Abstract
Breast cancer is the most common malignancy among women, while invasive ductal carcinoma is the most common type of invasive breast cancer. Metastatic spread to the colon and rectum in breast cancer is rare. This report describes a case of a 69-year-old woman with metastatic ductal breast cancer to the rectosigmoid, presenting as an incidental finding on screening colonoscopy. The breast carcinoma was first diagnosed 2 years prior. Colonic biopsies from colonoscopy confirmed metastatic adenocarcinoma consistent with a breast primary. Ultimately her clinical condition worsened as she developed malignant ascites, a small bowel obstruction, and new bone metastases, and the patient succumbed to her illness. Cases of metastatic breast cancer to the gastrointestinal tract have predominantly been lobular breast carcinoma. Increased awareness of colonic metastasis may lead to more accurate diagnosis and earlier systemic treatment.
Keywords: breast surgery, breast cancer, endoscopy
Background
Breast cancer is the most common malignancy among women, and invasive ductal carcinoma, not otherwise specified (invasive mammary carcinoma of no special type under WHO classification), is the most common type of invasive breast cancer. Metastasis of breast cancer to the gastrointestinal (GI) tract is rare, with the most common sites of metastases being bone, lung, liver and brain. Of the metastases to the GI tract from a breast primary, the small bowel, oesophagus and stomach are more commonly reported compared with the colon or rectum. The most common type of breast cancer that has been found to metastasise to the colon or rectum is invasive lobular carcinoma, the histological subtype known to have a higher metastatic risk.
We present the case of a 69-year-old woman who was referred to our institution with a history of invasive ductal breast carcinoma who was incidentally found to have metastatic deposits to the colon on screening colonoscopy.
Case presentation
A 69-year-old woman presented to our institution in 2009 after a routine annual screening mammogram revealed a 1 cm BIRADS-5 lesion in the left breast at the 12 o’clock position. Her history revealed that she had undergone menarche at the age of 13, had no pregnancies and experienced menopause in her mid-40s. She had taken oral contraceptives for a total of 15 years and had been on hormone replacement therapy for a total of 10 years. Her