Abstract
Air pollution generated in urban areas is a global public health burden since half of the world’s population live in either cities, megacities or periurban areas. Its direct effects include initiating and exacerbating disease, with indirect effects on health mediated via climate change putting the basic needs of water, air and food at risk.
Keywords: respiratory
What is already known on this topic?
Air pollution is a global problem with negative health effects on the respiratory, cardiovascular and neurological systems.
There is robust evidence that the effects of air pollution span over the life course, with growing children being particularly vulnerable.
Diesel vehicles produce disproportionally more air pollution and should be a focus of exposure-mitigation policies.
What this study hopes to add?
The role of emissions from diesel in contributing to exposure of UK children is reviewed.
The adverse health effects of diesel emissions on UK children is reviewed.
Ways of reducing exposure of children to fossil-fuel-derived air pollution in the UK, on personal and national levels, are discussed.
Introduction
There is strong epidemiological evidence that air pollution is associated with a wide range of adverse health effects on the respiratory, cardiovascular and neurological systems.1–3 Indeed, in the UK, the combination of new-onset (incident) diseases associated with long-term exposure, and exacerbation of diseases once disease is established results in approximately 40 000 excess deaths a year that are attributable to air pollution, increasing health service and social costs by over £20 billion a year.2 Although deaths associated with air pollution are mainly in adults, there is also increasing concern that air pollution, especially from diesel vehicles, has major adverse effects in children and that this has long-term consequences.4–6 In this review, we report the evidence that underpins the need for exposure reduction policy to focus on diesel vehicles and the potential beneficial effects of such a policy on children’s health. Although this review focuses on the heavily dieselised UK environment, it is also relevant to countries where diesel vehicles remain a major source of emissions.
Components of air pollution
The major outdoor pollutants in urban areas are inhalable particulate matter (PM, measured as either PM less than 10 µm in aerodynamic diameter (PM10) or the even smaller PM2.5), nitrogen oxides (NOX, such as nitrogen dioxide, NO2), ozone (O3), sulfur dioxide (SO2), carbon monoxide (CO) and hydrocarbons (HC). Sources of these include gasoline-powered and diesel-powered engines from vehicles, trains and, in port towns, ships (proximately PM, NOX), vehicle tyre and brake wear (PM), power stations and factories from coal combustion and biomass burning (PM, NOX and SO2),7–9 and wood burning heating that is increasingly popular, contributing up to 9% of PM in London during winter.10 For diesel engines, an important component of emissions is black carbon, that is, the fraction of PM that most strongly absorbs light—a component that is often called ‘diesel soot’. Another pollutant, ozone, is formed by the reaction of NOX with carbon compounds called volatile organic compounds (VOCs) in the presence of sunlight. Two of the most important VOCs emitted by vehicles are benzene and 1,3-butadiene. For emissions from diesel, there is a strong correlation between locally emitted PM10 and NOX,11 and it is reasonable to assume that, where diesel vehicles predominate, either metric is a good marker of exposure to the locally generated pollutant mix in urban areas.
Why focus on diesel?
Many parts of the UK breach the EU legal limits and WHO guidelines (table 1) for pollutants on a regular basis.12 While London often exhibits the biggest breach of pollution limits, other parts of the UK are also affected. Indeed, a recent report from the Department of Environment Food and Rural Affairs and the Department of Transport showed 37 out of 43 reporting zones across the UK had maximum annual mean NO2 concentrations over the EU legal limit.13
Table 1.
