Abstract
Aims
Obesity is an increasing health problem and is an important risk factor for the development of atrial fibrillation (AF). We investigated the association of body mass index (BMI) on the safety and long-term efficacy of pulmonary vein isolation (PVI) for drug-refractory AF.
Methods
414 consecutive patients who underwent transcatheter PVI for AF between 2003 and 2013 were included. Successful PVI was defined as absence of atrial arrhythmia on Holter monitoring or ECG, without and with antiarrhythmic drugs during follow-up. Obesity was defined as BMI≥30 kg/m².
Results
Mean age was 56±10 years, 316 (76%) were male, 311 (75%) had paroxysmal AF and 111 (27%) were obese. After a mean follow-up of 46±32 months (1590 patient-years), freedom from atrial arrhythmia and antiarrhythmic drugs was significantly lower in patients with obesity compared with non-obese patients (30% vs 46%, respectively, P=0.005, log-rank 0.016). With antiarrhythmic drugs, freedom from atrial arrhythmia was 56% vs 68% (P=0.036). No differences in minor and major adverse events were observed between patients with obesity and non-obese patients (major 6% vs 3%, P=0.105, and minor 5% vs 5%, P=0.512). Sensitivity analyses demonstrated that BMI (as continuous variable) was associated with PVI outcome (HR 1.08, 95% CI 1.02 to 1.14, P=0.012).
Conclusion
Obesity is associated with reduced efficacy of PVI for drug-refractory AF. No relation between obesity and adverse events was found.
Keywords: atrial fibrillation, radiofrequency ablation (rfa), obesity
Key questions.
What is already known about this subject?
The recent ARREST-AF trial showed that aggressive risk factormanagement improves long-term outcomes of AF ablation. Also, if weight loss issustained at long-term follow-up, reduction of AF burden and maintenance ofsinus rhythm are significantly higher compared to patients with weightfluctuation.
What does this study add?
This studydemonstrates that obesity isassociated with lower long term success of PVI. Procedural safety was comparable between obese andnon-obese patients.
How might this impact on clinical practice?
Long-termefficacy of PVI seems reduced compared to non-obese patients. Therefore, inobese patients weight loss together with management of other risk factorsshould be considered to reduce AF burden and symptoms, before invasivetreatment modalities are deployed.
Introduction
Transcatheter pulmonary vein isolation (PVI) using radiofrequency energy is a widespread and well-established technique for the treatment of atrial fibrillation (AF).1–3 Current guidelines indicate that PVI should be considered even before antiarrhythmic drugs (AADs) have failed in patients with paroxysmal AF.2 Catheter ablation is superior to AADs for rhythm control in symptomatic paroxysmal AF4–6 and can also be performed successfully for persistent or long-standing persistent AF.7 However, radiofrequency PVI has only shown moderate success at long-term follow-up.8–10 Several comorbidities increase the risk for AF.11 Obesity is an independent risk factor for the development and perpetuation of AF11 and negatively influences success rates of PVI at 1-year follow-up.12 The recent ARREST-AF trial showed that aggressive risk factor management improves long-term outcomes of AF ablation.12 Also, if weight loss is sustained at long-term follow-up, reduction of AF burden and maintenance of sinus rhythm are significantly higher compared with patients with weight fluctuation.13 The aim of the present study was to investigate long-term outcome in consecutive patients undergoing a PVI strategy and to assess procedural safety in patients with obesity versus non-obese patients with AF.
Methods
Patient population
We retrospectively analysed all patients scheduled for a first PVI between 2003 and 2013 at the University Medical Center Groningen, the Netherlands. All consecutive patients had highly symptomatic AF and failed at least one AAD. Exclusion criteria for PVI were significant underlying heart diseases and age <18 years or >80 years and <12 months follow-up. Body mass index (BMI) was determined for all patients at the time of ablation. BMI was calculated by dividing bodyweight in kilograms by the square of the height in metres. Obesity was defined as BMI≥30 kg/m2. Patients provided informed consent to the ablation procedure. All data were collected following our institutional protocol (see Follow-up section) and concerned standard patient care. Due to the observational nature of the study, no further specific investigation was requested to the patients. Patients’ data privacy was granted by coding the database, according to the rules of Good Clinical Practice and Dutch Privacy Law.
