Abstract
Objective
This study examines the relative utility of a particular class of non-invasive functional biomarkers -- sensory functions -- for detecting those at risk of cognitive decline and impairment. Three central research objectives were examined including whether: (1) olfactory function, vision, and audition exhibited significant longitudinal declines in non-demented older adults, (2) multi-wave change for these sensory function indicators predicted risk of mild cognitive impairment, and (3) change within persons for each sensory measure shared dynamic time-varying associations with within-person change in cognitive functioning.
Method
A longitudinal sample (n=408) from the Victoria Longitudinal Study was assembled. Three cognitive status subgroups were identified: not impaired cognitively (NIC), single assessment mild cognitive impairment (SA-MCI), and multiple assessment mild cognitive impairment (MA-MCI).
Results
We tested independent predictive associations, contrasting change in sensory function as predictors of cognitive decline and impairment, utilizing both linear mixed models and logistic regression analysis. Olfaction and, to a lesser extent, vision were identified as the most robust predictors of cognitive status and decline; audition showed little predictive influence.
Conclusions
These findings underscore the potential utility of deficits in olfactory function, in particular, as an early marker of age- and pathology-related cognitive decline. Functional biomarkers may represent potential candidates for use in the early stages of a multi-step screening approach for detecting those at risk of cognitive impairment, as well as for targeted intervention.
Keywords: sensory function, olfaction, functional biomarkers, cognitive change, cognitive aging, mild cognitive impairment, Victoria Longitudinal Study
It is widely known that aging is marked by normative declines in sensory acuity including smell, sight, touch, sound, and taste (e.g., Anstey, 2008; Li & Lindenberger, 2002; Schneider & Pichora-Fuller, 2000; Scialfa, 2002; Wehling, Wollschlaeger, Nordin, & Lundervold, 2016). However, it is also recognized that underlying processes associated with aging-related changes in physiological, neurodegenerative, and brain injury effects can exacerbate sensory declines beyond ordinary levels (e.g., Anstey, 2008; Sigurdardottir et al., 2016). Three senses in particular -- olfaction, vision and audition -- have been highlighted as potential candidates for use as sensory markers of non-demented cognitive decline as well as mild cognitive impairment (MCI) status. Although sensory function indicators reflect once-removed proxies of the mechanisms linked to underlying cognitive impairment and dementia risk, such indicators are consistent with the predictive utility of functional biomarkers for identifying those at risk of cognitive decline (e.g., Albers et al., 2015; Barresi et al., 2012; Dixon, 2011; MacDonald et al., 2011). If it were determined that olfaction, audition or vision could be used as predictors of cognitive decline or impairment in non-demented older adults at risk of converting to Alzheimer’s disease (AD), such markers could facilitate early detection and allow for further testing as well as potential targeted intervention (Westervelt, Bruce, & Faust, 2016). In particular, to the extent that they improve predictive utility and sensitivity, such changes in sensory function are potential candidates for use in the early stages of a multi-step screening approach.
Olfaction, Age, and Cognitive Function
Many previous studies have documented decreases in olfactory function with increasing age (e.g., Doty et al., 1984; Mobley et al. 2014; Rawson et al., 2011; Schneider & Pichora-Fuller, 2000). Group trajectories of this decline have been shown to display large between-person differences, indicating that this decline does not occur evenly across populations (Wehling et al., 2016). Notably, those with higher rates of olfactory decline have also exhibited cognitive differences or decline linked to Alzheimer’s disease (e.g., Laakso et al., 2009; Schubert, Cruickshanks, Klein, Klein, & Nondahl, 2011; Thomann et al., 2009; Westervelt et al., 2016; Wilson et al., 2009). Key brain projection regions in the olfactory pathway include the olfactory bulb tract, entorhinal cortex, thalamus and hippocampal formation. These olfactory projection regions, particularly the entorhinal cortex and hippocampus, are significant reservoirs of neuropathology early in the AD process (Braak & Braak, 1991), and have been directly linked to memory function (Squire & Schacter, 2002). Furthermore, according to select studies (e.g., Devanand et al., 2010; Graves et al., 1999; Mobley et al., 2014; Westervelt et al., 2016), olfactory changes have been shown to better predict cognitive decline relative to (and in conjunction with) standard neuropsychological measures. In each study, the point is emphasized that some of the earliest changes in AD occur in the olfactory pathway, citing evidence such as high concentrations of plaques and tangles distributed throughout the olfactory system, as well as early neuropathological change in the medial temporal lobe, a key projection site associated with olfactory identification. Recently, the National Institute on Aging sponsored a workshop (“Sensory and Motor Dysfunctions in Aging and AD") attended by experts on sensory, motor, and cognitive function. A resulting consensus paper documented clear epidemiological, neuropathological, neuroscientific, genetic, and molecular evidence linking AD pathology to changes in sensory and motor regions of the CNS (Albers et al., 2015), with the expert panel advocating for future research directions that include the exploration of olfactory dysfunction as a biomarker for detecting preclinical AD.
Evidence from medical imaging has also linked deficits in olfactory function with risk of progression to Alzheimer’s disease. Hiroshi Matsuda (2007) used longitudinal PET and SPECT imaging to predict decline from MCI to AD, and found that changes in the entorhinal cortex, a critical brain region for both cognition and olfactory function, was the best predictor of conversion to AD. Similarly, using PET and MRI imaging, de Leon et al. (2007) found that declines in gray matter volumes in entorhinal cortex, hippocampus, and thalamus, as well as declines in glucose metabolism, were all linked to AD progression. Notably, Li, Howard, and Gottfried (2010) report that the posterior piriform cortex, located adjacent to the entorhinal cortex and centrally involved in odor perception, represents a potential mechanistic pathway that mediates olfactory deficits in early-stage Alzheimer’s disease. Using fMRI, the authors observed a disruption of functional activation patterns related to olfactory coding in the piriform cortex, and posit that the functional activation differences observed between controls and those with Alzheimer’s disease may serve as a non-invasive biomarker of early disease risk. Olfactory decline is also suggested to predict mild cognitive impairment (MCI), a status widely considered to be a prodromal stage preceding AD (Djordjevic et al., 2008; Wilson et al., 2009). As primary olfactory projections target the entorhinal cortex, early structural changes due to AD may disrupt not only cognition but olfaction as well, with changes in the latter potentially serving as a non-invasive biomarker of cognitive change and dementia risk (e.g., Stoub et al., 2010; Thomann et al., 2009). In support of this assertion, structural MRI findings have linked AD-related volumetric changes in hippocampus to impaired odor identification (e.g., Murphy, Jernigan, & Fennema-Notestine, 2003; see Albers et al., 2015).
Visual and Auditory Acuity, Age, and Cognitive Function
Beyond olfactory function, changes in both vision and audition also share known associations with age differences in cognitive function (e.g., MacDonald, Bunce, & Hultsch, 2006; Scialfa, 2002). Several theorists have postulated that changes in vision (e.g., contrast sensitivity) may represent early and sensitive markers of cognitive decline (e.g., Koronyo-Hamaoui et al., 2011; Saykin et al., 2010). McKee and colleagues (2006) were among the first to propose this notion, citing clinical reports and imaging studies demonstrating that over the pathological process of AD, the occipital lobe as well as part of the posterior temporoparietal lobe show accelerated atrophy. The association between atrophy in these regions, AD risk and vision suggests that individual differences in vision may serve as a sensitive early marker of impairment.
