Abstract
Objective
This review aimed to update the research and development of cellular senescence in the treatment of ovarian cancer. We discussed the current mechanisms of senescence and the major biomarkers of senescence, especially the methods of cellular senescence in the treatment of ovarian cancer.
Materials and Methods
We collected all relevant studies in PubMed from 1995 to 2017. The search terms included senescence and cancer, senescence and ovarian cancer, senescence-associated secretory phenotype, ovarian cancer and chemotherapy, radiotherapy, or biotherapy. PubMed search with the key words senescence and ovarian cancer lists approximately 85 publications. After excluding the duplicated articles, we selected 68 articles most relevant to senescence and ovarian cancer in this review.
Results
Cellular senescence plays a key role in various biological processes of ovarian cancer, which is closely related with the occurrence, development, and treatment of ovarian cancer. Cellular senescence on the one hand can reduce the dose of chemotherapy in ovarian cancer; on the other hand, it also can solve the problem of tumor resistance to apoptosis. Therefore, cellular senescence has been shown to be the third intracellular mechanism of ovarian cancer prevention followed by cellular DNA repair and apoptosis.
Conclusions
In the near future, cellular senescence therapy could be a powerful tool for ovarian cancer treatment.
Key Words: Senescence, Ovarian cancer, Chemotherapy, Radiotherapy
Ovarian cancer is the most lethal gynecologic malignancy at present. Despite the recent new diagnostic possibilities and medical advances, the 5-year survival rate of patients with advanced ovarian cancer is only 25% to 35%.1 Prognosis is usually very poor because of the late diagnosis. More than 70% of patients diagnosed with this cancer have reached late stage (stage III–IV), when cancer has already spread beyond the ovaries.2 Thus, there is an urgent need to develop effective therapies for ovarian cancer.
Cellular senescence is a process characterized by normal human cells losing their proliferative capacity and departing from the cell cycle into a relatively stable state, also named replicative senescence. In recent years, some studies have found that the phenotype of cellular senescence induced by factors such as DNA damage, oxidative stress, and oncogene stress is basically the same with replicative senescence, but differs in the specific mechanism. This form of cellular senescence is unrelated with the shortening of telomeres and the generations of proliferation; therefore, it is called premature senescence.3 Premature senescence has been shown to be the third intracellular mechanism of cancer prevention followed by cellular DNA repair and apoptosis, which is closely related with the occurrence, development, and treatment of tumors.4 Therefore, cellular senescence has been suggested as a novel therapy for ovarian cancer. In this article, we focus on the current research and development of cellular senescence in the treatment of ovarian cancer.
Cellular Senescence
The Mechanism of Cellular Senescence
Normal human cells have limited life under the conditions of in vitro culture. This phenomenon was first observed in fibroblasts cultured in in vitro human cells. According to Haiflick and Moorhead’s5 findings in 1961, the continuous culture of human diploid fibroblast cells would enter a state of senescence on 50 to 70 generations of in vitro proliferations. Under such circumstances, cells had lost the ability of continuous proliferation but were still alive. Even if the most suitable culture conditions were provided, the cells could not escape the fate of limited proliferation. This proliferative limit of cells is sometimes referred to as the Haiflick limit. The phenomenon that the normal human cells have limited potential of in vitro division is known by Haiflick as cellular senescence, or more precisely, replicative senescence. The hypothesis of telomere shortening is commonly recognized among the hypothesis of the replicative senescence mechanism. Muller6 proposed telomere in 1938 for the first time and found that it was easily connected between the ends of injured and ruptured chromosomes in Drosophila, thereby forming the various types of chromosome aberrations, such as the melted end and the formation of annularity or dicentric chromosomes. But the natural end of chromosomes seems neither connected to the ends of ruptured chromosomes nor combined with the natural end of chromosomes, just like a hat to maintain the stability of the end of the chromosome. Therefore, the fragment located at both ends