Transcatheter radiofreqency PVI strategy
The transcatheter wide circumferential PVI was performed as described previously.14 15 During the 10-year study period, the PVI procedure evolved according to technical modifications. Briefly, point-by-point ablation wide antral lines were created around the pulmonary veins. For the first procedures, radiofrequency energy was delivered with a non-irrigated ablation catheter, later on this was an irrigated tip. In the initial patients, PVI was assessed with pacing within the pulmonary veins to conform exit block. From 2011 a circular catheter was used to confirm entrance and exit block. During the first procedure, no additional ablation lines were made. In case the first PVI was unsuccessful, repeat PVI procedures were performed when symptomatic atrial arrhythmias were present (>3 months after initial PVI), in consultation with the patient and treating physician. Additional (linear) ablation was performed at the discretion of the treating electrophysiologist.
Following PVI, oral anticoagulation was immediately restarted after the procedure, and low-molecular-weight-heparin was stopped when International Normalized Ratio (INR) >2.0 was reached. Oral anticoagulation treatment was given for at least 3 months and thereafter continued based on the CHADS2-score and later on the CHADS2VA2Sc.1 2 AADs were discontinued after the first three months blanking period if the patient was free from AF recurrence.
Follow-up
Patients visited our clinic at 3, 6 and 12 months post PVI. Thereafter, patients were seen annually or on indication. To assess the occurrence of (a)symptomatic atrial arrhythmias, at 6 months 48–96-hour Holter monitoring was performed, and at 12 months 24-hour Holter monitoring was performed. At each visit a routine 12-lead ECG was performed, and when atrial arrhythmia was detected, a 12-lead rhythm strip (>30 s) was recorded. In case of symptomatic recurrence without documentation, event recording was performed to confirm and classify the atrial arrhythmia. Follow-up data were censored for patients who reached the primary endpoint or had been followed through 1 December 2015.
Endpoints
Primary endpoint was freedom of atrial arrhythmias, that is, no evidence of AF, atrial flutter or other atrial arrhythmias with a duration>30 s, without use of AADs at the end of follow-up. Procedural safety was investigated by reporting the occurrence of periprocedural and procedural minor or major adverse events. Major adverse events were defined as those that resulted in death or permanent injury. Also registered as major adverse events were temporarily injuries that required intervention or specific treatment, (eg, stroke, transient ischaemic attack, major bleeding requiring surgery or blood transfusion or >2.0 points haemoglobin decrease, cardiac tamponade and/or perforation, significant or symptomatic pulmonary vein stenosis>70%, pericarditis and/or pericardial effusion, myocardial infarction, phrenic nerve lesion, pneumothorax, pneumonia and other not predefined events). Minor adverse events were defined as bleeding from the femoral artery/vein, femoral aneurysm not requiring intervention, pericardial effusion not requiring intervention and asymptomatic pulmonary vein stenosis.16
Statistics
Baseline descriptive statistics are presented as mean±SD or median (range) for continuous variables, if appropriate, and counts with percentages for categorical variables. Differences between subgroups, in terms of patient characteristics at baseline, different follow-up times and end of study, were evaluated by Student’s t-test or the Mann-Whitney U test, depending on normality of the data. χ2 or Fisher’s exact test was used for comparison of categorical variables. By means of Cox-proportional hazard analyses the association of any increase in BMI with the primary outcome was assessed. Model 1 is adjusted for age and sex, model 2 for age, sex, self-reported obstructive sleep apnoea syndrome (OSAS), previous class I or III AAD use, LA diameter, AF duration, AF type, chronic heart failure and total number of PVI. Model 3 is adjusted for covariates of model 2 and also for the other components of the CHADS2VA2Sc, not included in model 2: hypertension, diabetes, vascular disease and stroke. No violations of the proportional hazards assumptions were found. All tests of significance were two-tailed, with p values <0.05 assumed to indicate significance.
