Supplemental digital content is available in the text.
Key Words: Ovarian neoplasms, Angiogenesis inhibitors, Chemotherapy, Systematic review, Meta-analysis
Abstract
Background
Angiogenesis inhibitors showed activity in ovarian cancer, but preliminary data could not accurately reflect the survival benefit. We thus did a systematic review and meta-analysis of randomized controlled trials to reassess the efficacy and safety of angiogenesis inhibitors combined with chemotherapy for ovarian cancer.
Methods
We searched PubMed, EMBASE, Cochrane, and ClinicalTrials.gov for randomized controlled trials comparing angiogenesis inhibitors containing therapy with conventional chemotherapy alone or no further treatment. Our main outcomes were the progression-free survival (PFS), overall survival (OS), and common adverse events.
Results
Fifteen trials were included (N = 8721 participants). For newly diagnosed ovarian cancer, combination treatment with angiogenesis inhibitors and chemotherapy yielded a lower risk of disease progression (hazard ratio [HR], 0.83; 95% confidence interval (CI), 0.71–0.97) and no improved OS (HR, 0.95; 95% CI, 0.86–1.05). In the high-risk progression subgroup, the addition of bevacizumab significantly improved PFS (HR, 0.72; 95% CI, 0.65–0.81) and OS (HR, 0.84; 95%CI, 0.74–0.96). In recurrent patients, the combined HR was 0.58 (95% CI, 0.52–0.65) for PFS, and for OS, the combined HR was 0.86 (95% CI, 0.79–0.94). We found no significant improvement for either PFS (HR, 0.80; 95% CI, 0.63–1.01) or OS (HR, 1.06; 95% CI, 0.88–1.28) in the pure maintenance therapy.
In the overall population, angiogenesis inhibitors increased the incidence of gastrointestinal perforation (risk ratio [RR], 2.57; 95% CI, 1.66–3.97), hypertension (RR, 7.60; 95% CI, 2.79–20.70), arterial thromboembolism (RR, 2.27; 95% CI, 1.34–3.84), proteinuria (RR, 4.31; 95% CI, 2.15–8.64), and complication of wound healing (RR, 1.72, 95% CI, 1.12–2.63).
Conclusions
Combination treatment with angiogenesis inhibitors and chemotherapy significantly improved PFS and OS in both patients with high-risk of progression and recurrent ovarian cancer, with an increased incidence of common adverse events. Conversely, we detected no statistically significant survival benefit in the pure maintenance setting. The main limitation of the review is clinical heterogeneity across the studies.
Description of the Condition
Worldwide, ovarian cancer is the leading cause of gynecological cancer–associated death.1 It is the fifth leading cause of cancer-related deaths in female patients in developed countries.2 The poor prognosis is usually attributed to advanced stage at diagnosis and treatment resistance.3 Approximately 60% of women are diagnosed with late-stage disease that has already spread within the abdomen.1,4
Platinum/taxane doublet chemotherapy is the upfront standard of care in advanced ovarian cancer and yields an objective response in up to 80% of patients,5 but almost all will experience multiple recurrences of disease, with ever shorter disease-free intervals.6,7
Given the therapeutic limitations of conventional chemotherapy, recent investigations have explored molecularly guided therapies to target pathways of oncogenesis. A number of studies have shown that tumor growth and progression are partly dependent on angiogenesis.8,9
Description of the Intervention
Angiogenesis is recognized as a hallmark of several types of tumors including ovarian cancer.10 One of the most important cytokines responsible for tumor-mediated angiogenesis is vascular endothelial growth factor (VEGF), which is secreted by tumor cells and binds to the VEGF receptor that is present on normal endothelial cells, stimulating new blood vessel formation.11 Hence, efforts to block this pathway, either by inhibiting VEGF or its receptor, have emerged as attractive strategies for cancer treatment.12,13
Why it is Important to do This Review?
The good news is that there were clinical trials suggesting that angiogenesis inhibitors showed activity in ovarian cancer. However, the survival benefit was different in these trials. It is important to establish whether the addition of these new drugs to conventional chemotherapy regimens has additional survival benefit, if so, at what cost, and additional harmful effects. Moreover, there remain a lot of controversies. Should they be used as part of first-line therapy, recurrent setting, or to maintain patients with stable disease later in the course of their disease?
