Abstract
A 21-year-old woman presented with a 2-week history of vomiting, diarrhoea and epigastric pain, with 9 kg weight loss over the last two months. Laboratory tests were normal with negative coeliac serology. Duodenal biopsies revealed total villous atrophy, crypt hypertrophy and intraepithelial lymphocytosis. A diagnosis of seronegative coeliac disease was made, and she started a gluten-free diet. However, she did not respond and her weight fell to 30.6 kg (body mass index 11), becoming dependent on parenteral nutrition. Her diagnosis was reconsidered and the histology reviewed. The histopathological features were of severe active chronic duodenitis. By diagnosis of exclusion, with the absence of other clear pathology, she was treated as Crohn’s disease. She responded to third-line therapy with biologics. In this case, the patient had refractory villous atrophy and the mucosal features, in addition to response with anti-tumour necrosis factor therapy, suggest inflammatory bowel disease, although not with complete diagnostic certainty.
Keywords: gastrointestinal system, crohn’s disease, malabsorption, malnutrition, parenteral / enteral feeding
Background
The symptoms of weight loss, change in bowel habit and abdominal pain commonly result in referral for specialist investigations such as upper gastrointestinal endoscopy and duodenal biopsies. The finding of villous atrophy is commonly caused by coeliac disease, but other causes must be considered in a timely manner.
We report a case of severe villous atrophy associated with lymphadenopathy and profound malnutrition which failed to respond to a gluten-free diet. The case highlights the importance of a broad differential diagnosis when a patient does not improve following initial treatment.
Case presentation
A 21-year-old Caucasian woman initially presented with a 2-week history of intractable vomiting, diarrhoea and epigastric pain on the background of 9 kg weight loss over the preceding two months (original weight 50 kg, body mass index (BMI) 18). Blood tests showed a normal full blood count, normal liver function tests and normal thyroid function tests. Coeliac serology (tissue transglutaminase antibody and anti-endomysial antibody) was negative despite a normal total IgA. Oesophagogastroduodenoscopy (OGD) during the acute admission revealed a structurally normal upper gastrointestinal tract. Duodenal biopsies revealed total villous atrophy, crypt hypertrophy and widespread moderately active chronic inflammation (figure 1). There was a moderate intraepithelial lymphocytosis, positive for CD3 and CD8 cells.
Figure 1.
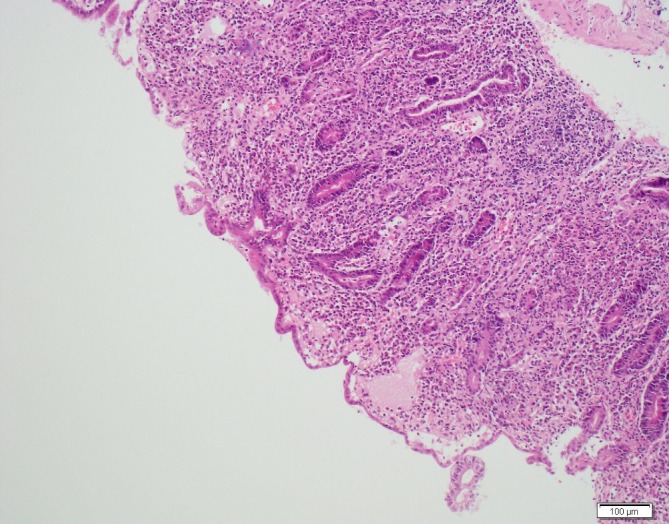
Histology from a duodenal 2 biopsy displaying total villous atrophy, crypt hypertrophy, widespread moderate active chronic inflammation and moderate intraepithelial lymphocytosis.
A diagnosis of seronegative coeliac disease was made, and she was initiated on a gluten-free diet which she followed strictly for the next seven months. However, during this time her weight fell to a nadir of 30.6 kg (BMI 11). She became extremely frail and unable to walk, mobilising in a wheelchair. She was admitted for initiation of parenteral nutrition.