Results
Patient population
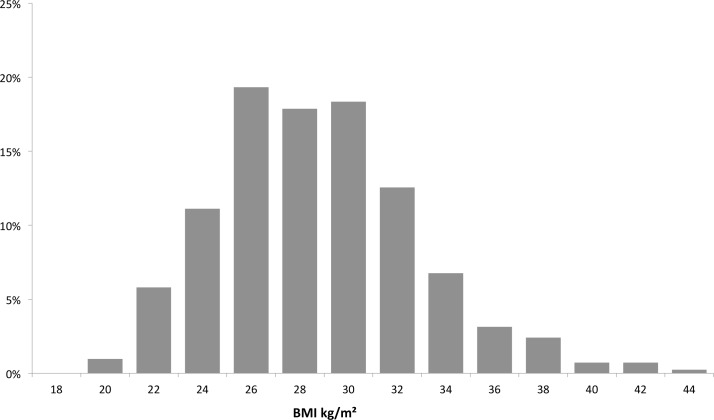
A total of 414 consecutive patients were included in this study. Patient characteristics are shown in table 1. Mean age was 56±10 years. Time since first AF diagnosis was 63 (IQR 29–118) months. AF was paroxysmal in 311 (75%), mean BMI was 27.8±4.1 kg/m². Among all patients, 111 (27%) were obese (BMI≥30 kg/m2) and 25 (6%) had a BMI≥35 kg/m2. Distribution of number of patients by BMI is shown in figure 1. Comparing patients with obesity (BMI≥30 kg/m2) versus non-obese patients (BMI<30 kg/m2), several differences were observed: chronic systolic heart failure (left ventricular ejection fraction (LVEF)≤35%), 10% versus 4%, P=0.034, hypertension 65% versus 46%, P=0.001, self-reported OSAS 7% versus 2%, P=0.013. Also left atrial diameter was larger in patients with obesity versus non-obese patients (44±5 mm vs 41±7 mm, P<0.001).
Table 1.
Baseline characteristics of patients undergoing transcatheter pulmonary vein isolation
| Total group N=414 |
BMI<30 N=303 |
BMI≥30 N=111 |
P values | |
| Age, mean±SD (years) | 56±10 | 56±10 | 56±10 | 0.859 |
| Males, n (%) | 316 (76%) | 236 (78%) | 80 (73%) | 0.298 |
| Chronic heart failure, n (%) | 24 (6%) | 13 (4%) | 11 (10%) | 0.034 |
| Diabetes mellitus, n (%) | 21 (5%) | 12 (4%) | 9 (8%) | 0.124 |
| Previous stroke, n (%) | 17 (4%) | 11 (4%) | 6 (5%) | 0.407 |
| Hypertension, n (%) | 213 (51%) | 141 (46%) | 72 (65%) | 0.001 |
| Vascular disease, n (%) | 47 (11%) | 36 (12%) | 11 (10%) | 0.726 |
| CHADS2VA2Sc score>1, n (%) | 142 (34%) | 94 (31%) | 48 (43%) | 0.019 |
| Hypercholesterolaemia, n (%) | 79 (19%) | 59 (19%) | 20 (18%) | 0.888 |
| Thyroid dysfunction, n (%) | 35 (9%) | 21 (7%) | 14 (13%) | 0.072 |
| Self-reported OSAS, n (%) | 13 (4%) | 5 (2%) | 8 (7%) | 0.013 |
| Time since first AF episode, median (IQR) (months) | 63 (29–118) | 66 (30–121) | 48 (23–108) | 0.073 |
| Paroxysmal AF, n (%) | 311 (75%) | 235 (77%) | 76 (68%) | 0.095 |
| Non-paroxysmal AF, n (%) | 103 (25%) | 69 (23%) | 34 (32%) | 0.095 |
| LA diameter parasternal, mm (mean±SD) | 42±6 | 41±7 | 44±5 | <0.001 |
| LVEF, mean±SD | 57±6 | 58±5 | 57±7 | 0.234 |
| AAD use | ||||
| Class I or III, n (%) | 275 (72%) | 200 (66%) | 75 (68%) | 0.921 |
| Amiodarone, n (%) | 93 (24%) | 58 (19%) | 35 (32%) | 0.010 |
AAD, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; LVEF, LA left atrial, left ventricular ejection fraction; OSAS, obstructive sleep apnoea syndrome.