The most recently published meta-analysis14 indicated that antiangiogenic therapy showed clear progression-free survival (PFS) benefit with increased toxicity, but its role in overall survival (OS) was undefined for ovarian cancer. We therefore did a systematic review and meta-analysis of RCTs comparing angiogenesis inhibitors containing therapy with conventional chemotherapy alone or no further treatment for ovarian cancer to reassess the efficacy and safety of angiogenesis inhibitors in different clinical setting, including newly diagnosed ovarian cancer, recurrent patients, and pure maintenance setting. In this present study, the final data and 3 new randomized controlled trials (RCTs)15–17 were included.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
We searched PubMed, EMBASE, Central (Cochrane clinical trials database) database, and clinicaltrial.gov. We searched the database from 1994 to March 2017. We sought articles in all languages and there were no translations necessary. We used the following combined text and MeSH terms: “Ovarian Neoplasms”, “Angiogenesis Inhibitors”, “Bevacizumab”, “Avastin”, “Pazopanib”, “GW786034”, “Votrient”, “Trebananib”, “AMG386”, “Nintedanib”, “vargatef”, “BIBF1120”, “cediranib”, “AZD2171”, “recentin”, “Sorafenib”, “BAY 545-9085”, “BAY43-9006”, “Nexavar”, “NSC724772”, “sunitinib”, and “SU11248”.
Study Selection and Data Extraction
We regarded studies as eligible for inclusion if they were RCTs in women with histologically proven epithelial ovarian cancer of any stage (age, ≥18 years), compared angiogenesis inhibitors plus conventional chemotherapy with conventional chemotherapy alone, or angiogenesis inhibitors to no further treatment.
Two investigators independently reviewed study titles and abstracts, and excluded those studies that clearly did not meet our inclusion criteria. We then obtained copies of the full text of potentially relevant references. Trials selected for detailed analysis and data extraction were analyzed by 2 investigators. We resolved disagreements by discussion between the 2 authors and documented the reasons for exclusion. We extracted the following data from each selected trial: participant characteristics, study interventions, and outcomes.
Assessment of Risk of Bias in Included Studies
Cochrane Collaboration’s tool was used to assess the risk of bias in included RCTs. We had presented results in both a risk of bias graph and a risk of bias summary.
Data Analysis and Statistical Methods
We assessed the effect and safety of angiogenesis inhibitors–containing therapy on 3 outcomes: OS, PFS, and incidence of adverse events. For time-to-event data (OS and PFS), we pooled the hazard ratios (HRs) and two-sided 95% confidence interval (CI) using the generic inverse variance facility of RevMan 5.3. For dichotomous outcomes (toxicity), we used the risk ratio (RR). The Karlan 201218 trail had multiple treatment groups (3-arm trial), and so we divided the control group between the treatment groups (with different dose), and treated comparisons between each treatment group and a split control group as independent comparisons.
The χ2 test and Cochran Q-test were used to evaluate heterogeneity among trials, and I2 > 50% indicated a moderate-to-high heterogeneity.19 We used random-effects models for PFS and toxicity based on the large heterogeneity among the different trials. We pooled OS in a fixed effect model. Subgroup analysis was adopted to determine whether there is clinical benefit for patients in the subgroup classified by prognostic factors or different response to platinum-containing therapy. The meta-analysis software RevMan 5.3 provided by the Cochrane library was used for the data analysis.
We assessed the possibility of publication bias by constructing a funnel plot. We assessed funnel plot asymmetry using Begg and Egger tests, and defined significant publication bias as a P < 0.1.20 We used Stata (version 12.0) for the statistical analysis.
RESULTS
We initially identified 5440 articles from all searched database of which 15 trials (with data for 8721 participants) were retained after a full-text screening for inclusion in our review after excluding duplicates, reviews, case report, and phase I trials (Fig. 1). Two16,17 of the references were conference abstracts that described RCTs that met our inclusion criteria. The 15 trials were all published between 2011 and 2016.
FIGURE 1.
