Abstract
Recent studies suggested that simian virus 40 (SV40) may cause malignant mesothelioma, although the pathogenic mechanism is unclear. We found that in SV40-positive malignant mesothelioma cells, the hepatocyte growth factor (HGF) receptor (Met) was activated. In human mesothelial cells (HMC) transfected with full-length SV40 DNA (SV40-HMC), Met receptor activation was associated with S-phase entry, acquisition of a fibroblastoid morphology, and the assembly of viral particles. Coculture experiments revealed the ability of SV40-HMC to infect permissive monkey cells (CV-1), HMC, and murine BNL CL cells. Cocultured human and murine SV40-positive cells expressed HGF, showed Met tyrosine phosphorylation and S-phase entry, and acquired a spindle-shaped morphology (spBNL), whereas CV-1 cells were lysed. Cocultured HMC inherited from SV40-HMC the infectivity, as they induced lysis in cocultured CV-1 cells. Treatment with suramin or HGF-blocking antibodies inhibited Met tyrosine phosphorylation in all large T antigen (Tag)-positive cells and reverted the spindle-shaped morphology of spBNL. This finding indicated that Met activation and subsequent biological effects were mediated by an autocrine HGF circuit. This, in turn, was causally related to Tag expression, being induced by transfection with the SV40 early region alone. Our findings suggest that when SV40 infects HMC it causes Met activation via an autocrine loop. Furthermore, SV40 replicates in HMC and infects the adjacent HMC, inducing an HGF-dependent Met activation and cell-cycle progression into S phase. This may explain how a limited number of SV40-positive cells may be sufficient to direct noninfected HMC toward malignant transformation.
Malignant mesothelioma (MM) is an aggressive and invasive cancer with high mortality because it is resistant to current therapies (1, 2). It has been estimated that one-quarter million people will die of MM in Europe in the next three decades (3). Prolonged exposure to asbestos is a well-known risk factor for MM, and the cooperation of other carcinogens with asbestos in the onset of this neoplasm seems possible (1, 2).
The simian virus 40 (SV40) oncoprotein large T antigen (Tag) plays a crucial role in the transformation of human cells (4) and causes cell-cycle derangement of human mesothelial cells (HMC) (5). The effects of Tag are caused by its ability to bind the tumor suppressor gene products p53 and retinoblastoma family (Rb) proteins (6, 7). The direct involvement of SV40 Tag expression in the growth of malignant mesothelioma cells has been described (8), and SV40 is associated with a shorter survival of MM patients (9). A synergistic action between SV40 and asbestos fibers has been suggested (10), and HMC have been shown to be highly sensitive to SV40-mediated transformation (11).
In an animal model, the high growth rate and in vivo tumorigenicity of the neoplastic cells from SV40-dependent MM were shown to be associated to insulin-like growth factor-1 release (12).
Several studies investigated the potential involvement of other growth factors, like platelet-derived growth factor A and B (13), insulin-like growth factor-1 (14), transforming growth factor β, fibroblast growth factor-2 (15), and hepatocyte growth factor (HGF) (16–19) in the onset of MM. High levels of HGF, in particular, were detected in pleural effusion from patients with MM (20).
HGF is a heterodimeric, glycosylated protein made of a heavy α chain and a light β chain, linked by an interchain disulfide bond. The active form is generated by cleavage of the biologically inactive monomeric precursor. The high affinity receptor of HGF is the MET protooncogene product (p190Met), a transmembrane receptor tyrosine kinase, made of a 145-kDa β subunit and a 50-kDa α subunit, synthesized as a single chain precursor of 170 kDa and linked by disulfide bridges. The α chain and the N-terminal portion of the β chain are exposed on the cell surface, whereas the C-terminal portion of the β chain is located in the cytoplasm and contains the tyrosine kinase domain and phosphorylation sites involved in the regulation of enzyme activity and signal transduction. HGF-induced Met oligomerization and activation leads to cell growth, motility, and morphogenesis in cells of different origin (21). It is worth noting that a fragile site for SV40 integration has been reported on chromosome 7 (22), where human HGF and its receptor, the Met tyrosine kinase, colocalize (23, 24).
In the present work, we investigated whether SV40 induced HGF expression in HMC and in turn could play a role in MM tumorigenesis.
Materials and Methods
Cell Cultures.
MM cells were derived from pleural effusion of MM patients, whereas HMC cell cultures were obtained from pleural effusion of patients with heart failure. The other cell lines were purchased from the American Type Culture Collection. MM and HMC cell lines were characterized as described (25, 26). Cells were cultured in RPMI 1640, DMEM, and Ham's F-10 medium supplemented with 10–15% FBS (GIBCO) and maintained at 37°C in a 5% CO2-humidified atmosphere. Stable transfectants were obtained by transfection of pSV3neo plasmid expressing Tag and SV40 full-length DNA by using the polycation compound Superfect (Qiagen, Chatsworth, CA). The following selection was performed in growth medium supplemented with 0.8 mg/ml G418-sulfate (GIBCO). Stable cell cultures were examined by immunoblotting, by using a mAb to Tag (Ab-1, Oncogene Science). Cocultures were performed in Transwell chambers (Costar). SV40-HMC cells were seeded on the upper side of a porous polycarbonate membrane (8.0-μm pore size), whereas target cells were seeded in the lower chamber.
