Abstract
Objective
To determine whether the 3-month, community-based early stimulation coaching and social support intervention ‘CASITA’, delivered by community health workers, could improve early child development and caregiver-child interaction in a resource-limited district in Lima, Peru.
Design
A controlled two-arm proof-of-concept study.
Setting
Six neighbourhood health posts in Carabayllo, a mixed rural/urban district in Lima. Sessions were held in homes and community centres.
Participants
Children aged 6–24 months who screened positive for risk of neurodevelopmental delay (using validated developmental delay tool) and poverty (using progress out of poverty tool) were enrolled with their caregivers. Dyads with children born >21 days early were excluded.
Intervention
12-week parenting/support intervention plus nutritional support (n=41) or nutrition alone (n=19).
Outcome measures
Development and home environment differences and mean changes from baseline to 3 months postintervention were evaluated using age-adjusted z-scores on the Extended Ages and Stages Questionnaire (EASQ) and the Home Observation Measurement of the Environment (HOME) scores, respectively.
Results
Development in CASITA improved significantly in all EASQ domains, whereas the control group’s z-scores did not improve significantly in any domain. The mean adjusted difference (MAD) in change in EASQ age-adjusted z-scores between the two study arms was 1.39 (95% CI 0.55 to 2.22); Cohen’s d effect size of 0.87 (95% CI 0.23 to 1.50). Likewise, intervention significantly improved global HOME scores versus control group (MAD change of 6.33 (95% CI 2.12 to 10.55); Cohen’s d of 0.85 (95% CI 0.28 to 1.41)).
Conclusions
An evidence-based early intervention delivered weekly during 3 months by a community health worker significantly improved children’s communication, motor and personal/social development in this proof-of-concept study.
Keywords: neurodevelopment, comm child health, health services research
What is already known on this subject?
Poverty and its psychosocial manifestations contribute to increased developmental risk in young children in resource-poor settings.
Parenting support programmes in low-income and middle-income countries are associated with increased scores in child cognitive, motor and psychosocial development.
However, access to early stimulation programmes for caregivers remains a significant challenge in resource-limited areas where often transportation is difficult and services few and costly.
What this study hopes to add?
Community health workers delivered CASITA, a 12-week, community-based early stimulation coaching and social support intervention, in Lima, Peru.
This pilot compared CASITA plus nutrition with nutrition alone in 60 children aged 6–24 months at risk for delay.
CASITA sessions significantly improved child development and caregiver behaviour compared with nutrition alone.
Background
Globally, 249.4 million children under age 5 were at risk of failing to reach full developmental potential1–3 as of 2010. Poverty and its psychosocial manifestations (including maternal depression and domestic violence) likely contribute to increased developmental risk among young children in resource-poor settings.4–8 Young children living in adversity suffer from ‘toxic stress’,9 10 which can interfere with early brain development by disrupting or slowing the growth of neuron connections.11 Regular contingent and responsive interactions between parents and their young children can mitigate the adverse consequences of living with poverty and stress.11–13
Teaching caregivers to practice stimulating and responsive interactions with their children is a simple way to promote healthy child development. Indeed, literature in high-income countries confirms the efficacy of early interventions that target nutritional supplements combined with caregiver stimulation coaching and support for children with neurodevelopmental delay.14–18 A recent comprehensive review found that parenting support programmes in low-income and middle-income countries were associated with increased scores in child cognitive, motor and psychosocial development.19 Yet, access to early stimulation programmes for caregivers remains a significant challenge in resource-limited areas.20 Trained community health workers (CHWs) can be a valuable resource to teach early interaction to caregivers, particularly where homes are distant, transportation is difficult, trained doctors and nurses are few and costly and stigma or discrimination are barriers to clinic attendance. Data from community-based early childhood interventions and home visiting programmes suggest they are effective in delivering early child stimulation interventions in resource-limited settings due to their low cost and high health return.21 22
Here, we report results of a pilot study of a community-based early child intervention (‘CASITA’) to assess its impact on early child development, home environment and caregiver behaviour.
Methods
Study location
Carabayllo District, Lima has both rural and urban areas, and a rapidly expanding population of >290 000 people due to immigration from the provinces, and 26.3% living in poverty. Carabayllo is the founding site of the non-governmental organisation Socios en Salud (SES, Partners In Health, Peru). Official estimates of the population under 2 years of age vary from 5000 (Ministry of Health) to 7500 (National Institute of Health). In Peru, CHWs reside in the community, work as volunteers and on average have completed high school.
