Abstract
DNA repair is critical to maintain genome stability. In eukaryotic cells, DNA repair is complicated by the packaging of the DNA ‘substrate’ into chromatin. DNA repair pathways utilize different mechanisms to overcome the barrier presented by chromatin to efficiently locate and remove DNA lesions in the genome. DNA excision repair pathways are responsible for repairing a majority of DNA lesions arising in the genome. Excision repair pathways include nucleotide excision repair (NER) and base excision repair (BER), which repair bulky and non-bulky DNA lesions, respectively. Numerous studies have suggested that chromatin inhibits both NER and BER in vitro and in vivo. Growing evidence demonstrates that histone modifications have important roles in regulating the activity of NER and BER enzymes in chromatin. Here, we will discuss the roles of different histone modifications and the corresponding modifying enzymes in DNA excision repair, highlighting the role of yeast as a model organism for many of these studies.
Keywords: DNA repair, chromatin, histone acetylation, NER, BER
This review surveys the role of histone post-translational modifications in DNA excision repair.
INTRODUCTION
DNA in all living organisms is under constant assault by DNA-damaging agents (Friedberg et al.2006). Many DNA lesions are caused by endogenous sources such as cellular reactive oxidative species and DNA replicative errors (Bont and van Larebeke 2004). Environmental factors, including solar ultraviolet (UV) radiation, cigarette smoke, alkylating agents and medical x-rays, can also induce various lesions in the genome. Some DNA lesions can block DNA and RNA polymerases, leading to stalling of DNA replication and transcription. Alternatively, if damaged DNA is replicated by error-prone DNA polymerases, the low fidelity of these polymerases gives rise to mutations in the daughter cells and increases the risk of cancer (Rattray and Strathern 2003). Cells must repair DNA lesions in a timely manner to avoid mutation or cell death, and so employ a plethora of DNA repair pathways to cope with different types of genotoxic DNA lesions. The major DNA repair pathways identified to date include nucleotide excision repair (NER), base excision repair (BER), mismatch repair, double-strand break (DSB) repair (including homologous recombination and non-homologous end-joining) and direct reversal of damage (Friedberg et al.2006). Each DNA repair pathway generally targets specific classes of DNA lesions, but functional overlap between different DNA repair pathways has also been observed. All the major DNA repair mechanisms are important for maintaining genome integrity, as demonstrated by high cancer incidence associated with defects in each DNA repair pathway (reviewed in Jeggo, Pearl and Carr 2016). Our discussion will focus on the two excision repair pathways: NER and BER.
NER is important for the repair of helix-distorting DNA lesions, such as UV-induced cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts (PPs). NER is a complex process requiring more than 30 different proteins. During NER, these repair proteins are recruited in a sequential manner to perform DNA damage recognition and verification, DNA incision on both sides of the lesion, excision of a single-stranded oligonucleotide (25–30 nt) containing the damage, repair synthesis and DNA ligation (Friedberg et al.2006). There are two subpathways in NER, transcription-coupled repair (TC-NER) and global genomic repair (GG-NER). The main difference between the two subpathways lies in the initial damage recognition step. In TC-NER, the elongating RNA polymerase II (RNAPII) functions as a damage sensor and DNA lesion-induced RNA Pol II stalling serves as the signal to initiate repair (Hanawalt and Spivak 2008). TC-NER is only responsible for repair of DNA lesions located on the transcribed strand of genes undergoing transcription. In contrast, GG-NER relies on specific damage-recognition proteins (i.e. XPC in mammals and Rad4 in yeast) to recognize DNA damage in non-transcribed regions (and DNA strands) throughout the genome (Friedberg et al.2006). The two subpathways merge after the recognition step and use the same set of enzymes for subsequent steps. Given the importance of NER in removing UV photolesions, defects in NER have been associated with serious human diseases, such as xeroderma pigmentosum (XP) and Cockayne syndrome (CS) (reviewed in Marteijn et al.2014). Both patients with XP and CS suffer from hypersensitivity to sunlight and patients with XP can exhibit orders of magnitude higher risk of skin cancer.
BER is the most frequently used DNA repair mechanism in cells, as BER targets the most ubiquitous base lesions in DNA: oxidation, alkylation, deamination and uracil misincorporation (reviewed in Krokan and Bjørås 2013). These base lesions usually cause little disruption to the DNA double helix, and thus are not targeted by NER. BER is initiated by DNA glycosylases. At least 11 distinct mammalian glycosylases have been identified (Krokan and Bjørås 2013), each specifically recognizing a few related base modifications. DNA glycosylases ‘flip out’ damaged bases during lesion recognition and hydrolyze the N-glycosidic bond, resulting in apurinic/apyrimidinic (AP) sites. The AP site is further processed by the AP endonuclease (APE1), which cleaves the DNA backbone 5′ to the AP site. However, some bifunctional DNA glycosylases (i.e. OGG1; Boiteux and Radicella 2000) have intrinsic AP lyase activity and can break the DNA backbone after removing the damaged base. In either case, the nicked DNA substrate is processed by a DNA polymerase (frequently DNA polymerase β, Pol β) for repair synthesis. Depending on the length of the synthesized product, BER can be divided into short-patch (a single nucleotide is replaced) and long-patch (2–10 nucleotides are replaced) BER. The mechanism for determining short- and long-patch repair is not completely understood. A variety of factors, such as the type of DNA glycosylase (Fortini et al.1999), ATP concentration (Petermann, Ziegler and Oei 2003) and the chromatin context of the lesion (Meas and Smerdon 2016), are thought to influence the choice between the two BER subpathways. BER is completed by the activity of DNA ligase, which seals the nick to restore the original DNA sequence.