Whereas potential mechanistic pathways linking declines in olfaction or vision to Alzheimer’s-related pathology have been identified, the potential link between audition and cognitive decline or impairment is less clear. Audition does not share the same degree of neural processing overlap with the areas impacted by Alzheimer’s-based pathology, and thus seems much less likely to provide sensitive prediction of those at risk for cognitive impairment. It is possible, for example, that sensory deprivation due to normative age-related degeneration at the end organ (e.g., cochlear degeneration), as opposed to atrophy in neural projection regions (e.g., for visual input), would exert a greater impact on cognitive performance (e.g., Humes, 2005; Li & Lindenberger, 2002). This hypothesis is reinforced by the fact that of the three senses, audition is the only one with bilateral redundancy, creating a buffer to pathophysiological decline. In contrast to the sensory deprivation hypothesis, however, various olfactory inputs remain largely intact during the course of normal aging, suggesting that any modest observed changes in olfactory function may be pathological in origin and thus be differentially sensitive for detecting those at risk of cognitive decline, AD, and related disorders. This view is supported by a number of theorists who suggest that auditory deficits are likely independent of incident dementia risk (e.g., Lin et al., 2011). Despite evidence for audition being the least clear of the three sensory factors being examined here, it remains an empirical question as to whether auditory deficits may present a risk factor for cognitive impairment either independent of, or in concert with, the other sensory markers.
The Present Study
The overall aim of the present investigation is to examine the relative utility of a particular class of non-invasive functional biomarkers—specifically, sensory functions—for detecting those at risk of cognitive decline and impairment (e.g., Anstey, 2008; Anstey, Lord, & Smith, 1996; Dixon, 2011; Dolcos et al., 2012; MacDonald et al., 2011). The present investigation employs data from the Victoria Longitudinal Study (VLS; Dixon & de Frias, 2004) to examine links between sensory functioning, cognitive change and cognitive status. Three primary research objectives were examined. First, using linear mixed models, we evaluated whether olfactory, visual and auditory function exhibited significant change over time, with an expectation of age-related declines for each sensory modality (cf., Scialfa, 2002). Second, we evaluated potential associations between change in sensory function and cognitive impairment status based on the classification concept of mild cognitive impairment (see Dixon et al., 2014). Based on aforementioned mechanistic pathways, we expected a pattern of associations characterized by olfaction representing the most sensitive predictor of MCI status, followed by changes in vision and then audition. Third, we examined whether change within an individual for each sensory measure shared a dynamic time-varying association with within-person change for selected measures of cognitive functioning including digit symbol substitution, episodic memory, and vocabulary. With increasing impairment (e.g., MCI vs. controls), we expected that underlying changes in brain structure and function related to both sensation and cognition (e.g., Thomann et al., 2009) would be manifest as stronger within-person coupling between change in cognition and change in sensory function. Although most studies have tested such associations at the population rather than individual level, the most conservative test of the hypothesis (changes in sensory and cognitive function travel together over time) should be demonstrated within-persons (see Sliwinski and Mogle, 2008). Given the centrality of episodic memory in mild cognitive impairment, we further expected that this cognitive function would be prominently associated with change in olfaction (cf., MacDonald et al., 2011).
Method
Participants
The Victoria Longitudinal Study (VLS) is a multi-cohort epidemiological study of biomedical, health, physiological, cognitive, and neurocognitive aspects of aging. Participants were community-dwelling older adults, originally recruited through advertisements in the public media and to community groups. Three independent samples of initially healthy older adults are followed at three-year intervals. The present study focuses on Sample 1 (Waves 6 and 7), Sample 2 (Waves 4 and 5), and Sample 3 (Waves 1-3). Exclusionary criteria at baseline included a history of Alzheimer’s Disease, psychiatric disturbance, head injury, and serious episodes of cardio/cerebrovascular disease. Demographic information is presented in Table 1. Analysis of variance (ANOVA) indicated that the not impaired cognitively (NIC) group had more years of education than either MCI group (ps < .01); there were no significant group differences for age or either measure of self-reported health. In addition, descriptive statistics by cognitive status group are reported for baseline sensory and neuropsychological function (see Table 2). Employing ANOVA, all mean group comparisons were significant (p < .05) for the neuropsychological tests save for the comparisons between MCI subgroups for the digit symbol and letter series tasks. For the sensory measures, a significant difference for olfaction (p < .05) was observed between the NIC and stable MCI subgroup. Ethical approval for this study was obtained from the Human Research Ethics Board of the University of Victoria; all participants provided written informed consent and were treated in accord with established ethical guidelines. See Dixon and de Frias (2004) for further detail on the VLS general design, measures, and procedures.
Table 1.
Sample demographics by cognitive status group.
| NIC (n=197) | SA-MCI (n=126) | MA-MCI (n=85) | |
|---|---|---|---|
| Age | 75.44 (7.28) | 76.71 (8.68) | 75.68 (7.23) |
| Gender (% women) | 64.5% | 59.5% | 51.8% |
| Education | 15.57 (3.08) | 14.63 (2.95) | 14.14 (2.93) |
| SR Health | 1.92 (0.70) | 1.80 (0.80) | 1.89 (0.62) |
| SR Health-P | 1.63 (0.69) | 1.49 (0.70) | 1.59 (0.62) |
NIC = not impaired cognitively; SA-MCI = single assessment mild cognitive impairment reflecting cross-sectional classification or unstable classification across measurement occasions; MA-MCI = multiple assessment mild cognitive impairment reflecting stable classification observed across consecutive measurement occasions; SR Health = self-reported health relative to a perfect state on a 5-point scale that ranged from 0 (very good) to 4 (poor); SR Health-P = self-reported health relative to peers on a 5-point scale that ranged from 0 (very good) to 4 (poor). Values in parentheses are SDs.
Table 2.
Baseline performance on neuropsychological and sensory function measures by cognitive status group.
| NIC (n=197) | SA-MCI (n=126) | MA-MCI (n=85) | |
|---|---|---|---|
| Word recall | 20.22 (3.14) | 16.61 (4.22) | 14.97 (3.86) |
| Digit symbol | 51.41 (9.64) | 44.39 (9.93) | 44.30 (8.78) |
| Letter series | 12.53 (4.03) | 9.07 (4.48) | 7.85 (4.52) |
| Controlled associations | 17.88 (5.60) | 13.14 (6.03) | 11.08 (5.19) |
| Vocabulary | 45.90 (4.21) | 42.54 (5.87) | 40.08 (7.04) |
| Olfaction | 7.19 (1.53) | 6.78 (1.76) | 6.80 (1.90) |
| Distance vision | 0.97 (0.11) | 0.96 (0.11) | 0.95 (0.13) |
| Audition | 30.31 (12.11) | 32.34 (12.61) | 31.77 (12.14) |
NIC = not impaired cognitively; SA-MCI = single assessment mild cognitive impairment reflecting cross-sectional classification or unstable classification across measurement occasions; MA-MCI = multiple assessment mild cognitive impairment reflecting stable classification observed across consecutive measurement occasions; values in parentheses are SDs.