Intervention
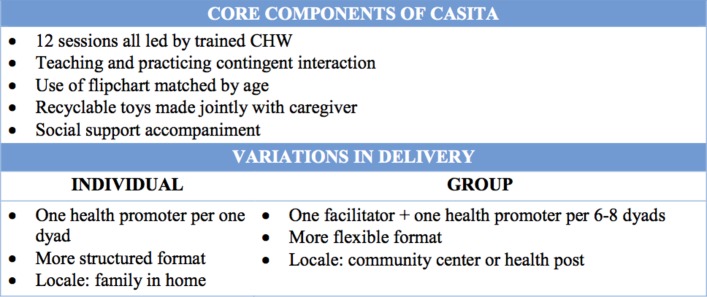
The CASITA intervention is a community-based early child intervention that was adapted from the SPARK Center at Boston Medical Center using the ADAPT-ITT (Assessment, Decision-making, Adaptaion, Production, Topical experts,-Integration, Training, Testing)guidelines for community participation in intervention adaptation.23 CASITA provides parents with skills, resources and support to stimulate child development. Two modes of CASITA delivery were piloted: individual (one-on-one with a CHW) and group (one CHW to approximately 10 caregivers). Although modalities were piloted separately, results were combined into one study arm (‘intervention’) for analysis. The following core components were maintained across both delivery models: coaching on early child stimulation, teaching and practising contingent interaction and social support accompaniment (figure 1). All sessions were led by a trained CHW. All CASITA participants received 12 weekly sessions, organised in four sequential segments: 1) child observation and discussion of general child development; 2) demonstration and initiation of cognitive stimulation and social interaction activities tailored to the child’s development; 3) encouragement of responsive parenting behaviour; 4) parent social support through referral assistance (eg, connection to Ministry of Health early child health visits), reassurance and discussion of parent concerns. Weekly sessions also included workshops to create toys from recyclable materials found in the home. Families received reimbursement for transportation costs. CASITA encounters were videotaped and assessed for fidelity using standardised forms with feedback to the CHW.
Figure 1.
Key components of individual and group modalities of CASITA. CHW, community health worker.
Study population
The CASITA pilot was conducted between April 2014 and October 2015. Individual sessions occurred from April 2014 to September 2014. Group sessions occurred from March 2015 to October 2015. Six health posts were selected based on prior collaboration with SES and greater need. We stratified health posts by urban (n=4) versus rural (n=2), then randomly allocated health posts (ratio 1:2) to receive either monthly nutritional support alone (control) or CASITA and monthly nutritional support (group intervention or individual intervention). Participants’ study arm was based on the assignment of their local health post. The four health posts in the intervention arm were each randomly assigned to individual or group intervention. Children between 6 and 24 months old were screened per routine care at participating health establishments, using the developmental screening instrument Escala de Evaluacion del Desarollo Psychomotor (EEDP).24 To reach children who did not attend health posts, CHWs also screened children using EEDP in the community. Primary caregivers of children who screened ‘at risk’ or ‘delayed’ in at least one of the four domains (motor, language, social and coordination) were invited to participate in the study and enrolled after providing informed consent. Exclusion criteria included dyads with: children born >21 days early; parents who declined participation; children who were screened ‘not delayed’ on the EEDP and households that screened above the poverty threshold as measured by the Progress Out of Poverty Index.25 Budgetary constraints limited enrolment sample size to 60 dyads; children were screened until 60 eligible dyads were enrolled. Group and individual intervention arms were analysed together as ‘intervention’ to increase statistical power.
Data collection
Primary child development outcomes were measured using the Extended Ages and Stages questionnaire (EASQ). The EASQ, which was validated in a national sample of Peruvian children aged 3–24 months, contains all ASQ-3 items in a continuous format, allowing for comparison across age groups without relying on Western-established cut-off scores.26 27 The ASQ-3 is a parent-reported screening tool available in Spanish, often administered by trained laypeople such as CHWs27 28 to assess developmental domains including communication, fine motor, gross motor, problem solving and personal/social. Secondary outcomes were measured using the Infant Toddler Home Observation Measurement of the Environment (HOME)questionnaire,29 which evaluates parenting and home influences on child development. Global HOME scores and subscores of responsivity and involvement (parenting behaviours most likely to be influenced by CASITA) were compared preintervention and postintervention.29 Baseline assessments included EASQ, HOME, sociodemographic and health characteristics for the caregiver and child; as well as caregiver depression and social support, using the Hopkins Symptom Checklist (HSC)30 and Duke UNC social support scale (DUSSC),31 32 respectively. The EASQ, HOME, HSC and DUSSC have all been translated into Spanish and successfully used in prior studies by our team.33 34 Interviews were conducted by trained study staff. EASQ and HOME data were collected on handheld devices and other data were collected on paper, double-entered into a database developed in the SES local informatics system. Data entry errors and conflicts were reconciled.
