Abstract
Low-intensity transcranial focused ultrasound (FUS) has emerged as a non-invasive brain stimulation modality that can reach deep brain areas with high spatial specificity. Previous studies have identified transient effects of FUS on the brain excitability and accompanying physiological responses. Yet the presence of long-lasting effects of FUS, which extend on the order of half an hour or more, has not been probed. We transcranially applied FUS to the somatosensory areas of the anesthetized rats for 10 min at a low duty cycle (5%) and intensity, far below the level that could alter the tissue temperature. Concurrently, we measured electroencephalographic (EEG) somatosensory evoked potentials (SEP) induced by the unilateral electrical stimulation of the hind limb before and after the sonication. Compared to the control sham condition that did not involve sonication, differential SEP features were evident and persisted beyond 35 min after the administration of FUS. The presence of this non-transient neuromodulatory effect may provide early evidence that FUS-mediated brain stimulation has the potential to induce neuroplasticity.
Keywords: electrophysiology, electroencephalography, evoked potentials, focused ultrasound, brain stimulation
Introduction
Low-intensity transcranial focused ultrasound (FUS) has been shown to stimulate deep brain areas with high spatial specificity in a non-invasive fashion,1–5 which cannot be readily achieved using existing brain stimulation methods, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Transcranial FUS was used to selectively modulate neural tissue excitability of the brain cortical regions, such as sensory,4,6,7 motor,2,5,8 and visual areas.5,9,10 The technique has also been applied to modulate the function of deep brain areas, for example, the hippocampus or the thalamus in rodents.11–14 Its utility has been extended to provide means for brain stimulation in the context of brain-to-brain interface.15,16
The presence of durable changes on neural excitability induced by a brain stimulation modality, assimilating the effects such as long-term potentiation (LTP) or long-term depression (LTD), is considered one of the main features that may ultimately lead to neuroplasticity. For example, TMS applied for extended duration has shown LTD-like effects,17 mediated by changes in activity of the N-methyl-D-aspartate (NMDA) glutamate receptor. On the other hand, most of the previous studies examining the effects of FUS have only shown transient and reversible changes in brain activity. We hypothesize that it is mainly due to the use of brief exposure (duration less than a few hundreds of millisecond per a stimulation event) to the non-thermal, low-intensity ultrasound pressure waves. Although several studies have explored the use of longer sonication duration (e.g. one or two minutes) to show stimulation effects that slightly outlasted the sonication itself,5,9 the investigation to probe the presence of non-transient neuromodulatory effects, which can last on the order of half an hour, has been lacking. Chu and colleagues18 recently investigated the presence of long-term effects from FUS sonication on cortical excitability and have found that the use of low duty cycle sonication (10 ms pulse duration at 1 Hz repetition, i.e. 1% duty cycle), specifically designed for disrupting the blood brain barrier (BBB), did not lead to persisting alterations in somatosensory evoked potentials (SEP). They hypothesized that the use of extremely low pulse repetition frequency might have been insufficient for inducing desired neuromodulation.
In the present study, we were motivated to examine whether the effects of neuromodulatory FUS could last for more than 30 min. A 30 min duration is known to be sufficient time to induce initial LTP/LTD-like synaptic behavior.19,20 We transcranially applied low-intensity FUS (210 mW/cm2 spatial-peak temporal-average intensity; Ispta) to the somatosensory areas of the rat brain for 10 min at a low duty cycle (5%; in a pulsed mode), far lower than the level that could alter the tissue temperature.21 Concurrently, we measured electroencephalographic (EEG) SEP induced by the unilateral electrical stimulation of the hind limb before and up to 35 min after the sonication to examine the presence of differential SEP features affected by FUS. The sham sessions (i.e. without sonication using the same experimental setup) were also conducted to provide the control condition.
