Abstract
Ocean acidification is expected to alter community composition on coral reefs, but its effects on reef community metabolism are poorly understood. Here we document how early successional benthic coral reef communities change in situ along gradients of carbon dioxide (CO2), and the consequences of these changes on rates of community photosynthesis, respiration, and light and dark calcification. Ninety standardised benthic communities were grown on PVC tiles deployed at two shallow-water volcanic CO2 seeps and two adjacent control sites in Papua New Guinea. Along the CO2 gradient, both the upward facing phototrophic and the downward facing cryptic communities changed in their composition. Under ambient CO2, both communities were dominated by calcifying algae, but with increasing CO2 they were gradually replaced by non-calcifying algae (predominantly green filamentous algae, cyanobacteria and macroalgae, which increased from ~30% to ~80% cover). Responses were weaker in the invertebrate communities, however ascidians and tube-forming polychaetes declined with increasing CO2. Differences in the carbonate chemistry explained a far greater amount of change in communities than differences between the two reefs and successional changes from five to 13 months, suggesting community successions are established early and are under strong chemical control. As pH declined from 8.0 to 7.8, rates of gross photosynthesis and dark respiration of the 13-month old reef communities (upper and cryptic surfaces combined) significantly increased by 10% and 20%, respectively, in response to altered community composition. As a consequence, net production remained constant. Light and dark calcification rates both gradually declined by 20%, and low or negative daily net calcification rates were observed at an aragonite saturation state of <2.3. The study demonstrates that ocean acidification as predicted for the end of this century will strongly alter reef communities, and will significantly change rates of community metabolism.
Introduction
The oceanic uptake of anthropogenic carbon dioxide (CO2) emissions is causing ocean acidification (OA) [1]. OA not only lowers seawater pH, but also reduces the saturation state (Ω) of calcium carbonate (CaCO3) minerals, and increases CO2 and bicarbonate ion concentration. Predicting how marine communities will respond to OA is complicated, as many of these chemical alterations can act as drivers of change [1,2]. For example, the inhibition of calcification from declining pH and Ω [3], or the stimulus of photosynthesis from the increases in dissolved inorganic carbon (CT) [2], may affect species performances. Such physiological responses may also cause disruptions of ecological interactions, further altering communities [4,5]. As OA is occurring progressively, the response of species and communities is likely to occur along a continuum as well. Individual species have displayed both linear responses [6,7], as well as non-linear thresholds or tipping points [8] along gradients of CO2, while the response of communities remains largely uninvestigated. To better predict how communities will be shaped under OA, there is thus a need for studies which investigate the response curves of communities to increasing CO2.
Coral reefs are likely to be among the ecosystems most affected by OA [9]. Predictions are based on a multitude of single-species physiological studies [10], and several that have investigated changes at the community level. Community scale studies have centred around naturally occurring high-CO2 analogues, such as volcanic CO2 seep sites [11–13] or other oceanographic features affecting their carbonate chemistry [14–16], as well as larger-scale multi-species tank experiments [17–19]. While there is substantial variation in the responses between taxa, the general consensus predicts declines in biodiversity, a retraction of many calcifying species (e.g. scleractinian corals, coralline algae and foraminifera), an expansion of non-calcifying phototrophs (e.g. algae and seagrasses), and increased bioerosion. [20].
Coupled with the predicted changes in community composition under OA will likely be changes in community metabolism. However, scaling up OA effects on metabolic processes from individuals and species to the community level has proven difficult, and our current understanding is poor [21]. To date the best inferences have been based on naturally occurring seasonal carbonate chemistry changes [22–24], or the manipulation of seawater carbonate chemistry on coral reefs in situ [25,26] and in experimentation [18,27], as well as larger-scale mesocosm experiments [17,19,28,29]. These studies generally predict that rates of community photosynthesis and respiration will remain relatively unchanged from the reefs of today, while calcification and net CaCO3 accumulation will decline. However, these investigations have mainly examined effects due to changes in seawater carbonate chemistry, without fully accounting for changes due to the longer-term shifts in benthic community composition that may occur under OA. For example, Ω declines may directly reduce calcification rates in numerous taxa [10], but if these taxa are then outcompeted by non-calcifers, community calcification rates may further decline. Similarly, OA can increase rates of community production by directly stimulating photosynthesis in some species [30,31], or indirectly by increasing the benthic cover of certain phototrophs [2]. To gain further insight into the community metabolic dynamics of coral reefs under OA, measurements must be made on communities that have developed in their entirety under altered seawater carbonate chemistries.
The frequency and severity of disturbances affecting coral reefs is increasing [32], and scleractinian coral cover is now often well below 30% [33]. Scleractinian corals eventually re-enter communities, however it is early-successional non-scleractinian taxa (e.g. algae, sponges, and other sessile invertebrates) that increasingly dominate light exposed benthic reef communities [34]. Furthermore, shade exposed cryptic taxa within crevices of the reef matrix can account for the largest fraction of biomass in reef systems [35]. Both of these communities—the early successional benthic taxa on illuminated and shaded surfaces—are often overlooked in reef community metabolism studies, although their metabolism co-determines the carbonate chemistry conditions for newly settling corals within the benthic boundary layer.
In this study we investigate how OA will shape the composition of early-successional benthic communities that live on the carbonate substrata of coral reefs, and the metabolic rates of the non-scleractinian components of reef communities that have developed under altered carbonate chemistries. To do so, non-carbonate settlement tiles were deployed in situ, under natural levels of light and shade, temperature and water flow, along CO2 gradients at two volcanic CO2 seep and two control sites in Papua New Guinea. Benthic communities developing on the upper light exposed tile sides, as well as the shaded crevice-dwelling taxa on the lower sides were investigated after five and 13 months, and their successional changes, and taxa-specific responses along the CO2 gradients were explored. Response curves in community photosynthesis, respiration and light and dark calcification were then determined after 13 months.
Materials and methods
Study site and carbonate chemistry
This study was conducted at two tropical shallow water (<5 m depth) CO2 seeps and adjacent control sites in Milne Bay Province, Papua New Guinea. Seep and control sites at Upa-Upasina and Dobu are located adjacent to Normanby and Dobu Islands, respectively, and are described in detail by Fabricius et al. [11]. At both seep sites there is an area where near-pure (~99%) CO2 gas emerges from the seafloor, locally altering the carbonate chemistry of the seawater without altering its temperature [31]. The seeping has resulted in an altered benthic community, with hard and soft coral diversity, calcifying algal cover and the abundance and diversity of numerous mobile invertebrate taxa declining, while non-calcifying algae and seagrass cover is increased [4,11]. This research was conducted under permits issued to Dr Katharina Fabricius from Papua New Guinea’s Department of Environment and Conservation, and the National Research Institute.
Data on the seawater carbonate chemistry for the sites has been published by Fabricius et al. [4,11], as have seawater measurements above settlement tiles used in this study [8,20,36]. Specifically, during four ~2-week expeditions between 2011 and 2013, 911 pH measurements were recorded across the 90 settlement tiles (median n = 8 per tile), and a further 625 samples (median n = 5 per tile) were taken for total alkalinity (AT) [8]. Given seeping intensity varies spatially and temporally within the mosaic of gas streams that comprises the seep sites, these samples were used to form long-term medians of the seawater carbonate chemistry at the exact location of each tile. Unless otherwise stated, all pH values are presented in the total scale (pHT).
Benthic community composition
In December 2011, 90 labelled settlement tiles were evenly distributed across seep and control sites at both Dobu and Upa-Upasina reefs (n = 15 control and 30 seep site tiles per reef). Tiles were 11.5 * 11.5 cm, made of 3 mm thick polyvinyl chloride (PVC) and roughened on both sides with sandpaper. Kennedy et al. [37] have shown that the percent coverage of benthic taxa settling on PVC tiles more closely matched adjacent reef substrata than other tile materials. Tiles were deployed horizontally on numbered baseplates, at ~3 m depth, ~2 cm from the reef substratum, >2m apart. The gap between the tile and the reef substrate allowed communities to not only develop on the upper light exposed tile side, but also for cryptic taxa to recruit to the lower shaded side. The tiles were first collected after five months (May 2012), photographed on both sides while being continuously submerged, and redeployed to their original location. In January 2013 (after 13 months deployment) the tiles were again collected, physiological measurements were made (details below), and they were rephotographed, before being dried and transported to the laboratories at the Australian Institute of Marine Science. Eighty-eight of the 90 settlement tiles were recovered during both census periods, with one missing from each seep site at Dobu and Upa-Upasina.
The benthic community composition of the upper and lower sides of the tiles, from the two census periods, was assessed on the tile photographs using the evenly spaced point analysis method [38]. To do so, photographs were imported into image editing software (Photoshop CS6, Adobe Systems, USA), and digitally overlaid with a grid consisting of 7 * 7 evenly spaced lines. The identity of the benthos occurring under the resultant 49 cross points was recorded and classified into 15 operational taxonomic units (OTUs, S1 Table), including seven groups of algae and cyanobacteria, six phyla of benthic invertebrates, bare tile space, and a category for any taxa that could not be identified. The point counts were converted into percent coverage data and some OTUs were further grouped into functional categories (non-calcifying algae, calcifying and non-calcifying invertebrates) for later statistical analysis (S1 Table). Photographs from 15 of the 30 seep tiles at Upa-Upasina at the 13 month census were lost (the camera memory card was accidently formatted prior to backup) and hence not included in analyses. The abundances of sessile tube-forming polychaetes and of coral recruits were counted directly on each tile once dried in the laboratory using a dissection microscope.