At 3 months (postintervention), all dyads were assessed using the EASQ and HOME. Primary outcomes measured the difference between intervention and control arms in: 1) children’s mean development postintervention indicators as measured by EASQ z-scores, 2) mean change from baseline to postintervention in children’s development, 3) home environment and parent behaviour postintervention as measured by HOME and 4) mean change from baseline to postintervention in home environment and parent behaviour.
Analysis
Data were analysed using Stata V.13.35 Two-by-two tables using Χ2 or Wilcoxon rank sum, t-tests and univariable and multivariable logistic and linear regressions were conducted for binary and continuous baseline covariates and outcomes, respectively (using robust SEs to account for clustering by district for outcomes). Mean differences and SD in EASQ and HOME score change from baseline to 3-month follow-up between the two study arms were calculated using linear regression. Baseline covariates significantly different (p<0.05) between intervention and control arms were adjusted for in the final model.
We calculated EASQ scores and converted to two sets of age-adjusted z-scores using age-category specific means and SD; the first z-score compares the CASITA study population with that of the World Bank normed Peruvian population (‘WB z-score’, valid for children aged 3–24 months), and the second z-score is based only on the study population (‘Internal z’, valid for children aged 3–31 months). The World Bank data only applies to children under 2 years so primary analysis was conducted on children under 2 years at the second data collection. HOME scores were calculated per the administration manual.29 EASQ and HOME results are presented as overall scores and subscale scores; subscales for EASQ include motor, communication and personal/social domains; subscales for HOME include parent responsivity, acceptance and involvement, household organisation, learning materials and variety. Effect sizes were calculated in SD using Stata’s esize command to calculate Cohen’s d.
The study was reviewed and approved by the Partners Institutional Review Board at Brigham and Women’s Hospital and by the Instituto Nacional de Salud del Niño (National Children’s Institute) in Peru. Partners IRB reviewed and approved the study and designated it as exempt from clinical trial registration under FDAAA (Food and Drug Administration Amendments Act 2007); nonetheless, the trial was registered in clinicaltrials.gov (ID# NCT03010306) to comply with ICMJE (International Committee of Medical Journal Editors) guidelines.
Results
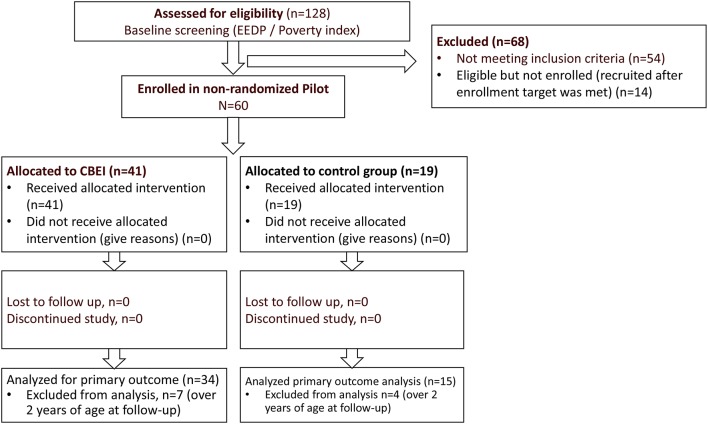
One hundred and twenty-eight children were screened. Of these, 68 were not enrolled; 54 (42.2%) were ineligible and 14 were being screened for eligibility when the enrolment target was reached (figure 2). No families refused participation.
Figure 2.
CASITA flow chart. EASQ, Extended Ages and Stages Questionnaire; EEDP, Escala de Evaluacion del Desarollo Psychomotor; HOME, Home Observation Measurement of the Environment.
Sixty mother/child dyads (all primary caregivers were mothers) met eligibility criteria and were enrolled into the study; 41 into the intervention arm and 19 into the control arm. No significant differences existed between the study arms at baseline on EASQ or HOME scores (table 1). A few baseline differences between study arms were noted: more male children were enrolled in the intervention arm (53.7% vs 36.8%, p<0.05); more caregivers in the intervention arm were married or living as married (97.6% vs 78.9%, p<0.05) and fewer children in the intervention arm had more than one primary caregiver (39.0% vs 68.4%, p<0.05). Also, caregivers in the intervention arm had lower mean HSC scores (indicating greater depression symptoms) than those in the control group (24.7 vs 28.4, p<0.05). Analyses of score changes were therefore adjusted for child sex, caregiver marital status, baseline HSC score and number of primary caregivers (one vs more than one).
Table 1.