EU limits, WHO guidelines and main sources of ambient (outdoor) air pollutants. Adapted from European Commission Air Quality Standards (updated September 2017), WHO Ambient (outdoor) air quality and health fact sheet (updated Sept 2016), and Lethal and Illegal, Solving London’s Air Pollution Crisis by Institute for Public Policy Research, November 2016
| Pollutants | EU legal limits (averaging period) |
WHO guidelines (averaging period) |
Main sources |
| Nitrogen dioxide (NO2) | 200 µg/m3 (1 hour) 40 µg/m3 (1 year) |
200 µg/m3 (1 hour) 40 µg/m3 (1 year) |
Transport, combustion |
| Ozone (O3) | 120 µg/m3 (8 hours) | 100 µg/m3 (8 hours) | Reaction of hydrocarbons, nitrogen oxides and volatile organic compounds in sunlight |
| Particulate matter (PM10) | 50 µg/m3 (24 hours) 40 µg/m3 (1 year) |
50 µg/m3 (24 hours) 20 µg/m3 (1 year) |
Transport (exhaust, tyre, brake wear), combustion, industrial processes and construction |
| Particulate matter (PM2.5) | 25 µg/m3 (1 year) | 10 µg/m3 (24 hours) 25 µg/m3 (1 year) |
|
| Sulfur dioxide (SO2) | 350 µg/m3 (1 hour) 125 µg/m3 (24 hours) |
500 µg/m3 (10 min) 20 µg/m3 (24 hours) |
Coal combustion and road transport |
While there are other sources of outdoor air pollution, the largest contributor to air pollution in urban areas in the UK is road traffic, which has been rising over the last 60 years. By contrast, active forms of transport such as walking and cycling have been on a decline.2 In the UK, approximately 50% of NO2 emissions come from the roads,14 with diesel engines powering half the cars and the majority of heavy vehicles.15 At a global level, diesel vehicles contribute about 20% of NOx.16 As discussed above, fossil-fuel-powered engines emit carbon monoxide (CO), hydrocarbons (HC), PM and NOx, all of which are associated with negative health effects.17 The reason why diesel engines should be a major target for exposure-reduction strategies is that they emit more PM and NOx than their petrol or hybrid counterparts, contributing to about 40% of all NOx emissions in inner cities.18 19 Furthermore, diesel, and not petrol, soot is categorised by WHO as carcinogenic20—a categorisation that implies that diesel PM is, mass for mass, more toxic than petrol PM. Vehicle emissions are regulated by the European Union (EU) Euro standards, currently at Euro 6 (table 2).
Table 2.
EU Euro emissions standards. Adapted from Lethal and Illegal, Solving London’s Air Pollution Crisis by Institute for Public Policy Research, September 2016, and SMMT Euro Standards for Cars (accessed March 2018)
| Euro emissions standards | Petrol cars | Diesel cars | ||
| NOX
(g/km) |
PM10
(g/km) |
NOX
(g/km) |
PM10
(g/km) |
|
| Euro 4 (2005) | 0.08 | – | 0.25 | 0.025 |
| Euro 5 (2009) | 0.06 | 0.005 | 0.18 | 0.005 |
| Euro 6 (2014) | 0.06 | 0.005 | 0.08 | 0.0045 |
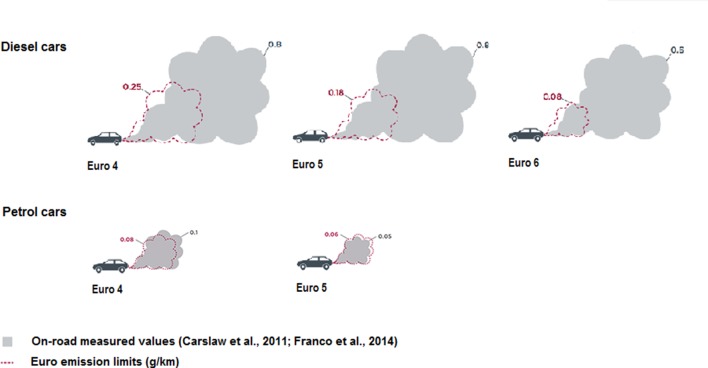
Compliance with Euro standards is assessed under laboratory conditions only and are less strict for diesel engines. But even given this leeway, recent measurements under real-life driving conditions have shown that diesel cars produce significantly more toxic emissions than the Euro standard, whereas petrol engines map closely to the laboratory Euro standard (figure 1); this phenomenon is observed globally, and Anenberg et al 16 reported approximately a third of heavy-duty and over half of light-duty diesel vehicle emissions breaching the certification limits, across 11 major vehicle markets. Thus, over 2000 education or childcare providers in England and Wales are located close to busy roads with concentrations of NOX that are regularly higher than legal limits (40 µg/m3 annual mean or 200 µg/m3 1 hour mean).14 21 22 In addition, children attending these schools are exposed to high concentrations of freshly generated diesel pollutants during the commute to and from school and during outdoor activities (figure 2).
Figure 1.
Real-life NOX emissions from diesel and petrol cars compared with Euro emissions standards. Adapted from the Impact of improved regulation of real-world NOX emissions from diesel passenger cars in the EU, 2015–2030 by the International Council on Clean Transportation, 2016.