Benthic community metabolism
To determine rates of community gross photosynthesis, dark respiration, and light and dark calcification, all settlement tiles were incubated in the light and dark on the day of their collection in January 2013. For the incubations, tiles were transferred from their holding containers in running seawater into clear rectangular-prism chambers (920 mL volume), atop spacers which left ~1.5 cm gap between the tile and the walls, bottom and lid of the chamber. Chambers were placed in black flow-through bins that served as water baths and were maintained at ambient in situ temperatures from a 2m deep intake (30°C). To minimise boundary layers, a 35 mm magnetic stirrer bar was placed into each chamber, activated with a custom made system of rotating magnets and pullies placed under the water baths. Communities from control sites were incubated in seawater at pHT 8.08, while those originating from the seep sites were placed in seawater with pHT 7.70. Four chambers per seep, and two per control site, were incubated with a blank settlement tile as a control. Water obtained from the seep sites was mixed with control seawater immediately prior to incubations to make a large batch at the target pH level, which was then used to fill the chambers. pH was determined with a portable pH meter (SG23, Mettler Toledo, USA) calibrated on the NBS scale. Measurements of pHNBS and AT from the incubation water were used to calculate carbonate chemistry parameters using the macro CO2SYS with the constraints set by Dickson and Millero [39], following Lewis et al. [40] (S2 Table).
Light incubations were conducted in black bins under four white fluorescent tubes (10 000K) at their maximum output of 180 μmol photons m-2 s-1. After ~90 minutes, the O2 concentration in each chamber was measured (meter: HQ30d; probe: LDO101 IntelliCAL, Hach, USA) and a sample of water was retained and fixed with saturated HgCl2 (>7 g L-1) for calcification assays. Tile communities were allowed >30 min dark adaptation in the water baths under black lids, before the chambers were closed again and the process was repeated in the dark. Light and dark calcification rates were determined with the alkalinity anomaly technique [41], using open cell titration (Metrohm 855 robotic titrosampler, Switzerland) fitted with a gran function following Vogel et al. [42]. The alkalinity anomaly technique assumes incubation water AT is only affected by the removal (calcification) or addition (dissolution) of CaCO3, however some organisms in the tile communities (e.g. molluscs) would have altered AT through the release or uptake of nutrients [43]. This would have added some unaccounted measurement error to calcification rate estimates, however the technique has been used successfully to estimate rates of coral reef community calcification [27,44], and is considered robust in coral reef environments when compared to other techniques [45]. The incubations of tile communities resulted in a change in AT from blank-tile control chamber values that ranged from -219 to -6 μmol kg-1 SW in the light, and -76 to +92 μmol kg-1 SW in the dark. Primary AT standards (CRMs; A. Dickson Laboratory, Scripps Institution of Oceanography) were titrated to calculate titrant concentration, and replicate secondary seawater standards (n = 15) were interspaced and titrated throughout incubation samples and were highly consistent (SE = 2.19 μmol kg-1 SW). Rates of gross photosynthesis and dark respiration (μg O2 cm-2 min-1), and light and dark calcification (μM CaCO3 cm-2 min-1), were calculated by subtracting the values of blank tile O2 or AT values, at the end of the incubation runs, from values of chambers with tile communities, and then standardised to incubation time and planar surface area of each tile. Changes in the surface area of the tiles due to differences in settling benthos were not accounted for. Estimates of daily net photosynthesis and calcification were calculated by combining light and dark rates, assuming a square profile of 11.5 hrs light and 12.5 hrs dark. This daily estimate equated to a cumulative daily light integral of 7.45 mol photon m-2 d-1, which is similar to those at the study site and depth [42].
Statistical analyses
Generalised linear models (GLM) were used to examine how percent cover of the different benthos OTUs on the tiles differed with median pHT, and across Reef (Dobu and Upa-Upasina) and Time (five vs 13 month censuses). Median pHT was used in models as it was directly measured many times at each tile (rather than being calculated), and correlated better with the other carbonate chemistry parameters than AT (which is not directly affected by CO2 addition). Percent cover data were fit using a quasibinomial distribution accounting for the larger proportion of values at the upper and lower bounds of the distribution. Non-significant (p > 0.05) main effects and interaction terms were removed in the final models. For comparison to control values (seawater pHT 8.05, or ΩAr = 3.84), percent coverages of taxa were also predicted for seawater pHT 7.80 or ΩAr = 2.50, using the predict function in R, as this value may be expected by the year 2100 in tropical oceans [46].
Redundancy analysis (RDA) was also conducted to visually examine how the community data separated out in two-dimensional space, and to further test the significance of each explanatory variable via permutation. The explanatory variables in the analysis included reef and census period as categorical factors, as well as the median pHT at each tile as a continuous quantitative variable.
GLMs were fitted to the metabolic measurements from all tiles, using Reef and median pHT of the tile origin as predictors for measurements of gross photosynthesis and respiration, and Reef and ΩAr for calcification. For the calcification assay, two chambers opened, and five outliers (with values >8-fold from the confidence intervals) were removed from final models (n = 81). A second series of GLMs was conducted on the metabolism measurements which incorporated the carbonate chemistry of the tiles, as well as the taxonomic benthic cover data (n = 66 due to the loss of 15 photos). An identity link function was used when response parameters approximated a Gaussian distribution. Quasipoisson distributions and log link functions were used for over-dispersed data. The appropriate model distribution was selected by comparing dispersion factors, preferring those which approached a value of 1. All statistical procedures were conducted with the statistical software R version 3.2.5 [47] using the packages vegan and gmodels.
Results
Carbonate chemistry
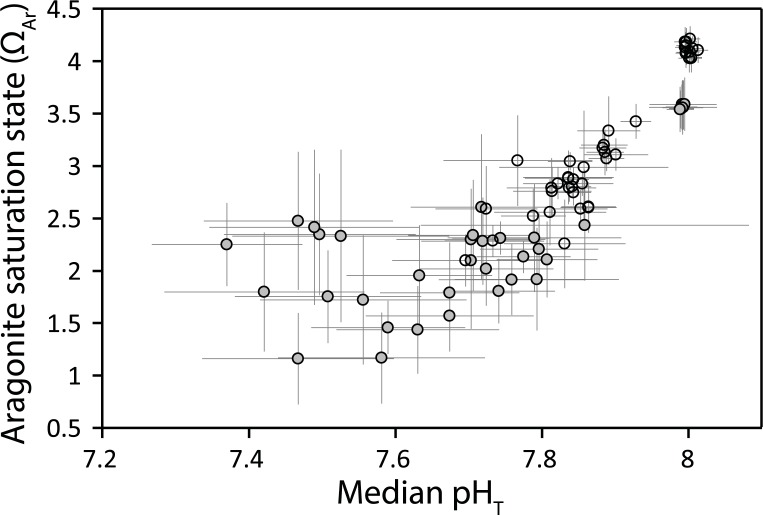
The settlement tile communities were developed along clear CO2 gradients at both the Dobu and Upa-Upasina seeps (Fig 1). Both control sites had similar seawater chemistry, however the pH gradient at the Dobu seep reached lower levels than at the Upa-Upasina seep. The median seawater pHT over the tiles ranged from 8.0 ± 0.001 (SE) at the control sites of both reefs, to 7.7 ± 0.10 as lowest values at the Upa-Upasina seep, and to 7.4 ± 0.10 at Dobu seep (Fig 1). Median aragonite saturation state (ΩAr) was 3.84 ± 0.05 at the control sites, declining to 2.10 ± 0.25 and 1.16 ± 0.43 at the Upa-Upasina and Dobu seeps, respectively (Fig 1). Similarly, calcite saturation state (ΩCa) values decreased from 5.75 ± 0.10 at the control sites along the carbonate chemistry gradient to 3.14 ± 0.38 and 1.73 ± 0.64 at the Upa-Upasina and Dobu seep, respectively. While none of the communities were exposed to median ΩAr values <1.0, 55% had median ΩAr <3.0, a value which has been suggested to be the limit for reef development [48].
Fig 1. Median pHT and saturation state of aragonite (ΩAr) from the 88 settlement tiles.
Median n = 8 pH and 5 ΩAr measures per tile. White points are from Upa-Upasina, grey are from Dobu. Error bars are standard errors.
Values of pCO2, CT and AT were also spread along the carbonate chemistry gradient. The daytime pCO2 at the control sites averaged 392.15 ± 9.32 μatm, increasing to 1008 ± 291.99 and 2253 ± 759.63 μatm at Upa-Upasina and Dobu seeps respectively. Control CT values averaged 1926 ± 0.70 μmol kg-1, and increased to >2100 at the seeps. Median AT values were slightly elevated within the seep sites (perhaps due to CaCO3 dissolution), and differed from control values by no more than 6%. AT values increased from an average of 2252.68 ± 3.33 μmol equivalents kg-1 at the controls to 2339.60 ± 16.29 and 2329.68 ± 18.89 μmol equivalents kg-1 within the Upa-Upasina and Dobu seeps, respectively (see also S2 Fig in Enochs et al. [20] as their experimental units were deployed alongside the settlement tiles of the present study). Carbonate chemistry parameters were more variable within the seeps compared to the control sites (Fig 1).