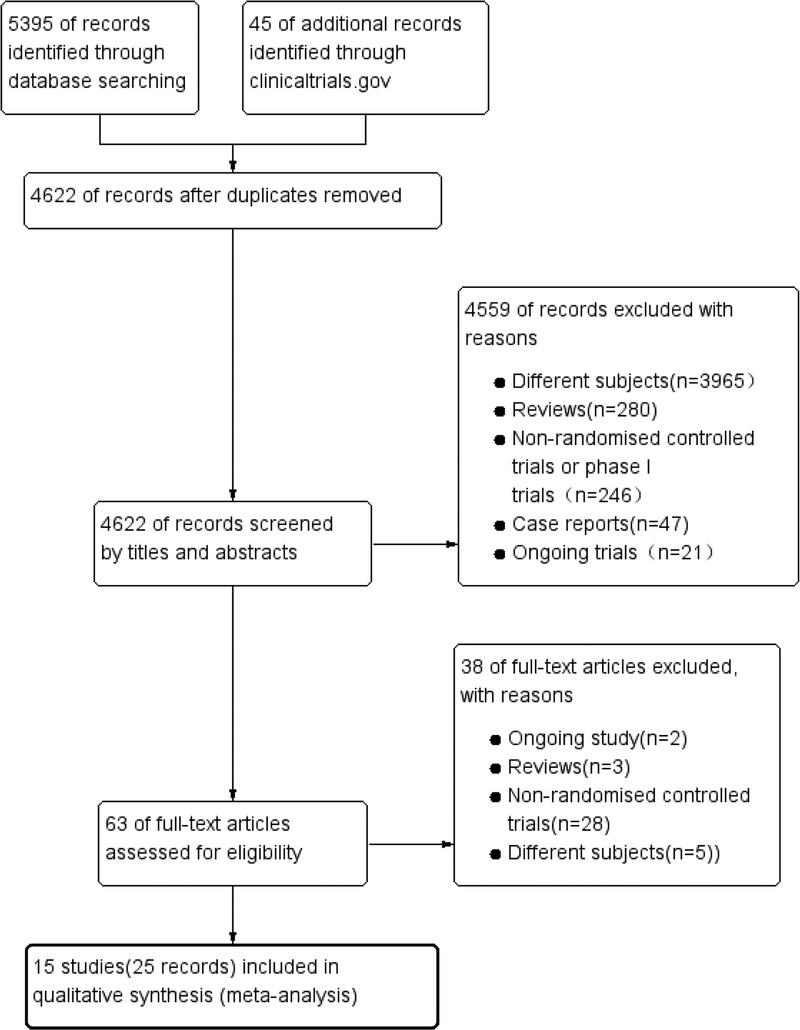
Flow chart indicating the study selection procedure.
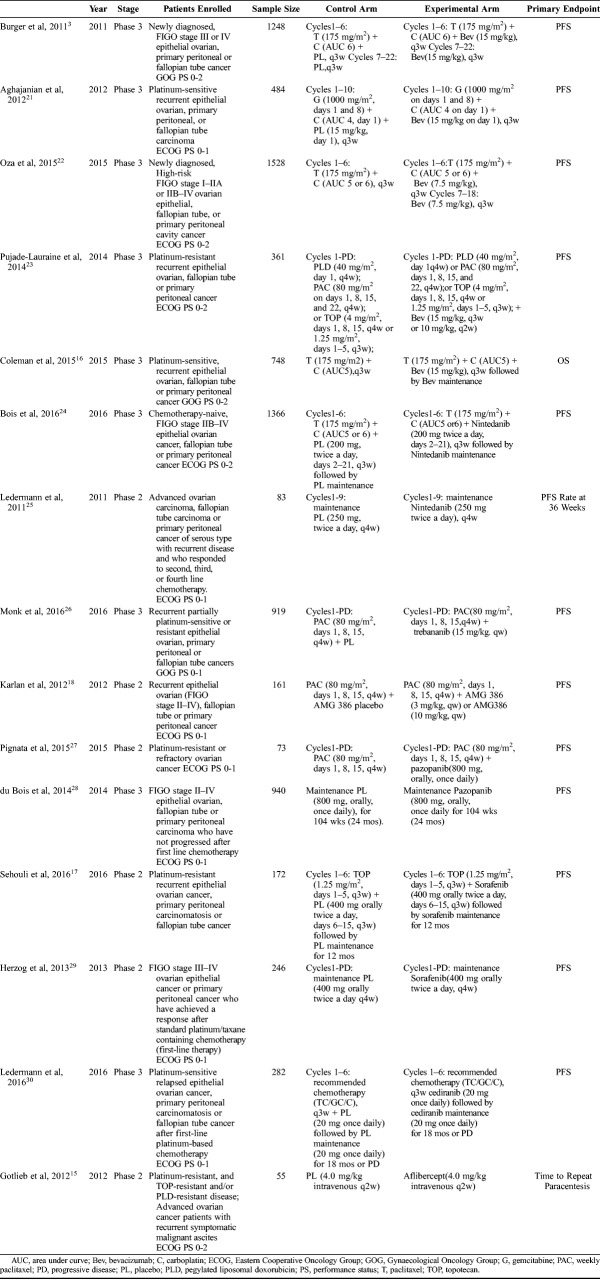
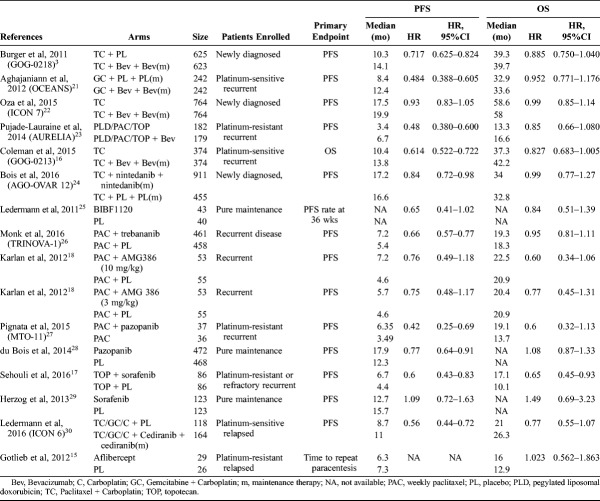
The main characteristics of 15 RCTs were summarized in Table 1, and the data of outcomes were summarized in Table 2.
TABLE 1.
Characteristics of included RCTs
TABLE 2.
Efficacy results of included RCTs
The assessment of risk of bias in the trials was shown in Figure 2. The risk of bias was unclear in the 2 studies that were published in an abstract form. Other RCTs reported sufficient information for randomization excluding 2 trials,28,29 for which “Randomize” was used