Biochemical Assays.
Immunoprecipitation and immunoblotting were performed as described (27). Proteins from cell lysates were immunoprecipitated with anti-Tag (Ab-1) and anti-Met (C-28, Santa Cruz Biotechnology) antibodies. The immunocomplexes were washed, and proteins from immunoprecipitates were solubilized in Laemmli buffer and run in reducing conditions. The proteins were separated on SDS/PAGE and transferred to nitrocellulose filters (Hybond, Amersham Pharmacia). Filters were probed with the appropriate antibodies and detected by the enhanced chemiluminescence system (ECL, Amersham Pharmacia). Tyrosine phosphorylation was evaluated by immunoblotting as described above on immunoprecipitates, by using phosphotyrosine monoclonal antibody (4G10, Upstate Biotechnology, Lake Placid, NY).
PCR and Reverse Transcriptase (RT)-PCR.
Genomic DNA (250 ng) were PCR-amplified (1 min at 95°C, 30 s at 56°C, 16 s at 72°C for 35 cycles). The oligomers used were SV5, 5′-TAGGTGCCAACCTATGGAACAGA-3′ and SV6, 5′-GAAAGTCTTTAGGGTCTTCTACC-3′.
Total RNA (400 ng), extracted with the guanidinium thiocyanate system (Rneasy Kit, Qiagen), was used for reverse transcription (Accept RT-PCR kit, Promega). The oligomers used were HGF forward, 5′-GGGGAGAGTTATCGAGGTCTC-3′ and HGF reverse, 5′-CAAACTAACCATCCATCCTATG-3′.
Southern hybridization of DNA or cDNA were performed as described (28). DNA/cDNA was transferred on nylon membranes (Hybond-N+, Amersham Pharmacia), and filters were hybridized with HGF or SV40 probes, radiolabeled with [α-32P]dCTP (specific activity 3,000 Ci/mmol, Amersham Pharmacia) by using the Random-Primed kit (Roche Molecular Biochemicals).
Cell-Cycle Analysis.
Ethanol-fixed cells were washed twice in PBS and stained for 30 min at room temperature with 10 mg/μl propidium iodide in 0.1 M PBS, pH 7.2, containing 100 units/ml RNase. Cell-cycle analysis was carried out by using a DAKO/Partec PAS III flow cytometer (Glostrup, Denmark) under the following conditions: Argon ion laser excitation power 50 mW at 488-nm, 610-nm long pass filter for the red fluorescence (propidium iodide) detector.
Scatter Assay.
Madin–Darby canine kidney (MDCK) cells (1.5 × 103) were seeded on 96-well dishes in DMEM supplemented with 5% FBS. After 12 h, cells were exposed to concentrated conditioned medium or control medium. Recombinant HGF was released by transiently transfected N-2A cells in the medium, harvested, and stored at 4°C. Serum-free culture media of mesothelioma cell lines and BNL CL cells were harvested after 4 days' culture, cleared by centrifugation, and concentrated 20×. HGF was activated in 5% FBS at 37°C for 1 h; these media were sterilized and added to MDCK cells.
Suramin Treatment and Blocking Antibodies.
Subconfluent cells were exposed to suramin (350 μg/ml) for 24 h, then washed three times with sterile PBS and trypsinized. After washing in PBS, the pellet was resuspended in culture medium containing 10 μg/ml blocking antibodies anti-HGF (mAb 294, R & D Systems). Cells were maintained in suspension for 1 h at 22°C before being plated again.
Electron Microscopy.
Cells were trypsinized and suspended in their medium, centrifuged, and suspended in sodium cacodilate-glutaraldehyde solution. Ultrastructural pictures were taken with a Hitachi 800 electron microscope.
Results
Met Activation and Tag Expression in MM Cell Lines.
To test whether Tag expression was associated with Met tyrosine kinase activity, we examined nine cell lines established from pleural effusions of nine individuals with MM.
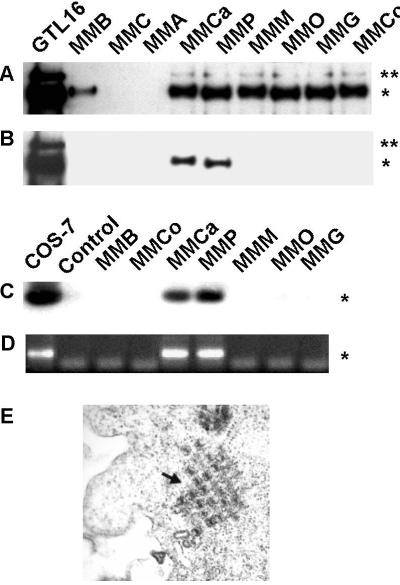
Expression and tyrosine phosphorylation of Met were investigated by immunoprecipitation followed by immunoblotting, using Met and phosphotyrosine-specific antibodies. Met was found expressed in seven of nine MM cell lines (Fig. 1A), and in two of them (MMP and MMCa) it was phosphorylated on tyrosine (Fig. 1B).