Much of our understanding of NER and BER comes from biochemical characterization using purified repair enzymes (or cell-free extracts) acting on naked DNA substrates. A central task in the excision repair field is to elucidate how NER and BER operate in intact cells. Genomic DNA in eukaryotic cells is wrapped around histone octamers (an H3-H4 tetramer and two H2A-H2B dimers) to form nucleosomes, which are further folded into compact chromatin structure with the help of histone H1 (Luger, Dechassa and Tremethick 2012). This DNA packaging mechanism is needed to fit a large eukaryotic genome into the relatively tiny volume of a nucleus. However, chromatin structure profoundly inhibits the access of DNA repair proteins to lesions. Indeed, even the first level of chromatin compaction, the nucleosome, is refractory to NER in vitro (Hara, Mo and Sancar 2000). This is supported by a recent genome-wide analysis of UV damage formation and repair, which demonstrated that repair of UV lesions by NER is inhibited within the centers of strongly positioned nucleosomes throughout the yeast genome (Mao et al.2016). Inhibition of BER activity by nucleosomes has also been observed in vitro, particularly when DNA damage (i.e. uracil incorporation) is rotationally positioned toward the histone core within nucleosomes (Cole, Tabor-Godwin and Hayes 2010; Hinz, Rodriguez and Smerdon 2010). These observations highlight the necessity of dynamically altering chromatin structure in response to DNA damage to enable efficient excision repair in living cells. Histone post-translational modifications have emerged as an important cellular mechanism for altering chromatin structure during excision repair. Several histone modifications have been characterized in DNA excision repair, through in vivo or in vitro studies. In this review, we will discuss the functions of different histone modifications in NER and BER.
DIVERSE ROLES OF HISTONE ACETYLATION IN EXCISION REPAIR
Overview
Acetylation of histone lysine residues is an important modification occurring in numerous cellular processes. Histone acetylation is generally associated with transcriptionally active euchromatin (reviewed in Roth, Denu and Allis 2001), although nucleosomes within the coding regions of actively transcribed genes are actively deacetylated to prevent cryptic transcription initiation (Carrozza et al.2005). Histone acetylation alters chromatin through at least two different mechanisms. The first mechanism emphasizes the chemical outcome of acetylation in neutralizing the positive charge of histone lysine residues. Histone acetylation is thought to reduce favorable electrostatic histone–DNA interactions, driving chromatin to a more open state. For example, acetylation of histone residues within the globular domain of H3 has been shown to impact nucleosome unwrapping dynamics and stability. Specifically, histone acetylation near the DNA entry/exit point (i.e. H3 lysine-56 [K56] acetylation) increases nucleosome dynamics (Neumann et al.2009), and acetylation near the nucleosomal dyad stimulates nucleosome disassembly (Manohar et al.2009). In the second mechanism, histone acetylation marks located in the flexible N-terminal tails of histones are recognized by bromodomain-containing proteins and function in recruiting downstream effectors. For example, acetylation of K14 in H3 tail is specifically recognized by the tandem bromodomains in RSC4 (Kasten et al.2004), a subunit of the ATP-dependent chromatin remodeler RSC (remodels the structure of chromatin). In this case, histone acetylation functions as a signal and indirectly regulates DNA accessibility in chromatin.
Role of histone acetylation in NER
Early experiments using the histone deacetylase inhibitor n-butyrate revealed that nucleosome hyperacetylation enhances repair of UV lesions in mammalian cells (Smith 1986; Ramanathan and Smerdon 1989). Although n-butyrate causes other side effects in mammalian cells (i.e. cell growth, cell cycle distribution), these observations imply an important role of histone acetylation in facilitating NER. Direct evidence comes from histone acetylation studies conducted in UV-irradiated yeast cells. Using antibodies specific for acetylated histones H3 and H4, Yu et al. (2005) showed that UV irradiation stimulates acetylation of lysine residues in the N-terminal tails of H3 (K9, K14) and H4 (K5, K8, K12, K16). Histone acetylation was induced within 30 min following UV irradiation, both globally and at the repressed MFA2 gene (Yu et al.2005). Furthermore, this study connected the Gcn5 histone acetyltransferase (HAT) with UV-induced histone hyperacetylation in yeast. In a gcn5Δ deletion mutant, UV-induced H3 acetylation and GG-NER are significantly reduced at the MFA2 locus (Teng, Yu and Waters 2002; Yu et al.2005). Intriguingly, although Gcn5 is important for acetylation and repair at MFA2, GCN5 deletion has little effect on global UV-induced H3 or H4 acetylation (Yu et al.2005), suggesting Gcn5 selectively acetylates a subset of genes during GG-NER. Consistent with the histone acetylation data, Gcn5 was shown to be important for efficient NER at the MFA2 promoter, but is not generally required for NER in yeast (Teng, Yu and Waters 2002). These findings suggest that other yeast HATs (reviewed in Roth, Denu and Allis 2001) likely play important roles in UV-induced histone acetylation and NER. Further studies are needed to characterize how other HATs coordinate with Gcn5 in the process of UV damage repair.
The mechanism by which Gcn5 functionally interacts with the yeast GG-NER machinery has been investigated. The general NER factors Rad4 and Rad14, which play essential roles in damage recognition and verification, are not required for UV-induced histone acetylation in yeast (Yu et al.2005). However, it was discovered that Rad16, which is specifically required for the GG-NER subpathway in yeast, is required for UV-induced histone acetylation (Teng et al.2008). Furthermore, it was shown that Rad16 regulates UV-induced histone acetylation at the repressed MFA2 locus by recruiting Gcn5 to MFA2 in response to UV irradiation (Teng et al.2008; Yu et al.2011). Rad16 is a member of the SWI/SNF family of chromatin remodeling factors and consists of two important functional domains: an ATPase domain and a RING (really interesting new gene) domain embedded in the ATPase domain (Guzder et al.1998). Rad16 associates with Rad7 and forms a tight heterodimer. Two distinct functions of the Rad7-Rad16 complex have been characterized in NER. First, Rad7-Rad16 specifically binds to UV-damaged DNA in an ATP-dependent manner (Guzder et al.1997). Second, Rad7 and Rad16 are components of an E3 ubiquitin ligase complex, which ubiquitylates the GG-NER protein Rad4 (Gillette et al.2006).