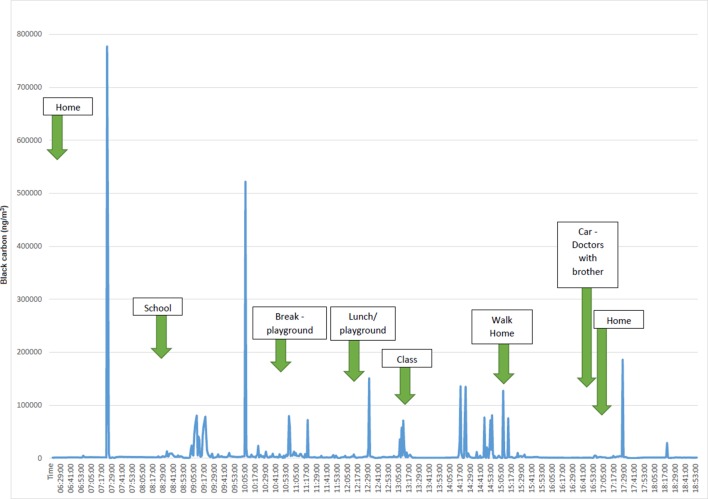
Figure 2.
Black carbon levels (ng/m3) from an aethalometer carried by a child in London on a typical school day. In London, diesel vehicles emit a disproportional amount of black carbon.
Health effects of diesel emissions on children
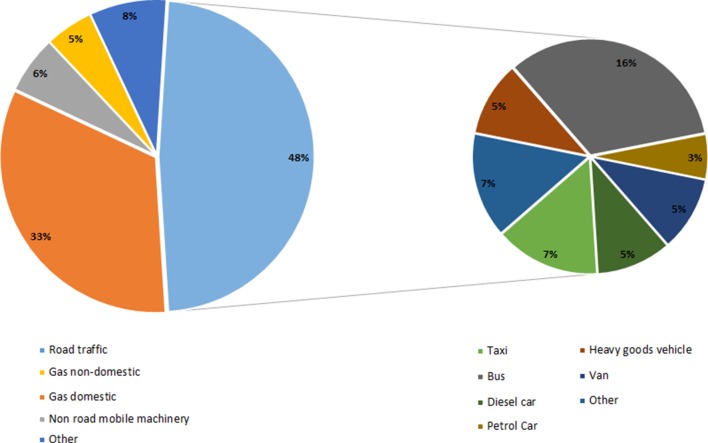
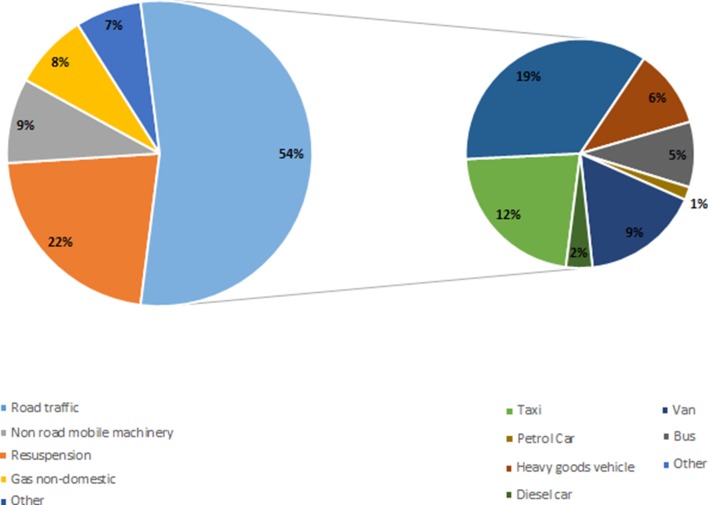
Few epidemiological studies address the effects of diesel emissions alone. However, it is reasonable to extrapolate from studies that have assessed exposure to either PM or NOX since (1) diesel PM is not less toxic than other types of PM, and (2) the adverse effects of gases such as NOX are independent of source. One way of estimating the health burden from diesel emissions alone is to use source apportionment data. For example, in London where most taxis, buses, heavy goods vehicles and vans are powered by diesel (table 3), 48% of NOX and 54% of PM10 is from road transport (figures 3 and 4).19 23 These vehicles, along with diesel cars, are responsible for 34% of total PM10 and 38% of total NOX emissions (figures 3 and 4).23
Table 3.
Fuel sources of vehicles in London, 2015 (adapted from Lethal and Illegal, Solving London’s Air Pollution Crisis by Institute for Public Policy Research, September 2016)
| Vehicles | Petrol (%) | Diesel (%) | Other (%) |
| Buses | 0 | 89 | 11 |
| Taxis | 0 | 100 | 0 |
| Private vehicles | 42 | 57 | 1 |
| Light goods vehicles | 2 | 97 | 1 |
| Heavy goods vehicles | 0 | 100 | 0 |
Figure 3.
