+2-evoked neurotransmitter release (24, 25). By raising extracellular Ca+2 from 400 μM to 6 mM, exocytosis in these mutants can be partially rescued (26). This implies that the divalent cation-binding machinery is still present in C2B, but the affinity for Ca+2 has been drastically altered. The marked phenotype of this mutation clearly illustrates that there is a direct link between Tyr-364 and Ca+2 affinity. To understand the relationship between our structure of human Syt 1 C2AB and this mutation in Drosophila, we computed a homology model of Drosophila Syt 1 C2B based on the primary structure alignment of all available Syt 1 orthologs and our X-ray structure of human Syt1 C2B (data not shown). In this model, Tyr-364 in Drosophila Syt 1 C2B is homologous to the Tyr-180 position in the human Syt 1 C2A domain. The model also predicts that Ser-423 (on loop 3) serves as the H-bond acceptor of Tyr-364 (β-sheet 3). We hypothesize that the phenotype of the AD3 mutation results from the removal of the hydrogen bond between Tyr-364 on β-sheet 3 and Ser-423 on Loop 3 of Drosophila C2B, thus collapsing Loop 3 of C2B. The collapse of Loop 3 repositions the Ca+2-binding residues, thereby increasing the amount of Ca+2 needed to rescue function. A homologous process may be occurring in human Syt 1 C2A by the close proximity of C2B. Since C2B reorients His-237, the H-bonding acceptor to Tyr-180, the same loss of Ca+2 affinity could be occurring in C2A (Figure 5B). Therefore, the AD3 mutation may mimic the behavior of the wild-type C2A domain under the influence of C2B.
Inter-domain interactions
The conformation of Loop 3 in C2A is restrained exclusively by the inter-domain interactions described below. As there are no significant contacts made by crystallographically related molecules, we conclude that the interactions we observe are possible in the native protein. Almost all of the amino acid interactions that link C2A with C2B are located on the H-A α-helix of C2B and Loops 1, 2 and 3 of the divalent cation binding pocket of C2A (Figure 3). Arg-388 on the H-A helix of C2B forms a salt-bridge interaction with Asp-178 located on Loop 1 of C2A. In previous structural analysis, Asp-178 contributes a single carbonyl oxygen to the highest-affinity Ca+2-binding site (9). When Asp-178 is mutated to Asn in the isolated C2A domain, Ca+2 binding is disrupted (27, 28). Since the observed interaction between Asp-178 and Arg-388 directly links C2B to calcium ion binding residues in C2A, it is tempting to conclude that this interaction may represent a switch or trigger to link Ca+2 occupancy to Syt 1 function. However, when this mutation was introduced into Drosophila, no significant phenotype was observed (29). While Asp-178 is certainly important to the overall function of Syt 1, it is clear that this residue is not solely responsible for the interactions with C2B. A second inter-domain interaction observed between C2A and C2B is contributed by Asp-392 of C2B. This residue forms a bifurcated salt-bridge between Asp-392 and Arg-199 on Loop 2 in C2A and between Asp-392 and Arg-233 on Loop 3. Arg-199 is also a notable interaction as it participates in several activities of C2A: interactions with phospholipid (12), SNARE components and is a contributor to the electrostatic switch of C2A (30).
Figure 3.