Benthic community composition
Both tile surfaces were covered by a significant amount of macrobenthos after five months, and after 13 months, the tiles were all but indistinguishable from the adjacent substrata (Fig 2). Communities on the upper tile sides, being exposed to higher light intensities and grazing, only included several algal OTUs and bare space, and no invertebrates. This contrasted with the lower sides of the tiles, where light and grazing intensities are low; these communities consisted of algal OTUs, as well as many invertebrate taxa and bare space (Fig 2). Coral recruits were observed on the lower tile sides [36], however, due to their small size (typically <2mm diameter), they failed to contribute to community cover estimates.
Fig 2.
Settlement tiles from control sites (a, b and c) and volcanic CO2 seep sites (d, e and f) in Papua New Guinea after 13 months deployment. Tiles in situ (a and d), upper sides (b and e), and lower sides (c and f).
The communities underwent some successional changes between the two census periods. Pioneering algal taxa, including green filamentous algae on the upper tile side and turf algae and cyanobacteria on the lower tile side, all recorded significantly lower percent cover after 13 months compared to the five month census (GLM significant main effect of Time, all p < 0.05; Table 1). Turf algae on the upper side displayed the opposite pattern, increasing in cover between the five and 13 month censuses (S3 Table). At 13 months, several of the slower growing taxa had increased in abundance: Peyssonnelia spp. on the upper sides and macroalgae and sponges on the lower sides had all increased in cover, as did the combined cover of both the calcifying and the non-calcifying invertebrate groups (Table 1). The amount of unoccupied space on both the upper and lower tile sides similarly declined between the census periods as available space was progressively occupied (Table 1).
Table 1. Changes in percent cover of the main benthic taxonomic and functional categories, in response to pHT (median per tile), Reef (contrasting Dobu against Upa-Upasina) and Time (contrasting 13 against five months of deployment).
| Estimate | SE | t | p | |
|---|---|---|---|---|
| Non-calcifying algae up | ||||
| Intercept | 18.88 | 3.13 | 6.04 | <0.001 |
| pH | -2.35 | 0.39 | -5.94 | <0.001 |
| Reef | -0.45 | 0.13 | -3.49 | <0.001 |
| Non-calcifying algae low | ||||
| Intercept | 40.36 | 4.42 | 9.13 | <0.001 |
| pH | -5.20 | 0.56 | -9.20 | <0.001 |
| Time | 16.61 | 6.79 | 2.45 | 0.016 |
| pH:Time | -2.17 | 0.87 | -2.50 | 0.014 |
| Green filaments up* | ||||
| Intercept | 10.91 | 2.59 | 4.21 | <0.001 |
| pH | -1.60 | 0.33 | -4.88 | <0.001 |
| Reef | 0.79 | 0.13 | 6.15 | <0.001 |
| Time | -0.45 | 0.11 | -4.02 | <0.001 |
| Turf up | ||||
| Intercept | -0.68 | 0.13 | -5.15 | <0.001 |
| Reef | -0.93 | 0.17 | -5.48 | <0.001 |
| Time | 0.51 | 0.17 | 3.01 | 0.003 |
| Turf low | ||||
| Intercept | -1.38 | 0.07 | -18.43 | <0.001 |
| Time | -1.15 | 0.14 | -7.82 | <0.001 |
| Macroalgae low* | ||||
| Intercept | 26.84 | 4.85 | 5.54 | <0.001 |
| pH | -4.18 | 0.61 | -6.83 | <0.001 |
| Reef | 2.57 | 0.59 | 4.31 | <0.001 |
| Time | 1.55 | 0.25 | 6.17 | <0.001 |
| Cyanobacteria low* | ||||
| Intercept | 67.31 | 12.79 | 5.26 | <0.001 |
| pH | -8.79 | 1.63 | -5.40 | <0.001 |
| Reef | -31.41 | 16.50 | -2.33 | 0.021 |
| Time | -0.32 | 0.17 | -1.91 | 0.057 |
| pH: Reef | 3.99 | 1.72 | 2.32 | 0.022 |
| Peyssonnelia up | ||||
| Intercept | 14.50 | 4.27 | 3.40 | <0.001 |
| pH | -2.38 | 0–55 | -4.33 | <0.001 |
| Time | 1.74 | 0.26 | 6.65 | <0.001 |
| Peyssonnelia low | ||||
| Intercept | 26.73 | 11.26 | 2.37 | 0.019 |
| pH | -3.64 | 1.43 | -2.54 | 0.012 |
| Reef | -33.07 | 12.82 | -2.58 | 0.011 |
| Time | 17.11 | 16.46 | 1.04 | 0.300 |
| pH: Reef | 4.15 | 1.63 | 2.54 | 0.012 |
| pH: Time | -2.02 | 2.08 | -0.97 | 0.334 |
| Reef: Time | -72.00 | 19.88 | -3.62 | <0.001 |
| pH: Reef: Time | 9.04 | 2.52 | 3.59 | <0.001 |
| CCA up | ||||
| Intercept | -64.69 | 6.86 | -9.43 | <0.001 |
| pH | 7.89 | 0.86 | 9.13 | <0.001 |
| Reef | 1.65 | 0.21 | 7.75 | <0.001 |
| Time | 27.24 | 8.96 | 3.04 | 0.003 |
| pH: Time | -3.37 | 1.13 | -2.99 | 0.003 |
| Reef: Time | -0.95 | 0.30 | -3.17 | 0.002 |
| CCA low | ||||
| Intercept | -53.013 | 8.45 | -6.28 | <0.001 |
| pH | 6.47 | 1.06 | 6.08 | <0.001 |
| Reef | -93.28 | 23.06 | -4.04 | <0.001 |
| Time | -0.26 | 0.17 | -1.56 | 0.122 |
| pH: Reef | 11.64 | 2.89 | 4.03 | <0.001 |
| Reef: Time | 1.08 | 0.27 | 4.03 | <0.001 |
| Non-calcifying invertebrates low* | ||||
| Intercept | -20.90 | 5.71 | -3.66 | <0.001 |
| pH | 2.16 | 0.72 | 3.00 | 0.003 |
| Reef | 1.29 | 0.37 | 3.48 | <0.001 |
| Time | 1.34 | 0.37 | 3.59 | <0.001 |
| Reef: Time | -1.36 | 0.46 | -2.95 | 0.004 |
| Calcifying invertebrates low* | ||||
| Intercept | -50.90 | 15.48 | -3.29 | 0.001 |
| pH | 6.00 | 1.95 | 3.08 | 0.002 |
| Reef | 42.69 | 16.64 | 2.57 | 0.011 |
| Time | 1.02 | 0.29 | 3.47 | <0.001 |
| pH: Reef | -5.35 | 2.10 | -2.55 | 0.012 |
| Reef: Time | -1.03 | 0.41 | -2.50 | 0.013 |
| Ascidians low* | ||||
| Intercept | -98.37 | 33.45 | -2.94 | 0.004 |
| pH | 12.00 | 4.20 | 2.86 | 0.005 |
| Reef | 77.74 | 34.19 | 2.27 | 0.024 |
| pH: Reef | -9.65 | 4.29 | -2.25 | 0.026 |
| Polychaetes low (counts per tile)* | ||||
| Intercept | -1395.59 | 436.92 | -3.19 | 0.002 |
| pH | 188.78 | 55.35 | 3.41 | 0.001 |
| Reef | -39.26 | 17.84 | -2.20 | 0.031 |
| Unoccupied space up | ||||
| Intercept | -0.66 | 0.09 | -7.41 | <0.001 |
| Reef | -0.62 | 0.14 | -4.51 | <0.001 |
| Time | -1.49 | 0.20 | -7.53 | <0.001 |
| Reef: Time | 1.11 | 0.25 | 4.43 | <0.001 |
| Unoccupied space low | ||||
| Intercept | -13.83 | 4.42 | -3.13 | 0.002 |
| pH | 1.61 | 0.56 | 2.88 | 0.004 |
| Reef | -0.78 | 0.19 | -4.04 | <0.001 |
| Time | -1.76 | 0.27 | -6.47 | <0.001 |
| Reef: Time | 0.98 | 0.37 | 2.65 | 0.009 |
Responses are for the upper (up) and lower (low) sides of the settlement tiles. Parameter estimates from the best fitting generalised linear models, with quasibinomial distributions. Non-significant terms removed from final models.
* indicates taxa only found on one tile side.