Sources of NOX emissions in London, 2010. Adapted from Lethal and Illegal, Solving London’s Air Pollution Crisis by Institute for Public Policy Research, November 2016.
Figure 4.
Sources of PM10 emissions in London, 2010. Adapted from Lethal and Illegal, Solving London’s Air Pollution Crisis by Institute for Public Policy Research, November 2016.
Antenatal exposure
When considering effects measured in later childhood, it is difficult to separate the effect of maternal exposure to air pollution from postnatal effects—since there is a strong correlation between exposure to traffic-derived air pollutants (TRAPs) of pregnant women and their children. But independent associations between antenatal exposure to NO2 and reduced FEV1 later in childhood are reported. For example, Morales et al 24 reported that an IQR increase in NO2 exposure during the second trimester was associated with an estimated change in childhood FEV1 by −28 mL, while the relative risk of having FEV1 <80% predicted was 1.30. By contrast, effects on the fetus or on the newborn infant must be due to maternal exposure. These epidemiological studies report that maternal exposure to TRAP has adverse effects on the fetus leading to increased infant mortality, reduced fetal growth, low birth weight at term and premature birth.25 26 Indeed, increased risk for the low birth weight for term metric is found at maternal PM2.5 exposure lower than the EU recommended annual limit of 25 µg/m3.26 27 It is likely that these antenatal effects synergise with postnatal pollution exposures to increase susceptibility to common respiratory conditions such as wheeze, bronchiolitis and asthma.28–30
Childhood exposure
Air pollutants, particularly NOX (reflecting exposure to both NOX and PM), are associated with reduced lung function in children—for both FVC and FEV1.5 Urman et al 5 showed that an increase of 17.9 ppb of NOX exposure was associated with a 1.56% deficit in FVC and 1.1% deficit in FEV1, and similar findings were seen in children with or without asthma. Residing in areas with high concentrations of PM and NO2 can also lead to suppression of lung function growth in school children.4 31 This reduction can potentially be halted and reversed with better air quality. For example, Gauderman et al 32 showed that reducing the levels of NO2, PM10 and PM2.5 were associated with improvements in FEV1 and FVC growth in adolescents over 4 years—mean 4-year growth in FEV1 increased by 91.4 mL per 14.1 ppb of NO2 reduction, and 65.5 mL per 8.7 µg/m3 of PM10 reduction, and 65.5 mL per 12.6 µg/m3 of PM2.5 reduction, with comparable findings in FVC. Children with existing chronic illnesses, particularly respiratory conditions, are most vulnerable. Air pollution can predispose individuals to new-onset asthma; preschool children are more prone to new onset of wheeze. A meta-analysis concluded that exposure to NO2 is linked to new-onset asthma, while exposure to PM is linked to new-onset wheeze.33 An effect of diesel PM per se on reactivity to inhaled allergens is supported by the association between long-term traffic pollution exposure and allergies.34–36 Asthma exacerbations are also closely associated with short-term variations in PM2.5.37 Although increasing inhaled corticosteroids prior to high pollution days may seem logical,38 it is unclear whether this strategy is effective.
There is emerging evidence that air pollution impacts on children’s neurological system and development. For example, associations between exposure to air pollutants and reduced IQ and neurocognitive ability such as working memory, autism and reduced brain-derived neurotrophic factor are widely reported.39–41 In particular, Basagaña et al 39 reported that traffic-related PM2.5 was more strongly associated with reduction in cognitive function compared with fine particulates from other sources such as mineral, heavy oil combustion or road dust. In addition, exposure to high levels of traffic-induced pollutants may delay maturation of the brain.42 An additional emerging link is between air pollution and the endocrine system. For example, Thiering et al 43 reported an association between insulin resistance and either NO2 or PM exposure in healthy children.