Methods
Wearable transcranial FUS transducer and animal preparations
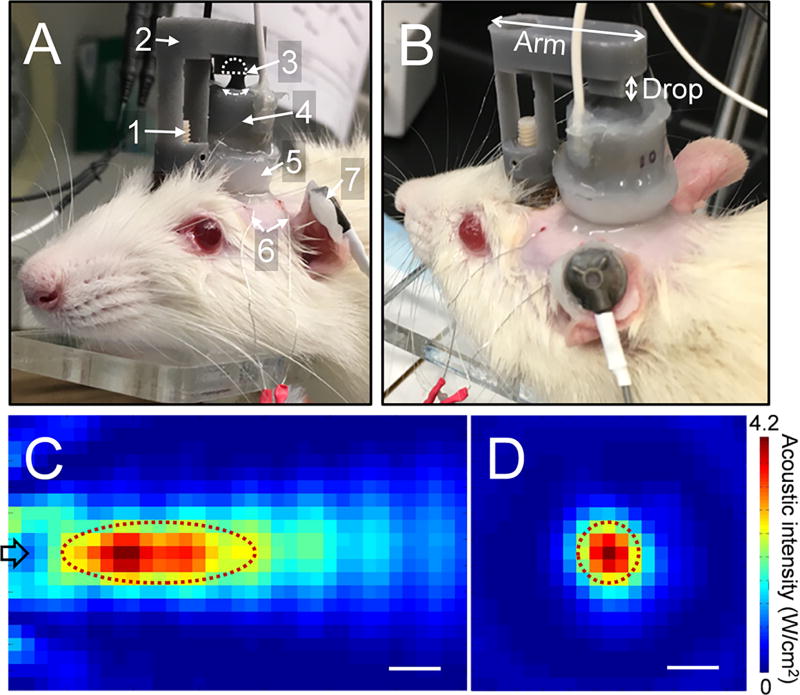
All experiments reported herein were conducted according to the guideline set forth by a local Institutional Animal Care and Use Committee. To transcranially deliver FUS to the somatosensory area of the rat, we used a custom-made, miniature FUS transducer that can be worn over the pedestal implanted over the rats’ skull. A piezo-ceramic disc (American Piezo Ceramics, Mackeyville, Pennsylvania, USA) was coupled with a ceramic acoustic lens (Edmund Optics, Barrington, New Jersey, USA), and packaged in a custom-made housing (cylindrical shape, 16 mm in diameter, 12 mm in height) capable of being mounted and carried by the rats (Fig. 1A and B). The overall weight of the transducer unit was 9 g. An applicator was designed to hold the transducer using a ball-and-socket joint and connected to a pedestal that was implanted on the surface of the rat skull. A fundamental ultrasound frequency of 650 kHz was used and the acoustic focus was formed 10.5 mm away from the exit plane of the transducer. The size of the focus was 7.5 mm in length and 2.5 mm in diameter, being measured at full-width at half-maximum (FWHM) of acoustic intensity profile (Fig. 1C and D).
Fig. 1.
The experimental setup and the acoustic intensity profile of the wearable FUS transducer. (A) The transducer worn by a rat during sonication. 1: skull-mounted pedestal, 2: detachable applicator, 3: ball-and-socket joint, 4: FUS transducer, 5: coupling gel, 6: subdermal EEG electrodes, 7: ground EEG electrode. (B) the detachable applicator with customizable dimension. The acoustic intensity profile across the (C) longitudinal plane and (D) transversal plane at 10.5 mm from the exit plane of the transducer. Dotted lines in panels (C) and (D) depict the FWHM of the intensity profile. The arrow in panel (C) indicates the direction of acoustic beam. Scale bar = 2 mm.