A CT abdomen revealed no intra-abdominal masses but did demonstrate multiple subcentimetre mesenteric lymph nodes. Laparoscopic lymph node biopsy was taken and histology did not show features of lymphoma. At the time the surgeon commented that the remaining small bowel appeared normal. A small bowel MRI revealed no evidence of bowel wall thickening or abnormal enhancement. Colonoscopy was macroscopically normal, but villous atrophy was noted in terminal ileal biopsies. HLA DQ2 and DQ8 gene testing was negative.
Investigations
At this point she was transferred to a tertiary intestinal failure centre for a period of nutritional assessment. A faecal elastase test showed moderate pancreatic insufficiency and a SeHCAT (tauroselcholic acid) scan demonstrated bile salt malabsorption. She was started on Creon and Colesevelam, respectively, but her symptoms persisted. Further blood tests revealed laboratory signs of malnutrition including an albumin of 16. She suffered complications of parenteral nutrition and malnutrition including catheter-related line sepsis and lumbar vertebral collapse following a fall. A dual-energy X-ray absorptiometry scan confirmed osteoporosis, requiring bisphosphonates. Immunoglobulins were normal throughout. Anti-enterocyte and anti-goblet cell antibodies were negative.
Repeat endoscopic examinations confirmed persistent villous atrophy without positive coeliac serology. Small bowel capsule endoscopy showed loss of the normal villous architecture and superficial ulcers in the antrum and first part of the duodenum (figure 2).
Figure 2.
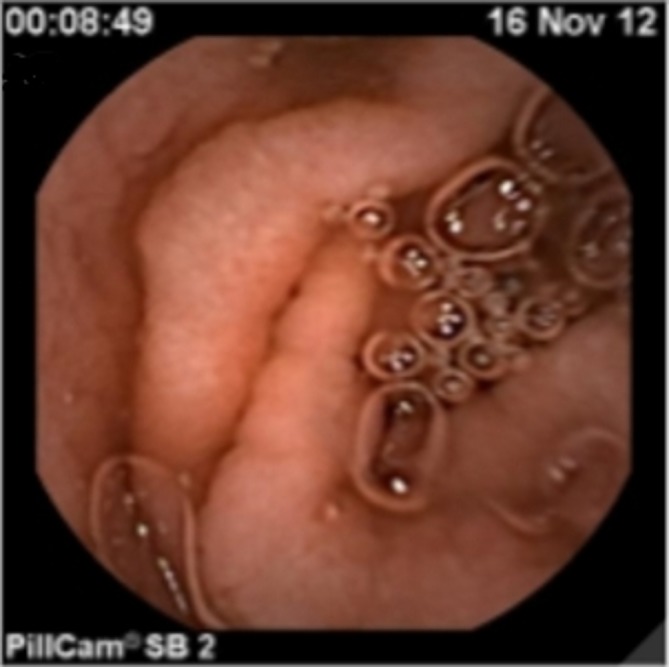
Capsule endoscopy pre-adalimumab demonstrating loss of normal villous architecture.
Differential diagnosis
Following the above investigations, a specialist histopathologist reviewed the histology to date and commented the overall features were of severe active chronic duodenitis. The differential diagnosis included an infective aetiology, drug-induced enteritis or an inflammatory bowel-related enteritis. There were no eosinophils on histology and no parasites/ova on stool examination for evidence of an infective cause. The severe inflammation was unusual for Helicobacter pylori infection, excluding an infective aetiology. The features were not typical for non-steroidal anti-inflammatory drug enteritis either. Therefore, following multidisciplinary discussion, a decision was made to treat as Crohn’s disease, by diagnosis of exclusion.
Treatment
Parenteral nutrition was established and her weight improved to 49 kg and stabilised. Initial treatment with prednisolone and azathioprine failed to reduce her dependency on parenteral nutrition. Sixteen months after her initial presentation she was started on anti-tumour necrosis factor (TNF) therapy and she was given a choice between infliximab and adalimumab, the licensed therapies at the time. Due to her poor mobility and thus difficulty in attending for an infusion, she opted for adalimumab given it can be self-administered at home.