of the chromosome was named telomere by Muller.6 The hypothesis of telomere shortening refers to the correlation between the division ability and the telomere length of the fibroblast and more division times of cells with longer telomeres than those with shorter telomeres. Studies have shown that the presence of telomere shortening is seen in a variety of primary human cells during culture. These results suggest that telomere shortening plays a direct role in the number of division times that may be experienced by primary cells and may serve as a marker of cellular senescence. Therefore, telomere shortening or telomere structural destruction is considered as the main mechanism of senescence.7
With the deepening of this study, the researchers found that telomere shortening was not the only factor to induce the senescence phenotype (irreversible growth arrest, apoptosis, and changes in cell functions). Some of the stimuli that were unrelated with telomere, including DNA damage and the expression of some oncogenes, could also induce normal cell growth arrest and exhibit a senescence phenotype. The inducers other than telomeres had one thing in common that they had the potential to cause or promote tumorigenesis. Cellular senescence seems to be a safe mechanism to prevent tumorigenesis through inhibition of cell proliferation. Cellular senescence involves the signal regulation factor of cell cycle and complex approach.8 When cellular DNA encounters damage attacks, it can activate proteins such as p16, p21, p53, and retinoblastoma (RB) protein related to G1/S arrest, and proliferation arrest can occur to cells.9,10 Among them, the signaling pathways of p53-p21-pRB and p16-pRB are the most important. The tumor suppressor gene p53 in induction of cell cycle arrest is realized by induction of high expression of p21. And high-expression p21 can inhibit the binding of cyclin-dependent kinase (CDK) 2 and type E cell cycle protein, thereby inhibiting the phosphorylation of the RB. The RB that is in the form of nonphosphorylation or low phosphorylation can be combined with several transcription factors to inhibit their transcription activation functions and further inhibit the expression of downstream genes needed from S phase into G1 phase, which causes growth arrest so as to control the cell cycle progression and differentiation. The expression of p16 gene plays an important role in the maintenance of cellular senescence. Overexpression of p16 can inhibit the binding of CDK4/6 and type D cell cycle protein to block the phosphorylation of CDK4/6 on RB and prevent cells from entering the S phase for induction of cellular senescence (Fig. 1). Whether p16-pRB or p53-p21-pRB pathway activation is involved in the induction of senescence seems to depend on the cell type and origin of the species.11
FIGURE 1.
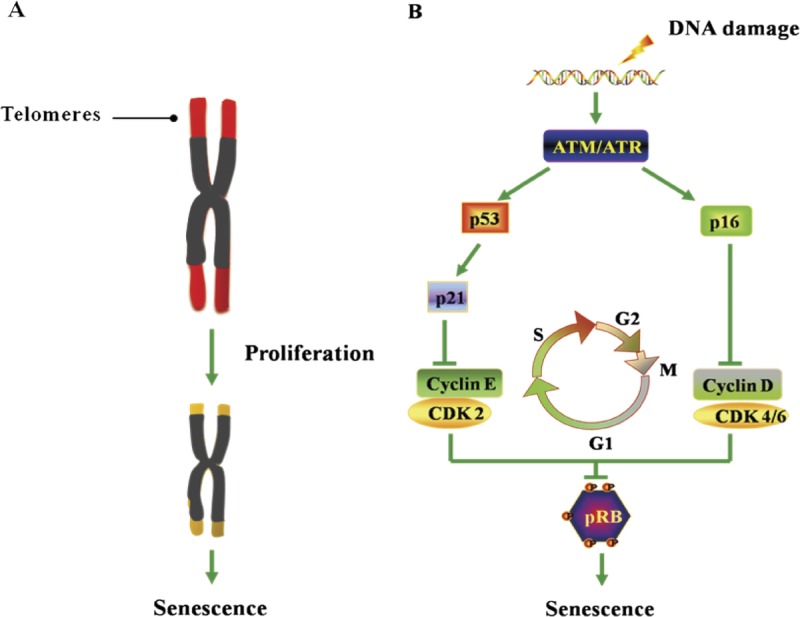
Overview of replicative senescence and premature senescence. A, Telomere shortening–induced replicative senescence. B, Accumulation stress–induced premature senescence.
Detection Biomarkers of Cellular Senescence
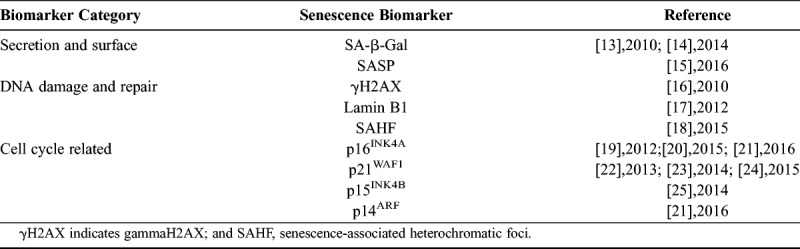
At present, there are many ways to detect cellular senescence. In addition to the morphological changes of senescent cells observed by using an optical microscope, senescence-associated β-galactosidase (SA-β-gal) staining is a common way to show the senescence cells. In 1995, Dimri et al12 found that when the pH value of culture medium was 6, the positive rate of β-galactosidase staining for the diploid fibroblasts cultured in vitro was gradually increased with the increase of the age. This neutral β-galactosidase was defined by them as SA-β-gal, namely the SA-β-gal. The β-galactosidase produced by senescent cells or tissues can catalyze the substrate X-Gal to form a dark blue product, which is easily observed under an optical microscope. The SA-β-gal can also be found to be increased with the increase of the age in the stratum corneum cells of human epidermis. In addition, SA-β-gal can differentiate between DNA cells and quiescent cells, which are independent of the replication process. The SA-β-gal is a kind of biological marker that can be used in vivo and in vitro. Because the method of detecting SA-β-gal is simple and easy to use, it has been widely applied in the detection of senescent cells. At present, more than 2300 articles have applied this method. But as we know, there is no single biomarker that can robustly identify senescent cells. Recently, multiple studies proved that several classes of biomarkers can be identified as potential senescence indicators, which are summarized in Table 1.13–25
TABLE 1.