medical history revealed hypertension and hypercholesterolaemia. She had previously undergone a total abdominal hysterectomy in 1990 for fibroids but did have her ovaries sparred at that time. Her family history was significant for breast cancer, with a sister diagnosed with breast cancer at age 45 years and a paternal aunt with breast cancer in her 50s. She was a lifetime non-smoker and consumed alcohol occasionally. She had worked as an educational consultant and was married with no children. At the time of her screening mammogram, she had no palpable axillary or breast disease. She was fully functional and able to carry out all activities without any limitations.
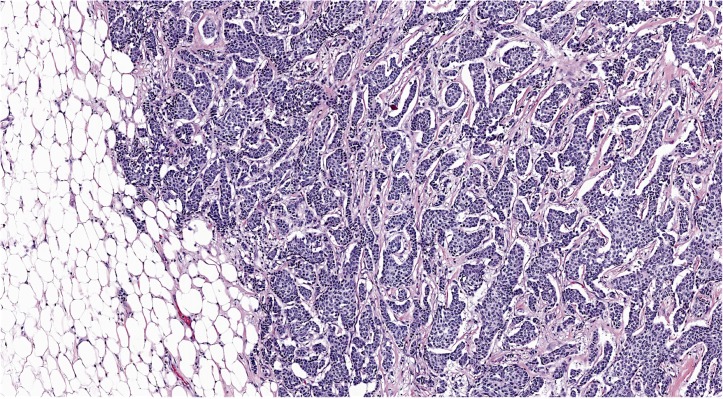
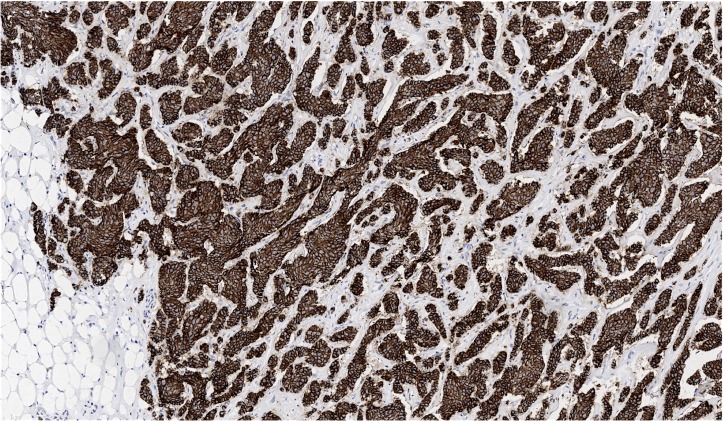
Ultrasound-guided core biopsies of the left breast showed invasive ductal carcinoma in all five cores with positive lymph node metastasis. The Nottingham histological grade was II (6/9; nuclear grade: 2; tubule formation score: 3; mitotic score: 1). A subsequent wire-guided lumpectomy revealed a greater than 5 cm invasive ductal carcinoma with prominent lobular features (figure 1). Axillary lymph node dissection found 14 of 18 nodes positive for metastatic carcinoma, making the pathological stage T3N3a. The largest metastatic lymph node measured 4.3 cm, with extranodal extension present in almost all of the nodes. E-cadherin was positive (figure 2). Hormone receptors showed oestrogen receptor (OR) as strongly positive (more than 80% of nuclei staining), progesterone receptor (PR) positive (50%–60% of nuclei staining weakly to moderately strong) and no evidence of human epidermal growth factor receptor 2 (HER2) overexpression.
Figure 1.
Wire-guided breast resection showing invasive ductal carcinoma (100×).
Figure 2.
Wire-guided breast resection showing invasive ductal carcinoma positive for E-cadherin (100×).
She received adjuvant radiation therapy to the left breast and left axillary and supraclavicular nodal areas along with six cycles of adjuvant chemotherapy (fluorouracil, epirubicin, cyclophosphamide and docetaxel). This was followed by antihormonal therapy using letrozole, which was later switched to exemestane in 2012. Postoperatively, she had no clinical concerns, and follow-up mammograms and bone scans were normal.
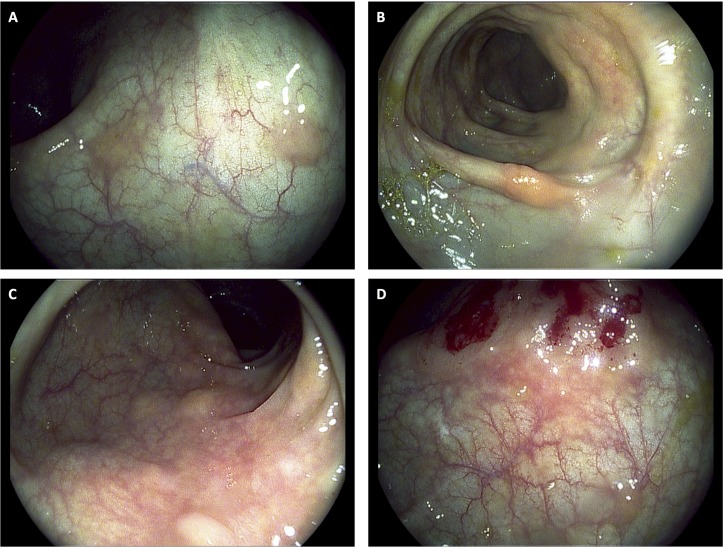
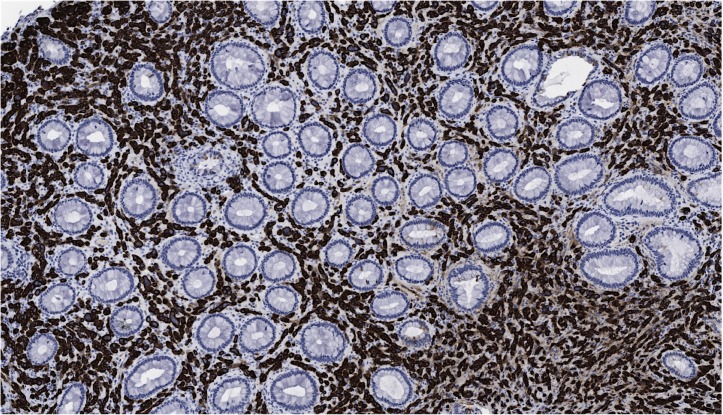
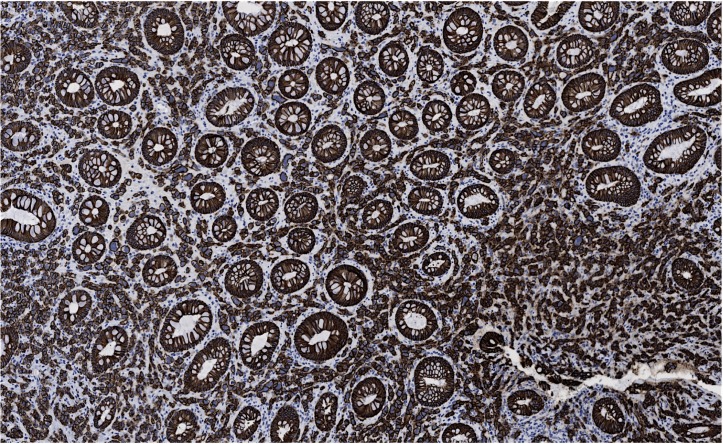