Outcome and follow-up
Within weeks of starting adalimumab her symptoms were significantly improved and she gained weight rapidly. After 6 months of treatment, her weight had stabilised at 61 kg (BMI 23) and she was able to come off parental nutrition. She regained her ability to mobilise and was able to return to work. A capsule endoscopy 18 months after starting adalimumab demonstrated a complete clinical response, with no evidence of significantly active Crohn’s disease and near-total regeneration of the villous mucosa (figure 3).
Figure 3.
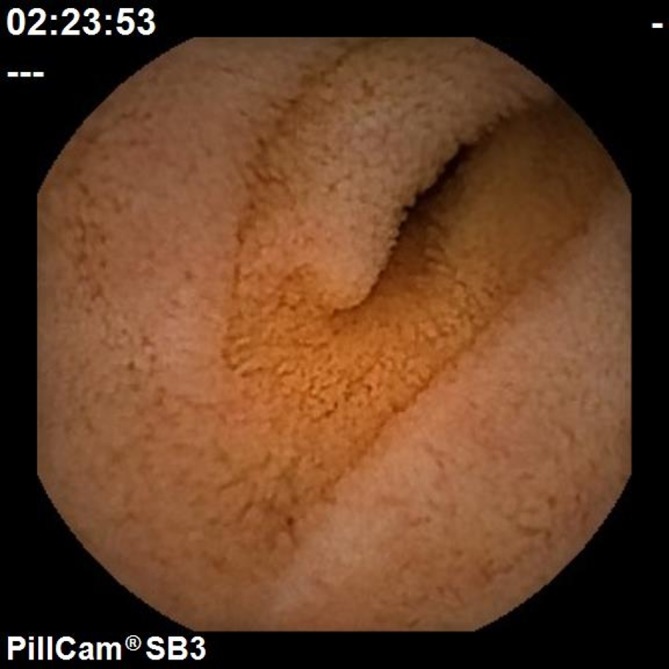
Capsule endoscopy of the jejunum 18months post starting adalimumab demonstrating regeneration of villous architecture.
Discussion
The presence of villous atrophy and negative coeliac disease serology poses a diagnostic and therapeutic challenge. Seronegative villous atrophy can be broadly categorised as seronegative coeliac disease and seronegative non-coeliac disease. In this case, the absence of the HLA DQ2 and DQ8, which are present in the vast majority of patients with coeliac disease, effectively excludes seronegative coeliac disease.1 The causes of seronegative non-coeliac disease are extensive but include infective, drug related, inflammatory and immune mediated. In some cases, no cause can be found and are thus labelled unclassified sprue.2
Although rare events, medications including the angiotensin 2 receptor blockers olmesartan and telmisartan have been associated with seronegative villous atrophy.3 4 However, there was no relevant drug history in this case.
A recent large prospective study in the UK evaluated 200 new patients with seronegative villous atrophy over a 15-year period. Seronegative coeliac disease was diagnosed in 31% of cases with the remaining 69% due to seronegative non-coeliac disease. Of the 138 cases with seronegative non-coeliac disease, 6 were caused by Crohn’s disease. The largest subgroup of seronegative non-coeliac disease was secondary to infective causes (27%), with other causes including peptic duodenitis (11.5%), drug induced (6%), systemic immune mediated (2%), radiation enteritis (0.5%) and eosinophilic enteritis (0.5%). A significant majority (18%) were identified as unclassified sprue despite extensive investigation.2
It is important to continue to pursue a diagnosis in seronegative villous atrophy. A US retrospective study of 72 patients evaluated complex cases of seronegative villous atrophy, in which a definitive aetiology was found in 85% of cases. After seronegative coeliac disease (which was defined by histological improvement on a gluten-free diet), the next most common causes were medication-related villous atrophy (26%), common variable autoimmune deficiency (6%), autoimmune enteropathy (4%) and Giardia (4%). In that series, only one patient had a final diagnosis of Crohn’s disease.5
Another US case series reviewed 30 patients with seronegative villous atrophy with persistent histological change despite a gluten-free diet. The most common final diagnosis was peptic duodenitis (16.6%), and this entire group responded to proton pump inhibitors. Others included collagenous sprue (10%), small intestinal bacterial overgrowth (10%) and eosinophilic gastroenteritis (7%). Crohn’s disease affected two patients in this series (7%). One of these patients was initially diagnosed as seronegative coeliac disease, but was found to have granulomatous inflammation on repeat biopsy. In the other patient, the development of colonic inflammation led to the correct diagnosis. Both responded to budesonide.6
In the current case, the patient presented with symptoms that can be associated with a number of gastrointestinal pathologies and appropriately underwent an OGD with duodenal biopsies. Given that coeliac disease remains the most common cause of villous atrophy with raised intraepithelial lymphocytes, the initial management with a gluten-free diet was logical. When she did not respond to this initial management, alternative causes were investigated, but her physical and nutritional deterioration necessitated parenteral nutritional support.