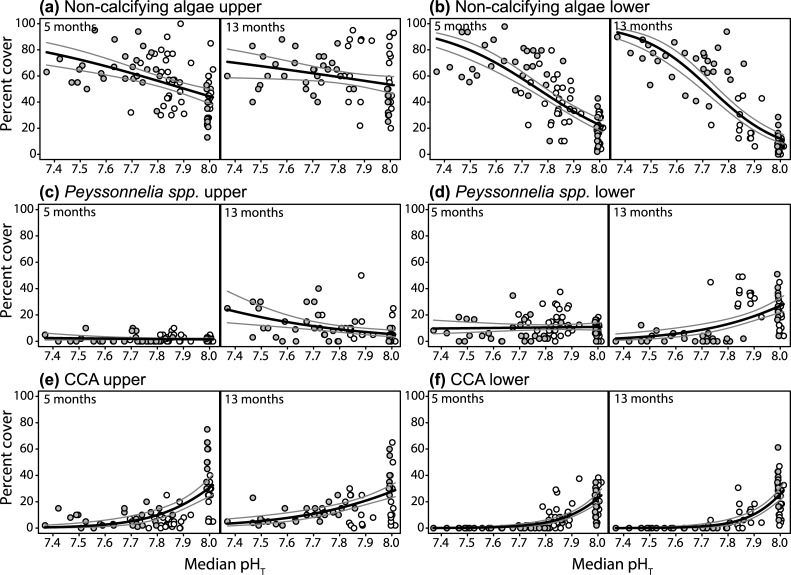
Changes in pH explained two and 50 times more variation in the cover of non-calcifying algae on the upper and lower tile sides, respectively, compared to the explanatory variables Reef and Time (GLM F ratios, S3 Table), indicating many patterns in the tile communities were established within five months and were largely consistent between reefs. Non-calcifying algal cover increased as pH declined from relatively low control values to ~80% at the lower end of pH gradients, without a clear threshold at which point non-calcifying algae came to dominate communities (Fig 3A and 3B). On the upper tile side the cover of non-calcifying algae increased from control values of 40.3 ± 1.7 (SE) to 56.3 ± 2.0% at pH 7.8 after five months, and 49.4 ± 2.07 to 59.2 ± 2.3% at pHT 7.8 after 13 months (Fig 3), predominantly due to green filamentous algae (S1 Fig). Similarly, on the lower sides, non-calcifying algae increased from control values of 19.3 ± 1.7% to 45.1 ± 2.1% by pH 7.8 at five months, and from 8.6 ± 0.6 to 37.4 ± 2.5% by pHT 7.8 at 13 months (Fig 3). Lower side communities were dominated by cyanobacteria at both seeps, and also by macroalgae at Dobu (S1 Fig).
Fig 3. Shifts in settlement tile community composition along the pH gradients.
Percent cover of non-calcifying algae on the upper (a) and lower (b) settlement tile sides, Peyssonnelia spp. from the upper (c) and lower (d) sides, and crustose coralline algae (CCA) from the upper (e) and lower (f) sides in relation to pHT. Left and right panels per plot represent the five and 13 month old communities, respectively. White points are tiles from Upa-Upasina and grey are from Dobu. The black lines represent the modelled means, while the grey lines are confidence intervals.
The different taxa of calcifying algae contrasted in response to changes in pH. The very mildly calcifying red alga Peyssonnelia spp. on the upper surface increased in cover between census periods. In 13 month old communities, upper side Peyssonnelia spp. cover also increased with declining pH from control values of 4.1 ± 0.3 to 9.1 ± 1.2% at pHT 7.8 (Table 1, Fig 3C and 3D). On the lower sides, Peyssonnelia spp. cover showed no clear patterns, indicating light limitation or competition with other benthos was co-limiting their distribution (Fig 3). The cover of the heavily calcified crustose coralline algae (CCA) declined steeply along the pH gradient on both the upper and lower tile sides (Fig 3E and 3F), and the effects of pH changes were again far stronger than Reef or Time (GLM F ratios, S3 Table). On the upper sides, CCA cover declined from control values of 35.2 ± 2.4 to 9.9 ± 1.8% by pHT 7.8 in five month old communities, and from 29.6 ± 1.8 to 15.2 ± 1.5% by pHT 7.8 in 13 month old communities. An apparent threshold in CCA cover was observed on the lower tile sides at pHT 7.8, where CCA was virtually absent below pHT 7.8, while they continued to persist with low cover on the upper sides below this pH level (Fig 3).
Patterns in the invertebrate communities were less clear compared to those seen in the algae. The cover of both calcifying and non-calcifying groups declined with pH at both reefs after five months, and at Upa-Upasina but not Dobu after 13 months (S2 Fig). Ascidian cover and the number of polychaetes significantly declined with pH at both reefs and both times, but cover differed between the reefs (Table 1). No distinct patterns were observed in the cover of bivalves, foraminifera or bryozoa (S3 Table).
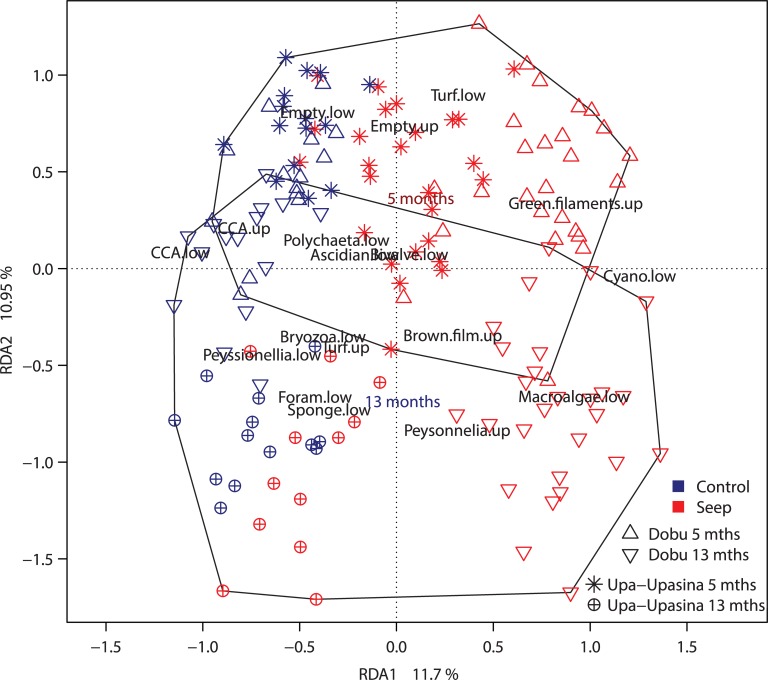
Results of the RDA agreed with the GLM analyses: all explanatory variables accounted for significant variation in benthic communities (ANOVA, all p = 0.001), and tile pH explained more of this variation than differences between census periods or reef (F ratios of 22.37, 19.29 and 14.67 for pH, census period and reef, respectively). In the ordination, the first RDA axis separated communities between seep and control sites. This was primarily driven by high cover of cyanobacteria, macroalgae, and green and brown filamentous algae in the high CO2 communities, and high CCA cover in control sites (Fig 4). Communities from Upa-Upasina clustered closer together between high CO2 and control tiles in comparison to Dobu, perhaps reflecting the greater intensity of CO2 exposure at Dobu seep. The second RDA axis divided communities between census periods: the five month old communities on the lower sides were associated with more empty space and turf algae, and on the upper sides with green filamentous algae (Fig 4). At 13 months, the communities were associated with an increased cover of Peyssonnelia spp., sponges, foraminifera (on the lower sides of the control tiles), and macroalgae (on the lower sides of high CO2 tiles).
Fig 4. Redundancy analysis ordination of the settlement tile benthic communities at the seep and control sites.
Red points represent tiles from the seeps, and blue from the controls, of Dobu and Upa-Upasina Reefs. Points in the upper polygon are five month (mths) old communities, while those in the lower polygon are at 13 months.
Benthic community metabolism
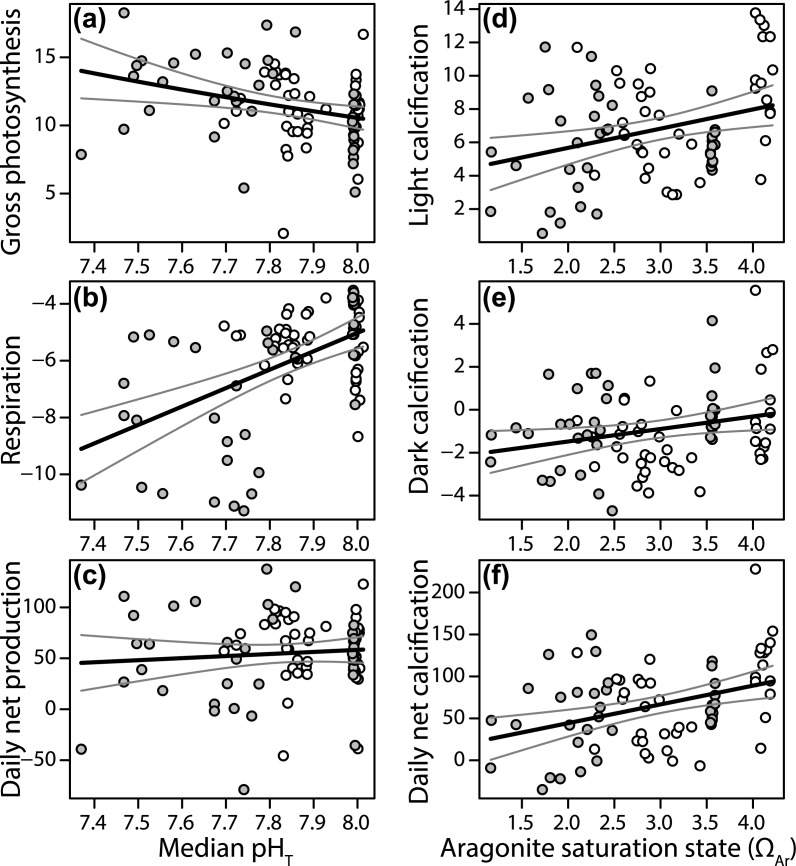
Rates of community metabolism were related to both changes in the seawater carbonate chemistry and changes in benthic communities. All metabolic measurements gradually changed along the carbonate chemistry gradient, and no abrupt changes or threshold responses were detected (Fig 5). Gross photosynthetic rates increased linearly as pH declined along the gradient (Fig 5A, Table 2). On average, control tiles produced 10.37 ± 0.4 μg O2 cm-2 hr-1, increasing by 10% to 11.54 ± 0.3 μg O2 cm-2 hr-1 at pHT 7.8. Increased gross photosynthetic rates were also positively related to the increasing cover of non-calcifying algae in the upper communities, but declined with the increasing cover of non-calcifying invertebrates along the pH gradient (Table 2).