Biomarkers of senescence
Cellular Senescence in the Treatment of Ovarian Cancer
In the recent researches, it has been confirmed that inducing tumor cellular senescence to prevent the unlimited proliferation of tumor cells can be an important treatment. Normal cells accumulate the alterations from DNA damage, oxidative stress, and oncogene stress that trigger an initial phase of aberrant cell proliferation. This aberrant proliferation can give rise to preneoplastic lesions; to parallel this aberrant situation, ovarian cancer cell–intrinsic fail-safe mechanisms such as senescence and apoptosis are activated. During ovarian cancer progression, the accumulation of damage is acquired to override these protective mechanisms, giving rise to ovarian cancer.26 Experimental researches have confirmed that conventional treatments such as chemotherapy and radiotherapy can induce cellular senescence; at the same time, research about the treatment of target biological treatment on ovarian cancer has also made certain progress. Chemotherapy-, radiotherapy- and biotherapy-induced cellular senescence permanently arrests growth. In cancer cells, chemotherapy-induced senescence is an important alternative cell fate to apoptosis.27 The study reported an apoptosis-independent function to p53 in cancer and showed that murine lymphomas overexpressing Bcl-2 respond to chemotherapy by engaging a senescence program controlled by p53 and p16INK4a rather than apoptosis.28 Conversely, upregulating the expression of DNA methyltransferase (DNMT) DNMT3a in colorectal cancer cells subject to DNA damage induced by doxorubicin switches senescence to apoptosis.29 When senescent cells were challenged with p53-dependent apoptotic stimuli, they underwent necrosis in response to the treatments.30 Muñozespín et al31 proposed that cellular senescence, followed by macrophage-dependent clearance of senescent cells or by the overgrowth of nearby cells, alters cellularity, resulting in tissue remodeling. Interestingly, the activation of senescence in ovarian cancer cells was associated with a strong senescence-associated secretory phenotype (SASP), which can attract and activate immune system cells to alter the ovarian cancer microenvironment. On immune dysfunction, the SASP’s proinflammatory nature can recruit immune cells, which can kill and clear senescent cells or other surviving cancer cells.32 All in all, chemotherapy-, radiotherapy- and biotherapy-induced cellular senescence can be explored as alternative or complement treatments to ovarian cancer (Fig. 2).
FIGURE 2.
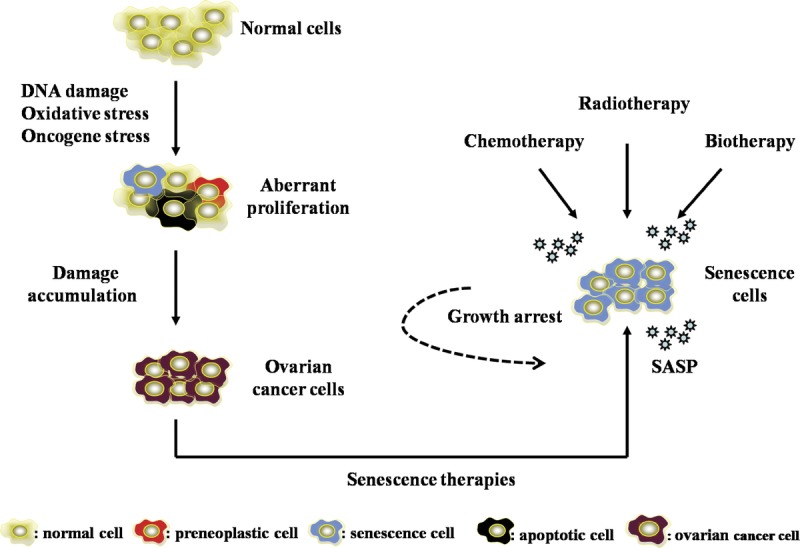
Overview of cellular senescence in the treatment of ovarian cancer.