Sprague-Dawley rats (all male; n = 11) underwent surgery to implant a pedestal (Plastics One, Roanoke, Virginia, USA) to the skull using a cyanoacrylate adhesive (Loctite 4541; Loctite Corporation, Rocky Hill, Connecticut, USA) with two screws to anchor the pedestal to the skull. Rats were allowed to heal for at least 2 weeks prior to FUS sessions. For the FUS session, detachable applicators having different combinations of the arm length and drop distances (Fig. 1B) were 3D-printed with 0.5 mm intervals (Form 2, Formlabs, Somerville, Massachusetts). One applicator was then selected according to the distance between the pedestal site and the inter-aural line appropriate for targeting the individual rat’s somatosensory area (of the left hind limb). The pivoting mechanism using the ball-and-socket joint further allowed for the adjustment of the acoustic focus to be approximated to the somatosensory area using the stereotactic guidance as previously described.11 FUS was given with 100 Hz pulse repetition frequency, 0.5 ms tone burst duration (i.e. 5% duty cycle) at 4.2 W/cm2 spatial-peak pulse-average intensity (Isppa; i.e. 210 mW/cm2 Ispta) based on the parameters that have shown transient suppressive effects on cortical excitability.5 The parameter setting does not raise tissue temperature.5
SEP measurement and analysis
Rats were anesthetized (ketamine/xylazine = 80:10 mg/kg, i.p.) and the fur over the scalp was shaved. A cone-shaped polyvinyl alcohol (PVA) cryogel (8% weight/volume in degassed water, 2 freeze-thaw cycles) coupled the scalp with the transducer (detailed method described in Lee et al.22). Ultrasound gel was also applied to all the interfaces to provide an uninterrupted sonication path. The rat was then placed on a plastic platform which allowed its limbs to freely-hang. SEP was measured from the head using needle EEG electrodes (SWE-L-25; Ives EEG Solutions, Newburyport, Massachusetts, USA) inserted ~4 mm post-laterally to the bregma and a reference electrode inserted 2–3 mm posterior to the lambda while the ground cup electrode was placed over the skin of the ear (Fig. 1A). Electrical stimulation (15 mA constant current, 50 µs duration) was delivered to the left hind limb muscle (biceps femoris) 240 times at a rate of 2 Hz using a surface electrode stimulator (MLADDF30; ADInstruments, Colorado Springs, Colorado, USA). 15 mA was chosen based on the level that reliably generated EEG responses for about an hour from a pilot study involving a few rats (the results not reported). The corresponding EEG was acquired in a time-locked fashion covering 50 ms before and 200 ms after the onset of the electrical stimulation (sampling rate of 10 kHz, Mains filter, and 100 Hz low pass filter, PowerLab and LabChart; ADInstruments). The skin at the electrical stimulation site was exposed by carefully combing the furs after applying electrically conductive gel, and the stimulator was affixed against the skin using a tape around the lower body.
Each SEP measurement took 2 min. SEP was measured twice (labeled ‘Base 1’ and ‘Base 2’) 5 min and 2 min before the start of the sonication to establish the baseline signal level, and again five times, which acquired at 5, 10, 15, 25, and 35 min after the end of the sonication (labeled ‘Post 1’–‘Post 5’). In order to maintain a similar level of anesthetic conditions in relation to the sonication timing across the animals, FUS was given around 25 min after the induction of the anesthesia. Considering the 10 min-long sonication and 37 min-long post-SEP sessions, each experiment took approximately 72 min. During the sonication, EEG signals were contaminated with pulse repetition frequency of FUS (i.e. 100 Hz), and therefore, excluded from analysis. We also conducted the sham FUS session (i.e. no sonication, otherwise using the same experimental settings) for each of the animals on different days (with a 2–3 days gap between the sessions). The sequence of FUS and sham sessions were randomized and balanced across the animals. We did not re-dose the anesthesia to ensure an equivalent plane of anesthesia across the animals (the timing and amount of re-dosing creates another study confounder). The obtained time-averaged SEP data from each rat underwent direct current (DC) signal offset correction and was subjected to time-wise paired t-tests comparing FUS and sham sessions using MATLAB (Mathworks, Natick, Massachusetts, USA). The time segments that showed differential signal magnitude (P < 0.05; two-tailed t-test, d.f. = 10) were noted. Due to the presence of signal artifact associated with the electrical stimulation, the data from 0–5 ms time segment post-SEP stimulation was excluded from comparisons.