Baseline characteristics, EASQ, HOME and psychosocial scores, by study arm, n=60
| Variable | Control arm (n=19) N (%) or mean (SD) | Intervention arm (n=41) N (%) or mean (SD) | P values |
| Child’s age in months* | 13.4 (5.9); range: 6–22 | 15.9 (4.2), range: 6–23 | 0.10 |
| Male sex | 7 (37) | 22 (54) | 0.22 |
| Ever breast fed | 16 (84) | 35 (85) | 0.91 |
| In utero substance-use exposure (maternal self-report) | 0 | 0 | NA |
| Mean number of weeks gestation at birth | 39.4 (0.9) | 38.8 (1.3) | 0.19 |
| More than one primary caregiver | 13 (68) | 16 (39) | 0.03 |
| Parent has HIV | 0 | 0 | NA |
| Mother’s education level higher than primary school | 14 (73.6) | 30 (73.2) | 0.97 |
| Mother’s occupation is non-remunerative (housewife, student or not employed)** | 17 (89.5) | 34 (82.9) | 0.71 |
| Mother is married or living as married** | 15 (79) | 40 (98) | 0.23 |
| Maternal history of depression or attempted suicide** | 2 (10.5) | 5 (12.2) | 1.0 |
| Hopkins Symptom Checklist score | 28.4 (6.8) | 24.6 (5.9) | 0.04 |
| Social Support score | 27.1 (5.9) | 26.7 (6.5) | 0.82 |
*P value calculated using Wilcoxon rank sum test.
†P value calculated using Fisher’s exact test.
EASQ, Extended Ages and Stages Questionnaire; HOME, Home Observation Measurement of the Environment; NA, not available.
Child development outcomes
The mean baseline EASQ score was 602 (SD 195) and mean WB z-score was −1.38 (SD 0.93) (table 1). This compares with a mean score of 603 (SD 222) for the normed Peruvian population.26 Analyses comparing the normed Peruvian population were limited to the 49 children (81.7%) who were under age 2 at study completion: 15/19 in the intervention arm, 34/41 in the control arm. The intervention group improved significantly in all domains, whereas the control group’s z-scores did not improve significantly in any domain (table 2). The mean adjusted difference in change in WB z-scores between the two study arms was 1.39 (95% CI 0.49 to 2.29) (table 3), confirming that the differences in the intervention arm were significantly improved over differences in the control arm. Cohen’s d effect size was 0.87 (95% CI 0.23 to 1.50). Local z-scores and mean changes for all 60 children demonstrated similar findings: no difference at baseline, but significantly higher raw and adjusted mean differences in the CASITA arm.
Table 2.
Mean baseline and 3-month EASQ z-scores, and comparison of z-score changes, by study arm, n=60
| EASQ z-scores vs World Bank normed Peruvian data (limited to children aged 6–24 months at follow-up) | ||||||||
| Variable | Baseline (n=49) |
3-month follow-up (n=49) |
Difference in differences, intervention-control (n=49) | |||||
| Control arm mean (robust SE) (n=19) | Intervention arm mean (robust SE) (n=41) | Robust P values | Control arm mean (robust SE) (n=15) | Intervention arm mean (robust SE) (n=34) | Robust P values | Unadjusted mean difference (95% CI; P values) | Adjusted mean difference (95% CI; P values) | |
| Total EASQ z-scores | −1.26 (0.26) | −1.52 (0.16) | 0.39 | −1.36 (1.67) | −0.55 (0.98) | 0.08 | 1.06 (0.13 to 2.00; 0.025) | 1.39 (0.49 to 2.29; 0.003) |
| EASQ communication domain z-scores | −1.76 (0.21) | −1.82 (0.17) | 0.81 | −1.42 (1.45) | −0.83(0.77) | 0.07 | 0.65 (−0.15 to 1.46; 0.11) | 0.97 (0.23 to 1.71; 0.01) |
| EASQ motor domain z-scores | −0.59 (0.25) | −0.52 (0.15) | 0.82 | −0.57 (1.27) | 0.07 (0.91) | 0.08 | 0.57 (− 0.13 to 1.27; 0.11) | 0.67 (−0.09 to 1.43; 0.08) |
| EASQ personal/social domain z-scores | −0.85 (0.35) | −1.41 (0.21) | 0.17 | −1.31 (1.52) | −0.48 (1.07) | 0.06 | 1.40 (0.42 to 2.39; 0.006) | 1.72 (0.77 to 2.67; 0.001) |
| ‘Internal’ EASQ z-scores from the complete population of enrollees | ||||||||
| Baseline (n=60) |
3-month follow-up (n=49) |
Difference in differences, intervention-control (n=49) | ||||||
| Control arm mean (robust SE) (n=19) | Intervention arm mean (robust SE) (n=41) | Robust P values | Control arm mean (robust SE) (n=19) | Intervention arm mean (robust SE) (n=41) | Robust P values | Unadjusted mean difference (95% CI; P values) | Adjusted mean difference (95% CI; P values) | |
| EASQ total z-scores in local cohort | −0.39 (0.17) | −0.33 (0.14) | 0.78 | −0.31 (0.29) | 0.36 (0.14) | 0.04 | 0.67 (0.02 to 1.32; 0.04) | 0.74 (0.13 to 1.35; 0.019) |
| EASQ communication domain z-scores | −0.33 (0.13) | −0.46 (0.13) | 0.51 | −0.20 (0.29) | 0.31 (0.14) | 0.11 | 0.67 (0.00 to 1.33; 0.05) | 0.88 (0.26 to 1.51; 0.007) |
| EASQ motor domain z-scores | −0.38 (0.22) | −0.24 (0.14) | 0.59 | −0.33 (0.27) | 0.22 (0.13) | 0.07 | 0.46 (-0.16 to 1.07; 0.14) | 0.37 (−0.23 to 0.98; 0.22) |
| EASQ personal/social domain z-scores | −0.23 (0.21) | −0.14 (0.16) | 0.73 | −0.12 (0.24) | 0.39 (0.15) | 0.07 | 0.50 (−0.13 to 1.14; 0.11) | 0.54 (−0.12 to 1.21; 0.10) |
EASQ, Extended Ages and Stages Questionnaire.