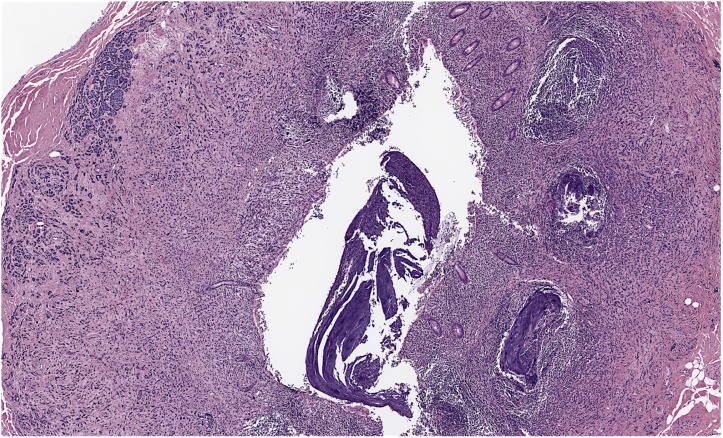
A screening colonoscopy was performed in 2011, which was prompted by a family history of colon cancer, and a rectosigmoid polyp was noted and resected endoscopically. At that time, she was still fully functional without any limitations in her activities and was asymptomatic. The rectosigmoid biopsy showed metastatic adenocarcinoma with features favouring breast as the origin based on immunohistochemical stains. No primary colonic carcinoma was identified. Hormone receptor results showed the OR as positive to the same extent as the original case, but the PR was negative and HER2 remained negative. Further colonic biopsies were performed 15 and 31 months later (figure 3), again showing metastatic adenocarcinoma consistent with a breast primary (figure 4), including the transverse colon, sigmoid and rectum. Histology showed a malignant infiltrate in the lamina propria that surrounded the residual colonic glands. The malignant cells stained positively for cytokeratin (CK)-7 (figure 5) and E-cadherin (figure 6) as well as focal positivity with antibodies to gross cystic disease fluid protein (GCDFP) and mammoglobin. Staining with CK-20 and CDX-2 was negative. Hormone receptor status remained the same.
Figure 3.
Endoscopic photos from colonoscopy completed in 2014 wherein biopsies revealed metastatic adenocarcinoma consistent with breast primary. (A) Metastatic polyps in the sigmoid colon. (B) Metastatic polyps in the proximal descending colon. (C) Numerous sessile colonic polyps in the rectosigmoid colon. (D) Postbiopsy image of sessile colonic polyps in the rectosigmoid colon.
Figure 4.
Colon biopsy showing metastatic breast carcinoma (200×).
Figure 5.
Colon biopsy showing metastatic breast carcinoma positive for CK-7 (100×).
Figure 6.
Colon biopsy showing metastatic breast carcinoma positive for E-cadherin (100×).
Investigations
CT and positron emission tomography scanning identified no radiological evidence of malignancy, though she had a slowly rising cancer antigen 125 level. A peritoneal fluid sample obtained in 2014 showed yellow cloudy fluid with all cells showing enlarged nuclei but still respecting each other with a cellular window reminiscent of mesothelial cells. Immunostaining showed weak staining with BerEp4 and positive staining with CK-7 and mammoglobin. The diagnosis was atypical cells favouring metastatic breast carcinoma.