Implications for adult life
It is increasingly recognised that impaired fetal well-being is a substrate for adult-onset cardiovascular disease such as atherosclerosis.44 Prolonged exposure to air pollutants may increase mean pulmonary arterial pressure and diastolic blood pressure,45 46 predisposing to cardiovascular events and premature death in adulthood. The effect on cognition lingers onto adulthood, where associations with dementia and Parkinson’s disease have been found.47 48
Although the epidemiological evidence for the health effect of fossil-fuel-derived pollution is very strong, there are important confounders that must be considered. For example, in England, increased exposure to mean annual NO2 concentrations is higher in areas of increased social deprivation and reduced access to healthcare.49 Furthermore, children from more deprived areas are also more likely to be exposed to other sources of pollution such as second-hand cigarette smoking.50
Mechanisms
Many of the mechanisms underlying the robust epidemiological associations between air pollution and health across the life course remain to be defined. Effects on organs distant from the lung are likely to be facilitated by mediators released in the airway subsequently leaching out into the systemic circulation.51 A key cell for release of mediators is alveolar macrophage (AM) since phagocytosis of PM by AM stimulates release of cytokines such as interleukin-6, interleukin-8 and tumour necrosis factor.52 53 PM that reaches the most distal airways is phagocytosed in a dose-dependent manner by airway macrophages54 55 (figure 5). Indeed, Kulkarni et al 56 reported that in healthy children, the amount of carbon in AM (as a marker of long-term personal exposure) is inversely associated with lung function. Phagocytosis of inhaled diesel PM by AM is also essential for normal removal of PM from the lungs, which minimises exposure of other airway cells. Conditions that impair AM phagocytosis will increase the proportion of PM impacting on and penetrating airway epithelial cells, further worsening the release of inflammatory mediators.57–59 Indeed, a recent study found significantly lower amounts of diesel soot in AM, compatible with abnormal clearance of inhaled PM, in children with moderate-to-severe asthma compared with healthy controls—despite similar levels of personal exposure to black carbon.60
Figure 5.
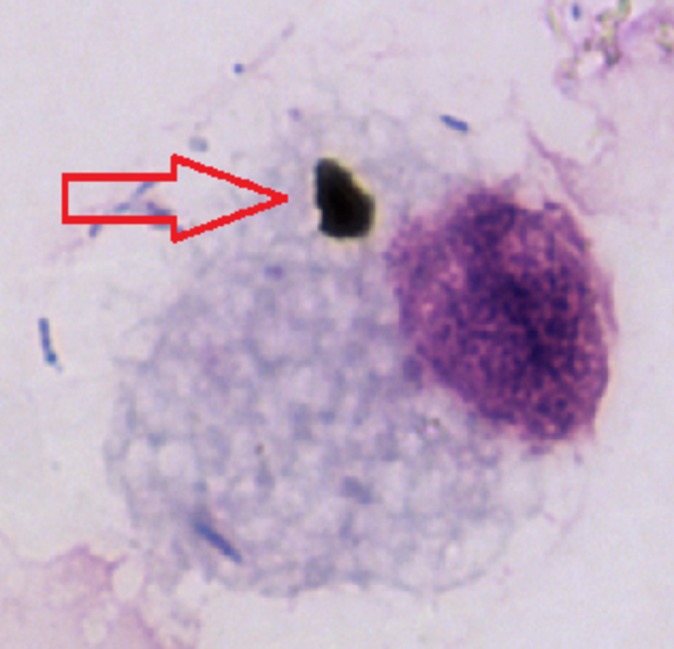
Phagocytosed black carbon (arrow) within an airway macrophage from a healthy child in one of the major cities in the UK.
Increased exposure of airway epithelial cells to PM increases the potential for PM to translocate into the systemic circulation and directly cause adverse effects in distant organs, including the fetus where transplacental transfer of nanomaterials up to 240 nm is possible.61
What can we do about diesel pollution?
National level
In London, air pollution is mostly caused by road traffic, of which diesel vehicles are a major contributor, as discussed above. With an estimated 9400 premature deaths attributable to air pollution, it has the second biggest impact on public health.19 These highly polluting vehicles should therefore be phased out to comply with legal limits of pollutants—and cleaner alternatives encouraged. Tougher national regulations on traffic emissions such as the expansion of Ultra Low Emission Zones and scrappage schemes for older generations of diesel vehicles should be considered. Indeed, the 2016 report from the Institute for Public Policy Research23 estimated that phasing out diesel-powered vehicles in London would lead to large reductions in NOX and NO2 levels, ultimately lowering NO2 levels to comply with EU standards. This report estimated that with a 45% reduction in NOX and 56% reduction in NO2, 1.4 million life-years would be gained along with a financial benefit of up to £800 million.
Planting trees can reduce air pollution by acting as a physical barrier to intercept PM and absorbing gaseous pollutants such as O3,62 although the effect on pollution concentrations at schools is, to date, unclear. However, the amount of pollutants removed by these organic barriers will be proportional to the extent of plantation. Therefore, vast tall hedges around nurseries and schools should be encouraged, but this does not provide protection against pollution exposure during travel to and from schools.