Although ultimately labelled Crohn’s disease, this would be an unusual phenotypical presentation of inflammatory bowel disease. Furthermore, the absence of any characteristic perianal or colonic changes of Crohn’s disease, the low burden of macroscopic changes (minor ulceration only on capsule endoscopy) and subsequent failure to respond to steroids and immunomodulatory therapy led to diagnostic uncertainty. Once initiated, anti-TNF therapy rapidly reversed the dependency on parenteral nutrition. While we appreciate it is not with complete diagnostic certainty, the combination of refractory seronegative villous atrophy, the mucosal features as well as response to anti-TNF therapy led to a clinical diagnosis of Crohn’s disease. There was no serological evidence for the main differential diagnoses, common variable immunodeficiency and autoimmune enteropathy. Ulcerative jejunitis was considered, but felt unlikely due to the low burden of ulceration in the visualised small bowel and the absence of structuring disease on cross-sectional imaging. We also appreciate that there is a possibility of unclassified sprue, and we are therefore closely following her up given the diagnostic dilemma.
In summary, this case of severe malnutrition was caused by persistent seronegative villous atrophy. A clinical diagnosis of Crohn’s disease was made by exclusion. The symptoms were entirely reversed with anti-TNF therapy. The case highlights the need to keep an open mind about alternative diagnoses in patients who present with seronegative villous atrophy. Failure to do so can lead to significant nutritional and functional deficiencies.
Learning points.
The causes of seronegative villous atrophy are extensive.
Crohn’s disease can manifest as duodenitis with villous atrophy.
Villous atrophy can result in malabsorption and intestinal failure requiring parenteral nutrition.
Anti-tumour necrosis factor therapy should be considered in treatment-resistant Crohn’s.
Acknowledgments
Many thanks to Dr Simon Gabe, Leonard Jones Intestinal Failure Unit, St Mark’s Hospital for his support in managing the case and
Footnotes
Contributors: JEM collected the data, analysed the data and drafted the bulk of the case report, including adding revisions. MB contributed to the discussion. RF is the senior author and corresponding author who began the case report and made critical revisions for the final version. AL contributed to the critical revisions.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pisapia L, Camarca A, Picascia S, et al. HLA-DQ2.5 genes associated with celiac disease risk are preferentially expressed with respect to non-predisposing HLA genes: implication for anti-gluten T cell response. J Autoimmun 2016;70:63–72. 10.1016/j.jaut.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Aziz I, Peerally MF, Barnes JH, et al. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000-2015). Gut 2017;66:1563–72. 10.1136/gutjnl-2016-312271 [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012;87:732–8. 10.1016/j.mayocp.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basson M, Mezzarobba M, Weill A, et al. Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut 2016;65:1664–9. 10.1136/gutjnl-2015-309690 [DOI] [PubMed] [Google Scholar]
- 5.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol 2013;108:647–53. 10.1038/ajg.2013.45 [DOI] [PubMed] [Google Scholar]
- 6.Pallav K, Leffler DA, Tariq S, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther 2012;35:380–90. 10.1111/j.1365-2036.2011.04938.x [DOI] [PubMed] [Google Scholar]