Figure 1.
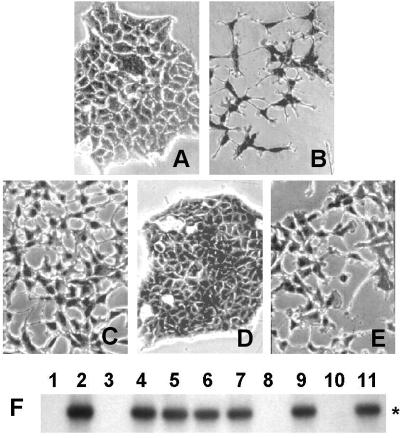
Expression of Met and Tag by MM cell lines. (A) Equal amounts of solubilized proteins were immunoprecipitated with Met antibodies, separated by SDS/PAGE, transferred to nitrocellulose filter, and probed with the same antibodies. Asterisks on the right indicate the pr170MET precursor (**) and the mature β-chain (p145MET, *). (B) The same filter was reprobed with antiphosphotyrosine antibodies. (C) Tag sequence (*, nucleotides 4402–4574) was amplified by PCR from genomic DNA. (D) RT-PCR was performed on MM cells and COS-7 cells to amplify a fragment of Tag cDNA (172 bp), indicated by the asterisk on the right. (E) Electron microscopy of MMP cells. (Original magnification: ×40,000.)
PCR and Southern hybridization analysis of genomic DNA performed on these MM cells using nucleotides 4402–4574 of SV40 genome (4) revealed the presence of the SV40 early region sequences only in those cell lines that expressed Met in the activated (phosphorylated) form (MMP and MMCa). These viral sequences were transcriptionally active because we demonstrated Tag expression by RT-PCR in the same cells. Tag was not detected in the other seven cell lines, suggesting a specific association with phosphorylated Met (Fig. 1D).
The presence of SV40 was confirmed by the detection of viral particles by electron microscopy in MMP cells (Fig. 1E).
Met Activation and S-Phase Entry Are Induced by Tag Expression in Mesothelial Cells.
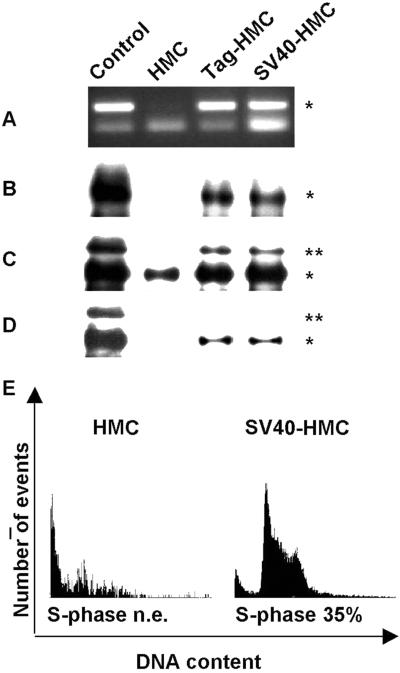
HMC expressing the Met receptor were transfected either with Tag-encoding cDNA (Tag-HMC) or SV40 DNA (SV40-HMC). Immunoprecipitation and immunoblotting performed on Tag-positive clones showed that both types of transfectants displayed Met tyrosine phosphorylation in association with the expression of SV40 Tag (Fig. 2 A–D). Interestingly, also the expression of the endogenous Met receptor was increased in Tag-expressing HMC (Fig. 2C).
Figure 2.
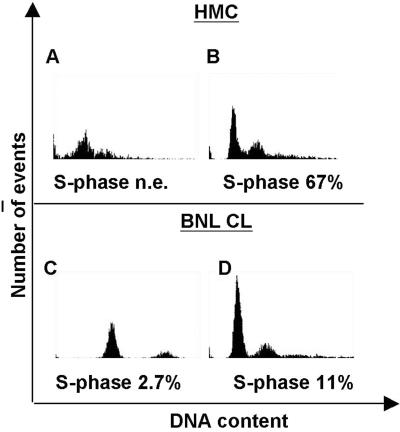
Transfection of HMC cells with Tag and SV40 DNA. (A) Tag sequence (*) was amplified by PCR from genomic DNA of COS-7 (control), HMC, Tag-HMC, and SV40-HMC cells. (B) Tag protein (*) was detected in cell lysates immunoprecipitated and probed with Tag antibodies. (C) Immunoblotting was performed on Met immunoprecipitates with Met antibodies. GTL-16 cell line was used as a control of Met expression and phosphorylation. Asterisks on the right of the blot indicate the positions of the pr170MET precursor (**) and p145MET (*). (D) The same filter was reprobed with antiphosphotyrosine antibodies. (E) Citofluorimeter analysis of HMC cells before (Left) and after (Right) transfection with SV40 DNA.
To investigate the effects of Tag-induced Met activation on the cell cycle, mesothelial cells were also analyzed by cytofluorimetry. Baseline cell-cycle analysis of HMC showed a very slow pattern of growth. Cell-cycle phases were not clearly distinguishable, with few proliferating cells. After SV40 transfection, a clear cell-cycle distribution was detected, with a large percentage of cells (35%) in the S phase (Fig. 2E). Significant entry in the S phase was obtained also with Tag-HMC (data not shown).