Cellular Senescence and Ovarian Cancer Chemotherapy
Therapies such as surgical adjuvant chemotherapy are the most commonly used methods in treatment of ovarian cancer, which are difficult to significantly prolong the 5-year survival rate (approximately 20%).33 In particular, the chemotherapy resistance and strong ability of invasion and metastasis of ovarian cancer result in severe reduction of the long-term curative effect and survival rate for ovarian cancer patients. The chemotherapy of ovarian cancer is intended to induce the apoptosis of ovarian cancer cells. However, the tumor cells have a barrier in apoptosis generally, as a consequence of which apoptosis resistance is seen as a difficult problem in the treatment of ovarian cancer. As we know, the tumor cells have the ability of proliferation without restriction, and it was once thought that the tumor cells had lost the ability to enter the senescent state. However, with the deepening of research, Studies have confirmed that chemotherapy drugs can induce cellular senescence in tumor cells.34 Senescent cells are in permanent growth arrest and, after repairing DNA damage, still undergo cell cycle arrest.27 Chemotherapy can induce cell cycle arrest and senescence, but cell cycle arrest is not a synonym of senescence. One type of quiescence is simple quiescence, a reversible arrest. Cells are arrested because of lack of growth factors, and addition of growth factors causes proliferation. Another type of quiescence is locked quiescence, an irreversible arrest. Differentiated cells are put on the brakes, and excessive stimulation induces senescence.35 Many studies have found that low doses of chemotherapy drugs can cause tumor cellular senescence36 and further proved that induction of tumor cellular senescence is a feasible strategy to reverse drug resistance.37 Senescence-inducing chemotherapy, on the one hand, can reduce the dose of chemotherapy; on the other hand, it also can solve the problem of tumor resistance to apoptosis.38 Studies have shown that low doses of chemotherapy drugs can induce A2780 ovarian carcinoma cellular senescence, which resulted in prolonged cell cycle arrest predominantly in the G1 phase of the cell cycle.39 In addition, according to Schmitt’s28 findings in the study of chemotherapy in mice, chemotherapy drugs can cause senescence changes in tumor cells, and cellular senescence pathway directly affects the effect of chemotherapy, which confirms that the expression rate of senescent cells is closely related to prognosis. At present, it is believed that the cycle arrest of cell proliferation induced by chemotherapeutic agents is related to DNA damage. If the DNA damage caused by low-dose chemotherapy cannot be repaired, ovarian cancer cells tend to undergo senescence rather than apoptosis. Therefore, senescence plays an important role in the in vivo response to chemotherapy.
Cellular Senescence and Ovarian Cancer Radiotherapy
The property of photon radiation (x-ray, γ-ray) and special ray (α, neutron, proton, and electron) that can lead to ionization effect is made full use of by radiotherapy to destroy the genetic material structure of tumor cells and kill tumor cells. Compared with surgical treatment, radiotherapy can avoid the traumatic injury of organ tissue, ensure the integrity of organ structure, and significantly prolong the survival time for cancer patients.
In ovarian cancer, debulking surgery and adjuvant chemotherapy are still fundamental treatments. Compared with debulking surgery and chemotherapy, the application of radiotherapy is relatively less in the treatment of ovarian cancer, but it still has a certain position. Ovarian cancer is a radiosensitive tumor40; although radiotherapy is not the main treatment for ovarian cancer, it can be used as an adjuvant therapy after surgery and palliative therapy of advanced and recurrent lesions.41,42 In 2010,43 International Federation of Gynecology and Obstetrics implemented a phase 1 clinical trial, which confirmed the clinical feasibility of intensity-modulated whole abdominal radiotherapy for patients with advanced ovarian carcinoma after surgery and adjuvant chemotherapy. Phase 2 clinical trial was conducted in 2011, further suggesting that intensity-modulated whole abdominal radiotherapy therapy can be used as an effective consolidation treatment for the advanced ovarian carcinoma patients after adjuvant chemotherapy.44 Until now many researches have confirmed that radiotherapy has a therapeutic effect on ovarian cancer. In epithelial ovarian cancer, radiotherapy can be used to relieve the symptoms of pain, bleeding, or oppression caused by advanced ovarian cancer.45 Yahara et al45 selected 27 patients with limited recurrence after complete remission and assessed the efficacy and toxicity of definitive radiotherapy for the recurrence of epithelial ovarian cancer. They found that radiotherapy for limited recurrence of epithelial ovarian cancer could achieve a better local control rate without severe toxicity.46 Wei et al46 also confirmed that radiotherapy combined with chemotherapy had good curative effect on platinum-resistant recurrent epithelial ovarian cancer.47 Albuquerque et al47 reported that long-term follow-up confirmed the benefit of involved field radiation therapy in localized recurrent ovarian cancer. In ovarian clear cell carcinoma, radiotherapy can improve disease-free survival by 20% at 5 years (relative risk, 0.5) for patients with early-stage (stage IC and stage II).48 Macrie et al49 evaluated the role of pelvic radiotherapy in ovarian clear cell carcinoma. They found that the patients with this disease exhibit a propensity for pelvic recurrence after surgery and chemotherapy and pelvic radiotherapy could effectively sterilize microscopic tumor cells and suggested that pelvic radiotherapy might benefit patients with ovarian clear cell carcinoma.