Table 3.
Mean baseline and 3-month HOME scores, and comparison of changes, by study arm, n=60
| Variable | Baseline (n = 60) | 3-month follow-up (n=60) | Difference in differences, intervention-control | |||||
| Control arm mean (robust SE) (n = 19) | Intervention arm mean (robust SE) (n = 41) | Robust P values | Control arm mean (robust SE) (n=19) | Intervention arm mean (robust SE) (n = 4) | Robust P values | Unadjusted mean difference (95% CI; P values) | Adjusted mean difference (95% CI; P values) | |
| HOME total scores | 21.90 (1.39) | 22.20 (0.78) | 0.86 | 24.10 (1.44) | 30.12 (0.90) | 0.001 | 5.76 (1.77 to 9.83; 0.006) | 6.33 (1.93 to 10.73; 0.006) |
| HOME responsivity | 5.89 (0.48) | 5.58 (0.30) | 0.58 | 6.37 (0.54) | 8.61 (0.31) | 0.001 | 2.55 (1.15 to 3.94; 0.001) | 2.69 (1.19 to 4.18; 0.001) |
| HOME acceptance | 4.89 (0.37) | 5.19 (0.24) | 0.50 | 5.26 (0.39) | 5.87 (0.21) | 0.17 | 0.31 (-0.99 to 1.61; 0.63) | 0.52 (−0.82 to 1.87; 0.44) |
| HOME involvement | 1.74 (0.28) | 2.02 (0.20) | 0.41 | 2.15 (0.43) | 3.85 (0.25) | 0.001 | 1.40 (0.43 to 2.39; 0.006) | 1.54 (0.42 to 2.66; 0.008) |
HOME, Home Observation Measurement of the Environment.
Home environment and parent behaviour outcomes
Mean baseline HOME scores were 22.1 (SD 5.27) (table 3). No significant baseline differences existed for any of the subdomains between study arms. HOME scores improved in both study arms at 3 months, with the intervention arm reporting both a significantly higher mean HOME score than the control arm (30.1 vs 24.1, p<0.01) and significantly greater improvement than the control arm after the intervention period (table 3), with a Cohen’s d effect size of 0.85 (95% CI 0.28 to 1.41). The mean change in scores for both involvement and responsivity subscales were significantly greater in the intervention versus control arm (table 3).
Discussion
CASITA plus nutritional supplementation was highly successful compared with nutritional supplementation alone at improving child development, the home environment and parenting behaviour. Findings are consistent with and generally better than those of other studies of comparable interventions.36–39 A recent community randomised trial in Uganda assessed a 12-session group parenting intervention (plus individual home visits). Children (aged 12–36 months) in the intervention group had significantly higher effect sizes of cognitive (Cohen’s d=0.36) and language scores (d=0.27) and mothers had lower depression after the intervention, compared with controls.40 CASITA was based on successful programmes in other settings, so while this finding is not surprising, these results are encouraging in this challenging setting of urban poverty.