Figure 1.
Body mass index (BMI) distribution of the total patient population.
PVI outcome in the total population
After a mean follow-up of 46±32 months (1590 patient-years; median 37, IQR 19–67), a total of 733 procedures were performed, with a median of 2.0 (range 1–5) ablations per patient. Of all patients, 56% underwent multiple ablation procedures. Overall long-term freedom from atrial arrhythmia and AAD was 42% (172/414 patients). With AAD this was 65% (268/414 patients).
PVI outcome according to obesity
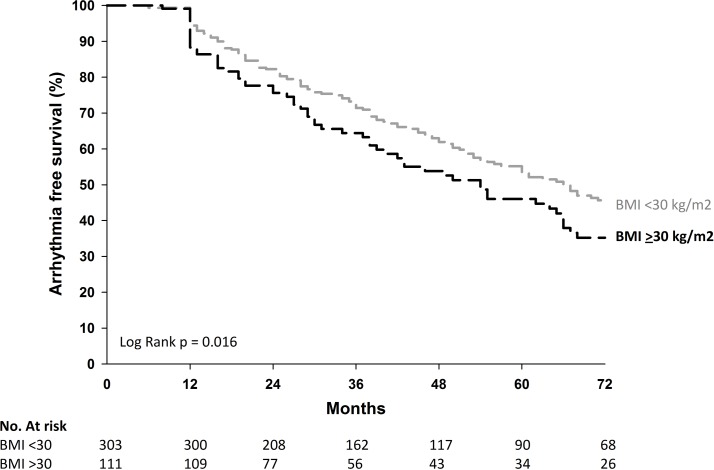
After a mean follow-up of 46±32 months (1590 patient-years), freedom from atrial arrhythmia and AADs was significantly lower in patients with obesity compared with non-obese patients (30% vs 46%, respectively, P=0.005, log-rank 0.016) (table 2 and figure 2). With AADs, freedom from atrial arrhythmia was 56% versus 68% (P=0.036) (table 2).
Table 2.
Efficacy and safety outcomes of multiple procedure follow-up
| Total | BMI<30 | BMI≥30 | P values | |
| Total n, PVI median (range) | 2.0 (1–5) | 2.0 (1–4) | 2.0 (1–5) | 0.505 |
| Multiple procedure success | ||||
| 12 months FU no AAD, n (%) | 119 (29%) | 93 (31%) | 26 (23%) | 0.178 |
| 12 months with and without AAD, n (%) | 221 (53%) | 163 (54%) | 58 (52%) | 0.911 |
| Long-term FU no AAD, n (%) | 172 (42%) | 139 (46%) | 33 (30%) | 0.005 |
| Long-term FU with and without AAD, n (%) | 268 (65%) | 206 (68%) | 62 (56%) | 0.036 |
| Major adverse events | ||||
| Procedure-related death | 0 | 0 | 0 | |
| Cardiac tamponade/perforation | 9 | 5 | 4 | |
| Thromboembolic event | 4 | 2 | 2 | |
| Air-embolic event | 2 | 2 | 1 | |
| Total (multiple procedures) | 16 (4%) | 9 (3%) | 7 (6%) | 0.105 |
| Minor adverse events | ||||
| Femoral bleeding/aneurysm/AVF | 14 | 9 | 5 | |
| Pericardial effusion no intervention | 4 | 3 | 1 | |
| Phrenic nerve lesion | 1 | 1 | 0 | |
| Pulmonary vein stenosis (asymptomatic) | 1 | 1 | 0 | |
| Pericarditis | 1 | 1 | 0 | |
| Total (multiple procedures) | 21 (5%) | 15 (5%) | 6 (5%) | 0.512 |
| Major or minor adverse events (multiple procedures) | 37 (9%) | 24 (8%) | 13 (12%) | 0.158 |
AAD, antiarrhythmic drugs; AVF, arterial venous fistula; BMI, body mass index; FU, follow-up; PVI, pulmonary vein isolation.
Figure 2.