As we know, the key to successful radiotherapy is to improve the sensitivity of tumor cells to radiotherapy. Some data demonstrate that large-scale radiation (x-ray) can induce tumor cellular senescence and further alter x-ray sensitivity in tumor cells.50 Penha et al reported that different dose and time of radiation exposure dictated different impacts on tumor cells. In particular, low-dose ionizing radiation-induced senescence might be a promising therapeutic target to induce growth inhibitory response in both early and late steps of carcinogenesis and might offer an opportunity to reduce the toxicity of radiotherapy.51 As senescence plays a more and more important role in anti-cancer strategies, senescence-inducing radiotherapy has been used widely in thyroid cancer,52,53 lung cancer,54 breast cancer,55 head and neck cancer,56 etc. Although senescence-inducing radiotherapy is quickly developed, this new therapy has not yet been used widely in ovarian cancer in the hope that this new therapy can provide a useful tool in treatment of ovarian cancer.
Cellular Senescence and Ovarian Cancer Biotherapy
The traditional tumor therapies such as radiotherapy and chemotherapy mainly achieve the treatment goals through causing tumor cell death. Owing to the advantage of good short-term therapeutic effect, the tumor mass shrinks rapidly. However, occurrence of the adverse conditions such as metastasis and recurrence results in less satisfactory therapeutic effect of the traditional tumor treatment mode. Biotherapy can significantly prolong patients’ survival time without great adverse reactions, unlike traditional chemotherapy and radiotherapy. Therefore, it is a promising new therapeutic target to restore the senescence pathway and induce senescence of tumor cells. There are some researches that provide evidence for senescence features not only in ovarian cancer cell lines, but also specimens.57 The SA-β-gal and p16 have been identified as cellular senescence markers; in Konecny et al’s study, they found that the overexpression of p16 in primary clinical ovarian cancer specimens could drive senescence.58 Mikułapietrasik et al59 showed that the expression of SA-β-Gal was significantly increased in ovarian cancer metastases specimens which had been induced senescence. In recent years, the researchers have made useful attempts in this direction, which are summarized in Table 2.60–66
TABLE 2.
Senescence-inducing factors as targets of ovarian cancer biotherapy
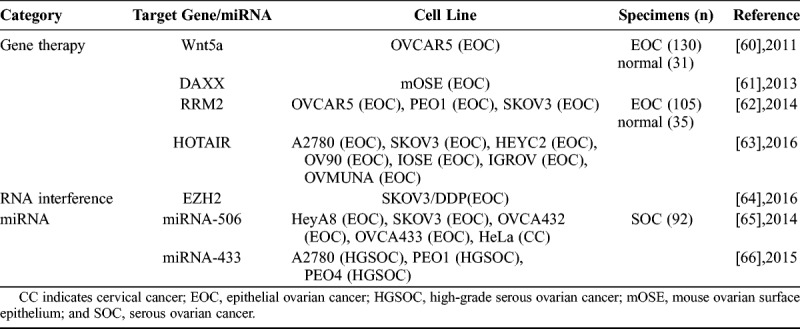
In the aspect of gene therapy, Bitler et al were the first to show that Wnt5a activation induced senescence of human epithelial ovarian cancer cells by promoting the histone cell cycle regulator/promyelocytic leukemia senescence pathway. It is worth noting that the different Wnt5a expression correlates with different tumor stage and overall survival in epithelial ovarian cancer patients. These data were consistent with the idea that Wnt5a signaling to drive senescence of human epithelial ovarian cancer cells was a potentially novel strategy for developing ovarian cancer therapeutics.60 Pan et al confirmed that Daxx deletion could accelerate mouse ovarian surface epithelium cells senescence in a p53/p21-dependent manner and induced the high expressions of DNA damage-related proteins (p-H2AX and p-CHK2) and cell cycle–related gene (p21 and p27). These results suggest that DAXX may play a role in epithelial ovarian carcinoma tumorigenesis and may be an anticancer target.61 Aird et al found that ribonucleotide reductase M2 (RRM2) expression was