Treatment
She was taken to the operating room for laparoscopy, which revealed carcinomatosis on the peritoneum, diaphragm, liver and bowel. Biopsies were taken of the pelvic wall, adnexa and appendix, all showing metastatic carcinoma morphologically and immunophenotypically consistent with metastatic mammary ductal carcinoma (figure 7). Malignant cells were positive for pankeratin, mammoglobin, E-cadherin and GCDFP.
Figure 7.
Appendix showing metastatic breast carcinoma (40×).
Outcome and follow-up
Her condition worsened with the development of malignant ascites, small bowel obstruction and new bone metastases within the next year, and she succumbed to her illness in 2015.
Discussion
The GI tract is not a common metastatic site for breast carcinoma, especially for the ductal histological subtype. However, a series of surgical resection cases that demonstrated metastatic tumours within the GI tract showed that the most common tumours were melanoma, ovary, bladder, breast and lung.1 In addition, the most common primary tumour identified in an autopsy series was lung, gynaecological malignancies, breast and pancreas.1 A large case series of breast cancer metastases to the GI tract revealed the distribution of metastases was small intestine (28%), oesophagus (25%), stomach (25%), colon (19%) and rectum (4%).2
There tends to be a histological preference for the site of distant breast metastasis.3 Histopathologically, most GI metastases from breast cancer are due to infiltrating lobular carcinoma, despite the much greater prevalence of infiltrating ductal type among women with breast cancer.4–6 A case series of colorectal metastases of primary breast carcinoma by Taal et al7 revealed a significant predominance of invasive lobular breast carcinoma. The majority of case studies have also reported metastatic lobular breast cancer to the colon8–13 and rectum.14–17 The reason is unknown, but some authors believe it could be related to a particular tropism of lobular cells.3 18 19 In a series of autopsies reported by Harris et al20, metastatic lobular carcinoma was again most predominantly found to involve the GI tract. The infiltration of metastatic lobular carcinoma was found to be of a diffuse pattern through the bowel wall and a linitus plastica-like thickening within the stomach, whereas invasive ductal metastases were nodular in nature.20 Similarly, in a case report by Okido et al21, a metastatic lobular breast cancer presented as linitus plastica of the colon. Few case reports have documented metastatic ductal breast carcinoma to the colon22–27 and even rarer to the rectum.28 The change in hormone receptor subtype between the primary tumour and metastatic lesions has been seen in previous studies.29
Histological diagnosis of metastatic lesions can be difficult. The lack of dysplasia or atypia of the colonic epithelium and the glands surrounding the malignant cells is often helpful in distinguishing between a primary and a metastatic lesion.3 A direct comparison of the resected colonic tumour with the primary breast lesion, as well as immunohistochemical staining can help identify the primary tumour site.3 4 Helpful markers include GCDFP-15, OR and PR. The GCDFP-15, OR and/or PR are positive in metastatic lesions of the breast, in contrast to most colorectal or gastric carcinomas, which are usually negative.3 30 However, one report showed that primary gastric carcinomas can express OR in approximately 20%–28% of cases.31 Another report stated that 30%–70% of primary colonic cancers are OR positive.32 The expression of CK-7 and the absence of CK-20 commonly occur in breast ductal carcinoma as well.4 Our case is in the minority, since it was a ductal rather than lobular breast carcinoma. The immunohistochemical profile was consistent with the literature, with positivity towards GCDFP, mammoglobin, OR and CK-7, while being negative for CK-20 and CDX-2.
In patients with a history of breast cancer who present with GI symptoms, the possibility of metastatic disease to the GI tract should be considered. Due to the non-specific nature of symptoms33 and rarity of the disease, the diagnosis can be challenging. Early detection is important as survival after the development of GI involvement in most patients is less than 1 year.1 Differentiating metastatic lesions from primary malignancies of the GI tract is important to allow for appropriate and timely treatment.
Learning points.
Common sites of breast cancer metastasis include bone, lung, liver and brain.