Estimation and actual measurement of temperature change at the FUS focus
We assessed the existence of potential tissue heating by FUS, first by estimating the theoretical temperature increase (per minute; assuming there is no perfusion and heat exchange) using the equation reported in previous studies2,25,26 (i.e., ΔT = 2αIt/ρbCp = 2 × 0.014 cm−1 × 4.2 W·cm−2 × (60 s × 0.05)/3.796 J·cm−3·°C−1; where α = the absorption coefficient,23 I = the attenuated AI at the FUS focal area, t = the ultrasound pulse duration given for 1 min at 5% duty cycle, ρb = the density of brain tissue,24 and Cp = the specific heat capacity of the brain tissue24). The estimated temperature rise of 0.093 °C (ΔT), which is negligible due to the heat dissipation through conduction and perfusion, will not affect neural excitability or cause thermal damage.27,28 Secondly, the temperature at the sonication focus was also measured in a separate experimental session using a metal thermocouple (Traceable, Control Company, Friendswood, TX) placed at the focal location at room temperature (20 °C) for ten minutes.
Results
The onset time of the sonication from the induction of anesthesia (FUS : Sham = 25.0 ± 1.8 min : 25.0 ± 1.8 min) as well as the weight of the animals (FUS : Sham = 401.4 ± 37.7 g : 399.7 ± 32.3 g) were similar between FUS and sham conditions (all n = 11, P > 0.5, two-tailed t-test, d.f. = 10). We found that the temperature measured at the focus in a separate session on thermocouple did not change.
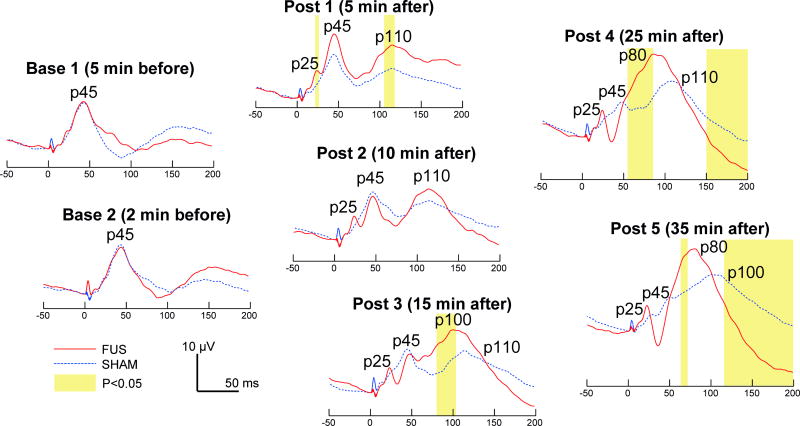
Fig. 2 shows the comparisons between grand-average SEPs from the FUS sessions (red line) and those from the sham sessions (dotted blue line) at different measurement points. We found that SEP measured prior to FUS (‘Base 1’ and ‘Base 2’) showed a distinctive peak (with amplitude of ~10 µV) at 45 ms after electrical stimulation (i.e. we define it as p45 component). 5 min after the sonication (‘Post 1’), SEP showed additional peaks at 25 ms and 110 ms latency (i.e. altogether p25, p45, p110 components), which differed from the control condition that showed a single peak at 45 ms latency. The p45 and p110 components progressively decreased in their amplitudes after the FUS, and a bigger single peak started to appear (p100 in ‘Post 3’). This peak became prominent, with 80 ms latency, 25 min after the sonication (p80 in ‘Post 4’). The signal from this peak continued to decrease until the end of the measurement window (i.e. 200 ms post electrical stimulation). The measurement window did not capture the complete recovery of the signal back to the baseline level. The p25 component that appeared in ‘Post 1’ remained throughout the post sonication sessions.
Fig. 2.
Time course of the grand-average (n = 11 rats) SEP comparing the FUS and sham conditions. The time segments that showed the differential signal features (two-tailed t-test, P < 0.05) are highlighted in yellow.