Long-term freedom from atrial arrhythmia and antiarrhythmic drugs for patients with obesity versus non-obese patients following multiple procedures. BMI, body mass index.
There was no difference between both groups in median number of procedures (P=0.500).
Adverse event according to obesity
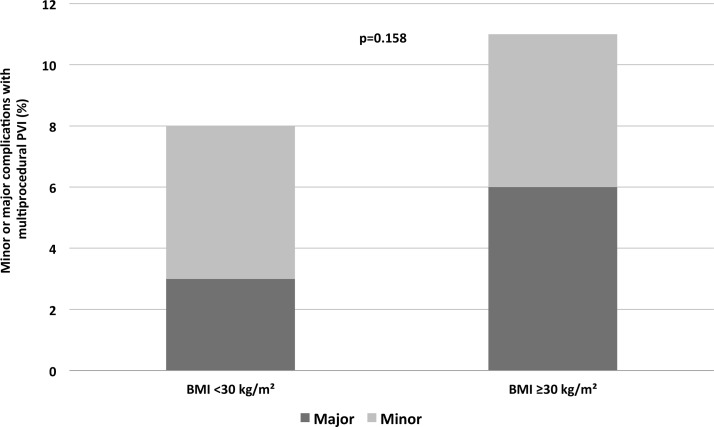
Table 2 shows the periprocedural and procedural minor or major adverse events. In 37 (9%) patients, adverse events occurred, being major in 16 (4%) patients and minor in 21 (5%) patients. There was no in-hospital mortality. No differences in minor and major adverse events were observed between patients with obesity and non-obese patients (major 6% vs 3%, P=0.105, and minor 5% vs 5% P=0.512, figure 3).
Figure 3.
Major and minor adverse events. BMI, body mass index; PVI, pulmonary vein isolation.
Association of BMI and PVI outcome
As sensitivity analyses, we performed multivariate Cox-proportional hazard analyses and assessed whether an increase in BMI (modelled as continuous covariate) was associated with an increased risk atrial arrhythmia recurrence. No violations of the proportional hazards assumptions were found. Table 3 shows the outcome of different models. When adjusting for the covariates included in model 2, any increase in BMI was associated with failure of PVI with an HR of 1.09 (95% CI 1.02 to 1.16), P=0.039. Model 3 showed that any increase of BMI was associated with failure of PVI with an HR of 1.09 (95% CI 1.01 to 1.16), P=0.017.
Table 3.
Sensitivity analyses of the association of body mass index and long-term outcome after multivariable adjusted analyses
| HR (95% CI) | P values | |
| Model 1 | 1.08 (1.02 to 1.14) | 0.012 |
| Model 2 | 1.09 (1.02 to 1.16) | 0.039 |
| Model 3 | 1.09 (1.01 to 1.16) | 0.017 |
Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, obstructive sleep apnoea syndrome, previous class I or III antiarrhythmic drug use, left atrial diameter, atrial fibrillation duration, atrial fibrillation type, chronic heart failure and total number of pulmonary vein isolation procedures. Model 3 adjusted for all factors mentioned previously and also hypertension, diabetes, vascular disease and stroke.
Discussion
This retrospective and observational study demonstrates that obesity is associated with lower >1-year success of PVI. Procedural safety was comparable between patients with obesity and non-obese patients.
Obesity as cause of AF
Obesity is an important health problem with an increasing prevalence. There is abundant evidence for the involvement of obesity in the development of AF. Individuals with obesity have up to 2.4-fold increased risk for new-onset AF.17 Several mechanisms may underlie the relation between obesity and new-onset AF. It might be related to structural and electrophysiological remodelling caused by elevated end-diastolic pressure, inflammation and increased plasma volume.18 Epicardial adipose tissue might cause a paracrine effect, acting through inflammatory markers and adipokines lead to enhanced fibrosis. Fatty infiltration of the atrium can shorten action potential duration. On top of this, local fat depots can cause an arrhythmogenic effect on the atria. In general, obesity is a known cause of hypertension and diabetes, which are both known to promote atrial fibrosis. Animal models of obesity demonstrated increased levels of atrial fibrosis and higher susceptibility and sustainability of AF. In humans, electro-anatomical mapping in patients with obesity showed areas of low voltages indicative of increased atrial fibrosis.18 Weight loss has been associated with a decrease of the AF burden in patients.19 Also, in human studies cardiac fat quantity has been proven to be related to AF severity and negatively influence ablation results. Following weight reduction, lower levels of inflammatory markers were measured and electro-anatomical mapping demonstrated recovery of atrial voltages.13 In our study, hypertension, chronic heart failure and an enlarged atrial size, all parameters associated with a lower success rate of rhythm control, were more frequently present in patients with obesity.3 This is in accordance with the literature; the relationship between diastolic impairment and obesity has been established by other groups.