Fig 5. Metabolic rates from benthic communities on settlement tiles across the seawater carbon chemistry gradients.
Rates of gross photosynthesis (a), respiration (b) and net daily production (c) in 13 month old communities are plotted against the median pHT from the tiles. Gross photosynthesis and respiration are displayed in μg O2 cm-2 hr-1, and daily net production in μg O2 cm-2 day-1. Rates of light (d), dark (e) and net daily calcification (f) are plotted against tile median aragonite saturation state (ΩAr) from the tiles. Light and dark calcification rates are displayed in μg CaCO3 cm-2 hr-1, and daily net calcification in μg CaCO3 cm-2 day-1. White points are from Upa-Upasina and grey are from Dobu. The black lines represent the modelled mean, while the grey lines are confidence intervals.
Table 2. Changes in community metabolism in response to median pH (gross photosynthesis; respiration) or the saturation state of aragonite (ΩAr: Calcification) and Reef (contrasting Dobu against Upa-Upasina).
| Estimate | SE | t | p | |
|---|---|---|---|---|
| Gross photosynthesisQ | ||||
| Intercept | 6.39 | 1.25 | 1.48 | 0.142 |
| pH | -1.57 | 0.16 | -2.81 | 0.006 |
| Gross photosynthesis benthosQ | ||||
| Intercept | -6.64 | 0.09 | -20.21 | <0.001 |
| Non-calc invert low | -1.01 | 0.002 | -3.78 | <0.001 |
| Non-calc algae up | 1.00 | 0.001 | 3.84 | <0.001 |
| Respiration^0.25G | ||||
| Intercept | 0.16 | 0.48 | 0.34 | 0.735 |
| pH | 0.05 | 0.06 | 0.80 | 0.427 |
| Reef | 1.75 | 0.53 | 3.29 | 0.001 |
| pH: Reef | -0.22 | 0.07 | -3.28 | 0.002 |
| Respiration benthos^0.25G | ||||
| Intercept | 1.77 | 0.18 | 9.61 | <0.001 |
| pH | -0.16 | 0.02 | -6.69 | <0.001 |
| Bivalve | 0.01 | 0.001 | 3.43 | 0.001 |
| Non-calc invert low | 0.001 | <0.001 | 4.32 | <0.001 |
| Net daily production benthosG | ||||
| Intercept | 78.10 | 6.48 | 12.04 | <0.001 |
| Bivalve | -4.97 | 1.70 | -2.92 | 0.005 |
| Non-calc invert low | -1.35 | 0.31 | -4.37 | <0.001 |
| Light calcificationG | ||||
| Intercept | 0.06 | 0.02 | 2.76 | 0.007 |
| ΩAr | 0.02 | <0.01 | 2.89 | 0.005 |
| Dark calcificationG | ||||
| Intercept | -0.06 | 0.01 | -4.27 | <0.001 |
| ΩAr | 0.01 | <0.01 | 3.15 | 0.002 |
| Reef | 0.02 | 0.01 | 2.33 | 0.023 |
| Net daily calcificationG | ||||
| Intercept | -0.37 | 19.47 | -0.02 | 0.985 |
| ΩAr | 22.34 | 6.34 | 3.52 | <0.001 |
Models were then run again but the cover of the main community members was additionally included as co-variates (benthos). Parameter estimates from the best fitting generalised linear models (G: Gaussian; Q: quasipoisson distributions), with estimates for the quasipoisson models back-transformed to aid interpretation. Non-significant terms were removed from final models.
Respiration rates increased 20% from control site values (5.18 ± 0.6 μg O2 cm-2 hr-1 consumption) to those at pHT 7.8 (6.21 ± 0.2), and continued to increase along the pH gradient (Fig 5B). The increase in respiration with pH was stronger at Dobu, however the pH main effect explained three times the variation in respiration compared to the interaction between pH and Reef (S4 Table, F = 33 and 10 for pH and the interaction, respectively). Respiration rates also increased with the cover of bivalves and non-calcifying invertebrates on the lower sides of the tiles (Table 2). Since declining pH elevated rates of both gross photosynthesis and respiration, no difference was detected in daily net production, which averaged 55 ± 13.2 μg O2 cm-2 day-1 across all tiles (Fig 5C). Daily net production was instead reduced by an increase in the cover of bivalves and non-calcifying invertebrates on the lower sides of the tiles (Table 2). Mean gross photosynthetic rates were on average twice that of respiration, and 90% of tiles recorded positive daily net production values.
Rates of community calcification in the light and dark, and net 24-h community calcification, all declined along the ΩAr gradient (Table 2). Mean light calcification rates averaged 7.73 ± 1.3 μg CaCO3 cm-2 h-1 at the control sites and decreased by 20% to 6.25 ± 0.4 μg CaCO3 cm-2 h-1 by ΩAr 2.5. None of the communities recorded net decalcification in the light, despite median ΩAr reaching as low as 1.16, while 70% of control and 80% of seeps communities showed net decalcification in the dark. Dark calcification declined from -0.01 ± 0.3 μg CaCO3 cm-2 h-1 at the control sites to -1.19 ± 0.2 μg CaCO3 cm-2 h-1 by 2.5 ΩAr, and continued to decrease along the gradient (Fig 5E, Table 2). Dark calcification rates were also lower at Upa-Upasina compared to Dobu (Table 2). Daily net calcification rates of 89.14 ± 19.3 μg CaCO3 cm-2 day-1 at the control sites declined by 38% to 55.49 ± 5.9 at ΩAr 2.5 (equalling annual CaCO2 deposition of 325 vs 204 g m-2 yr-1). Given rates of light calcification were greater than those in the dark, 90% of the tiles recorded positive daily net calcification, with negative rates all observed at <2.3 ΩAr (Fig 5F). Interestingly, the declines in calcification were not significant when calcification was modelled against tile pH. Tile pHT and ΩAr were highly correlated until the lower end of the pH gradient (Fig 1), where ΩAr increased relative to pH. This decoupling is likely driven by CaCO3 dissolution in the seeps, raising AT and subsequently ΩAr, and may explain the differences between model results. The inclusion of the benthic OTUs did not improve the GLM fits to the carbonate chemistry parameters for rates of light, dark or daily net calcification. No relationships were detected between gross photosynthesis and light calcification rates, or between respiration and dark calcification rates (linear regressions, all p > 0.05).
Discussion
Ocean acidification is predicted to fundamentally alter benthic marine communities. Here we report a drastic shift in the composition and metabolism of early successional benthic coral reef communities along seawater carbonate chemistry gradients. The carbonate chemistry explained a far greater amount of change in communities than successional changes from five to 13 months, and differences between the two reefs. Shifts were more pronounced in the algae compared to the invertebrate communities, where a suite of non-calcifying algal groups largely replaced CCA on seep site tiles. Changes in CO2 and community composition also affected community metabolism; rates of gross photosynthesis and respiration increased with increasing CO2, and with the cover of certain taxonomic groups, while 24-h net calcification decreased to low or even negative values.
The present study adds to the mounting body of evidence predicting ecosystem-wide changes in benthic communities under OA. Here we found rapid increases in non-calcifying algal cover as pH declined along the carbonate chemistry gradients, and little evidence of threshold responses for these taxa. This pattern was largely consistent between light exposed and cryptic communities, with green filaments establishing dominance on the upper sides, and cyanobacteria and macroalgae on the lower sides. While results are not universal [14], patterns in benthic communities at CO2 seeps in the temperate Mediterranean [5,49–51], as well as multiple tropical sites in the Indo-Pacific [11,12], concur with the present study. These studies similarly predict an increase in non-calcifying algae under OA, and there are suggestions that other non-calcifying phototrophs, such as seagrasses [11] and anemones [30], may also thrive. Interestingly, non-calcifying algae have increased abundances in the wider community at the seep sites in Milne Bay [11], however they do not dominate the benthos like on the settlement tiles of the present study, or at another tropical seep [12]. Grazers are diverse and abundant at the Milne Bay seeps [52], which may prevent the proliferation of algae on upper surfaces in the wider community. Similarly, longer-term competition with benthos that had not developed fully on the tiles (e.g. the scleractinian corals) may also constrain algal growth.