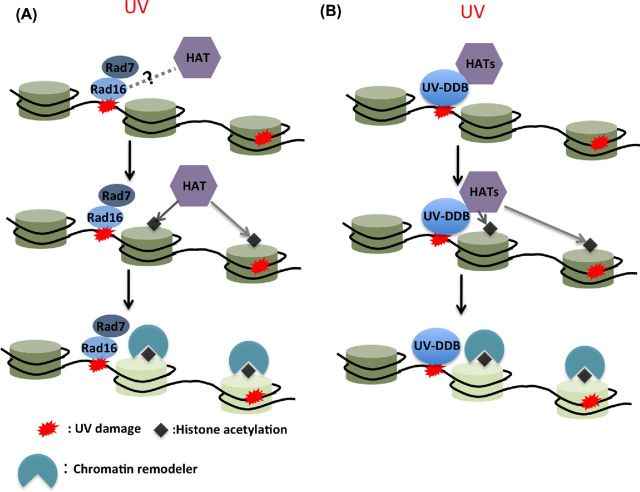
These and other studies suggest a mechanism for chromatin remodeling during GG-NER in yeast, as illustrated in Fig. 1A. In response to UV irradiation, Rad7–Rad16 may first translocate to DNA damage in nucleosome linker regions, as suggested by a previous study conducted in yeast (Lettieri et al.2008), or land on DNA damage located on the accessible nucleosomal surface. Rad7–Rad16 may be able to initiate chromatin remodeling directly, as seen with other SWI/SNF-related chromatin remodelers. However, direct evidence for the chromatin-remodeling activity of Rad7-Rad16 is lacking. The more important function of Rad7–Rad16 is likely to recruit histone modifying enzymes such as Gcn5 to the damaged chromatin, which acetylates histones and stimulates chromatin remodeling to facilitate subsequent repair (Yu et al.2011). A key question is how Rad16 recruits Gcn5 in response to UV irradiation. Does Rad16 specifically recruit Gcn5 (i.e. through a physical interaction) or does Rad16-initiated chromatin remodeling indirectly facilitate recruitment of downstream factors, including Gcn5?
Figure 1.
Regulation of GG-NER by HATs in yeast and mammalian cells. (A) GG-NER protein complex Rad7-Rad16 translocates to UV damage, where it recruits yeast Gcn5, through an unidentified mechanism. Gcn5 acetylates histone H3, which may facilitate the recruitment of downstream bromodomain-containing effectors such as ATP-dependent chromatin remodelers to disassemble nucleosomes (light color). Disruption of nucleosomes exposes UV damage to GG-NER machinery for efficient repair. (B) Mammalian UV damage recognition protein complex UV-DDB physically interacts with several HATs (i.e. CBP, p300, TFTC and STAGA). UV-DDB complex directs HATs to UV-damaged nucleosomes for histone acetylation. Presumably, acetylated histones are recognized by mammalian bromodomain-containing chromatin remodelers, which allows GG-NER proteins to access UV damage.
The involvement of Gcn5 and H3 acetylation in UV damage repair has been confirmed by studies conducted in mammalian cells. In this case, human Gcn5 is recruited to UV-damaged chromatin in human normal fibroblast cells, through its physical interaction with the transcription factor E2F1 (Guo et al.2011). Gcn5 stimulates H3K9 acetylation at the damaged sites and increases chromatin accessibility for NER factor recruitment (Guo et al.2011). A recent study indicates that Gcn5-mediated H3K9 acetylation is also important for the DNA translesion synthesis (TLS) pathway in response to UV irradiation, through regulating transcription of the TLS polymerase η (POLH) gene (Kikuchi et al.2012), suggesting that Gcn5 plays multiple roles in the cellular response to UV damage.
Studies in mammalian cells have revealed that HATs participate in GG-NER process through physically interacting with the UV-damaged DNA-binding protein (UV-DDB) complex. UV-DDB is the damage recognition factor functioning prior to XPC (ortholog of yeast Rad4) in mammalian GG-NER (Sugasawa et al.2005). A previous study demonstrated that DDB2 (the small subunit of UV-DDB) physically interacts with both CBP and p300 (Datta et al.2001), two transcription coactivators with HAT activities. Intriguingly, CPB/p300 also physically interacts with the essential TC-NER protein CSB (cockayne syndrome group B), and is recruited to the UV damage-stalled RNAPII (Fousteri et al.2006). Together, these findings suggest that CBP/p300 HATs participate in both GG-NER and TC-NER through interacting with the key factors in each repair subpathway. Although the exact function of CBP/p300 in UV damage repair is not fully understood, it is likely that the HAT activities of CBP/p300 play a role in acetylating histones at UV-damaged chromatin to facilitate NER. Similarly, two GCN5-containing protein complexes, TFTC [TBP-free TAF(II) complex] and STAGA (SPT3-TAFII31-GCN5L acetyltransferase), associate with DDB1 (the large subunit in UV-DDB complex) in mammalian cells (Brand et al.2001; Martinez et al.2001). In vitro data indicate that UV-DDB1 directs TFTC to UV-damaged nucleosomes, where GCN5 acetylates histone H3 (Brand et al.2001), suggesting that UV damage recognition and histone acetylation are coupled by TFTC. These studies suggest a chromatin remodeling mechanism at UV-damaged chromatin in mammalian cells, in which UV-DDB plays a central role in initiating chromatin remodeling through recruiting various HATs (Fig. 1B). Functional similarities can be drawn between UV-DDB and yeast Rad7–Rad16. For example, they both bind to UV-damaged DNA and recruit HATs to damage sites. Additionally, UV-DDB was shown to ubiquitylate XPC in response to UV irradiation (Sugasawa et al.2005), in a manner similar to Rad7–Rad16 in ubiquitylating Rad4.
In addition to in vivo studies, the function of histone acetylation in UV damage repair has been characterized in vitro, using recombinant histones acetylated at specific sites. For example, the function of H3K14 acetylation in UV damage repair (by CPD photolyase) has been examined in ‘designed’ nucleosomes. Histone H3 protein with homogeneous K14 acetylation was produced in a genetically engineered Escherichia coli strain (Neumann et al.2009), and the acetylated H3 was reconstituted into a strongly positioned nucleosome (Duan and Smerdon 2014). Interestingly, on its own H3K14 acetylation did not alter nucleosome unfolding dynamics or affect UV damage repair. However, in the presence of the chromatin remodeler RSC, H3K14 acetylation facilitated repair of UV lesions in the nucleosome by stabilizing the binding of RSC to the nucleosome substrate (Duan and Smerdon 2014).