SV40-HMC Are Virus Reservoirs and Cause Morphological Changes and Cell-Cycle Progression in Target Cells.
To verify whether SV40 transfection could influence neighboring cells, we performed coculture experiments by using the Transwell system. Transwell allows cell growth in two chambers, separated by a porous set that can be crossed by solutes and particles of definite size, including SV40. As targets, we used CV-1 monkey kidney epithelial cells, murine BNL CL, and HMC.
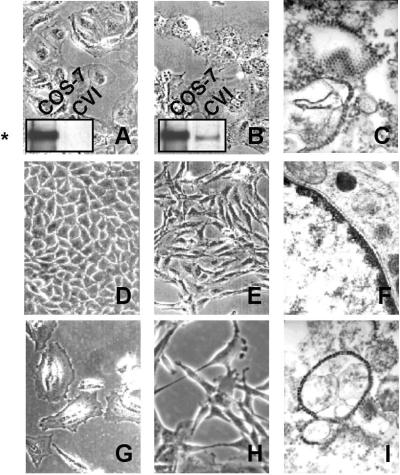
CV-1 cells are permissive to SV40 infection. CV-1 cells were cocultured with SV40-HMC transfectants and monitored every 24 h. After 4 days, early symptoms of cell crisis (vacuolization, loss of adherence, and death) in CV-1 cocultured with SV40-HMC were detected. After 5 additional days, all cells died (Fig. 3B), and viral particles were visible in the cell debris fraction (Fig. 3C). The molecular identity of the virus was assessed by PCR amplification of SV40 DNA early region (nucleotides 4402–4574), both in infected CV-1 cells and their conditioned medium (Fig. 4A).
Figure 3.
Morphology after cocultures with SV40-HMC. The morphology of target cells CV-1 (A), BNL CL (D), and HMC (G) changed after coculture with SV40-HMC cells (B, E, and H, respectively). SV40 particles were observed by electron microscopy (C and I). (F) No virions were detected in nonpermissive BNL CL cells. CV-1 cells underwent vacuolization and massive lysis, accompanied by Tag expression. Tag protein (*) was detected in cell lysates immunoprecipitated and probed with Tag antibodies. (Original magnifications: A, B, D, E, G, and H, ×320; C, ×50,000; F and I, ×40,000.)
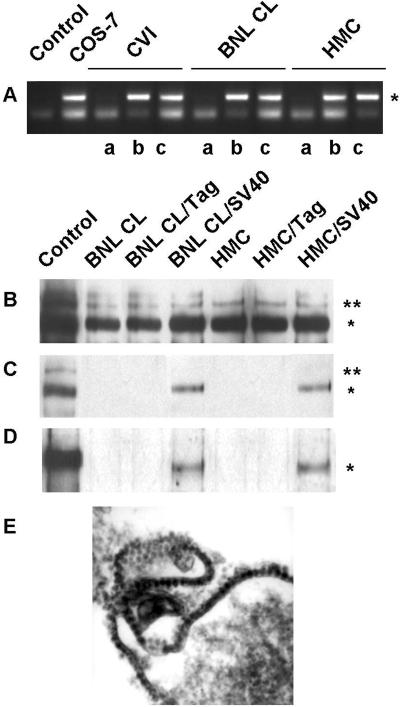
Figure 4.
Coculture experiments. (A) Tag sequence (*) was amplified by PCR from genomic DNA of CV-1, BNL CL, and HMC. PCR without DNA (lane 1) and with COS-7 DNA (lane 2) were also performed as controls. Shown is the product of PCR amplification performed on lysates from cells cocultured with Tag-HMC (a) and SV40-HMC (b). The same PCR amplification was also performed on the SV40-HMC coculture medium (c). (B) Solubilized proteins from cell lysates were immunoprecipitated with Met antibodies and probed with the same Met antibodies and (C) with the antiphosphotyrosine antibodies. Asterisks on the right indicate the positions of the pr170MET precursor (**) and p145MET (*). (D) Tag protein (*) was detected in cell lysates immunoprecipitated and probed with Tag antibodies. (E) Electron microscopy of SV40-HMC cells. (Original magnification: ×60,000.)
Moreover, immunoblotting confirmed Tag expression in these cells at early stages of infection, i.e., 4 days after the onset of the coculture experiment (Fig. 3B).
Murine BNL CL cells are nonpermissive, as they can be infected but do not allow SV40 replication. In addition, these cells express the Met receptor, which can be activated in an HGF-dependent manner, leading to growth, scatter, and migration (29). Coculture with SV40-HMC induced a clear-cut morphological change in epithelial BNL CL target cells (BNL CL/SV40), which lost their original morphology and became elongated and spindle-shaped; we identified these cells as spBNL to distinguish them from the parental cells (Fig. 3E). The morphological changes were caused by viral replication in SV40-HMC in which viral particles were detected (Fig. 4E) because Tag-HMC (with Tag expression but no viral replication) did not exert any morphological effects on BNL CL cells (data non shown). In spBNL, PCR amplification of genomic DNA revealed the presence of SV40 DNA sequences (Fig. 4A), and immunoblotting demonstrated expression of Tag protein (Fig. 4D). As expected, no viral particles were detected in these cells (Fig. 3F) because murine cells are not permissive to SV40 replication.