significantly higher in epithelial ovarian cancer cell lines and specimens than normal controls, and knockdown of RRM2 expression inhibited the growth of human epithelial ovarian cancer cells through a cellular senescence mechanism. These data suggest that inhibition of RRM2 to induce senescence is a novel therapeutic strategy for epithelial ovarian cancer patients.62 Özeş et al demonstrated the key role that HOTAIR in DNA damage contributes to cellular senescence. They reported that the overexpression of HOTAIR could alter the tumor microenvironment and induced the tumor growth slow. They suggested that HOTAIR may represent a target for a novel therapeutic strategy of drug resistance in ovarian cancer.63
In the aspect of RNA interference inhibitor, Sun et al found that decreased enhancer of zeste homolog 2 (EZH2) expression could induce SKOV3/DDP cell cycle arrest in the G0/G1 phase; at the same time, some expressions of the senescence signaling proteins (p14ARF, p16INK4a, p53, and p21) were significantly increased, and other proteins (CDK1, CDK2, and H3K27me3) were lower expressed. These findings suggest that interfering with EZH2 expression can be a new candidate that could possibly reverse the cisplatin resistance of ovarian cancer cells.64
In the aspect of miRNA: Liu et al demonstrated that overexpression of miR-506 could inhibit the CDK4/CDK6-FOXM1 signaling pathway, consequently decrease proliferation, and promote senescence in ovarian cancer cells and specimens. This newly recognized miR-506-CDK4/6-FOXM1 axis provides further insight into the pathogenesis of ovarian cancer, and miR-506 could be a potential therapy for ovarian cancer.65 Weinergorzel et al showed that the aberrant expression of miR-433 in ovarian cancer cells could result in the induction of cellular senescence and affect intracellular signaling to mediate chemoresistance. They guessed that the high miR-433 expression might act as a protective mechanism to affect the progression of the cancer and patient survival.66
CONCLUSIONS AND PERSPECTIVES
In conclusion, cellular senescence can effectively prevent the uncontrolled cell growth and carcinogenesis caused by cytokine DNA damage or oncogene; therefore it is an important tumor suppression mechanism. The research of cellular senescence in ovarian cancer treatment is mainly related to chemotherapy, radiotherapy, and biotherapy, etc. Chemotherapy/radiotherapy– induced cellular senescence is not intended to kill the ovarian cancer cells; the required medication/radiation dose and their toxic reactions are likely to be less than traditional chemotherapy and radiotherapy. What’s more, chemotherapy/radiation–induced cellular senescence is not dependent on telomere length and telomerase activity; the related study will possibly promote the advances in tumor treatment. Biotherapy mainly includes gene therapy, RNA interference, and medication miRNA treatment, etc, which can further clarify the molecular mechanism of ovarian cancer cellular senescence. At present, the research of cellular senescence in ovarian cancer treatment is still in progress; the mechanism of cellular senescence is unclear, and many senescence-related genes have not been studied. As a promising ovarian cancer therapy, induction of ovarian cancer cellular senescence is facing great opportunities and challenges.
Footnotes
This study was supported by the National Key R&D Program of China (2016YFC1303100) and the National Natural Science Foundation of China (31570803, 81773090, 81272879, 81402151).
The authors declare no conflicts of interest.