Influence of obesity on PVI outcome
More and more data become available on obesity and atrial arrhythmia recurrences following PVI. A report of 226 patients with symptomatic, drug-refractory paroxysmal and persistent AF (mean BMI 26.6±3.5 kg/m²) showed that BMI was not predictive for AF recurrence at a mean follow-up of just over 1 year, although a trend to a higher AF recurrence was found in patients with higher BMI.20 Cha et al showed similar results in their study of 523 symptomatic, medication-refractory patients with AF (58% paroxysmal, 42% persistent or permanent AF) undergoing PVI. The study showed no difference in success of catheter ablation between the groups of BMI>25 (18%), BMI 25–29.9 kg/m² (44%) and BMI≥30 (38%) at 12–24 months follow-up.21 However, the main finding of our study is that we observed a lower success rate of PVI in patients with obesity versus non-obese patients during >1-year follow-up. Differences between these studies may be explained by differences in clinical characteristics of the patients and follow-up duration. Of note, we also observed no difference in efficacy during the first year of follow-up, but only after long-term follow-up. The results of the present study seem to be in accordance with the recently published data by Sanders et al, who demonstrated that aggressive risk factor reduction including weight loss improves the outcome of PVI in patients with obesity.12 The >1-year freedom from atrial arrhythmias in our study is comparable to long-term efficacy rates reported by others.7–9 22 Also, the reported adverse events rates are comparable.16
Clinical relevance
Since both obesity and AF pose an epidemic threat, it is important to recognise that AF is not only more frequent in patients with obesity but also that long-term efficacy of PVI seems reduced compared with non-obese patients. In order to improve long-term results of PVI, patient selection is pivotal.23 Therefore, as stated in the new AF guidelines, in patients with obesity, weight loss, together with management of other risk factors, should be considered to reduce AF burden and symptoms before invasive treatment modalities are deployed.3 Risk factor modification and weight loss might even result in higher treatment success by reducing substrate and slowing down AF progression.
Strengths and limitations
Our study was retrospective, precluding definite conclusions about cause–effect relations of obesity and PVI outcome. However, strength of our study was that we had a >1500 patient-years follow-up in most patients with extensive Holter recordings, which increased the probability of observing any atrial arrhythmia recurrence. First, short and asymptomatic episodes of AF might be undetected. Second, obesity is often accompanied by more comorbidities, so obesity may reflect a clustering of cardiovascular risk factors that may impact PVI outcome, though even after multivariable adjustment the association of BMI with PVI outcome remained. Third, the incidence of OSAS was low and may been caused by the fact we only collected self-reported OSAS, and no structural polysomnography was performed in our cohort. Fourth, the present analysis did not offer the opportunity to look into temporal associations between weight gain of loss and success of PVI. Finally, as described above, the transcatheter ablation strategy evolved over time with the introduction of improved equipment and was applied equally in all patients, regardless of the body mass composition.
Conclusion
Obesity is associated with reduced efficacy of PVI for drug-refractory AF. No relation between obesity and procedural adverse events was found. This emphasise that risk factor reduction before ablation including weight loss should be implemented in the work-up of patients with symptomatic AF referred for AF ablation.
Footnotes
Contributors: GedM and BM conducted the analysis. GEdM, BM, MAM and MR wrote the manuscript. YB and IVG designed the study. MIHA-J and WLB assisted in data gathering, processing and statistical analysis. YEST, ACPW, IVG and YB revised the manuscript critically for important intellectual content.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained for the procedure.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All available data are reported in the article.