HMC are semipermissive cells because they can be infected and allow replication at low titer (11). After 20 days of coculture with SV40-HMC transfectants, viral particles were detected in HMC target cells (HMC/SV40, Fig. 3I), and these cells underwent morphological changes (Fig. 3H). This cells also acquired the ability to infect nearby cells as confirmed by the massive lysis of CV-1 cells, induced by coculturing together targeted HMC/SV40 and CV-1 cells (data not shown).
Cell-cycle analysis demonstrated that cocultured HMC/SV40 and spBNL cells displayed a higher rate of S-phase entry compared with cells not exposed to SV40-HMC culture medium (Fig. 5).
Figure 5.
Cell cycle of HMC and BNL CL cocultured cells. Cytofluorimeter analysis before (A and C) and after (B and D) coculture with SV40-HMC cells.
SV40-Mediated Tag Expression Induces an HGF Autocrine Circuit.
Immunoprecipitation and immunoblotting showed that the Met receptor became spontaneously tyrosine-phosphorylated in HMC and in spBNL cocultured with SV40-HMC because of SV40 infection (Fig. 3 B and C).
The conditioned medium from nonpermissive spBNL displayed scatter activity on MDCK cells, revealing the acquired ability to express and release HGF (Fig. 6E). The scatter activity was also detected in conditioned medium of human MMP and MMCa cells (Fig. 6C) but not in MMM (Fig. 6D). This finding suggests that in MM cells the Met activation can be explained by an HGF-autocrine loop. To test this hypothesis, RT-PCR using oligonucleotides specific for HGF sequences was performed on MM cells, as well as on spBNL and HMC/SV40. The results revealed the presence of HGF transcripts in these cells, suggesting that an autocrine circuit underlies Met activation in MM cells as well as in cocultured models (Fig. 6F).
Figure 6.
Met/HGF autocrine loop. Scatter assay was performed on the MDCK cell line. Shown are untreated MDCK cells (A) and MDCK stimulated with recombinant HGF (B), with MMP-conditioned medium (C), with MMM-conditioned medium (D), and with spBNL-conditioned medium (E). (Original magnification: ×320.) (F) RT-PCR and Southern hybridization specific for HGF (nucleotides 646-1533). Cell lines expressing activated Met (MMP, lane 4; MMCa, lane 5; Tag-HMC, lane 6; SV40-HMC, lane 7; HMC/SV40, lane 9; and spBNL, lane 11) display HGF expression (*), whereas HGF cDNA was not amplified in MMM (lane 3), HMC/Tag (lane 8), and BNL CL (lane 10) cells as well as in RT-PCR control without RNA (lane 1). MRC5 cell line (lane 2) was used as a control of HGF expression.
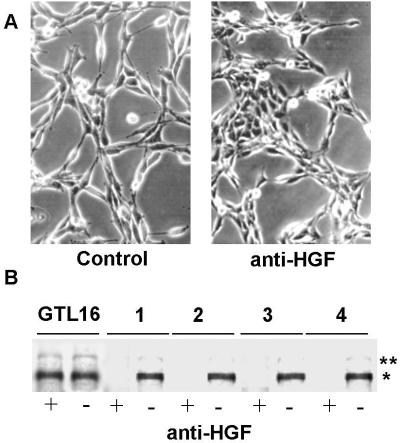
To confirm the existence of the HGF-mediated autocrine loop, we performed inhibition experiments using HGF-blocking antibodies. Cells in suspension were incubated for 1 h with commercial antibodies certified for blocking the HGF-receptor interaction. Then, cells were seeded and grown in the continuous presence of the antibodies. After 48 h, the reversion of the dissociated (scattered) phenotype was already evident, and cell islets remained detectable during the next 3 days (Fig. 7A). The interference on the autocrine loop was confirmed by immunoblotting with phosphotyrosine antibodies that revealed inhibition of Met tyrosine phosphorylation only in anti-HGF-treated cells and not in cells treated with control antibodies (Fig. 7B). Similar results were obtained by cell treatment with suramin, a well-known chemical agent that minimizes protein–protein interactions on the cell surface (data not shown).
Figure 7.
Met/HGF autocrine loop. (A) spBNL cells were photographed before (Left) and after (Right) treatment with anti-HGF blocking antibodies. (Original magnification: ×320.) (B) Met phosphorylation in MMP (lane 1), SV40-HMC (lane 2), HMC/SV40 (lane 3), and spBNL cells (lane 4) with (+) and without (−) treatment with anti-HGF blocking antibodies. Equal amounts of proteins from cell lysates were immunoblotted with antiphosphotyrosine antibodies. GTL-16 cell line was used as a control of Met expression and phosphorylation. Asterisks on the right indicate the positions of the pr170MET precursor (**) and p145MET (*).