Calcifying algae on the settlement tiles displayed dissimilar results between taxa along the CO2 gradient. The cover of the lightly calcifying algae Peyssonnelia spp. increased at lower pH, albeit only in high-light environments. Some Peyssonnelia species have increased in abundance at other seep sites [49,51], suggesting that certain species of calcifying algae may be resilient to or even benefit from OA [42,53], perhaps due to the use of aragonite over high magnesium-calcite in their skeletons [51], and by using the additional CT for photosynthesis. The steep decline in CCA cover is consistent with data from multiple seep sites [8,12] and in experimentation [54] with potentially profound effects on coral reef communities [36]. The steeper decline in CCA on the lower tile surfaces, as well as the increase in Peyssonnelia spp. on the upper tile surfaces, indicates light intensity is playing a role in the response of these taxa to OA.
Invertebrate responses to carbonate chemistry changes varied between taxa, and overall their cover and abundances did not respond as strongly as the algae did. It is important to note that the invertebrate communities in the present study were in relatively early successional stages, and consisted of shade-adapted communities without scleractinian corals (no invertebrates were found on the upper tile surfaces). The number of tube-dwelling polychaetes per tile declined with pH, as did the cover of ascidians. Similar declines were seen in tube-dwelling polychaete species at a Mediterranean seep [55], possibly due to reduced calcification in the juvenile stage [56]. Little is known about why ascidians appear to respond negatively to elevated CO2, however a previous study found the abundances of ascidians on natural reef substrata also declined with CO2 exposure at the Milne Bay seeps [4]. Our study found no apparent effect of carbonate chemistry on the cover of the diverse groups of bivalves, bryozoans or sponges. Both bivalves and bryozoans are calcifying and considered sensitive to carbonate chemistry changes, but their CaCO3 skeletons are somewhat protected from the surrounding seawater through external tissue layers [10,57]. Sponges have shown a mixed response to elevated CO2, with some species negatively responding, while species with phototrophic symbionts or siliceous spicules may respond positively [58].
Patterns in the 13 month old tile communities were largely established in the first five months, and successional changes between census periods were considerably weaker than the influence of the carbonate chemistry gradient for the majority of taxa. While the cover of some ephemeral (turf and green filamentous algae and cyanobacteria) and slower growing taxa (Peyssonnelia spp., other macroalgae and the invertebrate groups) changed between census periods, this did not significantly alter the patterns in the rest of the tile communities. For example, patterns along the CO2 gradients in the cover of non-calcifying algae and CCA, which accounted for the majority of the tile communities, were largely consistent between census periods. This consistency between census periods contrasts a similar study at Mediterranean seeps, where Kroeker et al. [5] found commonalities between early settlement seep and control communities progressively diverged as competitive hierarchies were disrupted. Fabricius et al. [8], who closely examined patterns in CCA distributions on the tiles of the present study, concluded that it was recruitment limitation in the CCA at lower pH, rather than competition with other taxa, that established the patterns seen here.
It is unknown to what extent the successional tile communities reflect the surrounding mature benthic communities. After 13 months, the tile communities blended in and greatly resembled the surrounding benthos (personal observation). Previous work at the Milne Bay seeps has similarly shown higher turf and macroalgae cover, and lower CCA cover, on natural substrate within the seep reefs compared to control reefs [11]. Regardless of any disparities between tile and mature benthic communities, early successional communities may become increasingly prevalent on coral reefs as the frequency and severity of disturbances increases [32], making larger contributions to overall reef composition and metabolic signals, with potentially important complications for the carbonate chemistry newly settling corals will experience within the benthic boundary layer.
The present study presents the first investigation of metabolic changes for combined surface and cryptic subsurface reef communities that have developed entirely in situ under high CO2. Here we documented a 10% increase in gross photosynthesis and a 20% increase in respiration at pHT 7.8 compared to control sites with a pHT 8.0, but no change in net community production. Gross photosynthesis may increase under OA by directly stimulating photosynthesis [30,31], and/or by increasing the benthic cover of phototrophs [2]. Studies that have investigated metabolic changes under OA at the reef community scale are few and from quite different communities, however they have not observed the increases in gross photosynthesis reported here [17,20,27,28]. In the present study, models which included benthic community cover indicated that increases in gross photosynthesis were predominantly due to increases in the cover of non-calcareous algae, rather than the changing seawater carbonate chemistry per se. Respiration may increase as a consequence of increasing biomass or increased metabolism. Biomass estimates are unavailable for the tiles, however our models indicated that declining seawater pH, and increasing invertebrate cover, both significantly contributed to the observed increase in respiration at lower pH.
OA is likely reducing reef calcification rates on coral reefs [29,26]. Light, dark and net calcification rates on the tiles all declined along the ΩAr gradient, and our models indicated that it was these changes in ΩAr, rather than shifts in tile communities, that were responsible. While results are not universal, OA is widely reported to reduce calcification in individual coral reef organisms [6,10] and at the community scale [17–20,29]. For example, Enochs et al. [20] found net daily calcification rates of light exposed coral reef communities on CaCO3 substrata decreased linearly along CO2 gradients, and became negative by pHT 7.8. The stronger response found by Enochs et al. [20] compared to the present study is thought to be because their study used CaCO3 blocks as settlement substrata, and attracted many macro-boring organisms, yet did not include cryptofauna on the lower surfaces.
When predicting OA effects on coral reef calcification, one must also take the permeable carbonate matrix and sediments into consideration. These are the largest sources of reef CaCO3 [59], and they are more vulnerable to dissolution than calcifying organisms as they lack tissue layers to buffer them from the surrounding seawater [19,23,59,60]. For example, Comeau et al. [61] documented a 60% decline in the calcification of experimental coral reef communities at 1300 μatm pCO2, with half of this being attributed to sediment decalcification. Some estimates suggest that even if coral calcification rates are maintained under OA, the dissolution of carbonate sediments alone would result in reef loss [59,62]. Results from the calcification assays in the present study, lacking sediments and a CaCO3 substrata, are thus likely to considerably under-estimate reef-wide dissolution rates expected under OA. Instead, they provide insight into how the calcification dynamics of a part of the biological components of coral reef communities may respond.
There are several factors that preclude CO2 seep sites from perfectly representing the future of the world’s oceans. Firstly, they are relatively small, and scaling up predictions to the world’s coral reefs will undoubtedly introduce some uncertainty. Secondly, the altered carbonate chemistry at the seeps is occurring in isolation from the warming that is also predicted for a high CO2 world [46], and the combined effect of these two stressors can be greater than either in isolation [10]. Thirdly, the seep site AT is slightly elevated (by ≤ 6% of control values), which may increase calcification rates at the seep sites [26]. Increased dissolution of carbonate sediments under OA may locally increase AT, however sediment dissolution- and dilution-rates from the surrounding seawater are largely unknown [59]. And finally, the seep seawater carbonate chemistry is characteristically variable over short (i.e. hourly) time-scales [63] with uncertain consequences for coral reef communities. Scleractinian corals have been shown to be largely robust, or to even benefit from increased pH variability (when compared to static lowered pH), while other coral reef organisms (e.g. CCA) can be negatively affected [64]. On the other hand, community scale studies, which allow for interactions between species and their environment, are not easily conducted in laboratory settings. While seep sites studies are not definitive, they do provide unique opportunities to overcome some issues with laboratory-based OA studies (i.e. organism acclimation and species/environment interactions) and provide further contributions to scientific consensus about the severe effects ocean acidification is afflicting on marine communities.
Results from the two seep sites investigated here, as well as other naturally occurring high CO2 analogues [12–14], generally agree with a plethora of experimental work from the small- [6,10,65] to large-scale [17–19,25,29,66], in situ seasonal comparisons [23,44] and quantitative models [67–69]. All these generally predict considerable changes for coral reefs under ‘business as usual’ carbon emissions scenarios and that we will likely see shifts in community composition, with the proliferation of non-calcifying taxa and a retraction of many calcifiers. Increases in non-calcifying algae may lead to increases in community gross production, however gross gains may be balanced by increased respiration. Ecosystem-wide calcification and CaCO3 accumulation rates will likely decline, owing to the carbonate chemistry changes and the dissolution of carbonate sediments and increased bio-erosion [14,20]. Unfortunately neither this study, meta-analyses [1,10], nor experimental comparisons of pre-industrial to present-day conditions [17,26] have shown signs that ecosystem acclimation will prevent the expected changes. This study further shows that many changes expected on coral reefs under increasing OA will occur along a continuum, indicating that the less CO2 emitted into the atmosphere, the less deviation we will see from the reefs of today.
Supporting information
Percent cover of the non-calcifying algal groups responsible for the increases seen along the pH gradient at Upa-Upasina (white points) and Dobu (grey points): green filamentous algae on the upper tile sides (a) and cyanobacteria (b) and macroalgae (c) on the lower tile sides. Left and right hand panels represent the five and 13 month census periods, respectively. The black lines represent the modelled means, while the grey lines are confidence intervals.