References
- 1. Camm AJ, Kirchhof P, Lip GY, et al. . Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the european society of cardiology (ESC). Europace 2010;12:1360–420. 10.1093/europace/euq350 [DOI] [PubMed] [Google Scholar]
- 2. Camm AJ, Lip GY, De Caterina R, et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. 10.1093/europace/eus305 [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof P, Benussi S, Kotecha D, et al. . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Kuck KH, Cappato R, et al. . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. 10.1093/europace/eus027 [DOI] [PubMed] [Google Scholar]
- 5. Wilber DJ, Pappone C, Neuzil P, et al. . Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. 10.1001/jama.2009.2029 [DOI] [PubMed] [Google Scholar]
- 6. Morillo CA, Verma A, Connolly SJ, et al. . Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 7. Mont L, Bisbal F, Hernández-Madrid A, et al. . Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. 10.1093/eurheartj/eht457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ouyang F, Tilz R, Chun J, et al. . Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368–77. 10.1161/CIRCULATIONAHA.110.946806 [DOI] [PubMed] [Google Scholar]
- 9. Tilz RR, Rillig A, Thum AM, et al. . Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the hamburg sequential ablation strategy. J Am Coll Cardiol 2012;60:1921–9. 10.1016/j.jacc.2012.04.060 [DOI] [PubMed] [Google Scholar]
- 10. Weerasooriya R, Khairy P, Litalien J, et al. . Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57:160–6. 10.1016/j.jacc.2010.05.061 [DOI] [PubMed] [Google Scholar]
- 11. Vermond RA, Geelhoed B, Verweij N, et al. . Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–7. 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 12. Pathak RK, Middeldorp ME, Lau DH, et al. . Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 13. Pathak RK, Middeldorp ME, Meredith M, et al. . Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 14. De Maat GE, Van Gelder IC, Rienstra M, et al. . Surgical vs. transcatheter pulmonary vein isolation as first invasive treatment in patients with atrial fibrillation: a matched group comparison. Europace 2014;16:33–9. 10.1093/europace/eut208 [DOI] [PubMed] [Google Scholar]
- 15. Tan ES, Mulder BA, Rienstra M, et al. . Pulmonary vein isolation of symptomatic refractory paroxysmal and persistent atrial fibrillation: a single centre and single operator experience in the Netherlands. Neth Heart J 2009;17:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappato R, Calkins H, Chen SA, et al. . Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. 10.1161/CIRCEP.109.859116 [DOI] [PubMed] [Google Scholar]
- 17. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 2005;118:489–95. 10.1016/j.amjmed.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 18. Nalliah CJ, Sanders P, Kottkamp H, et al. . The role of obesity in atrial fibrillation. Eur Heart J 2016;37 10.1093/eurheartj/ehv486 [DOI] [PubMed] [Google Scholar]
- 19. Pathak RK, Elliott A, Middeldorp ME, et al. . Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol 2015;66:985–96. 10.1016/j.jacc.2015.06.488 [DOI] [PubMed] [Google Scholar]
- 20. Letsas KP, Siklódy CH, Korantzopoulos P, et al. . The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int J Cardiol 2013;164:94–8. 10.1016/j.ijcard.2011.06.092 [DOI] [PubMed] [Google Scholar]
- 21. Cha YM, Friedman PA, Asirvatham SJ, et al. . Catheter ablation for atrial fibrillation in patients with obesity. Circulation 2008;117:2583–90. 10.1161/CIRCULATIONAHA.107.716712 [DOI] [PubMed] [Google Scholar]
- 22. Teunissen C, Kassenberg W, van der Heijden JF, et al. . Five-year efficacy of pulmonary vein antrum isolation as a primary ablation strategy for atrial fibrillation: a single-centre cohort study. Europace 2016;18:1335–42. 10.1093/europace/euv439 [DOI] [PubMed] [Google Scholar]
- 23. Gorenek B, Pelliccia A, Benjamin EJ, et al. . European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 2016;19:190–225. 10.1093/europace/euw242 [DOI] [PMC free article] [PubMed] [Google Scholar]