REFERENCES
- 1.Scholz HS, Tasdemir H, Hunlich T, et al. Multivisceral cytoreductive surgery in FIGO stages IIIC and IV epithelial ovarian cancer: results and 5-year follow-up. . 2007;106:591–595. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. . 2006;95(suppl 1):S161–S192. [DOI] [PubMed] [Google Scholar]
- 3.Lin AW, Barradas M, Stone JC, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. . 1998;12:3008–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornsby PJ. Senescence as an anticancer mechanism. . 2007;25:1852–1857. [DOI] [PubMed] [Google Scholar]
- 5.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. . 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 6.Muller HJ. The remaking of chromosomes. . 1938;13:181–198. [Google Scholar]
- 7.Hayflick L. Mortality and immortality at the cellular level. A review. . 1997;62:1180–1190. [PubMed] [Google Scholar]
- 8.Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy. . 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soto Martínez JL, Cabrera Morales CM, Serrano Ortega SS, et al. Mutation and homozygous deletion analyses of genes that control the G1/S transition of the cell cycle in skin melanoma: p53, p21, p16 and p15. . 2005;7:156–164. [DOI] [PubMed] [Google Scholar]
- 10.Hong SY, Chen X, Yang VW. Krüppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. . 2003;278:2101–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surova O, Zhivotovsky B. Various modes of cell death induced by DNA damage. . 2012;32:3789–3797. [DOI] [PubMed] [Google Scholar]
- 12.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. . 1995;92:9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng YQ, Guan JT, Xu XH, et al. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. . 2010;396:866–869. [DOI] [PubMed] [Google Scholar]
- 14.Sosińska P, MikułaPietrasik J, Ryżek M, et al. Specificity of cytochemical and fluorescence methods of senescence-associated β-galactosidase detection for ageing driven by replication and time. . 2014;15:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matjusaitis M, Chin G, Sarnoski EA, et al. Biomarkers to identify and isolate senescent cells. . 2016;29:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX as a molecular marker of aging and disease. . 2010;5:129–136. [DOI] [PubMed] [Google Scholar]
- 17.Freund A, Laberge RM, Demaria M, et al. Lamin B1 loss is a senescence-associated biomarker. . 2012;23:2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster MR, Xu M, Kinzler KA, et al. Wnt5A promotes an adaptive, senescent-like stress response, while continuing to drive invasion in melanoma cells. . 2015;28:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcoux S, Le ON, Langlois-Pelletier C, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. . 2012;8:2261–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pustavoitau A, Barodka V, Sharpless NE, et al. Role of senescence marker p16(INK4a) measured in peripheral blood T-lymphocytes in predicting length of hospital stay after coronary artery bypass surgery in older adults. . 2015;74:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pare R, Shin JS, Lee SC. Increased expression of senescence markers p14(ARF) and p16 (INK) (4a) in breast cancer is associated with increased risk of disease recurrence and poor survival outcome. . 2016;69:479–491. [DOI] [PubMed] [Google Scholar]
- 22.Aravinthan A, Pietrosi G, Hoare M, et al. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. . 2013;8:e72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aravinthan A, Mells G, Allison M, et al. Gene polymorphisms of cellular senescence marker p21 and disease progression in non-alcohol-related fatty liver disease. . 2014;13:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caliò A, Zamò A, Ponzoni M, et al. Cellular senescence markers p16INK4a and p21CIP1/WAF are predictors of Hodgkin lymphoma outcome. . 2015;21:5164–5172. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Guo L, Xing P, et al. Increased expression of oncogene-induced senescence markers during cervical squamous cell cancer development. . 2014;7:8911–8916. [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. . 2012;22:211–219. [DOI] [PubMed] [Google Scholar]
- 27.Childs BG, Baker DJ, Kirkland JL, et al. Senescence and apoptosis: dueling or complementary cell fates? . 2015;15:1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. . 2002;109:335–346. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Gao Y, Zhang G, et al. DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells. . 2011;128:551–561. [DOI] [PubMed] [Google Scholar]
- 30.Seluanov A, Gorbunova V, Falcovitz A, et al. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. . 2001;21:1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñozespín D, Cañamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. . 2013;155:1104–1118. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez LC, Ghadaouia S, Martinez A, et al. Premature aging/senescence in cancer cells facing therapy: good or bad? . 2016;17:71–87. [DOI] [PubMed] [Google Scholar]
- 33.Ataseven B, Chiva LM, Harter P, et al. FIGO stage IV epithelial ovarian, fallopian tube and peritoneal cancer revisited. . 2016;142:597–607. [DOI] [PubMed] [Google Scholar]
- 34.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. . 2010;10:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blagosklonny MV. Cell cycle arrest is not senescence. . 2011;3:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taschner-Mandl S, Schwarz M, Blaha J, et al. Metronomic topotecan impedes tumor growth of MYCN-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence. . 2016;7:3571–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Q, Wajapeyee N. Exploiting cellular senescence to treat cancer and circumvent drug resistance. . 2010;9:166–175. [DOI] [PubMed] [Google Scholar]