It is not clear to what extent helix-distorting lesions other than UV PPs induce histone acetylation in yeast and mammalian cells. However, a recent study suggested that cisplatin damage, which is also repaired by the NER pathway, induces histone H3K14 acetylation in yeast (Powell et al.2015). Surprisingly, histone acetylation changes due to cisplatin mirrored acetylation changes due to UV across the yeast genome, even though the underlying distributions of damage for cisplatin and UV differed (Powell et al.2015). Clearly, further studies are needed to clarify the role of histone acetylation in NER of other classes of helix-distorting DNA lesions.
The ambiguous role of histone acetylation in BER
The role of histone acetylation in BER is not well understood. Biochemical studies using strongly positioned nucleosomes have shown that the structural location of DNA lesions in the nucleosome strongly influences BER efficiency, especially for the glycosylase and APE1 steps (Rodriguez and Smerdon 2013). If the base lesion (i.e. uracil) is rotationally positioned away from the histone core (i.e. is solvent exposed), the lesion can be readily recognized by the glycosylase without the need for chromatin remodeling (Cole, Tabor-Godwin and Hayes 2010; Hinz, Rodriguez and Smerdon 2010). Alternatively, if the damage is located at translational positions near the nucleosomal DNA ends (i.e. distant from the central nucleosome dyad), intrinsic nucleosome unwrapping dynamics allows access of glycosylases to the DNA damage (Rodriguez and Smerdon 2013). However, repair of inwardly oriented base lesions near the nucleosome dyad is still significantly hindered in vitro (Rodriguez and Smerdon 2013), and may require assistance from chromatin remodelers or histone-modifying (e.g. HAT) enzymes.
Limited in vivo studies in mammalian cells indicate that CBP/p300 acetyltransferase associates with thymine DNA glycosylase (TDG) and forms a protein complex (Tini et al.2002). TDG is important for repair of G/T and G/U mismatches. TDG is acetylated by CBP/p300 in the complex, and this acetylation prevents the interaction between TDG and the downstream DNA endonuclease APE1 (Tini et al.2002). It is possible that the association between TDG and CPB/p300 may facilitate the recruitment of CPB/p300 to mismatch sites to acetylate histones and stimulate BER, but this has yet to be conclusively demonstrated. HATs such as CBP/p300 have also been shown to associate with and acetylate other BER glycosylases (e.g. OGG1 and NEIL2), APE1 and Pol β (reviewed in Carter and Parsons 2016). Again, it is not clear if these interactions lead to increased histone acetylation at sites of DNA lesions or BER intermediates.
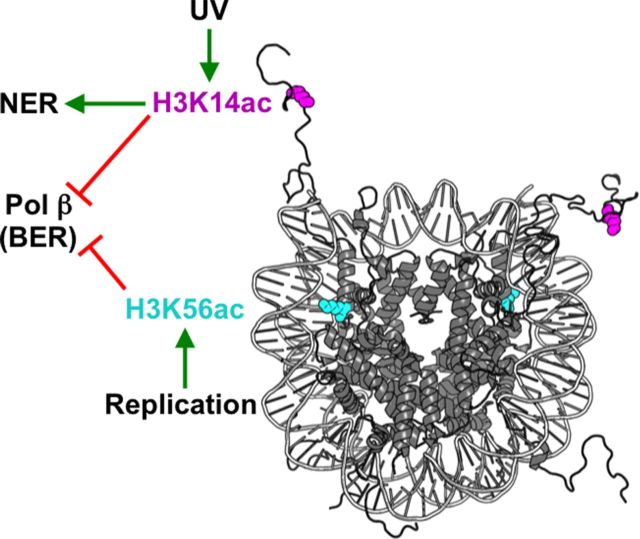
A recent study has suggested that acetylation of certain histone lysine residues can inhibit the repair synthesis step in the BER pathway (Rodriguez et al.2016). This study characterized the impact of site-specific histone acetylation of histone H3 on BER of damaged nucleosome substrates in vitro. Rodriguez et al. generated nucleosome core particles (NCP) containing homogenous site-specific acetylation at H3K14 or H3K56. They found that neither H3K14ac nor H3K56ac affects the removal of uracil by uracil DNA glycosylase (UDG) in nucleosomes (Rodriguez et al.2016). However, it is not known if histone acetylation would enhance UDG activity on nucleosome substrates in the presence of an ATP-dependent chromatin remodeler (e.g. RSC), as was previously observed for UV damage repair (Duan and Smerdon 2014). Intriguingly, Rodriguez et al. (2016) found that acetylation at either H3K14 or K56 inhibits repair synthesis by Pol β at specific locations in reconstituted NCPs. While the mechanism by which histone acetylation inhibits Pol β activity is not yet clear, these results suggest that histone acetylation does not necessarily facilitate repair activity, as is commonly assumed. These specific acetylation sites are associated with non-BER pathways, namely NER (H3K14ac) and DNA replication (H3 K56ac; Han et al.2007), while Pol β primarily functions in gap-filling repair synthesis in BER. Because Pol β is a relatively low-fidelity DNA polymerase (Sweasy 2003), it is tempting to speculate that acetylation at certain histone residues may block Pol β in favor of alternative gap filling DNA mechanisms (e.g. employing the replicative DNA polymerases δ or ɛ) for improved repair synthesis fidelity (Fig. 2). If this model is correct, it will be important to test whether histone acetylation also affects SP versus LP repair synthesis in BER, perhaps by regulating DNA polymerase activity.
Figure 2.
Acetylation of certain histone residues inhibits repair synthesis by Pol β associated with BER. Acetylation of H3K14, which is induced by UV damage and promotes NER in yeast (along with H3K9 acetylation [not depicted]), also inhibits Pol β polymerase activity during BER in vitro. Similarly, H3K56 acetylation, which is associated with new histone assembly during DNA replication, inhibits Pol β activity on certain nucleosome substrates. Inhibition of Pol β activity by histone acetylation may represent a mechanism to prevent Pol β from performing repair synthesis during NER or gap filling during DNA replication, and to instead promote gap filling by higher fidelity replicative polymerases (e.g. Pol δ or ɛ). Image of nucleosome was created using Pymol of pdb id 1k×5.