A key VLS research theme concerns a focus on patterns and predictors of cognitive status and decline (Dixon et al., 2014). To identify cognitive status subgroups, we have utilized the standard and fully objective (non-subtyped and non-clinical) multi-step classification procedure used consistently in other research and consensus reports conducted by the VLS (de Frias et al., 2009; DeCarlo et al., in 2016; Dixon et al., 2007; Dolcos et al., 2012, Dixon et al. 2014). At each wave of testing, MCI status was determined by identifying individuals who scored 1.0 standard deviations (SD) below their age- and education-referenced group means on five cognitive reference measures including the theoretical domains of perceptual speed (digit symbol substitution), inductive reasoning (letter series), episodic memory (immediate free recall), verbal fluency (controlled associations), and semantic memory (vocabulary). These five standard measures from the cognitive reference battery are widely available and used, and their psychometric properties have been regularly documented as acceptable according to conventional standards (e.g., Hultsch et al., 1998). The 1.0 SD criterion was previously established and represented an approach that provided a degree of differentiation appropriate to the goal of detecting early or established signs of cognitive impairment (de Frias et al., 2009; Dixon et al., 2007). Once individuals were identified who scored one or more standard deviations (SD) below their own age x education group means on one or more of the cognitive tasks, the final step involved coding for classification stability over time (cf., Vandermorris, Hultsch, Hunter, MacDonald, & Strauss, 2011). Specifically, participants without any scores below 1.0 SD on the 5 cognitive measures, consistently across any combination of measurement waves, were classified as NIC. To address limitations in MCI identification procedures, we further differentiated between two MCI subtypes. Single assessment mild cognitive impairment (SA-MCI) individuals exhibited a 1.0 SD deficit on one of five reference cognitive domains relative to age and education matched peers when measured on a single occasion (e.g., wave 1 assessment only) or a change in classification status over time (e.g., completed 2 measurement waves, but did not exhibit a persistent 1.0 SD deficit). In contrast, multiple assessment mild cognitive impairment (MA-MCI) individuals exhibited a persistent 1.0 SD deficit for two or more reference cognitive domains relative to age and education matched peers across at least two consecutive assessments, without reverting to NIC status. The frequencies associated with each MCI group are NIC n = 197, SA-MCI n = 126 and MA-MCI n = 85.
Cognitive Measures
Five cognitive measures sensitive to aging as well as cognitive changes were used in this study to measure perceptual processing speed, inductive reasoning, verbal fluency, vocabulary and episodic memory. This subset of indicators was consistent with those from previous VLS analyses (Hertzog, Dixon, Hultsch and MacDonald, 2003), and was selected in order to span a continuum of fluid and crystallized facets of cognition. These five domains were employed as cognitive reference measures for the classification of MCI, with digit symbol, word recall, and vocabulary used as outcome measures for the time-varying analyses.
Episodic memory
The word recall task (Hultsch, Hertzog, & Dixon, 1990) was used to assess episodic memory. For this assessment, two distinct lists of 30 English words selected from a total set of six lists were presented. The participant was given two minutes to study the lists and five minutes to write as many words as they could recall in any order. The number of correctly recalled words, averaged over the two lists, was used as the final score (range = 0-30).
Inductive reasoning
The Letter Series test (Thurstone, 1962) was used to assess inductive reasoning. In this test a series of letters following a distinct pattern was presented and the participant was asked to decipher the pattern in the target string and to then provide the next letter in the sequence according to the pattern. The maximum score is 20 correct sequences (range = 0-20).
Perceptual speed
The WAIS-R Digit Symbol Substitution task (Wechsler, 1958) was used to assess perceptual processing speed. It consists of symbol search sections as well as digit symbol-coding. The final score reflected total correct responses produced in 90 seconds.
Verbal fluency
The Controlled Associations test from the ETS kit of factor-referenced cognitive tests (Ekstrom, French, Harman, & Dermen, 1976) was used to assess verbal fluency. The participant was given six minutes to generate as many synonyms as possible in response to a set of target words. The final score was generated by summing the number of correct synonyms.
Vocabulary
A recognition vocabulary test, combining three 18-item multiple-choice tests from the ETS kit of factor-referenced cognitive tests (Ekstrom et al., 1976), was used to assess vocabulary. The participant was given 15 minutes to complete the 54 items, with the total number of correct answers summed to yield the final vocabulary score (range = 0-54).
Sensory Function
Olfaction
Olfactory function was tested via olfactory identification using the brief sensory identification (B-SIT) test (Sensonics. Inc., Haddon Heights, USA). This test has been widely employed for the determination of olfactory-related clinical group differences (e.g., Westervelt et al., 2016) and cognitive performance changes over time (e.g., Kjelvik et al., 2007). It is comprised of either 9 or 12 items (standardized in this study to 9 items). Each participant received a B-SIT booklet that, on each individual page, contained a scratch and sniff strip embedded with a specific odor. Each scent is released using a pencil, with participants required to identify the correct odor from a list of four multiple-choice options. Correctly identified odors on the B-SIT are summed to yield a final score ranging from 0-9. The B-SIT was derived from the University of Pennsylvania Smell Identification Test (UP-SIT) to be an effective and rapid screen for olfactory deficits and has been shown to be effective across cultures (Doty, 1995).
Vision
Binocular-corrected distance visual acuity was measured at 3 meters using the Snellen chart. Testing began at the largest print line (20/200), with the participant asked to read the characters down to the smallest line possible at which they could read 100 percent of the letters. The Snellen acuity fraction (e.g., 20/200 vision = 0.1, 20/20 vision = 1) at which the subject maintained their ability to read all characters with perfect accuracy was recorded for the vision score. In the present sample, Snellen acuity fractions ranged from 0.20 to 1.
Audition
Audition was measured using decibels (db) required to hear an audiometer tone. Specifically, we used a MAICO MA39 portable pure tone audiometer. Decibel thresholds were attained for both the left and the right ears. Specific measurement points were 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. In this study, only data within the range of speech frequency (specifically 500-2000 Hz) were analyzed in accordance with previous research (MacDonald et al., 2004). Hearing aids were not worn during the audiometry assessment. The decibels required to hear each tone ranged from 1.67 to 105 db. For each frequency, all participants were able to hear the tone below the maximum decibel (110 dB). The outcome measure reflected the mean db level required to hear the tone averaged across all speech range frequencies (500-2000 Hz) and across the left and right ears.
Statistical Procedure
Linear mixed models of change, using Hierarchical Linear Modeling (HLM) 6.08 software, were implemented to assess change in sensory function with increasing age. Multilevel models facilitate the examination of within-person change (Level 1), as well as between-person differences (Level 2) in trajectories of change. For example, to examine change in olfaction, olfactory identification performance for a given individual (i) at a given time of assessment (j) was modeled as a function of the individual’s performance centered at the grand mean of age (the intercept), plus their average rate of change per additional year increase in age (the slope), plus an error term (εij: see equation 1).
| (1) |
Comparable models were estimated for vision and audition. For all linear mixed models, variance components for both intercept and slope were estimated.