The CASITA intervention was successfully delivered both individually and in groups. For both modalities of delivery, well-trained and supervised CHWs consistently delivered the intervention with high fidelity, requiring minimal support from health professionals. The use of CHWs to identify at-risk children in the community and deliver a structured intervention is a strength of this study. Our data add to existing literature of CHW-delivered early child interventions in resource-poor settings. In a 2002 study, CHWs in South Africa delivered a home-based intervention for maternal depression and parenting skills, resulting in significant increases in maternal sensitivity and positive affect during feeding, and infant physical development.36 Community health aides in Jamaica provided a parenting and development coaching intervention to the caregivers of undernourished children aged 9–30 months, resulting in improved child development and greater maternal knowledge and childrearing practices.41
This was a small pilot study in one urban setting in Peru; findings may not be generalisable elsewhere. Although most baseline characteristics did not significantly differ between arms, caregiver marital status and number of caregivers did differ, with potential bias in favour of the intervention group. Although we controlled for these baseline factors, unmeasured or residual confounding remains a possibility. Because of resource constraints, our sample size is small; we did not conduct a priori power calculations and did not randomise at the level of the individual. Although we used robust standard errors during regression to mitigate the possible effects of clustering at the clinic level, it is possible that non-independence may have biased our results. Combining group and individual CASITA in the analysis, while weakening our ability to compare the effects of different modalities, increased our sample size; of note, outcomes did not differ significantly between individual and group study arms.
This pilot demonstrates that community-based early parenting and support interventions can improve child development and home environment characteristics in this resource-limited setting. CASITA is well-poised to scale to a wider area, given its low cost and reliance on CHWs to identify vulnerable children and deliver the structured intervention. Expansion to all of Caraballyo is underway, including screening of 6000 children and a randomised controlled trial of >350 children. This larger research study will, we hope, determine how effective and cost-effective CASITA is at scale and over longer periods of time.
Acknowledgments
The authors would like to acknowledge the assistance of Dr Patricia Kariger, PhD (University of California, Berkeley, provided access to data to calculate age-adjusted z-scores. Shared EASQ instrument. No compensation was given) and Dr Lia Fernald, PhD, MBA (University of California, Berkeley School of Public Health, provided access to data to calculate age-adjusted z-scores. Shared EASQ instrument. No compensation was given) for their help with understanding the EASQ, Carmen Contreras, BA (Socios En Salud, Partners In Health, Sucursal Peru, provided budget and programmatic guidance), Director of Intervention Projects for Socios En Salud for discussions and manuscript review, the data collectors and the participants of the project and their families.
Footnotes
AKN and ACM contributed equally.
Contributors: AKN helped to design the study, co-wrote the protocol, provided training and data collection oversight during the project, helped to analyse data, co-drafted the initial manuscript and co-wrote the final manuscript. ACM helped to design the analysis plan and the study design, collaborated on the protocol, conducted all quantitative analysis and provided interpretation, co-drafted the initial manuscript and co-wrote the final manuscript. MM oversaw the implementation of the project as Program Manager and Co-Principal Investigator, helped to design the CASITA protocol, trained the community health workers, oversaw data collection reviewed and approved the final manuscript as submitted. NR as Project Coordinator coordinated and supervised all study activities including data collection and quality control in Peru, assisted with early drafts of the manuscript, reviewed and revised all versions of the manuscript and approved the final manuscript as submitted. BK contributed to the selection of instruments, conducted training and data collection oversight, provided interpretation of data analysis, reviewed and revised all versions of the protocol and manuscript and approved the final manuscript as submitted. MV helped to design the initial intervention, helped to select tools for evaluation, participated in the adaptation of the intervention to the local setting, reviewed and revised all versions of the manuscript and approved the final manuscript as submitted. SL contributed to the selection of instruments, conducted training and data collection oversight, provided interpretation of data analysis, reviewed and revised all versions of the protocol and manuscript and approved the final manuscript as submitted. GS contributed significantly to the design of the CASITA intervention and the fidelity and monitoring activities of the CHWs, assisted in data collection, reviewed and revised the manuscript and approved the final manuscript as submitted. LL is the Co-Principal Investigator for the CASITA study. He collaborated on the protocol, contributed significant knowledge of the political landscape in Carabayllo to the study, reviewed and revised the final version of the manuscript and approved it as submitted. AC: collaborated on the protocol and helped to adapt and implement study instruments such as the HOME and EASQ in Carabayllo to the local setting, provided interpretation of results and reviewed and revised the final version of the manuscript and approved it as submitted. YV: contributed to data collection, assisted to modify study instrument application to suit local context, contributed to the early draft sections of the manuscript, reviewed and revised the final version of the manuscript and approved it as submitted. SAA designed statistical programs for analysis, assisted in conducting analyses and reviewed and revised all versions of the manuscript and approved the final manuscript for submission. SSS conceptualised and designed the study, wrote the protocol, provided analysis interpretation, co-wrote the first version of the manuscript and reviewed and revised all versions of the manuscript.
Funding: Support for this study was provided by Grand Challenges Canada, Saving Brains Seed Grant 0351-03. ‘Community-based Family Coaching for Children with Developmental Risks in Lima, Peru’. MV was also supported by the Griffin Foundation, for SPARK global knowledge exchange activities.