Individual level
Various measures such as walking along less busy roads, cycling, use of public transport and carpooling may reduce exposure to air pollution,63 but the evidence base for whether this is achievable over the long term, and is sufficient to improve health, is limited. The Department for Environment Food and Rural Affairs website provides information and forecast on UK air quality, while the British Lung Foundation provides information on various measures to take according to air pollution levels (table 4).
Table 4.
Advice on measures to take according to air pollution levels (adapted from British Lung Foundation website (https://www.blf.org.uk/support-for-you/air-pollution/what-can-i-do))
| Pollution level | Measures |
| Low |
|
| Moderate |
|
| High |
|
Air cleaning systems are available commercially claiming to reduce indoor pollution—these can either remove particles and gaseous pollutants or have ultraviolet light technology to destroy indoor pollutants.64 All have their limitations, for example, large particles tend to settle before reaching filters, while gaseous pollutant filters may have short lifespans.64 These systems also use electricity—which may not be from sustainable sources. Improvement in our air quality will benefit the whole population with lasting health and economic advantages. We should aim to build cities in order to promote and improve the health of the population.
In conclusion, in the UK, the phasing out of the current diesel car, van and taxi fleet, and replacing this fleet with greener alternatives must be a pillar of exposure-reduction strategy. Changes that would support such an initiative are (1) more active travel supported by better public transport infrastructure, (2) providing electric charging points on residential streets, and (3) providing clinicians with the tools to discuss personal exposure reduction strategies with their patients.
Acknowledgments
We thank the International Council on Clean Transportation (ICCT) for their permission to adapt infographics from their website.
Footnotes
Contributors: All authors contributed equally to this review article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Newby DE, Mannucci PM, Tell GS, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015;36:83–93. doi:10.1093/eurheartj/ehu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holegate S, Grigg J, Raymond A, et al. Every breath we take: the lifelong impact of air pollution, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Review of evidence on health aspects of air pollution—REVIHAAP Project. 2013http://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report-final-version.pdf?ua=1 [PubMed]
- 4. Chen Z, Salam MT, Eckel SP, et al. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. J Thorac Dis 2015;7:46–58. doi:10.3978/j.issn.2072-1439.2014.12.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urman R, McConnell R, Islam T, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 2014;69:540–7. doi:10.1136/thoraxjnl-2012-203159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Künzli N, McConnell R, Bates D, et al. Breathless in Los Angeles: the exhausting search for clean air. Am J Public Health 2003;93:1494–9. doi:10.2105/AJPH.93.9.1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sources of air pollutants. IARC monographs on the evaluation of carcinogenic risks to humans, No 109, 2016. [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer JJ, Rogge WF, Hildemann LM, et al. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmos Environ 1996;30:3837–55. doi:10.1016/1352-2310(96)00085-4 [Google Scholar]
- 9. Nunes RAO, Alvim-Ferraz MCM, Martins FG, et al. Assessment of shipping emissions on four ports of Portugal. Environ Pollut 2017;231(Pt 2):1370–9. doi:10.1016/j.envpol.2017.08.112 [DOI] [PubMed] [Google Scholar]
- 10. Fuller GW, Tremper AH, Baker TD, et al. Contribution of wood burning to PM10 in London. Atmos Environ 2014;87:87–94. doi:10.1016/j.atmosenv.2013.12.037 [Google Scholar]
- 11. Fuller G, Green D. Evidence for increasing concentrations of primary PM10 in London. Atmos Environ 2006;40:6134–45. doi:10.1016/j.atmosenv.2006.05.031 [Google Scholar]
- 12. World Health Organization. WHO global urban ambient air pollution database. http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/.