Multinomial logistic regression (utilizing the software package PASW 18) was used to examine individual differences in change for the various sensory measures as predictors of cognitive status (NIC vs. SA-MCI and NIC vs. MA-MCI) across the longitudinal retest interval. Specifically, individual slopes reflecting age-related change in sensory function for each indicator were estimated (see equation 1), saved, and then subsequently employed as predictors of cognitive status in the multinomial logistic regression. Additional covariates in all models included age at baseline and time in study (cf., DeCarlo et al., 2016).
Finally, linear mixed models were also used for examining the time-varying covariation between corresponding changes in cognitive and sensory function across multiple occasions; as the cognitive outcomes were part of the MCI cognitive reference battery, the time-varying analyses were computed within each cognitive classification subgroup (NIC, SA-MCI, and MA-MCI) to avoid biasing the results. Equation 2 shows an example for modeling the time-varying covariation between change in episodic memory and olfactory function. Specifically,
| (2) |
independent of an individual’s chronological age, these models evaluated whether episodic memory function was higher (vs. lower) on occasions when olfactory function was also higher. To decompose the influence of sensory function into within- vs. between-person components (see Hoffman & Stawski, 2009), we person-mean centered the level 1 predictor for each sensory measure; for each individual, we subtracted the person-mean (PM) for a given measure of sensory function computed across all times of testing from the corresponding raw sensory function estimate at each time point. Employing this person-mean centering approach, the γ20 model parameter represents variation about an individual’s own mean sensory level irrespective of between-person differences (e.g., age, cognitive status), with the γ02 parameter of PM sensory function reflecting a pure estimate of between-person differences in olfaction, vision, and audition. Given our a priori hypotheses and clear expectations regarding pattern and direction of effects, we report corresponding one-tailed levels of significance.
Results
Multilevel models of change in vision, hearing, and olfaction
Consistent with expectation, each sensory modality exhibited significant age-related decline (see Table 3). Specifically, olfactory function declined -0.04 units of odor recognition accuracy per additional year increase in age beyond the grand mean (M=74.17 years; SD=9.20). Distance visual acuity was shown to decline by 0.004 Snellen ratio units per year (about .04 units per decade). Finally, the decibels required to hear an audiometer tone within the speech frequency range increased by almost 0.8 db per each additional year of age older than the grand mean. Having demonstrated that each sensory modality exhibited expected declines with increasing age, we proceeded to examine change in sensory function as a predictor for research objectives two and three.
Table 3.
Age-related change in sensory function.
| Variables | Intercept γ00 | Slope γ10 | SE Slope | p |
|---|---|---|---|---|
| Olfaction | 7.05 | -0.043 | 0.008 | < .001 |
| Distance Vision | 0.946 | -0.004 | 0.001 | < .001 |
| Audition | 32.84 | 0.796 | 0.059 | < .001 |
γ00 = Average sensory function centered at the grand mean of age (74.17 years; SD=9.20); γ10 = slope reflecting the average rate of linear change per additional year of age; SE = standard error. All intercept values were significantly different from 0 (p>.001), as were variance components for both intercept (ps<.01) and slope (ps<.05).
Change in sensory function as predictors of MCI Status
Using multinomial logistic regression, we evaluated whether individual change slopes for each sensory marker independent of age at baseline and time in study predicted risk of being classified in either MCI status subgroup, as compared with NIC. To facilitate direct comparison, we standardized each sensory predictor prior to entry in the logistic regression analyses. The overall models were significant, respectively, for olfaction (χ2 (6) = 83.62, p < .001, Nagelkerke R2 = 0.212), distance vision (χ2 (6) = 73.30, p < .001, Nagelkerke R2 = 0.188), and audition χ2 (6) = 67.66, p < .001, Nagelkerke R2 = 0.175). Figure 1 displays the odds ratios and p-values for change in each sensory predictor in relation to subgroup classification, independent of age at baseline testing or number of years in the study. As shown in the figure, results indicated a 1.75-fold increased risk of being classified persistently as MA-MCI relative to NIC, with a 1.38-fold increased risk of being classified as unstable MCI (SA-MCI), per each standardized unit of decline in olfactory function. Decline in distance vision (Snellen ratio reflecting ability to see at various distances what an average individual can see at 20 feet) was also linked to increased risk (OR=1.38) of being classified as persistent MCI (MA-MCI). Finally, auditory change (decibels required to hear the audiometer tone in the speech frequency range) did not predict cognitive impairment risk for either severity group.
Figure 1. Risk of being classified as unstable (SA) or stable (MA) MCI based on declining sensory function.
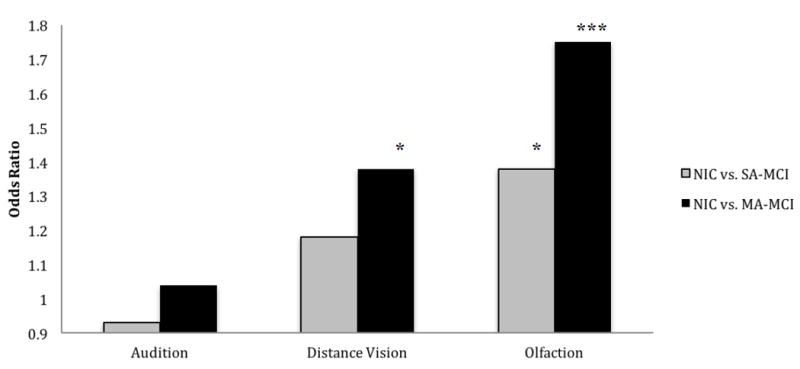
The reference category is the not impaired cognitively (NIC) group. Odds ratios reflect the risk of being classified as either SA-MCI (single assessment mild cognitive impairment) or MA-MCI (multiple assessment mild cognitive impairment). SA-MCI individuals exhibited a 1.0 SD deficit on one of five reference cognitive domains relative to age and education matched peers for a single point in time or change in classification status over time. MA-MCI individuals exhibited a 1.0 SD deficit for two or more reference cognitive domains relative to age and education matched peers across at least two consecutive assessments, without reverting to NIC status. All multinomial logistic regression models control for age at baseline and time (years) in study. To facilitate comparison across domain, the sensory predictors were standardized as Z-scores.
*p < .05 **p<.01 ***p<.001
In order to directly compare the relative predictive contributions of the sensory slopes, a multivariate prediction model was estimated. The overall model was significant, χ2 (10) = 90.03, p < .001, Nagelkerke R2 = 0.226. For differentiating NIC from SA-MCI, only change in olfactory function exerted a unique influence (OR=1.38, p<.05) independent of distance vision and audition. In contrast, both change in olfaction (OR=1.74, p<.001) and change in visual acuity (OR=1.37, p<.05) predicted increased risk of being classified as persistently impaired over time (MA-MCI). In summary, these patterns suggest that risk of being classified as SA-MCI or MA-MCI shared the strongest association with declines in olfactory function. Decline in visual acuity was also a notable predictor, but no predictive associations were observed for auditory decline.