When compared to the sham condition, SEP measured prior to FUS (‘Base 1’ and ‘Base 2’) were not different. 5 min after the administration of FUS (i.e. ‘Post 1’ in Fig. 2), a time-course difference was evident at the time segments around p25 and p110 components; however, the difference was not apparent at 10 min post-FUS session (‘Post 2’). As time passed, 15 min after the FUS, p100 peak from the FUS condition incrementally increased, so as the difference in the 80–102 ms time segment (during ‘Post 3’). 25 min after the FUS, differences in the time segments of 59–90 ms and 151–200 ms became apparent (see ‘Post 4’). When measured at 35 min after the sonication, FUS condition showed the distinctive p80 component (22 µV in amplitude), having the differential magnitude in 65–72 ms and in 117–200 ms compared to the sham condition. Interestingly, we found that the SEP acquired 35 min after the sham sonication condition (in dotted blue, ‘Post 5’) markedly resembled the SEP acquired at 15 min after the FUS sonication (in red, ‘Post 3’), showing peaks at p25, p45, p100 with similar signal amplitudes (P > 0.05).
Discussion
We reported the temporal progression of SEP affected by the FUS stimulation of the somatosensory areas in ketamine/xylazine anesthetized rats. A positive SEP peak at 45 ms latency elicited by the electrical stimulation of the unilateral hind limb was shown in both FUS and control (sham) group during the baseline conditions, which bears similarity with previous studies.29,30 During the course of emergence from the anesthesia (~72 min from the induction), SEP features varied over time. For example, p100/p110 components, which were not obvious during the early phase of anesthesia, started to show increased amplitude. The pre-FUS peaks in the control group gradually changed over the course of the experiment. Although we could not isolate the definite causes for this observation, we conjecture that the longer latency peak may be associated with higher-order sensory processing (such as from secondary somatosensory areas) that starts to take place during the arousal from anesthesia. We note that, however, there were also differences in the number of peaks and their signal amplitudes, for example, negative peaks that were identified in All et al.29 or Chu et al.18 were not seen from our study. We hypothesize that these discrepancies may be attributed to differences in experimental settings, such as duration, location, and intensities of electrical stimulations, animal sub-species, and the choice of anesthetic agents used.
The application of FUS appeared to result in differential SEP, with respect to the control condition, a short time after the end of sonication (5 min). This difference became less apparent after the 10 min time window. However, the FUS condition progressively gave rise to distinctively different SEP signals compared to the control condition, and these differential SEP features persisted even 35 min after the sonication. We believe that this is the first finding of FUS-mediated neuromodulatory effects lasting more than 35 min, which suggests the presence of non-transient, extended time-scale (on the order of half an hour) effects of FUS. This finding suggests that FUS-mediated brain stimulation may be able to induce initial LTD/LTP-like synaptic behavior,19,20 although we could not determine whether our results have a specific link to the direction of excitability (i.e. either potentiation or depression). The tissue temperature, a factor that is known to affect the neural excitability,8 was ruled out as the cause for the effects due to the use of low-intensity ultrasound (see the estimation and actual measurement of the temperature using the same sonication parameter). Instead, potential contributions from either neurovascular coupling with sonication or anesthesia-dependent modification of neural excitability should be considered as confounders. The evidence for unequivocal LTP/LTD-like neural responses awaits further investigation using animal or in vitro cell models.
We found that the SEP acquired at the 35 min mark, during the sham condition, markedly resembled the SEP acquired at 15 min after the sonication. This resemblance may be coincidental; alternatively it may suggest that the FUS expedited the SEP features to appear 20 min early (i.e. expediting the arousal from the anesthesia). If true, this would bear similarity with our previous observation that FUS sonication to the thalamus in ketamine/xylazine anesthetized rats shortened the time of arousal from the anesthesia.13 We conjecture that the effects of ketamine, as NMDA receptor antagonist, might have been modified (reduced) by the application of the FUS. Based on NMDA-mediated long-term effects of neuromodulation and neuroplasticity (example from a TMS study by Muller et al.17), the conjunctive use of non-NMDA anesthetic agents (such as pentobarbital or urethane) and NMDA receptor-specific antagonists (such as (2R)-amino-5-phosphonovaleric acid, i.e. AP5) with FUS would reveal a more detailed mechanism of action on long-term modulation of cortical excitability. As the mechanism of FUS-mediated changes on the neural tissue excitability is not sufficiently understood, the use of other brain stimulatory modalities, such as paired TMS,17 with FUS may also be used to examine the presence of LTD/LTP-like behavior. This constitutes subjects for future studies.