- 13. Department for Environment, Food and Rural Affairs. UK Plan for tackling roadside nitrogen dioxide concentrations. Technical report, 2017. [Google Scholar]
- 14. Air Quality Expert Group. Nitrogen dioxide in the United Kingdom, 2004. [Google Scholar]
- 15. New Car CO2 Report 2017. 16th edn, 2017. [Google Scholar]
- 16. Anenberg SC, Miller J, Minjares R, et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017;545:467–71. doi:10.1038/nature22086 [DOI] [PubMed] [Google Scholar]
- 17. Reşitoğlu İbrahim Aslan, Altinişik K, Keskin A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol Environ Policy 2015;17:15–27. [Google Scholar]
- 18. Environmental Committee London Assembly. Driving away from diesel: reducing air pollution from diesel vehicles, 2015. [Google Scholar]
- 19. Quilter-Pinner H, Laybourn-Langton L. Lethal and illegal: London’s air pollution crisis: IPPR, 2016. [Google Scholar]
- 20. Internation Agency for Research on Cancer WHO. IARC: diesel engine exhaust carcinogenic, 2012. [PubMed] [Google Scholar]
- 21. Air Quality Guidelines for Europe. WHO regional publications, European Series, 2000. No. 91. [PubMed] [Google Scholar]
- 22. Greenpeace. More than 1,000 nurseries nationwide close to illegally polluted roads. 2017. https://unearthed.greenpeace.org/2017/04/04/air-pollution-nurseries/
- 23. Laybourn-Langton L, Quilter-Pinner H, Ho H. Lethal and Illegal: solving London’s Air Pollution Crisis, 2016. [Google Scholar]
- 24. Morales E, Garcia-Esteban R, de la Cruz OA, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015;70:64–73. doi:10.1136/thoraxjnl-2014-205413 [DOI] [PubMed] [Google Scholar]
- 25. Proietti E, Röösli M, Frey U, et al. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv 2013;26:9–23. doi:10.1089/jamp.2011.0932 [DOI] [PubMed] [Google Scholar]
- 26. Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Respir Med 2013;1:695–704. doi:10.1016/S2213-2600(13)70192-9 [DOI] [PubMed] [Google Scholar]
- 27. European Commission. Air Quality Standards. http://ec.europa.eu/environment/air/quality/standards.htm.
- 28. Carrillo G, Perez Patron MJ, Johnson N, et al. Asthma prevalence and school-related hazardous air pollutants in the US–México border area. Environ Res 2018;162:41–8. doi:10.1016/j.envres.2017.11.057 [DOI] [PubMed] [Google Scholar]
- 29. Lee JY, Leem JH, Kim HC, et al. Effects of traffic-related air pollution on susceptibility to infantile bronchiolitis and childhood asthma: a cohort study in Korea. J Asthma 2018;55:223–30. doi:10.1080/02770903.2017.1313270 [DOI] [PubMed] [Google Scholar]
- 30. Girguis MS, Strickland MJ, Hu X, et al. Exposure to acute air pollution and risk of bronchiolitis and otitis media for preterm and term infants. J Expo Sci Environ Epidemiol 2017. doi:10.1038/s41370-017-0006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hwang BF, Chen YH, Lin YT, et al. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ Res 2015;137:382–90. doi:10.1016/j.envres.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 32. Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med 2015;372:905–13. doi:10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gasana J, Dillikar D, Mendy A, et al. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res 2012;117:36–45. doi:10.1016/j.envres.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 34. Krämer U, Koch T, Ranft U, et al. Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology 2000;11:64–70. doi:10.1097/00001648-200001000-00014 [DOI] [PubMed] [Google Scholar]
- 35. Morgenstern V, Zutavern A, Cyrys J, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 2008;177:1331–7. doi:10.1164/rccm.200701-036OC [DOI] [PubMed] [Google Scholar]
- 36. Pénard-Morand C, Raherison C, Charpin D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J 2010;36:33–40. doi:10.1183/09031936.00116109 [DOI] [PubMed] [Google Scholar]
- 37. Bouazza N, Foissac F, Urien S, et al. Fine particulate pollution and asthma exacerbations. Arch Dis Child 2017:archdischild-2017-312826 doi:10.1136/archdischild-2017-312826 [DOI] [PubMed] [Google Scholar]
- 38. Hasunuma H, Yamazaki S, Tamura K, et al. Association between daily ambient air pollution and respiratory symptoms in children with asthma and healthy children in western Japan. J Asthma 2018;7:1–7. doi:10.1080/02770903.2017.1369988 [DOI] [PubMed] [Google Scholar]
- 39. Basagaña X, Esnaola M, Rivas I, et al. Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environ Health Perspect 2016;124:1630–6. doi:10.1289/EHP209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saenen ND, Plusquin M, Bijnens E, et al. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE Birth Cohort Study. Environ Health Perspect 2015;123:834–40. doi:10.1289/ehp.1408549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bos I, De Boever P, Int Panis L, et al. Physical activity, air pollution and the brain. Sports Med 2014;44:1505–18. doi:10.1007/s40279-014-0222-6 [DOI] [PubMed] [Google Scholar]