Time-varying covariation between change in sensory modalities and change in cognition
As hypothesized, the predictive importance both between- and within-persons increased in a dose-response fashion as a function of increasing cognitive impairment. For analyses conducted within the NIC and SA-MCI groups, few significant between- or within-person effects were observed for sensory function predicting individual differences in cognition (intercepts) or cognitive change (slopes). For the NIC group, significant between-person effects were observed for olfaction on word recall (γ02 = 0.31, p < .05) and digit symbol (γ02 = 0.69, p < .05 one-tailed). These coefficients from the multilevel analyses reflect raw score units of cognitive change. For example, per each additional one unit increase of person-mean olfactory function, cognitive performance increased by 0.31 units on word recall and by 0.69 units on digit symbol. A significant within-person coupling effect (γ20 = 14.56, p < .01) was also observed for the NIC group. Specifically, for every unit increase in distance vision above an individual’s own mean, digit symbol performance would be expected to improve by 14.56 units (about 1.5 more symbols recalled per 1/10th unit improvement in Snellen decimal units). A single significant finding was observed for the unstable SA-MCI group. At the between-person level, a per unit increase of person-mean olfactory function was linked to a corresponding increase of 0.42 units (p < .05) on the word recall task.
The MA-MCI group had the largest number of significant effects in general, and the largest number for the episodic memory measure (word recall) in particular (see Table 4). Specifically, at the within-person level, change over time in word recall was associated with change in olfactory function as well as change in distance vision. Relative to an individual’s own mean performance, a 1 unit decline in olfactory accuracy was associated with a 0.36 unit decline in word recall (γ20 = 0.356, p < .05 one-tailed, range = 0.28 – 0.42, 95% CI = 0.30 - 0.41), with a 1 unit decline in distance vision linked to a 2.54 unit word-recall decline (γ20 = 2.539, p < .05 one-tailed, range = 0.28 – 5.64, 95% CI = 0.36 - 4.72). A third significant within-person coupling effect was observed for the association between audition and vocabulary (γ20 = -0.13, p < .05, range = -0.30 - 0.09, 95% CI = -0.29 - 0.03); vocabulary performance declined by 0.13 units per additional db required to hear a tone in the speech frequency range. As the B-SIT olfactory assessment used in the present study requires odor identification (i.e., the cross-referencing of odors to words describing them), it is possible that the observed coupling of olfaction with episodic memory is verbally mediated (e.g., Murphy et al., 2003). To examine this possibility, we included change in vocabulary as a time-varying covariate. The result was virtually no impact on the within-person coupling association between word recall and olfaction: coupling slope = 0.356 unadjusted (see Table 4) vs. 0.361 adjusted. Finally, at the between-person level, significant individual differences in distance vision were associated with differences in baseline cognitive performance for word recall; each additional unit lower in Snellen acuity was associated with a 6.69 unit lower score on word recall. Similarly, each additional unit increase in db required during the auditory acuity test was associated with a 0.14 unit lower score on digit symbol.
Table 4.
Time-varying covariation models for MA-MCI subsample: Change in cognition as a function of change in age (γ10) and change in sensory function (γ20).
| Variables | Intercept γ00/γ02 | Slope γ10/γ20 | SE (slope) | p-value (slope) |
|---|---|---|---|---|
|
| ||||
| Digit Symbol | ||||
|
| ||||
| Olfaction | 41.73 | -0.75 | 0.113 | < .001 |
| 0.19 | 0.384 | 0.378 | n.s. | |
|
| ||||
| Distance vision | 38.62 | -0.728 | 0.112 | < .001 |
| 4.80 | -2.109 | 3.397 | n.s. | |
|
| ||||
| Audition | 38.15 | -0.753 | 0.127 | < .001 |
| 0.14* | 0.019 | 0.071 | n.s. | |
|
| ||||
| Word Recall | ||||
|
| ||||
| Olfaction | 14.60 | -0.272 | 0.057 | < .001 |
| -0.02 | 0.356 | 0.189 | < .05# | |
|
| ||||
| Distance vision | 20.59 | -0.273 | 0.056 | < .001 |
| -6.69# | 2.539 | 1.386 | < .05# | |
|
| ||||
| Audition | 14.38 | -0.261 | 0.064 | < .001 |
| -0.01 | -0.040 | 0.037 | n.s. | |
|
| ||||
| Vocabulary | ||||
|
| ||||
| Olfaction | 42.23 | -0.178 | 0.086 | < .05 |
| -0.09 | -0.052 | 0.290 | n.s. | |
|
| ||||
| Distance vision | 39.49 | -0.201 | 0.089 | < .05 |
| 0.08 | -0.866 | 2.666 | n.s. | |
|
| ||||
| Audition | 41.20 | -0.043 | 0.099 | n.s. |
| -0.06 | -0.133 | 0.055 | < .05 | |
γ00 = Average cognitive function centered at baseline testing holding age and sensory function constant; γ02 = a person-mean centered predictor for sensory function reflecting the pure between-person effect of individual differences in olfaction, vision, and audition; γ10 = age slope (average rate of linear change per additional year of age, independent of change in sensory function); γ20 = change in sensory function slope (person-mean centered to represent pure within-person variation about one’s own mean sensory function, independent of the linear trend for age); SE = standard error. All variance components for intercept were significantly different from 0. The level-2 age predictor (γ01), centered at the grand mean (75.68 years; SD=7.23), was entered as a covariate for all models to adjust for between-person age heterogeneity upon entry into the study.
p < .05, one-tailed
p < .05
p<.01
p<.001
Discussion
The overall aim of this study was to examine and contrast the relative predictive patterns of olfaction, vision and audition on both cognitive status and cognitive performance decline in non-demented older adults. We completed a series of three primary research objectives, as discussed below. The main finding of this integrated set of analyses was that multi-year changes in sensory function, particularly for olfaction and vision, were associated with identification of cognitive impairment as well as systematic within-person change in cognitive function.
As expected, for the first research objective, we observed significant age-related declines for each of the three sensory modalities (Devanand et al., 2008; Djordjevic et al., 2006; Lehrner et al., 2009; McKee et al., 2006; Scialfa, 2002; Westervelt et al., 2007; Wilson et al. 2009). For the second research objective, we saved these slopes and used them as predictors of two levels of cognitive status. We observed significant associations between change in olfaction and change in vision in relation to SA-MCI and MA-MCI status. Individual differences in olfactory change predicted risk of both unstable and persistent MCI, whereas change in distance visual acuity was related solely to the stable MA-MCI classification. It has been shown (Scialfa, 2002) that both vision and audition show larger declines over chronological age compared to olfaction, a finding we found to be consistent with our results. When this knowledge is paired with the conclusion that olfaction is statistically shown to be a more sensitive predictor of cognitive status, the inference is that any observed changes in olfactory function, however modest, may be particularly informative about pathological processes that affect cognitive function (Westervelt et al., 2016; Wilson et al., 2013). Similarly, the dose-response pattern indicating more robust predictive effects for the persistent vs. unstable MCI classification is consistent with the perspective that because MCI is a variable, transitional and sometimes unstable classification it is best studied longitudinally (Albert et al., 2011). Due to the dynamic nature of MCI, single assessments can produce information that may be of somewhat limited clinical and research utility (e.g., Dixon et al., 2014; Palmer, Bäckman, Winblad, & Fratiglioni, 2003; Vandermorris et al., 2011).