The wearable FUS transducer used in this study provided a well-localized acoustic focus. However, due to the small skull cavity of rats, acoustic reverberation may occur and may obscure the acoustic focus.31 This would possibly stimulate different brain areas (other than designated primary somatosensory area) in its acoustic path, including the thalamus.11–13 Examination of the stimulatory effects in larger animals (with larger skull cavity) will help to reduce the study confounders.
Another limitation of the study was that our anesthetic protocol did not allow us to examine the effects of FUS more than 35 min. The use of anesthetic agents that allow for longer anesthetic time would be conducive to studying the longer-term effects of FUS. However, one should also account for the effects of anesthesia itself on neural excitability. The wearable FUS configuration, although not demonstrated in the present study, would eventually enable the experiment to be conducted in awake animals, eliminating the confounding effects from anesthesia.
In summary, application of the low-intensity pulsed FUS to the rat brain resulted in differential SEP being presented up to 35 min after sonication. We believe that this is the first report finding evidence for non-transient brain responses to FUS stimulation that far outlasted the application of the stimulation itself. Our results suggest that FUS may confer potential utility to induce long-term neural plasticity of the brain in a non-invasive fashion and to set a stage for developing therapeutic strategies. More detailed characterization of the mechanism of this observation would be needed to develop therapeutic FUS protocols.
Acknowledgments
This study was supported by the Focused Ultrasound Surgery Foundation (FUS 461, to W. Lee and S.S. Yoo) and NIH (RO1 MH111763, to S.S. Yoo). We thank Dr. Yongzhi Zhang and Mr. Michael Y. Park for the helpful technical advice on rat surgical procedures.
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- 1.Bystritsky A, Korb AS, Douglas PK, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4(3):125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Chiu A, Lee SD, Fischer K, Yoo S-S. Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain Stimul. 2014;7(5):748–756. doi: 10.1016/j.brs.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Taghados SJ, Fischer K, Maeng L-S, Park S, Yoo S-S. Noninvasive transcranial stimulation of rat abducens nerve by focused ultrasound. Ultrasound Med Biol. 2012;38(9):1568–1575. doi: 10.1016/j.ultrasmedbio.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legon W, Sato TF, Opitz A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17(2):322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- 5.Yoo S-S, Bystritsky A, Lee J-H, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56(3):1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee W, Chung YA, Jung Y, Song I-U, Yoo S-S. Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound. BMC Neurosci. 2016;17(1):68. doi: 10.1186/s12868-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W, Kim H, Jung Y, Song I-U, Chung YA, Yoo S-S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci Rep. 2015;5:8743. doi: 10.1038/srep08743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Lee SD, Chiu A, Yoo S-S, Park S. Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation. Neuroreport. 2014;25(7):475–479. doi: 10.1097/WNR.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Park MY, Lee SD, Lee W, Chiu A, Yoo S-S. Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound. Neuroreport. 2015;26(4):211–215. doi: 10.1097/WNR.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W, Kim H-C, Jung Y, et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep. 2016;6:34026. doi: 10.1038/srep34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min BK, Yang PS, Bohlke M, et al. Focused ultrasound modulates the level of cortical neurotransmitters: potential as a new functional brain mapping technique. Int J Imaging Syst Technol. 2011;21(2):232–240. [Google Scholar]