Disclaimer: The funders had no role in study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the manuscript for publication.
Competing interests: All authors (excepting SAA) had financial support from Grand Challenges Canada for the submitted work; MV is the executive director of the SPARK center; MM, NR, GS, LL, AC and YV are all employed by Socios en Salud. The authors declare no other relationships or activities that could appear to have influenced the submitted work.
Patient consent: Not required.
Ethics approval: Partners Institutional Review Board at Brigham and Women’s Hospital and by the Institute Nacional de la Salud del Nino (National Children’s Institute) in Peru.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Engle PL, Black MM, Behrman JR, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet 2007;369:229–42. doi:10.1016/S0140-6736(07)60112-3 [DOI] [PubMed] [Google Scholar]
- 2. Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. doi:10.1016/S0140-6736(07)60032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health 2016;4:e916–e922. doi:10.1016/S2214-109X(16)30266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernald L, Kariger P, Engle P, et al. Examining early child development in low-income countries: a toolkit for the assessment of children in the first five years of life. Washington DC: World Bank, 2009. [Google Scholar]
- 5. Servili C, Medhin G, Hanlon C, et al. Maternal common mental disorders and infant development in Ethiopia: the P-MaMiE Birth Cohort. BMC Public Health 2010;10:693 doi:10.1186/1471-2458-10-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bécares L, Nazroo J, Kelly Y. A longitudinal examination of maternal, family, and area-level experiences of racism on children’s socioemotional development: patterns and possible explanations. Soc Sci Med 2015;142:128–35. doi:10.1016/j.socscimed.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 7. Rahman A, Harrington R, Bunn J. Can maternal depression increase infant risk of illness and growth impairment in developing countries? Child Care Health Dev 2002;28:51–6. doi:10.1046/j.1365-2214.2002.00239.x [DOI] [PubMed] [Google Scholar]
- 8. Hanson JL, Chandra A, Wolfe BL, Pollak SD, et al. Association between income and the hippocampus. PLoS One 2011;6:e18712 doi:10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Closing the gap on what we know and what we do. The science of early child development. Boston: Center on the Developing Child Harvard University, 2007. [Google Scholar]
- 10. Herman-Smith R. Intimate partner violence exposure in early childhood: an ecobiodevelopmental perspective. Health Soc Work 2013;38:231–9. doi:10.1093/hsw/hlt018 [DOI] [PubMed] [Google Scholar]
- 11. Shonkoff JP, Garner AS, et al. Committee on Psychosocial Aspects of Child and Family Health. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232–e246. doi:10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- 12. Luby J, Belden A, Botteron K, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr 2013;167:1135–42. doi:10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garner AS. Home visiting and the biology of toxic stress: opportunities to address early childhood adversity. Pediatrics 2013;132(Suppl 2):S65–S73. doi:10.1542/peds.2013-1021D [DOI] [PubMed] [Google Scholar]
- 14. First LR, Palfrey JS. The infant or young child with developmental delay. N Engl J Med 1994;330:478–83. doi:10.1056/NEJM199402173300708 [DOI] [PubMed] [Google Scholar]
- 15. Grassi JR, La Morto-Corse A. Identification and remediation of basic cognitive deficits in disadvantaged children. J Learn Disabil 1979;12:483–7. doi:10.1177/002221947901200709 [DOI] [PubMed] [Google Scholar]
- 16. Law J, Garrett Z, Nye C. The efficacy of treatment for children with developmental speech and language delay/disorder: a meta-analysis. J Speech Lang Hear Res 2004;47:924–43. doi:10.1044/1092-4388(2004/069) [DOI] [PubMed] [Google Scholar]
- 17. Nelson HD, Nygren P, Walker M, et al. Screening for speech and language delay in preschool children: systematic evidence review for the US Preventive Services Task Force. Pediatrics 2006;117:e298–e319. doi:10.1542/peds.2005-1467 [DOI] [PubMed] [Google Scholar]
- 18. Grantham-McGregor SM, Powell CA, Walker SP, et al. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican Study. Lancet 1991;338:1–5. doi:10.1016/0140-6736(91)90001-6 [DOI] [PubMed] [Google Scholar]
- 19. Britto PR, Lye SJ, Proulx K, et al. Nurturing care: promoting early childhood development. Lancet 2017;389 doi:10.1016/S0140-6736(16)31390-3 [DOI] [PubMed] [Google Scholar]
- 20. Care. Promoting Early Childhood Development for OVC in Resource Constrained Settings: The 5x5 Model, 2008. [Google Scholar]
- 21. Nahar B, Hamadani JD, Ahmed T, et al. Effects of psychosocial stimulation on growth and development of severely malnourished children in a nutrition unit in Bangladesh. Eur J Clin Nutr 2009;63:725–31. doi:10.1038/ejcn.2008.44 [DOI] [PubMed] [Google Scholar]
- 22. Folger AT, Brentley AL, Goyal NK, Hall ES, et al. Evaluation of a community-based approach to strengthen retention in early childhood home visiting. Prev Sci 2016;17:52–61. doi:10.1007/s11121-015-0600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. SPARK Center BMC. SPARK Center (Supporting Parents and Resilient Kids). http://www.bmc.org/pediatrics-sparkcenter.htm (accessed 14 Jan 2016).