Finally, regarding the third research objective, we showed time-varying olfaction to be the modality with the greatest predictive power with respect to changes in episodic memory, digit symbol substitution, and vocabulary. Specifically, among the 3 sensory indicators, olfaction and distance vision were the only significant predictors at both the between- and within-person level, with olfaction exhibiting the largest number of significant effects. Further, among the cognitive constructs examined, the largest number of significant effects was observed for episodic memory in the MA-MCI group, potentially reflecting the impact of systematic changes in brain structure and function on both sensory and cognitive function (e.g., Braak & Braak, 1991; Thomann et al., 2009). Within this persistent MCI group, declines in episodic memory accuracy were predicted by within-person declines in both olfactory and visual acuity. Between-person differences in olfactory function were also linked to each of the cognitive outcomes, with lower olfaction linked to lower cognition. Notably, whereas between-person associations between sensory function and cognition may be an artifact of age heterogeneous samples (cf., Hofer & Sliwinski, 2001), the within-person time-varying models computed in the present study provide a more convincing indication that change in olfaction and episodic memory, for example, are unfolding synchronously within individuals. This coupling would be expected if olfactory deficits are indicative of underlying neural changes linked to cognitive decline and impairment. Indeed, these results are consistent with our knowledge of changes in the olfactory pathways projecting to entorhinal cortex and corresponding links to Alzheimer’s neuropathology (e.g., Westervelt et al., 2016). In contrast, both distance vision (Shayler, 2011) and audition encompass neural regions less affected by early Alzheimer’s disease (Tzekov & Mullan, 2013). In keeping with our dose-response expectation regarding the number and magnitude of associations observed, significant time-varying findings within the NIC and unstable MCI subgroups were largely absent, and most were between- rather than within-person. Such significant between-person associations are consistent with expected associations due to normative aging processes as opposed to pathology (e.g., Li & Lindenberger, 2002), as well as population mean confounds (e.g., Hofer & Sliwinski, 2001).
Our results support the view that changes in olfactory function may be a potentially important marker, particularly in early stages of age- and dementia-related cognitive impairment. Deficits in speeded performance, executive process, and episodic memory are among the first cognitive impairments to be manifest for those in the early stages of AD (Bäckman et al., 2005). The earliest neuropathological changes in AD are observed in the entorhinal cortex (Braak & Braak, 1991) which is a critical area in not only the olfactory pathway but also in the function of episodic memory (e.g., Graves et al., 1999; Stoub et al., 2010; Wilson et al., 2009), lending further support to the use of olfaction as a predictive measure. The present study extends these and other recent longitudinal findings (Wehling et al., 2016) by demonstrating that the predictive sensitivity of sensory deficit is especially significant for olfactory function. By both directly comparing change in various sensory domains and conducting process-specific tests of within-person change, the present patterns support the potential clinical utility of change in olfaction (e.g., Stoub et al., 2010; Thomann et al., 2009; Wehling et al., 2016) or perhaps visual ability (e.g., Saykin et al., 2010; Shayler, 2011) as an early predictor of accelerated cognitive decline or transitions into cognitive impairment.
Several limitations of the present study should be acknowledged. First, although the characterization of a persistent MCI classification represents a definitive improvement over previous single-assessment approaches to classifying cognitive impairment (e.g., Dixon et al., 2014; Vandermorris et al., 2011), the VLS study has an insufficient number of AD cases with which to evaluate the risk of transition to probable dementia. Future research should strive to isolate and determine the predictive influence of olfaction, for example, for not only cognitive decline and MCI, but also for the transition to mild or moderate Alzheimer’s disease (see Devanand et al. 2008; Stanciu et al., 2014). In addition, the ethnic composition of the VLS sample is predominantly caucasian, with future research required to assess the generalizability of these findings to other ethnic groups. Second, our classification of unstable (SA-MCI) and persistent (MA-MCI) mild cognitive impairment did not include formal clinical judgment; consequently, it is conceivable that our strategically chosen classification threshold (1 SD below a referenced peer group mean) may have incorrectly classified participants that could have been identified with supplementary clinical assessment (cf., Dixon et al., 2014; Dolcos et al., 2012). This limitation notwithstanding, the objective procedures employed yielded high stability rates for both MA-MCI and NIC – a classification procedure that may be transportable (and indeed advantageous) in both research as well as the clinic (Dixon et al., 2014; Vandermorris et al., 2011). The 1 SD classification threshold was chosen to maximize the likelihood of detecting emerging deficits in a non-demented and relatively healthy cohort, thus resulting in a conservative test of the hypotheses. Third, the within-person coupling analyses had to be conducted within each homogeneous classification subgroup in order to avoid biasing findings. As episodic memory performance levels were also employed as part of the cognitive status classification procedure, it was necessary to conduct tests within each subgroup. Specifically, within a given level of cognitive status, demonstrating a within-person association between change in olfaction and episodic memory represents a conservative test of the hypothesis. In contrast, conducting such analyses across cognitive classification subgroups may yield spurious or artefactual associations, at the between-person level in particular. Consequently, the sample sizes for the separate subgroup analyses were modest for the purposes of fitting linear mixed models, with the a priori hypothesized associations for these novel within-person analyses (p<.05, one-tailed) necessitating replication.
Although we establish that olfaction may occupy a privileged status among sensory modalities in predicting cognitive decline and status, further research is recommended. For example, an epidemiological study of olfaction in the combined or interactive context of markers of other domains of risk could be useful (see McFall, Wiebe, Vergote, Anstey, & Dixon, 2015; Westervelt et al., 2016). Candidates could be selected from domains such as imaging, genetic risk, biological markers, vascular or metabolic health, and other cognitive measures. In this case, the potential that sensory function serves as a proxy for neurological function underlying pathological change in cognitive function and status would be brought into sharper mechanistic focus (Masurkar & Devanand, 2014; Stamps et al. 2013). There is some evidence to suggest that genetic risk, in particular, may moderate the association between declines in olfaction and episodic memory (e.g., Larsson et al., 2016; Olofsson, Rönnlund, Nordin, Nyberg, Nilsson, & 2009; Olofsson et al., 2016). Olofsson and colleagues (2016) found that episodic memory decline across a period of up to 20 years was related to individual differences in odor identification at the last wave of assessment, but only for Apolipoprotein (APOE) ε 4 carriers. These findings suggest that genetic predispositions to AD, such as the APOE ε 4 allele, may be differentially linked to underlying atrophy in the mediotemporal lobe (MTL) that gives rise to observed associations between olfaction and episodic memory. It would also be of interest to determine when precisely olfaction begins to decline, in relation to the onset of cognitive impairment or Alzheimer’s disease, in order to gauge its utility vis-à-vis early detection. Ultimately, the clinical import of olfaction as an early marker will need to be determined within clinical settings by assessing how a given individual’s decline in olfactory function, relative to their own established baseline, may serve as a sensitive marker for subsequent cognitive decline or dementia risk.