- 12.Yang PS, Kim H, Lee W, et al. Transcranial focused ultrasound to the thalamus is associated with reduced extracellular GABA levels in rats. Neuropsychobiology. 2012;65(3):153–160. doi: 10.1159/000336001. [DOI] [PubMed] [Google Scholar]
- 13.Yoo S-S, Kim H, Min B-K, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport. 2011;22(15):783–787. doi: 10.1097/WNR.0b013e32834b2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, Yan J, Ma Z, Li X. Effect of noninvasive focused ultrasound stimulation on gamma oscillations in rat hippocampus. Neuroreport. 2016;27(7):508–515. doi: 10.1097/WNR.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 15.Lee W, Kim S, Kim B, et al. Non-invasive transmission of sensorimotor information in humans using an EEG/focused ultrasound brain-to-brain interface. PLoS One. 2017;12(6):e0178476. doi: 10.1371/journal.pone.0178476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo S-S, Kim H, Filandrianos E, Taghados SJ, Park S. Non-invasive brain-to-brain interface (BBI): establishing functional links between two brains. PLoS One. 2013;8(4):e60410. doi: 10.1371/journal.pone.0060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller PA, Dhamne SC, Vahabzadeh-Hagh AM, Pascual-Leone A, Jensen FE, Rotenberg A. Suppression of motor cortical excitability in anesthetized rats by low frequency repetitive transcranial magnetic stimulation. PLoS One. 2014;9(3):e91065. doi: 10.1371/journal.pone.0091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu P-C, Liu H-L, Lai H-Y, Lin C-Y, Tsai H-C, Pei Y-C. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci Rep. 2015;5:15477. doi: 10.1038/srep15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15(7 Pt 2):5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Yoo SH, Croce P, Margolin RW, Lee SD, Lee W. Pulsed focused ultrasound changes nerve conduction of earthworm giant axonal fibers. Neuroreport. 2017;28(4):229–233. doi: 10.1097/WNR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 22.Lee W, Lee SD, Park MY, Yang J, Yoo S-S. Evaluation of polyvinyl alcohol cryogel as an acoustic coupling medium for low-intensity transcranial focused ultrasound. Int J Imaging Syst Technol. 2014;24(4):332–338. [Google Scholar]
- 23.Goss SA, Frizzell LA, Dunn F. Ultrasonic absorption and attenuation in mammalian tissues. Ultrasound Med Biol. 1979;5(2):181–186. doi: 10.1016/0301-5629(79)90086-3. [DOI] [PubMed] [Google Scholar]
- 24.Elwassif MM, Kong Q, Vazquez M, Bikson M. Bio-heat transfer model of deep brain stimulation-induced temperature changes. J Neural Eng. 2006;3(4):306–315. doi: 10.1088/1741-2560/3/4/008. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien WD. Ultrasound—biophysics mechanisms. Prog Biophys Mol Biol. 2007;93(1–3):212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahab RA, Choi M, Liu Y, Krauthamer V, Zderic V, Myers MR. Mechanical bioeffects of pulsed high intensity focused ultrasound on a simple neural model. Med Phys. 2012;39(7):4274–4283. doi: 10.1118/1.4729712. [DOI] [PubMed] [Google Scholar]
- 27.Teschan P, Gellhorn E. Influence of increased temperature on activity of the cerebral cortex. Am J Physiol. 1949;159(1):1–5. doi: 10.1152/ajplegacy.1949.159.1.1. [DOI] [PubMed] [Google Scholar]
- 28.McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51(5):913–923. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- 29.All AH, Agrawal G, Walczak P, Maybhate A, Bulte JWM, Kerr DA. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurol Sci. 2010;31(5):595–601. doi: 10.1007/s10072-010-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herr DW, Chanda SM, Graff JE, Barone SS, Beliles RP, Morgan DL. Evaluation of sensory evoked potentials in Long Evans rats gestationally exposed to mercury (Hg0) vapor. Toxicol Sci. 2004;82(1):193–206. doi: 10.1093/toxsci/kfh246. [DOI] [PubMed] [Google Scholar]
- 31.Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, Aubry J-F. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med Phys. 2013;40(8):082902. doi: 10.1118/1.4812423. [DOI] [PubMed] [Google Scholar]