OTHER CLASSES OF HISTONE MODIFICATIONS IMPLICATED IN EXCISION REPAIR
Role of histone methylation in NER
Histone methylation is catalyzed by histone methyltransferases (HMTs), which covalently attach methyl groups to specific histone residues. Three families of HMTs have been identified so far: the SET-domain-containing proteins, DOT1-like proteins and members of the protein arginine N-methyltransferase family (reviewed in Greer and Shi 2012). These HMTs target basic residues (lysines and arginines) on histones for mono-, di- or trimethylation. Methyl groups can be removed by the activities of demethylases. Two families of histone demethylases, lysine-specific demethylase and Jumonji C demethylase, have been discovered in recent years (reviewed in Kooistra and Helin 2012). HMTs and demethylases dynamically regulate histone methylation levels in response to cellular stimuli, and play important roles in a broad range of cellular activities including DNA repair (reviewed in Martin and Zhang 2005).
Although numerous histone methylations have been identified, the only histone methylation that has been characterized in NER is H3K79 methylation. Methylation of H3K79 is catalyzed by Dot1 (disruptor of telomeric silencing-1) in yeast or its ortholog DOT1L in mammalian cells. Loss of H3K79 methylation by either deleting yeast Dot1 gene or mutating K79 at H3 leads to UV hypersensitivity (Bostelman et al.2007; Chaudhuri, Wyrick and Smerdon 2009). Consistent with this phenotype, two studies showed that H3K79 methylation is important for GG-NER at the silent HML locus (Chaudhuri, Wyrick and Smerdon 2009) and the non-transcribed strand of the actively transcribed gene Rpb2 in yeast (Tatum and Li 2011), respectively. Although Dot1 methylation is important for GG-NER in yeast, it is not required for TC-NER (Tatum and Li 2011). The mechanism by which H3K79 methylation regulates GG-NER is not fully understood, but it has been suggested that this modification may increase the accessibility of nucleosomal DNA at the silent HML chromatin (Chaudhuri, Wyrick and Smerdon 2009), or the methylation mark may function as a docking site on the nucleosome surface to recruit GG-NER machinery (Tatum and Li 2011). The ‘docking site’ mechanism of H3K79 methylation appears to have broad implications in the DNA damage response (DDR). For example, H3K79 methylation in yeast is important for DNA damage-induced cell cycle checkpoints, through recruiting the DDR protein Rad9 to damaged chromatin (Wysocki et al.2005).
Histone phosphorylation and NER
Several histone residues (serine, threonine, tyrosine) can be phosphorylated by protein kinases and dephosphorylated by phosphatases (reviewed in Rossetto, Avvakumov and Côté 2012). The best characterized DNA damage-induced histone phosphorylation is γ-H2AX, which occurs at serine-139 of the H2AX histone variant in mammals (Rogakou et al.1998), or serine-129 of canonical H2A in yeast (Downs, Lowndes and Jackson 2000). γ-H2AX is rapidly increased in chromatin adjacent to DNA DSBs due to the activity of the kinase ATM (ataxia telangiectasia mutated) in mammalian cells (Burma et al.2001) or Tel1 in yeast (Shroff et al.2004). In addition to DSBs, single-stranded DNA (ssDNA) is another signal for the induction of H2AX phosphorylation, through the activity of the kinase ATR (ataxia telangiectasia and Rad3 related) in mammals (Ward and Chen 2001) and Mec1 in yeast (Downs, Lowndes and Jackson 2000). Cellular ssDNA can be derived from various sources, including DNA replication stress, which occurs when the replication fork encounters DNA damage. Under replication stress, the uncoupling of replicative DNA helicase and stalled replication fork generates a significant length of ssDNA. The ssDNA is bound by RPA (replication protein A) and the ssDNA-RPA recruits ATR (or Mec1) to activate H2AX phosphorylation (Zou and Elledge 2003).
Although UV irradiation generally does not generate DSBs in the genome, UV photolesions induce γ-H2AX through at least three distinct mechanisms. First, because UV photolesions strongly block DNA polymerases, the resulting stalled DNA replication forks induce H2AX phosphorylation. Indeed, UV-induced γ-H2AX levels coincide with active DNA replication and are highest in S phase (Halicka et al.2005; Marti et al.2006). Second, the induction of γ-H2AX in non-dividing cells can be due to ssDNA generated during NER (Hanasoge and Ljungman 2007). One possibility is that the canonical 24–30 nucleotide stretches of ssDNA generated as NER intermediates are bound by RPA and act as the signal to recruit ATR/Mec1 (Hanasoge and Ljungman 2007). Recent studies indicate that exonuclease-1 (EXO1 in humans; Exo1 in yeast) is important for checkpoint signaling following UV irradiation in non-dividing yeast and human cells, due to its role in extending ssDNA gaps generated during NER (Giannattasio et al.2010; Sertic et al.2011). Third, γ-H2AX can be induced by R-loop formation associated with RNAP II stalling at UV lesion sites. A recent study demonstrated that arrested RNAP II at UV lesions triggers R-loop formation, which is a signal for activating ATM and H2AX phosphorylation (Tresini et al.2015). Alternatively, in vitro data suggest that ATR is an intrinsic UV damage sensor protein, and may directly bind to UV-damaged DNA to activate γ-H2AX (Ünsal-Kaçmaz et al.2002).
In addition to γ-H2A, other histone phosphorylations have been shown to be responsive to UV irradiation. A large-scale screening using antibodies against specific histone modifications revealed that phosphorylations on H3 (H3S10, H3S28 and H3.3S31) are repressed by UV irradiation in human cells (Tjeertes, Miller and Jackson 2009). However, these changes are likely caused by UV-induced changes in cell cycle progression (Tjeertes, Miller and Jackson 2009). A study using yeast as a model system identified a novel Mec1- and Tel1-catalyzed phosphorylation site at H2B T129 (termed γ-H2B) (Lee et al.2014), but it is not known if γ-H2B is induced by UV irradiation.