The SV40 Tag-Induced HGF Autocrine Circuit Is Rb-Dependent.
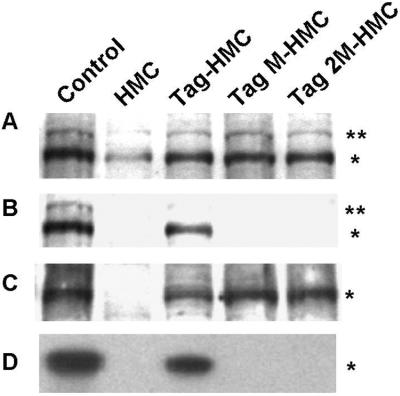
It has been reported that in MDCK cells, Tag may induce HGF expression in an Rb-dependent manner (30). As an attempt to understand the mechanism for HGF induction in SV40 Tag-expressing mesothelial cells, we used two defective SV40 Tag mutants, kindly provided by V. De Simone (University of Naples, Italy). One mutant, TagM, carries the E107→K amino acid substitution that impairs the binding of Tag to the Rb protein. The other mutant, Tag2M, has an additional D402→E substitution that blocks also the binding to p53. HMC were transfected either with TagM (TagM-HMC) or with Tag2M (Tag2M-HMC) cDNAs and compared with HMC transfected with wild-type Tag cDNA (Tag-HMC). Immunoprecipitation and immunoblotting performed on Tag and mutant-positive clones showed that all cells expressed the Met receptor (Fig. 8A), even though at relatively higher levels, as well as Tag proteins (Fig. 8C), but in cells expressing both types of Tag mutants, Met was not tyrosine phosphorylated (Fig. 8B). Consequently, RT-PCR analysis revealed the absence of HGF transcripts in Tag mutant-expressing cells (Fig. 8D), demonstrating that binding of Tag to Rb is an essential requisite for the onset of the HGF autocrine circuit, underlying Met activation in MM cells as well as in cocultured cells.
Figure 8.
Rb dependence of HGF autocrine circuit. (A) Solubilized proteins were immunoprecipitated with Met antibodies and probed with the same Met antibodies. Asterisks on the right of the blot indicate the positions of the pr170MET precursor (**) and p145MET (*). The same filter was reprobed with antiphosphotyrosine antibodies (B) and anti-Tag antibodies, using COS-7 as a control of Tag expression (C). (D) RT-PCR and Southern hybridization specific for HGF (nucleotides 646-1533). The MRC5 cell line was used as a control of HGF expression.
Discussion
Our results show that in MM-derived cell lines expressing the SV40 Tag protein, the Met receptor is constitutively phosphorylated. Activation of the Met receptor occurs in these cells through a Tag-induced HGF-dependent autocrine circuit. This is demonstrated by the presence of scatter activity in conditioned medium of the Tag-positive MM cells, expression of the HGF transcript, and inhibition of Met phosphorylation in these same cells, upon treatment with HGF-blocking antibodies or suramin.
Transfection of SV40 full-length DNA or Tag-encoding sequences in HMC cells caused alterations of growth rate and morphology. The consequent establishment of an HGF autocrine circuit, mimicking that occurring in SV40-positive MM cells, may reinforce or add to the known effects of Tag interaction with Rb or p53 oncosuppressor gene products (6, 7).
Coculture experiments between SV40-transfected HMC and target cells of different origin confirmed that SV40 does replicate in HMC, in agreement with recent similar data (11). The virus propagates to adjacent cells, which in turn undergo the same modifications of the original reservoir SV40-HMC cells, i.e., Met tyrosine phosphorylation, HGF release, cell-cycle progression, and mesenchymal conversion. Coculture of SV40-HMC with HMC that are permissive cells determines a new viral replication cycle in these latter cells, allowing a chain reaction leading to a dissemination of the same effects in the whole cell population.
We demonstrate here that in HMC, HGF synthesis and release strictly depends on Tag protein expression. Accordingly, the mere transfection of the Tag-encoding sequence (SV40 early region) is sufficient to cause HGF synthesis and release. Indeed, the HGF release by Tag-HMC can activate Met on the neighboring cells. A similar mechanism has been reported in myeloma caused by Kaposi's sarcoma-associated herpes virus infection, where an IL-6 paracrine release by virus-infected cells is responsible for malignant transformation of plasma cells (31).
Induction of an HGF autocrine loop has been obtained by SV40 Tag transfection in MDCK cells, where the involvement of Rb family protein inactivation has been suggested (30). This could be the mechanism that leads to HGF expression also in mesothelial cells, as demonstrated by our results. Transfection of HMC with Rb binding defective Tag mutants abolishes HGF production and tyrosine phosphorylation of Met in ligand absence. The establishment of an autocrine circuit for growth factors frequently confers a selective advantage to tumor cells as well. In particular, the occurrence of an HGF/Met autocrine loop has been reported in a number of cancers, including MM (16). Moreover, in MDCK, the expression of recombinant Tag has been reported to induce dissociation and motility (30). These and our results suggest the existence of a Tag-dependent mechanism leading to Met phosphorylation in different cell lineages.