(EPS)
Percent cover of the sum of non-calcifying (a) and calcifying (b) invertebrates on the lower tile sides at Upa-Upasina (white points) and Dobu (grey points) in relation to median pHT. Left and right hand panels represent the five and 13 month census periods, respectively. The black lines represent the modelled means, while the grey lines are confidence intervals.
(EPS)
Some OTUs were further classified into more general grouping for analysis (Group).
(DOCX)
Standard errors are shown in brackets. N = 2 and 4 per control and seep site.
(DOCX)
Up and low refer to the upper and lower sides of the tiles, respectively. Non-significant terms removed from final models. Caption as in Table 1. *indicates taxa which were only found on one tile side.
(DOCX)
Median pH was used as predictor in oxygen production and consumption models (Fig 4), while ΩAr was used in calcification models. For calcification, the inclusion of the biotic OTUs did not improve on the GLM fit to ΩAr. Generalised linear model results, with G and Q denoting Gaussian and Quasi Poisson distributions used respectively, and ^0.25 indicates square root transformation. Caption as in Table 1.
(DOCX)
Acknowledgments
We thank the communities at Dobu and Upa-Upasina for their ongoing support of this project. Thanks also go to the crew of the MV Chertan for their tireless efforts and to Qantas Link for logistical support.
Data Availability
The data underlying this study have been uploaded to figshare and are accessible using the following link: https://doi.org/10.6084/m9.figshare.6203396.v1.
Funding Statement
This study was funded by the Australian Institute of Marine Science and a grant by the National Geographic Society Committee for Research and Exploration to KF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Ann Rev Mar Sci. 2009;1: 169–192. doi: 10.1146/annurev.marine.010908.163834 [DOI] [PubMed] [Google Scholar]
- 2.Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos Trans R Soc Lond B Biol Sci. 2013;368: 20120442 doi: 10.1098/rstb.2012.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, et al. The effect of ocean acidification on calcifying organisms in marine ecosystems: An organism-to-ecosystem perspective. Annu Rev Ecol Evol Syst. 2010;41: 127–147. [Google Scholar]
- 4.Fabricius KE, De’ath G, Noonan SHC, Uthicke S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc R Soc London Ser B Biol Sci. 2014;281: 20132 http://dx.doi.org/10.1098/rspb.2013.2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroeker KJ, Micheli F, Gambi MC. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Chang. Nature Publishing Group; 2012;3: 156–159. doi: 10.1038/nclimate1680 [Google Scholar]
- 6.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Oceanogr. 2013;58: 388–398. doi: 10.4319/lo.2013.58.1.0388 [Google Scholar]
- 7.McCulloch M, Falter J, Trotter J, Montagna P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Chang. Nature Publishing Group; 2012;2: 623–627. doi: 10.1038/nclimate1473 [Google Scholar]
- 8.Fabricius KE, Kluibenschedl A, Harrington L, Noonan S, De’ath G. In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Sci Rep. 2015;5: 9537 doi: 10.1038/srep09537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, et al. Coral reefs under rapid climate change and ocean acidification. Science (80-). 2007;318: 1737–1742. doi: 10.1126/science.1152509 [DOI] [PubMed] [Google Scholar]
- 10.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol. 2013;19: 1884–1896. doi: 10.1111/gcb.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Chang. Nature Publishing Group; 2011;1: 165–169. Available: http://dx.doi.org/10.1038/nclimate1122 [Google Scholar]
- 12.Enochs IC, Manzello DP, Donham EM, Kolodziej G, Okano R, Johnston L, et al. Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat Clim Chang. 2015; 10.1038/nclimate2758. doi: 10.1038/nclimate2758 [Google Scholar]
- 13.Inoue S, Kayanne H, Yamamoto S, Kurihara H. Spatial community shift from hard to soft corals in acidified water. Nat Clim Chang. Nature Publishing Group; 2013;3: 683–687. doi: 10.1038/nclimate1855 [Google Scholar]
- 14.Barkley HC, Cohen AL, Golbuu Y, Starczak VR, Decarlo TM, Shamberger KEF. Changes in coral reef communities across a natural gradient in seawater pH. Sci Adv. 2015;1: e1500328 doi: 10.1126/sciadv.1500328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzello D. Ocean acidification hot spots: Spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnol Oceanogr. 2010;55: 239–248. Available: http://mediteran.aslo.net/lo/toc/vol_55/issue_1/0239.pdf [Google Scholar]
- 16.Crook ED, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan a. Calcifying coral abundance near low-pH springs: Implications for future ocean acidification. Coral Reefs. 2012;31: 239–245. doi: 10.1007/s00338-011-0839-y [Google Scholar]
- 17.Dove SG, Kline DI, Pantos O, Angly FE, Tyson GW, Hoegh-Guldberg O. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc Natl Acad Sci U S A. 2013;110: 15342–7. doi: 10.1073/pnas.1302701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langdon C, Takahashi T, Sweeney C, Chipman D, Atkinson J. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochem Cycles. 2000;14: 639–654. [Google Scholar]
- 19.Comeau S, Carpenter RC, Lantz C a., Edmunds PJ. Ocean acidification accelerates dissolution of experimental coral reef communities. Biogeosciences. 2015;12: 365–372. doi: 10.5194/bg-12-365-2015 [Google Scholar]
- 20.Enochs IC, Manzello DP, Kolodziej G, Noonan SHC, Valentino L, Fabricius KE. Enhanced macroboring and depressed calcification drive net dissolution at high-CO2 coral reefs. Proc R Soc London Ser B. 2016;283: 20161742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmunds PJ, Comeau S, Lantz C, Andersson A, Briggs C, Cohen A, et al. Integrating the effects of ocean acidification across functional scales on tropical coral reefs. Bioscience. 2016;66: 350–362. doi: 10.1093/biosci/biw023 [Google Scholar]
- 22.Andersson AJ, Yeakel KL, Bates NR, de Putron SJ. Partial offsets in ocean acidification from changing coral reef biogeochemistry. Nat Clim Chang. Nature Publishing Group; 2014;4: 56–61. doi: 10.1038/nclimate2050 [Google Scholar]
- 23.Bates NR, Amat A, Andersson AJ. Feedbacks and responses of coral calcification on the Bermuda reef system to seasonal changes in biological processes and ocean acidification. Biogeosciences. 2010;7: 2509–2530. doi: 10.5194/bg-7-2509-2010 [Google Scholar]
- 24.Albright R, Langdon C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob Chang Biol. 2011;17: 2478–2487. doi: 10.1111/j.1365-2486.2011.02404.x [Google Scholar]
- 25.Albright R, Takeshita Y, Koweek DA, Ninokawa A, Wolfe K, Rivlin T, et al. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature. Nature Publishing Group; 2018; doi: 10.1038/nature25968 [DOI] [PubMed] [Google Scholar]
- 26.Albright R, Caldeira L, Hosfelt J, Kwiatkowski L, Maclaren JK, Mason BM, et al. Reversal of ocean acidification enhances net coral reef calcification. Nature. Nature Publishing Group; 2016;531: 362–365. doi: 10.1038/nature17155 [DOI] [PubMed] [Google Scholar]
- 27.Langdon C, Broecker WS, Hammond DE, Glenn E, Fitzsimmons K, Nelson SG, et al. Effect of elevated CO2 on the community metabolism of an experimental coral reef. Global Biogeochem Cycles. 2003;17: 1011 doi: 10.1029/2002GB001941 [Google Scholar]
- 28.Leclercq N, Gattuso J-P, Jaubert J. Primary production, respiration, and calcification of a coral reef mesocosm under increased CO2 partial pressure. Limnol Oceanogr. 2002;47: 558–564. doi: 10.4319/lo.2002.47.2.0558 [Google Scholar]
- 29.Comeau S, Lantz CA, Edmunds PJ, Carpenter RC. Framework of barrier reefs threatened by ocean acidification. Glob Chang Biol. 2016;22: 1225–1234. doi: 10.1111/gcb.13023 [DOI] [PubMed] [Google Scholar]
- 30.Suggett DJ, Hall-Spencer JM, Rodolfo-Metalpa R, Boatman TG, Payton R, Tye Pettay D, et al. Sea anemones may thrive in a high CO2 world. Glob Chang Biol. 2012;18: 3015–3025. doi: 10.1111/j.1365-2486.2012.02767.x [DOI] [PubMed] [Google Scholar]
- 31.Noonan SHC, Fabricius KE. Ocean acidification affects productivity but not the severity of thermal bleaching in some tropical corals. ICES J Mar Sci. 2016; doi: 10.1093/icesjms/fsv127 [Google Scholar]
- 32.Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543: 373–377. doi: 10.1038/nature21707 [DOI] [PubMed] [Google Scholar]
- 33.De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci. 2012;109: 17995–17999. doi: 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, et al. Climate change, human impacts, and the resilience of coral reefs. Science (80-). 2003;301: 929–933. doi: 10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- 35.Richter C, Wunsch M, Rasheed M, Kötter I, Badran MI. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature. 2001;413: 726–730. doi: 10.1038/35099547 [DOI] [PubMed] [Google Scholar]