Metastasis of breast cancer to the gastrointestinal tract is rare; however, when it does occur, it is more common to the small bowel, oesophagus and stomach.
The most common histological subtype of breast cancer found to metastasise to the colon or rectum is invasive lobular breast carcinoma as it has a higher metastatic risk.
For patients with a history of breast cancer who present with gastrointestinal symptoms, the possibility of metastatic disease to the gastrointestinal tract should be considered.
Acknowledgments
We would like to thank the patient’s family for allowing us to share her story, allowing for the preparation of this case report for the educational benefit of others.
Footnotes
Contributors: AES completed the design of the case report, acquisition of data, the analysis and interpretation of this same content. She was essential in the drafting and revision of the article. MLW was involved in data acquisition, manuscript creation, manuscript revisions, coordinating amongst team members, final approval and manuscript submission. NB contributed to the acquisition of, analysis of and interpretation of all pathology content in the case report. PH was involved in all aspects of the case report from concept initiation to final revisions. All authors agree to be accountable for all aspects of the work included in this case report. All authors included above who have been given authorship with this case report fulfill the criteria for authorship. In addition, there are no authors who meet the criteria for authorship but have not been included here.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Washington K, McDonagh D. Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol 1995;8:427–33. [PubMed] [Google Scholar]
- 2.Asch MJ, Wiedel PD, Habif DV. Gastrointestinal metastases from crcinoma of the breast. Autopsy study and 18 cases requiring operative intervention. Arch Surg 1968;96:840–3. [DOI] [PubMed] [Google Scholar]
- 3.Arrangoiz R, Papavasiliou P, Dushkin H, et al. Case report and literature review: metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep 2011;2:301–5. 10.1016/j.ijscr.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Théraux J, Bretagnol F, Guedj N, et al. Colorectal breast carcinoma metastasis diagnosed as an obstructive colonic primary tumor. A case report and review of the literature. Gastroenterol Clin Biol 2009;33:1114–7. 10.1016/j.gcb.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer 2012;2012:1–8. 10.1155/2012/439023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637–42. [PubMed] [Google Scholar]
- 7.Taal BG, den Hartog Jager FCA, Steinmetz R, et al. The spectrum of gastrointestinal metastases of breast carcinoma. Gastrointest Endosc 1992;38:136–41. [DOI] [PubMed] [Google Scholar]
- 8.Nikkar-Esfahani A, Kumar BG, Aitken D, et al. Metastatic breast carcinoma presenting as a sigmoid stricture: report of a case and review of the literature. Case Rep Gastroenterol 2013;7:106–11. 10.1159/000348760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mistrangelo M, Cassoni P, Mistrangelo M, et al. Obstructive colon metastases from lobular breast cancer: report of a case and review of the literature. Tumori 2011;97:800–4. 10.1177/030089161109700619 [DOI] [PubMed] [Google Scholar]
- 10.Rabau MY, Alon RJ, Werbin N, et al. Colonic metastases from lobular carcinoma of the breast. Report of a case. Dis Colon Rectum 1988;31:401–2. 10.1007/BF02564897 [DOI] [PubMed] [Google Scholar]
- 11.Daniels IR, Layer GT, Chisholm EM. Bowel obstruction due to extrinsic compression by metastatic lobular carcinoma of the breast. J R Soc Promot Health 2002;122:61–2. [DOI] [PubMed] [Google Scholar]
- 12.Alves de Lima DC, Alberti LR. Breast cancer metastasis to the colon. Endoscopy 2011;43(suppl 2 UCTN):E143–4. 10.1055/s-0029-1215277 [DOI] [PubMed] [Google Scholar]