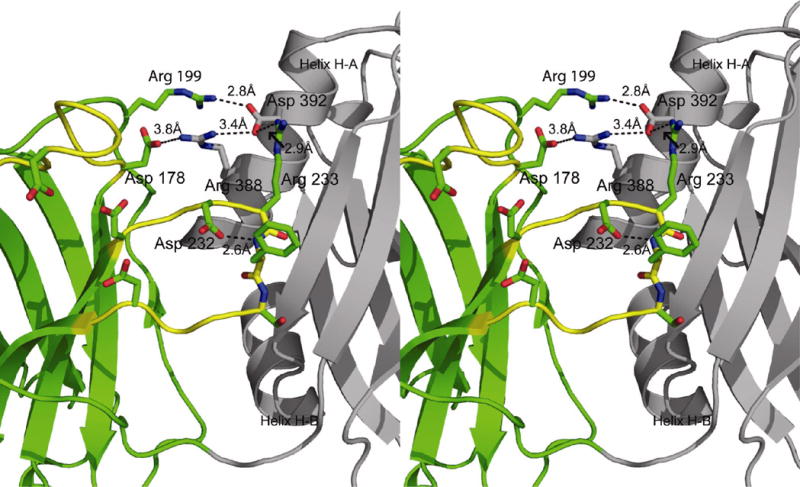
Stereoview of the C2A-C2B inter-domain interactions. C2A is rendered in green ribbons and C2B is rendered in grey on the right. Interacting residues are labeled according to the rat Syt1 numbering scheme. Also shown is the intra-domain interaction between Asp 232 in C2A and the backbone amide of Phe 234.
Consequences of the tandem arrangement
Most C2 domains share two common functions: first, as an adapter module that links a client protein or a neighboring motif to a phospholipid bilayer through the hydrophobic residues at the tips of the C2 domain (31), and the second function is mediated through the effector-binding polybasic regions (32, 33). In the closed conformation, the association with C2B affects residues that mediate both of these activities. The poly-basic region of C2A is composed of four contiguous Lys residues at the amino-terminus of β-stand and 3 basic amino acids at the carboxy-terminus of β-strand 4. While the biological function of the polybasic domain of C2A in Syt 1 function is not as clear as that of C2B, this region of C2A is almost completely occluded by its interaction with C2B. Upon forming the closed conformation, ~890 Å2 of solvent-accessible surface area are buried per C2 domain. This degree of domain contact is comparable to other observed protein dimer interfaces, and is indicative of associations that require weaker interactions (34). Thus, the closed conformation of Syt 1 may obstruct premature interactions with effectors such as phospholipids, syntaxin or the voltage-gated Ca+2-channel (30, 35, 36). Another notable feature of C2B is the highly conserved “WHxL” sequence in C2B. This motif has been implicated in docking events and neurexin binding of Syt 1 in the synapse (37). In the closed conformation of Syt 1 C2A-C2B, ~40% of the solvent exposed surface area of the …WHTL…” motif is occluded by the C2A-C2B interface. Most of this surface area is lost by burying Trp-405 within the interface. If this sequence does mediate the docking of synaptotagmin to the plasma membrane through a specific receptor, then it would be unable to do so while in this closed conformation.
Implications for other synaptotagmin orthologs
The human genome possesses genes for at least 17 distinct synaptotagmin proteins (Figure 4). The excessive redundancy of the Syt orthologs is either due to critical nature of Syt in regulated exocytosis (38) or as part of an intricate addressing system that allows for the temporal and spatial fine-tuning of fusion between specific vesicles and specific membrane targets in various cell types (39). The residues that we observe at the interface between C2A and C2B are present in many of the other isoforms, but not all. Therefore, it is conceivable that this C2A-C2B domain orientation is unique to Syt 1. The position of C2A relative to C2B may vary by homolog depending on the particular biological requirements the cell.
Conclusion
The C2A and C2B domains of Syt 1 are related by an ~108° rotation about the linker axis. This is in contrast to the more “open” conformation of Mg+2-bound Syt 3 C2A-C2B where there are no inter-domain interactions between domains, and the phospholipid binding loops directly face one another (Figure 1B) (6). NMR analysis of Syt 1 C2A-C2B concluded that, like Syt 3, the domains of Syt 1 can be separate and non-interacting (5). However, we clearly observe extensive interactions between domains. To rationalize these seemingly disparate results, we hypothesize that Syt 1 possesses an open and a closed conformation that is critical to its function. The closed form may be required for regulating the activity of Syt 1 prior to fulfilling its role in exocytosis, while the open form may be required for effector interaction during the final stages of exocytosis.