Implications of the present findings might entail incorporating olfactory assessments into clinical settings as part of a multi-step screening approach (e.g., odor identification, genotyping, screening for memory complaints, assessment of global cognitive function) for early identification of individuals at significant risk of cognitive decline. Evidence now clearly supports the view that sensory (e.g., olfaction) and motor (e.g., gait) changes actually precede AD-related cognitive changes (Albers et al., 2015). Further, the potential utility of olfaction as an early-stage indicator of AD and corresponding MTL cortical atrophy may be augmented by combining odor identification performance with knowledge of select genetic alleles such as APOE ε 4 (Olofsson et al., 2016). To the extent that such a multivariate screening approach can facilitate early detection of those at risk, physicians would be able to target available pharmacotherapy at the earliest stage possible, potentially delaying symptoms of the neuropathology. Finally, not only might assessing change in sensory function represent a non-invasive early marker of AD risk, but targeting and developing interventions to lessen well-documented AD-related sensory and motor deficits may improve function and quality of life for individual patients as the disease progresses (Albers et al., 2015).
In summary, we find that of the three sensory modalities examined in this study, olfaction (and to a lesser extent distance visual acuity) was the principal predictor systematically linked both to prediction of MCI risk, as well as non-demented cognitive decline. The differential importance of olfactory change as a predictor of persistent MCI is reflective of the neural pathways underlying each sensory modality and their connections to the Alzheimer’s disease pathology. The occipital and temporal lobes, each a primary processing center for vision and audition respectively, are less (or later) affected than the olfactory bulb tract, entorhinal cortex, thalamus and hippocampal formation. Thus, based upon these patterns, we see potential in employing change in sensory function; even modest change in olfactory abilities (e.g., as a proxy for the underlying neural areas involved) may be an early, inexpensive, and easily-administered-in-a-clinical-setting marker of age- and pathology-related cognitive decline.
Public Significance Statement.
Findings from the present study show that multi-year declines in olfaction, and to a lesser extent vision, are associated with cognitive declines as well as increased risk for mild cognitive impairment (MCI). These findings suggest that non-invasive, simple to administer, and inexpensive sensory assessments may be beneficial in clinical settings as part of a multi-step screening approach for detecting those at risk of MCI, and for targeting early interventions.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (National Institute on Aging) to Roger A. Dixon (R01 AG008235). Dr. Dixon was also supported by the Canada Research Chairs program. Stuart W.S. MacDonald was supported by a grant from the Natural Sciences and Engineering Research Council of Canada, as well as by the Royal Society of Canada College of New Scholars, Artists and Scientists.
Footnotes
Further information about the VLS may be accessed via: http://www.ualberta.ca/~vlslab/index.html, or by contacting Dr Dixon at rdixon@ualberta.ca.
References
- Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Zhang LI, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers & Dementia. 2015;11(1):70–98. doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K. Cognitive aging and functional biomarkers: what do we know, and where to from here? In: Hofer SM, Alwin DF, editors. Handbook of Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks: SAGE Publications; 2008. pp. 327–339. [Google Scholar]
- Anstey KJ, Lord SR, Smith G. Measuring human functional age: A review of empirical findings. Experimental Aging Research. 1996;22(3):245–266. doi: 10.1080/03610739608254010. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Barresi M, Ciurleo R, Giacoppo S, Foti CV, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. Journal of the Neurological Sciences. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- DeCarlo CA, MacDonald SWS, Vergote D, Jhamanda J, Westaway D, Dixon RA. Vascular health and genetic risk affect mild cognitive impairment status and 4-year stability: evidence from the Victoria Longitudinal Study. Journals of Gerontology: Psychological Sciences. 2016;71:1004–1014. doi: 10.1093/geronb/gbv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: from cognitively elite to cognitively impaired. Neuropsychology. 2009;23(6):778–791. doi: 10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF Studies in the preclinical diagnosis of Alzheimer’s disease. Annals of the New York Academy of Sciences. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biological Psychiatry. 2008;64(10):871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiological of Aging. 2010;31(9):1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA. Enduring theoretical themes in psychological aging: Derivation, functions, perspectives, and opportunities. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7. San Diego, CA: Elsevier; 2011. pp. 3–23. [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11(2-3):346–376. doi: 10.1080/13825580490511161. [DOI] [Google Scholar]
- Dixon RA, Garrett DD, Hultsch DF, Lentz TL, MacDonald SWS, Strauss E. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21(3):381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Dixon RA, DeCarlo CA, MacDonald SWS, Vergote D, Jhamandas J, Westaway D. APOE and COMT polymorphisms are complementary biomarkers of status, stability, and transitions in normal aging and early mild cognitive impairment. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00236. Article 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging. 2008;29(5):693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Dolcos S, MacDonald SWS, Braslavsky A, Camicioli R, Dixon RA. Mild cognitive impairment is associated with selected functional markers: integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012;26(2):209–223. doi: 10.1037/a0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. The Smell Identification Test Administration Manual. Haddon Heights, NJ: Sensonics Inc; 1995. [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor- referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53(7):1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SWS. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psychology and Aging. 2003;18(4):755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding Ageing: An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47(6):341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. doi: 10.1080/15427600902911189. [DOI] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychology and Aging. 1990;5(3):356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. New York: Cambridge University Press; 1998. [Google Scholar]
- Humes LE. Do ‘auditory processing’ tests measure auditory processing in the elderly? Ear and Hearing. 2005;26(2):109–119. doi: 10.1097/00003446-200504000-00001. [DOI] [PubMed] [Google Scholar]