- 42. Pujol J, Martínez-Vilavella G, Macià D, et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage 2016;129:175–84. doi:10.1016/j.neuroimage.2016.01.036 [DOI] [PubMed] [Google Scholar]
- 43. Thiering E, Cyrys J, Kratzsch J, et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia 2013;56:1696–704. doi:10.1007/s00125-013-2925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Risnes KR, Vatten LJ, Baker JL, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011;40:647–61. doi:10.1093/ije/dyq267 [DOI] [PubMed] [Google Scholar]
- 45. Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect 2007;115:1248–53. doi:10.1289/ehp.9641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bilenko N, van Rossem L, Brunekreef B, et al. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. Eur J Prev Cardiol 2015;22:4–12. doi:10.1177/2047487313505821 [DOI] [PubMed] [Google Scholar]
- 47. Sram RJ, Veleminsky M, Veleminsky M, et al. The impact of air pollution to central nervous system in children and adults. Neuro Endocrinol Lett 2017;38:389–96. [PubMed] [Google Scholar]
- 48. Forns J, Dadvand P, Esnaola M, et al. Longitudinal association between air pollution exposure at school and cognitive development in school children over a period of 3.5 years. Environ Res 2017;159:416–21. doi:10.1016/j.envres.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 49. Pye S, King K, Sturman J. Air quality and social deprivation in the UK: an environmental inequalities analysis—final report to Defra, 2006. [Google Scholar]
- 50. Wise J. UK survey confirms link between deprivation and smoking. BMJ 2014;348:g2184 doi:10.1136/bmj.g2184 [DOI] [PubMed] [Google Scholar]
- 51. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 2012;8:166–75. doi:10.1007/s13181-011-0203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rylance J, Fullerton DG, Scriven J, et al. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am J Respir Cell Mol Biol 2015;52:584–93. doi:10.1165/rcmb.2014-0188OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Imrich A, Ning Y, Lawrence J, et al. Alveolar macrophage cytokine response to air pollution particles: oxidant mechanisms. Toxicol Appl Pharmacol 2007;218:256–64. doi:10.1016/j.taap.2006.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boylen CE, Sly PD, Zosky GR, et al. Physiological and inflammatory responses in an anthropomorphically relevant model of acute diesel exhaust particle exposure are sex and dose-dependent. Inhal Toxicol 2011;23:906–17. doi:10.3109/08958378.2011.625454 [DOI] [PubMed] [Google Scholar]
- 55. Saxena RK, Gilmour MI, Hays MD. Isolation and quantitative estimation of diesel exhaust and carbon black particles ingested by lung epithelial cells and alveolar macrophages in vitro. Biotechniques 2008;44:799–805. doi:10.2144/000112754 [DOI] [PubMed] [Google Scholar]
- 56. Kulkarni N, Pierse N, Rushton L, et al. Carbon in airway macrophages and lung function in children. N Engl J Med 2006;355:21–30. doi:10.1056/NEJMoa052972 [DOI] [PubMed] [Google Scholar]
- 57. Geiser M, Stoeger T, Casaulta M, et al. Biokinetics of nanoparticles and susceptibility to particulate exposure in a murine model of cystic fibrosis. Part Fibre Toxicol 2014;11:19 doi:10.1186/1743-8977-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Becker S, Dailey L, Soukup JM, et al. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharmacol 2005;203:45–52. doi:10.1016/j.taap.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 59. Jeannet N, Fierz M, Schneider S, et al. Acute toxicity of silver and carbon nanoaerosols to normal and cystic fibrosis human bronchial epithelial cells. Nanotoxicology 2016;10:279–91. doi:10.3109/17435390.2015.1049233 [DOI] [PubMed] [Google Scholar]
- 60. Brugha RE, Mushtaq N, Round T, et al. Carbon in airway macrophages from children with asthma. Thorax 2014;69:654–9. doi:10.1136/thoraxjnl-2013-204734 [DOI] [PubMed] [Google Scholar]
- 61. Wick P, Malek A, Manser P, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect 2010;118:432–6. doi:10.1289/ehp.0901200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nowak DJ, Hirabayashi S, Bodine A, et al. Tree and forest effects on air quality and human health in the United States. Environ Pollut 2014;193:119–29. doi:10.1016/j.envpol.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 63. Sá TH, Tainio M, Goodman A, et al. Health impact modelling of different travel patterns on physical activity, air pollution and road injuries for São Paulo, Brazil. Environ Int 2017;108:22–31. doi:10.1016/j.envint.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. United States Environmental Protection Agency. Guide to air cleaners in the home, 2008. [Google Scholar]