We propose that, while still in this closed conformation, C2B·Ca+2 initially localizes to the membrane surface and may begin to associate with phospholipid membranes and other factors required for exocytosis (40). As the Ca+2/phospholipid binding loops of C2B are already in a conformation amenable to phospholipid interaction, it is reasonable to assume that C2B will behave as a phospholipid-binding module to initially localize the Ca+2-sensor to the target membrane. This may explain why C2B appears more essential to the Syt 1 protein, as it could be the first domain capable of responding to cytosolic Ca+2 gradients (41). At some point, the C2A domain must detach from C2B under the influence of a trigger. We predict that the trigger will be a threshold Ca+2 concentration, but phospholipid interaction, effector protein binding, or some combination of the three may initiate the transformation of Syt 1 molecule to an open conformation. Next, the divalent cation binding Loops 1 and 3 of C2A will attain their final conformation as calcium ions occupy binding sites (40). The final phase of synaptotagmin action is presently unclear, as it may involve a direct mechanism of phospholipid de-mixing that leads to fusion or the protein will set the stage for another component to facilitate the final step. This model of Syt1 C2AB also suggests a mechanism whereby the two C2 domains of synaptotagmin may act on the target membrane in series rather than in parallel, thereby providing a true structural trigger. Consequently, C2A may only attain its final Ca+2 occupied, membrane-binding competent structure during the final few microseconds prior to exocytosis. Defining the structural basis for the interactions of synaptotagmin 1, as well as other isoforms, with SNARE components and phospholipid bilayers will be the focus of future studies.
Supplementary Material
Acknowledgments
We thank Drs. Betsy Goldsmith and Andres Oberhauser for help with the manuscript. We would also like to acknowledge Jason Vertrees for help with the statistical plotting software “R”. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.
This work was supported by NIH grant MH-070589 to RBS. RBS is also supported by a Career Award in the Biomedical Sciences from the Burroughs-Wellcome Fund. KLF was supported by a training fellowship from the Houston Area Molecular Biophysics Program.
Abbreviations
- ASU
asymmetric unit
- EPR
Electron Paramagnetic Resonance
- GST
Glutathione-S-Transferase
- H-bond
hydrogen bond
- NMR
Nuclear Magnetic Resonance
- PCR
Polymerase Chain Reaction
- SNARE
SNAP receptor
- Syt
synaptotagmin
Footnotes
Coordinates and structure factors have been deposited in the Protein Data Bank under the accession number 2R83.
Supplementary Information
Representative electron density
http://xray.utmb.edu/~sutton/biochemistry_el_density_map.pdf)
2mFo-DFc Simulated Annealing (SA) composite omit map (at 1.0V contour level) of the interfacial residues between Syt 1 C2A and C2B. Residues in green residues localize to the C2A domain whereas the gray residues localize to the C2B domain.