- 36.Fabricius KE, Noonan SHC, Abrego D, Harrington L, De’ath G. Low recruitment due to altered settlement substrata as primary constraint for coral communities under ocean acidification. Proc R Soc London Ser B Biol Sci. 2017;284: 20171536. rspb.2017.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy E V., Ordoñez A, Lewis BE, Diaz-Pulido G. Comparison of recruitment tile materials for monitoring coralline algae responses to a changing climate. Mar Ecol Prog Ser. 2017;569: 129–144. doi: 10.3354/meps12076 [Google Scholar]
- 38.Meese RJ, Tomich PA. Dots on the rocks—a comparison of percent cover estimation methods. J Exp Mar Bio Ecol. 1992;165: 59–73. [Google Scholar]
- 39.Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep sea Res part A Oceanogr Res Pap. 1987;34: 1733–1743. [Google Scholar]
- 40.Lewis E, Wallace D. Program developed for CO2 system calculations, carbon dioxide information analysis center, Oak Ridge National Laboratory, Oak Ridge, Tenn: 1998; [Google Scholar]
- 41.Chisholm JRM, Gattuso J-P. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol Oceanogr. American Society of Limnology and Oceanography; 1991;36: 1232–1239. Available: http://www.jstor.org/stable/2837472 [Google Scholar]
- 42.Vogel N, Fabricius KE, Strahl J, Noonan SHC, Wild C, Uthicke S. Calcareous green alga Halimeda tolerates ocean acidification conditions at tropical carbon dioxide seeps. Limnol Oceanogr. 2014;60: 263–275. doi: 10.1002/lno.10021 [Google Scholar]
- 43.Gazeau F, Urbini L, Cox TE, Alliouane S, Gattuso JP. Comparison of the alkalinity and calcium anomaly techniques to estimate rates of net calcification. Mar Ecol Prog Ser. 2015;527: 1–12. doi: 10.3354/meps11287 [Google Scholar]
- 44.Albright R, Langdon C, Anthony KRN. Dynamics of seawater carbonate chemistry, production, and calcification of a coral reef flat, Central Great Barrier Reef. Biogeosciences Discuss. 2013;10: 6747–6758. doi: 10.5194/bgd-10-7641-2013 [Google Scholar]
- 45.Gattuso JP, Frankignoulle M, Smith S V. Measurement of community metabolism and significance in the coral reef CO2 source-sink debate. Proc Natl Acad Sci U S A. 1999;96: 13017–13022. doi: 10.1073/pnas.96.23.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.IPCC. Climate Change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al. , editors. Cambridge Univ. Press, Cambridge, UK, and New York; 2014. [Google Scholar]
- 47.R Development Core Team. A Language and Environment for Statistical Computing. R Found Stat Comput; Vienna; http://www.R-project.org. 2017; [Google Scholar]
- 48.Silverman J, Lazar B, Cao L, Caldeira K, Erez J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys Res Lett. 2009;36: 1–5. doi: 10.1029/2008GL036282 [Google Scholar]
- 49.Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. J Exp Mar Bio Ecol. 2011;400: 278–287. doi: 10.1016/j.jembe.2011.02.011 [Google Scholar]
- 50.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, et al. Volcanic carbondioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454: 96–99. doi: 10.1038/nature07051 [DOI] [PubMed] [Google Scholar]
- 51.Linares C, Vidal M, Canals M, Kersting DK, Amblas D, Aspillaga E, et al. Persistent natural acidification drives major distribution shifts in marine benthic ecosystems. Proc R Soc London Ser B Biol Sci. 2015;282: 20150587 doi: 10.1098/rspb.2015.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat Clim Chang. 2014;4: 487–492. doi: 10.1038/NCLIMATE2195 [Google Scholar]
- 53.Johnson VR, Russell BD, Fabricius KE, Brownlee C, Hall-Spencer JM. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob Chang Biol. 2012;18: 2792–2803. doi: 10.1111/j.1365-2486.2012.02716.x [DOI] [PubMed] [Google Scholar]
- 54.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT. Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci. 2007;1: 114–117. doi: 10.1038/ngeo100 [Google Scholar]
- 55.Cigliano M, Gambi MC, Rodolfo-Metalpa R, Patti FP, Hall-Spencer JM. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar Biol. 2010;157: 2489–2502. doi: 10.1007/s00227-010-1513-6 [Google Scholar]
- 56.Lane AC, Mukherjee J, Chan VBS, Thiyagarajan V. Decreased pH does not alter metamorphosis but compromises juvenile calcification of the tube worm Hydroides elegans. Mar Biol. 2013;160: 1983–1993. doi: 10.1007/s00227-012-2056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lombardi C, Rodolfo-Metalpa R, Cocito S, Gambi MC, Taylor PD. Structural and geochemical alterations in the Mg calcite bryozoan Myriapora truncata under elevated seawater pCO2 simulating ocean acidification. Mar Ecol. 2011;32: 211–221. doi: 10.1111/j.1439-0485.2010.00426.x [Google Scholar]
- 58.Morrow KM, Bourne DG, Humphrey C, Botte ES, Laffy P, Zaneveld J, et al. Natural volcanic CO2 seeps reveal future trajectories for host–microbial associations in corals and sponges. ISME J. 2015;9: 894–908. doi: 10.1038/ismej.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eyre BD, Andersson AJ, Cyronak T. Benthic coral reef calcium carbonate dissolution in an acidifying ocean. Nat Clim Chang. Nature Publishing Group; 2014;4: 969–976. doi: 10.1038/nclimate2380 [Google Scholar]
- 60.Rodolfo-Metalpa R, Houlbrèque F, Tambutté É, Boisson F, Baggini C, Patti FP, et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Chang. Nature Publishing Group; 2011;1: 308–312. doi: 10.1038/nclimate1200 [Google Scholar]
- 61.Comeau S, Carpenter RC, Edmunds PJ. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc R Soc London Ser B Biol Sci. 2012;280: 20130031 Available: http://rspb.royalsocietypublishing.org/content/280/1753/20122374.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cyronak T, Santos IR, Eyre BD. Permeable coral reef sediment dissolution driven by elevated pCO2 and pore water advection. Geophys Res Lett. 2013;40: 4876–4881. doi: 10.1002/grl.50948 [Google Scholar]
- 63.Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One. 2011;6: e28983 doi: 10.1371/journal.pone.0028983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivest EB, Comeau S, Cornwall CE. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr Clim Chang Reports. Current Climate Change Reports; 2017;3: 271–281. doi: 10.1007/s40641-017-0082-x [Google Scholar]
- 65.Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KRN. High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett. 2011;14: 156–162. doi: 10.1111/j.1461-0248.2010.01565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leclercq N, Gattuso J-P, Jaubert J. CO2 partial pressure controls the calcification rate of a coral community. Glob Chang Biol. 2000;6: 329–334. [Google Scholar]
- 67.Anthony KRN, Maynard JA, Diaz-Pulido G, Mumby PJ, Marshall PA, Cao L, et al. Ocean acidification and warming will lower coral reef resilience. Glob Chang Biol. Blackwell Publishing Ltd; 2011;17: 1798–1808. doi: 10.1111/j.1365-2486.2010.02364.x [Google Scholar]
- 68.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely R a, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437: 681–686. doi: 10.1038/nature04095 [DOI] [PubMed] [Google Scholar]
- 69.Ricke KL, Orr JC, Schneider K, Caldeira K. Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections. Environ Res Lett. 2013;8: 34003 doi: 10.1088/1748-9326/8/3/034003 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent cover of the non-calcifying algal groups responsible for the increases seen along the pH gradient at Upa-Upasina (white points) and Dobu (grey points): green filamentous algae on the upper tile sides (a) and cyanobacteria (b) and macroalgae (c) on the lower tile sides. Left and right hand panels represent the five and 13 month census periods, respectively. The black lines represent the modelled means, while the grey lines are confidence intervals.
(EPS)
Percent cover of the sum of non-calcifying (a) and calcifying (b) invertebrates on the lower tile sides at Upa-Upasina (white points) and Dobu (grey points) in relation to median pHT. Left and right hand panels represent the five and 13 month census periods, respectively. The black lines represent the modelled means, while the grey lines are confidence intervals.
(EPS)
Some OTUs were further classified into more general grouping for analysis (Group).
(DOCX)
Standard errors are shown in brackets. N = 2 and 4 per control and seep site.
(DOCX)
Up and low refer to the upper and lower sides of the tiles, respectively. Non-significant terms removed from final models. Caption as in Table 1. *indicates taxa which were only found on one tile side.
(DOCX)
Median pH was used as predictor in oxygen production and consumption models (Fig 4), while ΩAr was used in calcification models. For calcification, the inclusion of the biotic OTUs did not improve on the GLM fit to ΩAr. Generalised linear model results, with G and Q denoting Gaussian and Quasi Poisson distributions used respectively, and ^0.25 indicates square root transformation. Caption as in Table 1.
(DOCX)
Data Availability Statement
The data underlying this study have been uploaded to figshare and are accessible using the following link: https://doi.org/10.6084/m9.figshare.6203396.v1.