Histone ubiquitylation and ADP-ribosylation and excision repair
Other histone modifications, such as histone monoubiquitylation and ADP-ribosylation, are also responsive to DNA damage and play a role in DNA excision repair. The functions of H2A ubiquitylation (H2Aub) and H2B ubiquitylation (H2Bub) in transcription regulation have been extensively characterized. H2Aub is primarily associated with gene repression, whereas H2Bub is important for gene activation and transcription elongation (reviewed in Meas and Mao 2015). Interestingly, previous studies indicate that ubiquitylation of histone H2A, H3 and H4 is induced by UV; in contrast, H2B is deubiquitylated immediately after UV irradiation, and H2B ubiquitylation levels are restored following repair (reviewed in Meas and Mao 2015). Several ubiquitin ligases are known to ubiquitylate H2A in response to UV irradiation, including the E3 ubiquitin ligase complex UV-DDB-CUL4 (Kapetanaki et al.2006; Guerrero-Santoro et al.2008), RING1B (RING2) (Wang et al.2004; Gracheva et al.2016) and RNF8 (Marteijn et al.2009). Recently, it has been shown that RING1B interacts with UV-DDB-CUL4 and forms a stable complex (UV-RING1B), suggesting the two previously identified E3 ligases cooperate to ubiquitylate H2A in response to UV damage (Gracheva et al.2016). Although RNF8-mediated H2A ubiquitylation is important for DNA damage signaling by recruiting downstream factors such as 53BP1 and BRCA1 (Marteijn et al.2009), the function of H2A ubiquitylation catalyzed by the UV-RING1B complex remains elusive. UV-DDB-CUL4 also ubiquitylates histones H3 and H4, and ubiquitylated H3 and H4 are thought to promote histone eviction from UV-damaged nucleosomes (Wang et al.2006), thus rendering UV lesions more accessible to the NER machinery.
Histone H2B is deubiquitylated at the early stage of UV damage response, through the action of histone deubiquitylases (Ubp8 and Ubp10) associated with elongating RNAPII (Mao et al.2014). RNAPII stalling at UV lesions triggers H2B deubiquitylation, as deubiquitylation of H2B is significantly reduced in an RNAPII mutant (rpb1 E1103G) that can bypass UV lesions. Simultaneous deletion of UBP8 and UBP10 genes blocks UV-induced H2B deubiquitylation activity in yeast cells. The lack of H2B deubiquitylation in the ubp8Δubp10Δ double mutant leads to decreased TC-NER and increased RNAPII degradation (Mao et al.2014), suggesting that deubiquitylation of H2B is an important mechanism that facilitates rescue of UV-arrested RNAPII through TC-NER in chromatin. The H2B ubiquitylation level is gradually restored during NER, accompanying transcription resumption.
Histone ADP-ribosylation, which is the addition of one or more ADP-ribose moieties to histones by ADP-ribosyltransferases, occurs on all core histones and the linker histone H1 in mammals (reviewed in Messner and Hottiger 2011). As these topics have been recently reviewed (see Messner and Hottiger 2011; Meas and Mao 2015), they will not be discussed further.
CONCLUSIONS
An important but challenging task in DNA repair studies is to understand how repair factors are recruited and function in the context of compact chromatin. As suggested by the ‘access-repair-restore’ model (Polo and Almouzni 2015), chromatin needs to be remodeled or disassembled to increase the access of DNA repair proteins to damage, and chromatin structure must be restored in the wake of DNA repair in order to maintain important epigenetic marks. Many studies have revealed the important functions of histone modifications in DNA excision repair, particularly NER. Most of the understanding of NER and BER in the context of chromatin comes from repair studies conducted in vitro using reconstituted nucleosomes, or in vivo at specific loci with known nucleosome positioning. It is poorly understood how NER and BER proteins function in chromatin at the genomic level. Recent progress in developing genome-wide NER mapping methods has shed some light on the relationship between NER efficiency and different chromatin states (Adar et al.2016). Future analysis of genome-wide histone modifications after UV irradiation and correlation of a genome-wide map of NER activity with the genomic distributions of specific histone modifications should provide new insights in our understanding of how NER is regulated in the context of chromatin.
Acknowledgments
The authors would like to thank Dr Michael Smerdon and Amelia Hodges for helpful comments and suggestions.
FUNDING
This work was supported by a grant from the National Institute of Environmental Health Sciences [grant number ES002614]; and an internal grant from the College of Veterinary Medicine at Washington State University.
Conflict of interest.None declared.