References
- 1.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs Do Not Trigger Fusion between Synthetic Membranes but Do Promote PEG-Mediated Membrane Fusion. Biophys J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 3.Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 4.Rickman C, Archer DA, Meunier FA, Craxton M, Fukuda M, Burgoyne RD, Davletov B. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J Biol Chem. 2004;279:12574–12579. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- 5.Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 6.Sutton RB, Ernst JA, Brunger AT. Crystal structure of the cytosolic C2A-C2B domains of synaptotagmin III. Implications for Ca+2-independent snare complex interaction. J Cell Biol. 1999;147:589–598. doi: 10.1083/jcb.147.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damer CK, Creutz CE. Synergistic membrane interactions of the two C2 domains of synaptotagmin. J Biol Chem. 1994;269:31115–31123. [PubMed] [Google Scholar]
- 8.Hui E, Bai J, Chapman ER. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys J. 2006;91:1767–1777. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 10.Shao X, Davletov BA, Sutton RB, Sudhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 11.Chae YK, Abildgaard F, Chapman ER, Markley JL. Lipid binding ridge on loops 2 and 3 of the C2A domain of synaptotagmin I as revealed by NMR spectroscopy. J Biol Chem. 1998;273:25659–25663. doi: 10.1074/jbc.273.40.25659. [DOI] [PubMed] [Google Scholar]
- 12.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 13.Sutton RB, Sprang SR. Structure of the protein kinase C-β phospholipid-binding C2 domain complexed with Ca2+ Structure. 1998;6:1395–1405. doi: 10.1016/s0969-2126(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Sequeira SM, Malinina L, Tereshko V, Sollner TH, Patel DJ. Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin I C2B domain. Protein Sci. 2004;13:2665–2672. doi: 10.1110/ps.04832604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 16.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 17.Montes M, Fuson KL, Sutton RB, Robert JJ. Purification, crystallization and X-ray diffraction analysis of human synaptotagmin 1 C2A–C2B. Acta Cryst F. 2006 doi: 10.1107/S1744309106029253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In: Carter C, Sweet R, editors. Methods in Enzymology, Macromolecular Crystallography, part A. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 19.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Cryst D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 20.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 21.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; Palo Alto, CA, USA: 2002. [Google Scholar]
- 22.Shao X, Fernandez I, Sudhof TC, Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- 23.Strynadka NC, James MN. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T, Tamura T, Suzuki K, Kidokoro Y. External Ca2+ dependency of synaptic transmission in Drosophila synaptotagmin I mutants. J Neurophysiol. 2005;94:1574–1586. doi: 10.1152/jn.00205.2005. [DOI] [PubMed] [Google Scholar]
- 25.DiAntonio A, Parfitt KD, Schwarz TL. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 26.Littleton JT, Bai J, Vyas B, Desai R, Baltus AE, Garment MB, Carlson SD, Ganetzky B, Chapman ER. Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 28.Ubach J, Zhang X, Shao X, Sudhof TC, Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? Embo J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C(2)A domain. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- 30.Shao X, Li C, Fernandez I, Zhang X, Sudhof TC, Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- 31.Edwards AS, Newton AC. Regulation of protein kinase C βII by its C2 domain. Biochemistry. 1997;36:15615–15623. doi: 10.1021/bi9718752. [DOI] [PubMed] [Google Scholar]
- 32.Grass I, Thiel S, Honing S, Haucke V. Recognition of a basic AP-2 binding motif within the C2B domain of synaptotagmin is dependent on multimerization. J Biol Chem. 2004;279:54872–54880. doi: 10.1074/jbc.M409995200. [DOI] [PubMed] [Google Scholar]
- 33.Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- 34.Jones S, Thornton JM. Principles of protein-protein interactions. Proc Natl Acad Sci U S A. 1996;93:13–20. doi: 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charvin N, L'Eveque C, Walker D, Berton F, Raymond C, Kataoka M, Shoji-Kasai Y, Takahashi M, De Waard M, Seagar MJ. Direct interaction of the calcium sensor protein synaptotagmin I with a cytoplasmic domain of the alpha1A subunit of the P/Q-type calcium channel. EMBO J. 1997;16:4591–4596. doi: 10.1093/emboj/16.15.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen R, Schmitt BM, Atlas D. Molecular identification and reconstitution of depolarization-induced exocytosis monitored by membrane capacitance. Biophys J. 2005;89:4364–4373. doi: 10.1529/biophysj.105.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda M, Moreira JE, Liu V, Sugimori M, Mikoshiba K, Llinas RR. Role of the conserved WHXL motif in the C terminus of synaptotagmin in synaptic vesicle docking. Proc Natl Acad Sci U S A. 2000;97:14715–14719. doi: 10.1073/pnas.260491197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang ZP, Shin OH, Meyer AC, Rosenmund C, Sudhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 40.Kertz JA, Almeida PF, Frazier AA, Berg AK, Hinderliter A. The Cooperative Response of Synaptotagmin I C2A. A Hypothesis for a Ca2+-Driven Molecular Hammer. Biophys J. 2007;92:1409–1418. doi: 10.1529/biophysj.106.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