- 24. Rodriguez S, Escala de evaluacion del desarollo psicomotor: 0 a 24 meses. 12th edn Santiago, Chile: Galdoc, 1996. [Google Scholar]
- 25. Schreiner M. Progress out of Poverty Index: A Simple Poverty Score for Peru. St Louis, MO: Grameen Foundation, 2008. [Google Scholar]
- 26. Fernald LC, Kariger P, Hidrobo M, et al. Socioeconomic gradients in child development in very young children: evidence from India, Indonesia, Peru, and Senegal. Proc Natl Acad Sci U S A 2012;109(Suppl 2):17273–80. doi:10.1073/pnas.1121241109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Squires J, Bricker D. Ages and stages questionnaires in Spanish: a parent-completed child monitoring system. 3rd edn: Paul H, Brookes Publishing Company, 2009. [Google Scholar]
- 28. Bricker D, Squires J, Ages and stages questionnaires: a parent completed, child monitoring system. 2nd edn Baltimore, MD: Paul Brookes, 1999. [Google Scholar]
- 29. Caldwell B, Bradley R, Home Inventory Administration Manual. 3rd edn Little Rock, AR: University of Arkansas at Little Rock, 2001. [Google Scholar]
- 30. Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry 1974;7:79–110. [DOI] [PubMed] [Google Scholar]
- 31. Broadhead WE, Gehlbach SH, de Gruy FV, et al. The Duke-UNC functional social support questionnaire. measurement of social support in family medicine patients. Med Care 1988;26:709–23. [DOI] [PubMed] [Google Scholar]
- 32. Bellón Saameño JA, Delgado Sánchez A, Luna del Castillo JD, et al. [Validity and reliability of the Duke-UNC-11 questionnaire of functional social support]. Aten Primaria 1996;186:153–63. [PubMed] [Google Scholar]
- 33. Muñoz M, Nelson A, Johnson M, Godoy N, et al. Community-based needs assessment of neurodevelopment, caregiver, and home environment factors in young children affected by HIV in Lima, Peru. J Int Assoc Provid AIDS Care 2017;16 doi:10.1177/2325957416631625 [DOI] [PubMed] [Google Scholar]
- 34. Shin S, Muñoz M, Espiritu B, et al. Psychosocial impact of poverty on antiretroviral nonadherence among HIV-TB coinfected patients in Lima, Peru. J Int Assoc Physicians AIDS Care 2008;7:74–81. doi:10.1177/1545109708315326 [DOI] [PubMed] [Google Scholar]
- 35. Stata Statistical Software: Release 13. College Station, TX: StataCorp LLC, 2013. [Google Scholar]
- 36. Cooper PJ, Landman M, Tomlinson M, et al. Impact of a mother-infant intervention in an indigent peri-urban South African context: pilot study. Br J Psychiatry 2002;180:76–81. doi:10.1192/bjp.180.1.76 [DOI] [PubMed] [Google Scholar]
- 37. Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr 2013;163:1409–16. doi:10.1016/j.jpeds.2013.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gardner JM, Walker SP, Powell CA, et al. A randomized controlled trial of a home-visiting intervention on cognition and behavior in term low birth weight infants. J Pediatr 2003;143:634–9. doi:10.1067/S0022-3476(03)00455-4 [DOI] [PubMed] [Google Scholar]
- 39. Resnick MB, Eyler FD, Nelson RM, et al. Developmental intervention for low birth weight infants: improved early development outcome. Pediatrics 1987;80:68–74. [PubMed] [Google Scholar]
- 40. Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster randomised trial. Lancet Glob Health 2015;3:e458–e469. doi:10.1016/S2214-109X(15)00099-6 [DOI] [PubMed] [Google Scholar]
- 41. Powell C, Baker-Henningham H, Walker S, et al. Feasibility of integrating early stimulation into primary care for undernourished Jamaican children: cluster randomised controlled trial. BMJ 2004;329:89 doi:10.1136/bmj.38132.503472.7C [DOI] [PMC free article] [PubMed] [Google Scholar]