An issue that has been less clear is the constantly increased Met receptor protein levels in HMC expressing the Tag protein. This effect cannot be merely explained by the Met tyrosine kinase activation, which has been demonstrated to enhance the expression of the receptor itself (32), because Met expression is elevated also in the presence of the Tag mutants failing to bind Rb and to induce HGF. We postulate that some other mechanisms, presently unknown, are involved in this Tag-dependent transcriptional or posttranscriptional regulation. However, Met-enhanced expression does not seem to be essential for the observed biological effects because it is not associated to receptor tyrosine phosphorylation, and treatments with suramin and blocking antibodies reveal that Met activation and cell morphology changes rely on HGF release.
The level of expression of Tag protein required to elicit the biological effects observed in MM cells was quite low. This is in accordance with other reports of human cell growth (8). A possible explanation can be the simultaneous occurrence of different lesions in other molecules involved in the cell-cycle control (i.e., ARF proteins) (33). The low expression of ARF proteins makes the cells more susceptible to DNA damage, impairing their ability to repair any loss of genomic integrity.
On the whole, our study confirms that SV40, which is present in the United States and in Europe in at least 60% of human mesotheliomas (34, 35), plays a role of major importance in the development of MM, cooperating with other causative agents. Regarding the cancerogenic role of asbestos, the Tag-induced HGF-dependent autocrine loop can additively sustain cell transformation addressing HMC toward the neoplastic transformation.
The clinician should take in account of these findings whenever pleural plaques are detected in a patient because SV40 has also been detected in hyperplastic HMC (36). The induction of the HGF-dependent autocrine loop in these cells might render the SV40-positive hyperplastic lesion at higher risk for malignant transformation.
Acknowledgments
We thank Dr. C. Ponzetto for helpful discussion and suggestion, Dr. V. De Simone for Tag mutants, and I. Robuffo for electromicroscopy on MMP cells. P.C. is supported by a fellowship from Lega Tumori, Sezione di Alessandria. This work was supported by research grants from the Associazione Italiana per la Ricerca sul Cancro (to M.T. and G.G.) and from Consiglio Nazionale delle Ricerche “Target Project on Biotechnology 99.00373.PF49” and “Co-finanziamento MURST” 2000.
Abbreviations
- SV40
simian virus 40
- HGF
hepatocyte growth factor
- HMC
human mesothelial cells
- Rb
retinoblastoma
- RT
reverse transcriptase
- MDCK
Madin–Darby canine kidney
- MM
malignant mesothelioma
- Tag
T antigen
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Testa J R, Pass H I, Carbone M. In: Principle and Practice of Oncology. 6th Ed. De Vita V, Hellman S, Rosemberg S, editors. Philadelphia: Lippincott; 2000. pp. 1937–1943. [Google Scholar]
- 2.Robledo R, Mossman B T. J Cell Physiol. 1999;180:158–166. doi: 10.1002/(SICI)1097-4652(199908)180:2<158::AID-JCP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Decarli A, La Vecchia C, Levi F, Negr E. Br J Cancer. 1999;79:666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butel J, Lednicky J. J Natl Cancer Inst. 1999;91:119–134. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- 5.Levresse V, Renier A, Fleury-Feith J, Levy F, Moritz S, Vivo C, Pilatte Y, Jaurand M C. Am J Respir Cell Mol Biol. 1997;17:660–671. doi: 10.1165/ajrcmb.17.6.2854. [DOI] [PubMed] [Google Scholar]
- 6.Carbone M, Rizzo P, Grimley P M, Procopio A, Mew D J, Shridhar V, De Bartolomeis A, Esposito V, Giuliano M T, Steinberg S M, et al. Nat Med. 1997;3:908–912. doi: 10.1038/nm0897-908. [DOI] [PubMed] [Google Scholar]
- 7.De Luca A, Baldi A, Esposito V, Howard C M, Bagella L, Rizzo P, Caputi M, Pass H I, Giordano G G, Baldi F, et al. Nat Med. 1997;8:913–916. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 8.Waheed I, Guo Z S, Chen G A, Weiser T S, Nguyen D M, Schrump D S. Cancer Res. 1999;59:6068–6073. [PubMed] [Google Scholar]