- 13.Signorelli C, Pomponi-Formiconi D, Nelli F, et al. Single colon metastasis from breast cancer: a clinical case report. Tumori 2005;91:424–7. 10.1177/030089160509100509 [DOI] [PubMed] [Google Scholar]
- 14.Bamias A, Baltayiannis G, Kamina S, et al. Rectal metastases from lobular carcinoma of the breast: report of a case and literature review. Ann Oncol 2001;12:715–8. 10.1023/A:1011192827710 [DOI] [PubMed] [Google Scholar]
- 15.Cervi G, Vettoretto N, Vinco A, et al. Rectal localization of metastatic lobular breast cancer: report of a case. Dis Colon Rectum 2001;44:453–5. 10.1007/BF02234749 [DOI] [PubMed] [Google Scholar]
- 16.López Deogracias M, Flores Jaime L, Arias-Camisón I, et al. Rectal metastasis from lobular breast carcinoma 15 years after primary diagnosis. Clin Transl Oncol 2010;12:150–3. 10.1007/S12094-010-0481-0 [DOI] [PubMed] [Google Scholar]
- 17.Efthimiadis C, Kosmidis C, Fotiadis P, et al. Breast cancer metastatic to the rectum: a case report. Tech Coloproctol 2011;15(suppl 1):91–3. 10.1007/s10151-011-0740-2 [DOI] [PubMed] [Google Scholar]
- 18.Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol 2006;12:6219–24. 10.3748/wjg.v12.i38.6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol 2005;12:886–94. 10.1245/ASO.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 20.Harris M, Howell A, Chrissohou M, et al. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer 1984;50:23–30. 10.1038/bjc.1984.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okido M, Seo M, Hamada Y, et al. Metastatic breast carcinoma simulating linitis plastica of the colon: report of a case. Surg Today 2011;41:542–5. 10.1007/s00595-009-4305-1 [DOI] [PubMed] [Google Scholar]
- 22.Zhou XC, Zhou H, Ye YH, et al. Invasive ductal breast cancer metastatic to the sigmoid colon. World J Surg Oncol 2012;10:256 10.1186/1477-7819-10-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolić I, Ivković-Kapicl T, Kukić B, et al. Uncommon metastatic site from breast cancer. Vojnosanit Pregl 2012;69:806–8. 10.2298/VSP1209806N [DOI] [PubMed] [Google Scholar]
- 24.Tohfe M, Shami P, Aftimos G, et al. Gastrointestinal metastases from breast cancer: a case report. South Med J 2003;96:624–5. 10.1097/01.SMJ.0000053252.38588.B8 [DOI] [PubMed] [Google Scholar]
- 25.Feng CL, Chou JW, Huang SF. Colonic metastasis from carcinoma of the breast presenting with colonic erosion. Endoscopy 2009;41(suppl 2):E276–7. 10.1055/s-0029-1215066 [DOI] [PubMed] [Google Scholar]
- 26.Yokota T, Kunii Y, Kagami M, et al. Metastatic breast carcinoma masquerading as primary colon cancer. Am J Gastroenterol 2000;95:3014–6. 10.1111/j.1572-0241.2000.03238.x [DOI] [PubMed] [Google Scholar]
- 27.Law WL, Chu KW. Scirrhous colonic metastasis from ductal carcinoma of the breast: report of a case. Dis Colon Rectum 2003;46:1424–7. 10.1007/s10350-004-6762-3 [DOI] [PubMed] [Google Scholar]
- 28.Haubrich WS. Adenocarcinoma of the breast metastatic to the rectum. Gastrointest Endosc 1985;31:403–4. 10.1016/S0016-5107(85)72263-8 [DOI] [PubMed] [Google Scholar]
- 29.Cho DH, Jeon YS, Choi MY, et al. Ileal metastasis of breast cancer in a patient with a BRCA2 gene mutation: report of a case. Surg Today 2011;41:1665–9. 10.1007/s00595-011-4503-5 [DOI] [PubMed] [Google Scholar]
- 30.O’Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med 2005;129:338–47. [DOI] [PubMed] [Google Scholar]
- 31.Tokunaga A, Nishi K, Matsukura N, et al. Estrogen and progesterone receptors in gastric cancer. Cancer 1986;57:1376–9. [DOI] [PubMed] [Google Scholar]
- 32.Bracali G, Caracino AM, Rossodivita F, et al. Estrogen and progesterone receptors in human colorectal tumour cells (study of 70 cases). Int J Biol Markers 1988;3:41–8. [DOI] [PubMed] [Google Scholar]
- 33.Gifaldi AS, Petros JG, Wolfe GR. Metastatic breast carcinoma presenting as persistent diarrhea. J Surg Oncol 1992;51:211–5. 10.1002/jso.2930510317 [DOI] [PubMed] [Google Scholar]