- Kjelvik G, Sando SB, Aasly J, Engedal KA, White LR. Use of the brief smell identification test for olfactory deficit in a Norwegian population with Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2007;22(10):1020–1024. doi: 10.1002/gps.1783. [DOI] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in-vivo optical imaging of retinal plaques in a mouse model. NeuroImage. 2011;54:204–217. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Tervo S, Hanninen T, Vanhanen M, Hallikainen M, Soininen H. Olfactory identification in non-demented elderly population and in mild cognitive impairment: A comparison in clinical odor identification versus Boston Naming Test. Journal of Neural Transmission. 2009;116(7):891–895. doi: 10.1007/s00702-009-0235-8. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hedner M, Papenberg G, Seubert J, Bäckman L, Laukka EJ. Olfactory memory in the old and very old: relations to episodic and semantic memory and APOE genotype. Neurobiology of Aging. 2016;38:118–126. doi: 10.1016/j.neurobiolaging.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Lehrner J, Pusswald G, Gleiss A, Auff E, Dal-Bianco P. Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. The Clinical Neuropsychologist. 2009;23(5):818–830. doi: 10.1080/13854040802585030. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010;133(9):2714–2726. doi: 10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZH, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neuroscience and Biobehavioral Reviews. 2002;26(7):777–783. doi: 10.1016/s0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Lin FR, Metter JE, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Archives of Neurology. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: Towards improving characterizations of developmental time. Journal of Gerontology: Psychological Sciences. 2011;66(S1):59–70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12- year cognitive change in older adults: Findings from the Victoria Longitudinal Study. Gerontology. 2004;50(2):64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Bunce D. Intraindividual variability in vigilance performance: Does degrading visual stimuli mimic age related “neural noise”? Journal of Clinical and Experimental Neuropsychology. 2006;28(5):655–675. doi: 10.1080/13803390590954245. [DOI] [PubMed] [Google Scholar]
- Masurkar AV, Devanand DP. Olfactory dysfunction in the elderly: basic circuitry and alterations with normal aging and Alzheimer’s disease. Current Geriatrics Reports. 2014;3(2):91–100. doi: 10.1007/s13670-014-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Role of neuroimaging in Alzheimer’s disease with emphasis on brain perfusion SPECT. Journal of Nuclear Medicine. 2007;48(8):1289–1300. doi: 10.2967/jnumed.106.037218. [DOI] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Anstey KJ, Dixon RA. Alzheimer’s genetic risk intensifies neurocognitive slowing associated with diabetes in non-demented older adults. Alzheimer’s & Dementia: Diagnosis, Assessment, & Disease Monitoring. 2015;1(4):395–402. doi: 10.1016/j.dadm.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA. Visual association pathology in preclinical Alzheimer’s disease. Journal of Neuropathology and Experimental Neurology. 2006;65(6):621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- Mobley AS, Rodriguez-Gil DJ, Imamura F, Greer CA. Aging in the olfactory system. Trends in Neurosciences. 2014;37(2):77–84. doi: 10.1016/j.tins.2013.11.004. http://dx.doi.org/10.1097/00005072-200606000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Jernigan TL, Fennema-Notestine C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: a structural MRI study. Journal of the International Neuropsychological Society. 2003;9:459–471. doi: 10.1017/S1355617703930116. http://dx.doi.org/10.1017/S1355617703930116. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Josefsson M, Ekström I, Wilson D, Nyberg L, Nordin S, Nordin AA, Adolfsson R, Nilsson L-G, Larsson M. Long-term episodic memory decline is associated with olfactory deficits only in carriers of ApoE-∊4. Neuropsychologia. 2016;85:1–9. doi: 10.1016/j.neuropsychologia.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Rönnlund M, Nordin S, Nyberg L, Nilsson L-G, Larsson M. Odor identification deficit as a predictor of five-year global cognitive change: interactive effects with age and ApoE-ε4. Behavior Genetics. 2009;39:496–503. doi: 10.1007/s10519-009-9289-5. [DOI] [PubMed] [Google Scholar]
- Palmer K, Bäckman L, Winblad B, Fratiglioni L. Detection of Alzheimer’s disease and dementia in the preclinical phase: population based cohort study. BMJ. 2003;326:345. doi: 10.1136/bmj.326.7383.245. http://dx.doi.org/10.1136/bmj.326.7383.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson NE, Gomez G, Cowart BJ, Kriete A, Pribitkin E, Restrepo D. Age- associated loss of selectivity in human olfactory sensory neurons. Neurobiology of Aging. 2011;33(9):1913–1919. doi: 10.1016/j.neurobiolaging.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, Thompson PM, Stein JL, Moore JH, Farrer LA, Green RC, Bertram L, Jack CR, Weiner MW the Alzheimer’s Disease Neuroimaging Initiative. ADNI biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimer’s & Dementia. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B, Pichora-Fuller MK. Implications of sensory deficits for cognitive aging. In: Craik FIM, Salthouse T, editors. The Handbook of Aging and Cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 155–219. [Google Scholar]
- Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011;121(4):873–878. doi: 10.1002/lary.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialfa CT. The role of sensory factors in cognitive aging research. Canadian Journal of Experimental Psychology. 2002;56(3):153–163. doi: 10.1037/h0087393. http://dx.doi.org/10.1037/h0087393. [DOI] [PubMed] [Google Scholar]
- Shayler G. Vision dysfunction in Alzheimer’s disease. Optometry Today. 2011;51(3):41. [Google Scholar]
- Sigurdardottir S, Andelic N, Skandsen T, Anke A, Roe C, Holthe OO, Wehling E. Olfactory identification and its relationship to executive functions, memory, and disability one year after severe traumatic brain injury. Neuropsychology. 2016;30(1):98–108. doi: 10.1037/neu0000206. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle J. Time-based and process-based approaches to analysis of longitudinal data. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 477–491. [Google Scholar]
- Stamps JJ, Martoshuk LM, Heilman KM. A brief olfactory test for Alzheimer’s disease. Journal of the Neurological Sciences. 2013;333:19–24. doi: 10.1016/j.jns.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson L-G, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. Journal of the International Neuropsychological Society. 2014;20:209–217. doi: 10.1017/S1355617713001409. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipent and mid AD: Relation to memory function. Neurobiology of Aging. 2010;31(7):1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Schacter DL. The Neuropsychology of Memory. 3. New York: Guilford Press; 2002. [Google Scholar]
- Thomann PA, Dos Santos V, Toro P, Schonknecht P, Essig M, Schroder J. Reduced olfactory bulb and tract volume in early Alzheimer’s disease – A MRI study. Neurobiology of Aging. 2009;30(5):838–841. doi: 10.1016/j.neurobiolaging.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Thurstone TG. Primary Mental Abilities: Grades 9–12. Chicago: Science Research Associates; 1962. 1962 revision. [Google Scholar]
- Tzekov R, Mullan M. Vision function abnormalities in Alzheimer disease. Survey of Opthamology. 2013;59(4):414–433. doi: 10.1016/j.survophthal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Vandermorris S, Hultsch DF, Hunter MA, MacDonald SWS, Strauss E. Including persistency of impairment in mild cognitive impairment classification enhances prediction of 5-Year decline. Archives of Clinical Neuropsychology. 2011;26:26–37. doi: 10.1093/arclin/acq093. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The measurement and appraisal of adult intelligence. 4. Baltimore: Williams & Wilkins; 1958. [Google Scholar]
- Wehling EI, Wollschlaeger D, Nordin S, Lundervold AJ. Longitudinal changes in odor identification performance and neuropsychological measures in aging individuals. Neuropsychology. 2016;30(1):87–97. doi: 10.1037/neu0000212. [DOI] [PubMed] [Google Scholar]
- Westervelt HJ, Bruce JM, Faust MA. Distinguishing Alzheimer’s disease and dementia with Lewy bodies using cognitive and olfactory measures. Neuropsychology. 2016;30(3):304–311. doi: 10.1037/neu0000230. [DOI] [PubMed] [Google Scholar]
- Westervelt HJ, Carvalho J, Duff K. Presentation of Alzheimer’s disease in patients with and without olfactory deficits. Archives of Clinical Neuropsychology. 2007;22(1):117–122. doi: 10.1016/j.acn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Annals of the New York Academy of Sciences. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