- 38.Salminen A, Ojala J, Kaarniranta K. Apoptosis and aging: increased resistance to apoptosis enhances the aging process. . 2011;68:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.te Poele RH, Okorokov AL, Jardine L, et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. . 2002;62:1876–1883. [PubMed] [Google Scholar]
- 40.Rochet N, Sterzing F, Jensen AD, et al. Intensity-modulated whole abdominal radiotherapy after surgery and carboplatin/taxane chemotherapy for advanced ovarian cancer: phase I study. . 2010;76:1382–1389. [DOI] [PubMed] [Google Scholar]
- 41.Tinger A, Waldron T, Peluso N, et al. Effective palliative radiation therapy in advanced and recurrent ovarian carcinoma. . 2001;51:1256–1263. [DOI] [PubMed] [Google Scholar]
- 42.Fields EC, Mcguire WP, Lin L, et al. Radiation treatment in women with ovarian cancer: past, present, and future. . 2017;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochet N, Kieser M, Sterzing F, et al. Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III—The OVAR-IMRT-02 Study. . 2011;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.E C, Quon M, Gallant V, et al. Effective palliative radiotherapy for symptomatic recurrent or residual ovarian cancer. . 2006;102:204–209. [DOI] [PubMed] [Google Scholar]
- 45.Yahara K, Ohguri T, Imada H, et al. Epithelial ovarian cancer: definitive radiotherapy for limited recurrence after complete remission had been achieved with aggressive front-line therapy. . 2013;54:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei P, Zhang ZH, Li L, et al. Clinical observational study of conformal radiotherapy combined with topotecan chemotherapy in patients with platinum-resistant recurrent ovarian cancer. . 2015;14:3833–3842. [DOI] [PubMed] [Google Scholar]
- 47.Albuquerque K, Patel M, Liotta M, et al. Long-term benefit of tumor volume-directed involved field radiation therapy in the management of recurrent ovarian cancer. . 2016;26:655–660. [DOI] [PubMed] [Google Scholar]
- 48.Hoskins PJ, Le N, Gilks B, et al. Low-stage ovarian clear cell carcinoma: population-based outcomes in British Columbia, Canada, with evidence for a survival benefit as a result of irradiation. . 2012;30:1656–1662. [DOI] [PubMed] [Google Scholar]
- 49.Macrie BD, Strauss JB, Helenowski IB, et al. Patterns of recurrence and role of pelvic radiotherapy in ovarian clear cell adenocarcinoma. . 2014;24:1597–1602. [DOI] [PubMed] [Google Scholar]
- 50.Mendonca MS, Chinsinex H, Dhaemers R, et al. Differential mechanisms of X-Ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. . 2011;176:208–216. [DOI] [PubMed] [Google Scholar]
- 51.Tsai KK, Stuart J, Chuang YY, et al. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. . 2015;172:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podtcheko A, Namba H, Saenko V, et al. Radiation-induced senescence-like terminal growth arrest in thyroid cells. . 2005;15:306–313. [DOI] [PubMed] [Google Scholar]
- 53.Rambaldi GZ, Monari F, Fiorentino M, et al. Complete pathological response after chemo-radiation in anaplastic thyroid cancer: a report of two cases. . 2016;55:530–532. [DOI] [PubMed] [Google Scholar]
- 54.Luo H, Wang L, Schulte BA, et al. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. . 2013;43:1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu YC, Yang PM, Chuah QY, et al. Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways. . 2013;3:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marmary Y, Adar R, Gaska S, et al. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. . 2016;76:1170–1180. [DOI] [PubMed] [Google Scholar]
- 57.Mikuła-Pietrasik J, Uruski P, Matuszkiewicz K, et al. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. . 2016;39:473–481. [DOI] [PubMed] [Google Scholar]
- 58.Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. . 2011;17:1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mikuła-Pietrasik J, Uruski P, Pakuła M, et al. Oxidative stress contributes to hepatocyte growth factor-dependent pro-senescence activity of ovarian cancer cells. . 2017;110:270–279. [DOI] [PubMed] [Google Scholar]
- 60.Bitler BG, Nicodemus JP, Li H, et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. . 2011;71:6184–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan WW, Yi FP, Cao LX, et al. DAXX silencing suppresses mouse ovarian surface epithelial cell growth by inducing senescence and DNA damage. . 2013;526:287–294. [DOI] [PubMed] [Google Scholar]
- 62.Aird KM, Li H, Xin F, et al. Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. . 2014;13:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Özeş AR, Miller DF, Özeş ON, et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. . 2016;35:5350–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y, Jin L, Liu JH, et al. Interfering EZH2 expression reverses the cisplatin resistance in human ovarian cancer by inhibiting autophagy. . 2016;31:246–252. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Sun Y, Ji P, et al. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. . 2014;233:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinergorzel K, Dempsey E, Milewska M, et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. . 2015;4:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]