- 9.Procopio A, Strizzi L, Vianale G, Betta P, Puntoni R, Fontana V, Tassi G, Gareri F, Mutti L. Genes Chromosomes Cancer. 2000;29:173–179. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1019>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Mayall F G, Jacobson G, Wilkins R. J Clin Pathol. 1999;52:291–293. doi: 10.1136/jcp.52.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa J R, Pass H I, Rizzo P, Carbone M. Proc Natl Acad Sci USA. 2000;97:10214–10219. doi: 10.1073/pnas.170207097. . (First Published August 22, 2000; 10.1073/pnas.170207097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pass H I, Mew D J, Carbone M, Matthews W A, Donington J S, Baserga R, Walker C L, Resnicoff M, Steinberg S M. Cancer Res. 1996;56:4044–4048. [PubMed] [Google Scholar]
- 13.Versnel M A, Hagemeijer A, Bouts M J, Van der Kwast T H, Hoogsteden H C. Oncogene. 1988;2:601–605. [PubMed] [Google Scholar]
- 14.Lee T C, Zhang Y, Aston C, Hintz R, Jagirdar J, Perle M A, Burt M, Rom W N. Cancer Res. 1993;53:2858–2864. [PubMed] [Google Scholar]
- 15.Asplund T, Versnel M A, Laurent T C, Heldin P. Cancer Res. 1993;53:388–392. [PubMed] [Google Scholar]
- 16.Harvey P, Warn A, Dobbin S, Arakaki N, Daikuhara Y, Jaurand M C, Warn R M. Br J Cancer. 1998;77:1052–1059. doi: 10.1038/bjc.1998.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klominek J, Baskin B, Liu Z, Hauzenberger D. Int J Cancer. 1998;76:240–249. doi: 10.1002/(sici)1097-0215(19980413)76:2<240::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Tolnay E, Kuhnen C, Wiethege T, Konig J E, Voss B, Muller K M. J Cancer Res Clin Oncol. 1998;124:291–296. doi: 10.1007/s004320050171. [DOI] [PubMed] [Google Scholar]
- 19.Thirkettle I, Harvey P, Hasleton P S, Ball R Y, Warn R M. Histopathology. 2000;36:522–528. doi: 10.1046/j.1365-2559.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 20.Eagles G, Warn A, Ball R Y, Baillie-Johnson H, Arakaki N, Daikuhara Y, Warn R M. Br J Cancer. 1996;73:377–381. doi: 10.1038/bjc.1996.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigna E, Naldini L, Tamagnone L, Longati P, Bardelli A, Maina F, Ponzetto C, Comoglio P M. Cell Mol Biol. 1994;40:597–604. [PubMed] [Google Scholar]
- 22.Mishmar D, Rahat A, Scherer S W, Nyakatura G, Hinzmann B, Kohwi Y, Mandel-Gutfroind Y, Lee J R, Drescher B, Sas D E, et al. Proc Natl Acad Sci USA. 1998;95:8141–8146. doi: 10.1073/pnas.95.14.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean M, Park M, Le Beau M M, Robins T S, Diaz M O, Rowley J D, Blair D G, Vande Woude G F. Nature (London) 1985;318:385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- 24.Saccone S, Narsimhan R P, Gaudino G, Dalpra L, Comoglio P M, Della Valle G. Genomics. 1992;13:912–914. doi: 10.1016/0888-7543(92)90191-t. [DOI] [PubMed] [Google Scholar]
- 25.Mutti L, Valle M T, Balbi B, Orengo A M, Lazzaro A, Alciato P, Gatti E, Betta P G, Pozzi E. Int J Cancer. 1998;78:740–749. doi: 10.1002/(sici)1097-0215(19981209)78:6<740::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Orengo A M, Spoletini L, Procopio A, Favoni E, De Cupis A, Ardizzoni A, Castagneto B, Ribotta M, Betta P G, Ferrini S, Mutti L. Eur Respir J. 1999;13:527–534. doi: 10.1183/09031936.99.13352799. [DOI] [PubMed] [Google Scholar]
- 27.Santoro M M, Penengo L, Minetto M, Orecchia S, Cilli M, Gaudino G. Oncogene. 1998;16:741–749. doi: 10.1038/sj.onc.1201994. [DOI] [PubMed] [Google Scholar]
- 28.Hirvonen A, Mattson K, Karjalainen A, Ollikainen T, Tammilehto L, Hovi T, Vainio H, Pass H, I, Di Resta I, Carbone M, Linnainmaa K. Mol Carcinog. 1999;26:93–99. [PubMed] [Google Scholar]
- 29.Medico E, Mongiovì A M, Huff J, Jalinek M A, Follenzi A, Gaudino G, Parson J T, Comoglio P M. Mol Biol Cell. 1996;7:495–504. doi: 10.1091/mbc.7.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martel C, Harper F, Cereghini S, Noe V, Mareel M, Cremisi C. Cell Growth Differ. 1997;8:165–178. [PubMed] [Google Scholar]
- 31.Rettig M B, Ma H J, Vescio R A, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, et al. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 32.Boccaccio C, Gaudino G, Gambarotta G, Galimi F, Comoglio P M. J Biol Chem. 1994;269:12846–12851. [PubMed] [Google Scholar]
- 33.Chao H H, Buchmann A M, DeCaprio J A. Mol Cell Biol. 2000;20:7624–7633. doi: 10.1128/mcb.20.20.7624-7633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone M, Fisher S, Powers A, Pass H I, Rizzo P. J Cell Physiol. 1999;180:167–172. doi: 10.1002/(SICI)1097-4652(199908)180:2<167::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Carbone M, Rizzo P, Pass H. Anticancer Res. 2000;20:875–877. [PubMed] [Google Scholar]
- 36.Shivapurkar N, Wiethege T, Wistuba I I, Salomon E, Milchgrub S, Muller K M, Churg A, Pass H, Gazdar A F. J Cell Biochem. 1999;76:181–188. doi: 10.1002/(sici)1097-4644(20000201)76:2<181::aid-jcb2>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]