REFERENCES
- Adar S, Hu J, Lieb JD, et al. Genome-wide kinetics of DNA excision repair in relation to chromatin state and mutagenesis. P Natl Acad Sci USA. 2016;113:E2124–33. doi: 10.1073/pnas.1603388113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S, Radicella JP. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch Biochem Biophys. 2000;377:1–8. doi: 10.1006/abbi.2000.1773. [DOI] [PubMed] [Google Scholar]
- Bont RD, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–85. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- Bostelman LJ, Keller AM, Albrecht AM, et al. Methylation of histone H3 lysine-79 by Dot1p plays multiple roles in the response to UV damage in Saccharomyces cerevisiae. DNA Repair. 2007;6:383–95. doi: 10.1016/j.dnarep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Brand M, Moggs JG, Oulad-Abdelghani M, et al. UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation. EMBO J. 2001;20:3187–96. doi: 10.1093/emboj/20.12.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, et al. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, et al. Histone H3 methylation by set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–92. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Parsons JL. Base excision repair, a pathway regulated by posttranslational modifications. Mol Cell Biol. 2016;36:1426–37. doi: 10.1128/MCB.00030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, Wyrick JJ, Smerdon MJ. Histone H3 Lys79 methylation is required for efficient nucleotide excision repair in a silenced locus of Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:1690–700. doi: 10.1093/nar/gkp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HA, Tabor-Godwin JM, Hayes JJ. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J Biol Chem. 2010;285:2876–85. doi: 10.1074/jbc.M109.073544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bagchi S, Nag A, et al. The p48 subunit of the damaged-DNA binding protein DDB associates with the CBP/p300 family of histone acetyltransferase. Mutat Res. 2001;486:89–97. doi: 10.1016/s0921-8777(01)00082-9. [DOI] [PubMed] [Google Scholar]
- Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature. 2000;408:1001–4. doi: 10.1038/35050000. [DOI] [PubMed] [Google Scholar]
- Duan M-R, Smerdon MJ. Histone H3 lysine 14 (H3K14) acetylation facilitates DNA repair in a positioned nucleosome by stabilizing the binding of the chromatin remodeler RSC (remodels structure of chromatin) J Biol Chem. 2014;289:8353–63. doi: 10.1074/jbc.M113.540732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini P, Parlanti E, Sidorkina OM, et al. The type of DNA glycosylase determines the base excision repair pathway in mammalian cells. J Biol Chem. 1999;274:15230–6. doi: 10.1074/jbc.274.21.15230. [DOI] [PubMed] [Google Scholar]
- Fousteri M, Vermeulen W, van Zeeland AA, et al. Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol Cell. 2006;23:471–82. doi: 10.1016/j.molcel.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, et al. DNA Repair and Mutagenesis. Washington, D.C., USA: ASM Press; 2006. [Google Scholar]
- Giannattasio M, Follonier C, Tourrière H, et al. Exo1 competes with repair synthesis, converts NER intermediates to long ssDNA gaps, and promotes checkpoint activation. Mol Cell. 2010;40:50–62. doi: 10.1016/j.molcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Gillette TG, Yu S, Zhou Z, et al. Distinct functions of the ubiquitin–proteasome pathway influence nucleotide excision repair. EMBO J. 2006;25:2529–38. doi: 10.1038/sj.emboj.7601120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva E, Chitale S, Wilhelm T, et al. ZRF1 mediates remodeling of E3 ligases at DNA lesion sites during nucleotide excision repair. J Cell Biol. 2016;213:185–200. doi: 10.1083/jcb.201506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–57. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Santoro J, Kapetanaki MG, Hsieh CL, et al. The cullin 4B-based UV-damaged DNA-binding protein ligase binds to UV-damaged chromatin and ubiquitinates histone H2A. Cancer Res. 2008;68:5014–22. doi: 10.1158/0008-5472.CAN-07-6162. [DOI] [PubMed] [Google Scholar]
- Guo R, Chen J, Mitchell DL, et al. GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res. 2011;39:1390–7. doi: 10.1093/nar/gkq983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzder SN, Sung P, Prakash L, et al. Yeast Rad7-Rad16 complex, specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J Biol Chem. 1997;272:21665–8. doi: 10.1074/jbc.272.35.21665. [DOI] [PubMed] [Google Scholar]
- Guzder SN, Sung P, Prakash L, et al. The DNA-dependent ATPase activity of yeast nucleotide excision repair factor 4 and its role in DNA damage recognition. J Biol Chem. 1998;273:6292–6. doi: 10.1074/jbc.273.11.6292. [DOI] [PubMed] [Google Scholar]
- Halicka HD, Huang X, Traganos F, et al. Histone H2AX phosphorylation after cell irradiation with UV-B: relationship to cell cycle phase and induction of apoptosis. Cell Cycle Georget Tex. 2005;4:339–45. [PubMed] [Google Scholar]
- Hanasoge S, Ljungman M. H2AX phosphorylation after UV irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis. 2007;28:2298–304. doi: 10.1093/carcin/bgm157. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Bio. 2008;9:958–70. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- Han J, Zhou H, Horazdovsky B, et al. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–5. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- Hara R, Mo J, Sancar A. DNA damage in the nucleosome core is refractory to repair by human excision nuclease. Mol Cell Biol. 2000;20:9173–81. doi: 10.1128/mcb.20.24.9173-9181.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz JM, Rodriguez Y, Smerdon MJ. Rotational dynamics of DNA on the nucleosome surface markedly impact accessibility to a DNA repair enzyme. P Natl Acad Sci USA. 2010;107:4646–51. doi: 10.1073/pnas.0914443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer. 2016;16:35–42. doi: 10.1038/nrc.2015.4. [DOI] [PubMed] [Google Scholar]
- Kapetanaki MG, Guerrero-Santoro J, Bisi DC, et al. The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. P Natl Acad Sci USA. 2006;103:2588–93. doi: 10.1073/pnas.0511160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M, Szerlong H, Erdjument-Bromage H, et al. Tandem bromodomains in the chromatin remodeler RSC recognize acetylated histone H3 Lys14. EMBO J. 2004;23:1348–59. doi: 10.1038/sj.emboj.7600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H, Kuribayashi F, Imajoh-Ohmi S, et al. GCN5 protects vertebrate cells against UV-irradiation via controlling gene expression of DNA polymerase η. J Biol Chem. 2012;287:39842–9. doi: 10.1074/jbc.M112.406389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Bio. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- Krokan HE, Bjørås M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5:a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-S, Lee K, Legube G, et al. Dynamics of yeast histone H2A and H2B phosphorylation in response to a double-strand break. Nat Struct Mol Bio. 2014;21:103–9. doi: 10.1038/nsmb.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri T, Kraehenbuehl R, Capiaghi C, et al. Functionally distinct nucleosome-free regions in yeast require Rad7 and Rad16 for nucleotide excision repair. DNA Repair. 2008;7:734–43. doi: 10.1016/j.dnarep.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Luger K, Dechassa ML, Tremethick DJ. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat Rev Mol Cell Bio. 2012;13:436–47. doi: 10.1038/nrm3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M, Mooney AM, North JA, et al. Acetylation of histone H3 at the nucleosome dyad alters DNA-histone binding. J Biol Chem. 2009;284:23312–21. doi: 10.1074/jbc.M109.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P, Meas R, Dorgan KM, et al. UV damage-induced RNA polymerase II stalling stimulates H2B deubiquitylation. P Natl Acad Sci USA. 2014;111:12811–6. doi: 10.1073/pnas.1403901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P, Smerdon MJ, Roberts SA, et al. Chromosomal landscape of UV damage formation and repair at single-nucleotide resolution. P Natl Acad Sci USA. 2016;113:9057–62. doi: 10.1073/pnas.1606667113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteijn JA, Bekker-Jensen S, Mailand N, et al. Nucleotide excision repair-induced H2A ubiquitination is dependent on MDC1 and RNF8 and reveals a universal DNA damage response. J Cell Biol. 2009;186:835–47. doi: 10.1083/jcb.200902150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteijn JA, Lans H, Vermeulen W, et al. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Bio. 2014;15:465–81. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Bio. 2005;6:838–49. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- Martinez E, Palhan VB, Tjernberg A, et al. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol Cell Biol. 2001;21:6782–95. doi: 10.1128/MCB.21.20.6782-6795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti TM, Hefner E, Feeney L, et al. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. P Natl Acad Sci USA. 2006;103:9891–6. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meas R, Mao P. Histone ubiquitylation and its roles in transcription and DNA damage response. DNA Repair. 2015;36:36–42. doi: 10.1016/j.dnarep.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meas R, Smerdon MJ. Nucleosomes determine their own patch size in base excision repair. Sci Rep. 2016;6:27122. doi: 10.1038/srep27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner S, Hottiger MO. Histone ADP-ribosylation in DNA repair, replication and transcription. Trends Cell Biol. 2011;21:534–42. doi: 10.1016/j.tcb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Neumann H, Hancock SM, Buning R, et al. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol Cell. 2009;36:153–63. doi: 10.1016/j.molcel.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Ziegler M, Oei SL. ATP-dependent selection between single nucleotide and long patch base excision repair. DNA Repair. 2003;2:1101–14. doi: 10.1016/s1568-7864(03)00117-4. [DOI] [PubMed] [Google Scholar]
- Polo SE, Almouzni G. Chromatin dynamics after DNA damage: The legacy of the access–repair–restore model. DNA Repair. 2015;36:114–21. doi: 10.1016/j.dnarep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JR, Bennett MR, Evans KE, et al. 3D-DIP-Chip: a microarray-based method to measure genomic DNA damage. Sci Rep. 2015;5:7975. doi: 10.1038/srep07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan B, Smerdon MJ. Enhanced DNA repair synthesis in hyperacetylated nucleosomes. J Biol Chem. 1989;264:11026–34. [PubMed] [Google Scholar]
- Rattray AJ, Strathern JN. Error-prone DNA polymerases: when making a mistake is the only way to get ahead. Annu Rev Genet. 2003;37:31–66. doi: 10.1146/annurev.genet.37.042203.132748. [DOI] [PubMed] [Google Scholar]
- Rodriguez Y, Hinz JM, Laughery MF, et al. Site-specific acetylation of histone H3 decreases polymerase β activity on nucleosome core particles in vitro. J Biol Chem. 2016;291:11434–45. doi: 10.1074/jbc.M116.725788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Y, Smerdon MJ. The structural location of DNA lesions in nucleosome core particles determines accessibility by base excision repair enzymes. J Biol Chem. 2013;288:13863–75. doi: 10.1074/jbc.M112.441444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rossetto D, Avvakumov N, J Côté. Histone phosphorylation. Epigenetics. 2012;7:1098–108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Sertic S, Pizzi S, Cloney R, et al. Human exonuclease 1 connects nucleotide excision repair (NER) processing with checkpoint activation in response to UV irradiation. P Natl Acad Sci USA. 2011;108:13647–52. doi: 10.1073/pnas.1108547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R, Arbel-Eden A, Pilch D, et al. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14:1703–11. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ. n-Butyrate alters chromatin accessibility to DNA repair enzymes. Carcinogenesis. 1986;7:423–9. doi: 10.1093/carcin/7.3.423. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okuda Y, Saijo M, et al. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Sweasy JB. Fidelity mechanisms of DNA polymerase beta. Prog Nucleic Acid Re. 2003;73:137–69. doi: 10.1016/s0079-6603(03)01005-5. [DOI] [PubMed] [Google Scholar]
- Tatum D, Li S. Evidence that the histone methyltransferase Dot1 mediates global genomic repair by methylating histone H3 on lysine 79. J Biol Chem. 2011;286:17530–5. doi: 10.1074/jbc.M111.241570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Liu H, Gill HW, et al. Saccharomyces cerevisiae Rad16 mediates ultraviolet-dependent histone H3 acetylation required for efficient global genome nucleotide-excision repair. EMBO Rep. 2008;9:97–102. doi: 10.1038/sj.embor.7401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Yu Y, Waters R. The Saccharomyces cerevisiae histone acetyltransferase Gcn5 has a role in the photoreactivation and nucleotide excision repair of UV-induced cyclobutane pyrimidine dimers in the MFA2 gene. J Mol Biol. 2002;316:489–99. doi: 10.1006/jmbi.2001.5383. [DOI] [PubMed] [Google Scholar]
- Tini M, Benecke A, Um S-J, et al. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol Cell. 2002;9:265–77. doi: 10.1016/s1097-2765(02)00453-7. [DOI] [PubMed] [Google Scholar]
- Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009;28:1878–89. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresini M, Warmerdam DO, Kolovos P, et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature. 2015;523:53–8. doi: 10.1038/nature14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünsal-Kaçmaz K, Makhov AM, Griffith JD, et al. Preferential binding of ATR protein to UV-damaged DNA. P Natl Acad Sci USA. 2002;99:6673–8. doi: 10.1073/pnas.102167799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhai L, Xu J, et al. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22:383–94. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–62. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Wysocki R, Javaheri A, Allard S, et al. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol. 2005;25:8430–43. doi: 10.1128/MCB.25.19.8430-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Teng Y, Waters R, et al. How chromatin is remodelled during DNA repair of UV-induced DNA damage in Saccharomyces cerevisiae. PLOS Genet. 2011;7:e1002124. doi: 10.1371/journal.pgen.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Teng Y, Liu H, et al. UV irradiation stimulates histone acetylation and chromatin remodeling at a repressed yeast locus. P Natl Acad Sci USA. 2005;102:8650–5. doi: 10.1073/pnas.0501458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]