in abstract and text, but further details were not reported, and none was stopped early. Moreover, 3 studies22,23,27 lacked blinding to participants and personnel, the other 2 trials25,29 did not specify whether data collectors and outcome assessors were masked to treatment allocation, and only 43,22,27,30 were not funded by industry.
FIGURE 2.
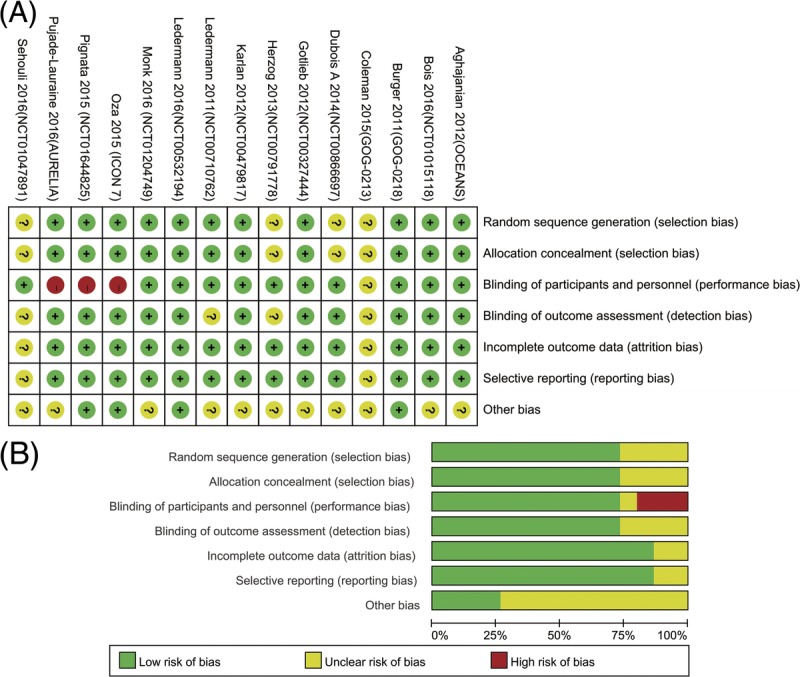
Risk of bias graph A, review of authors’ judgements about each risk of bias item presented as percentages across all included studies. Risk of bias summary B, review of authors’ judgements about each risk of bias item for each included study.
Overall Survival
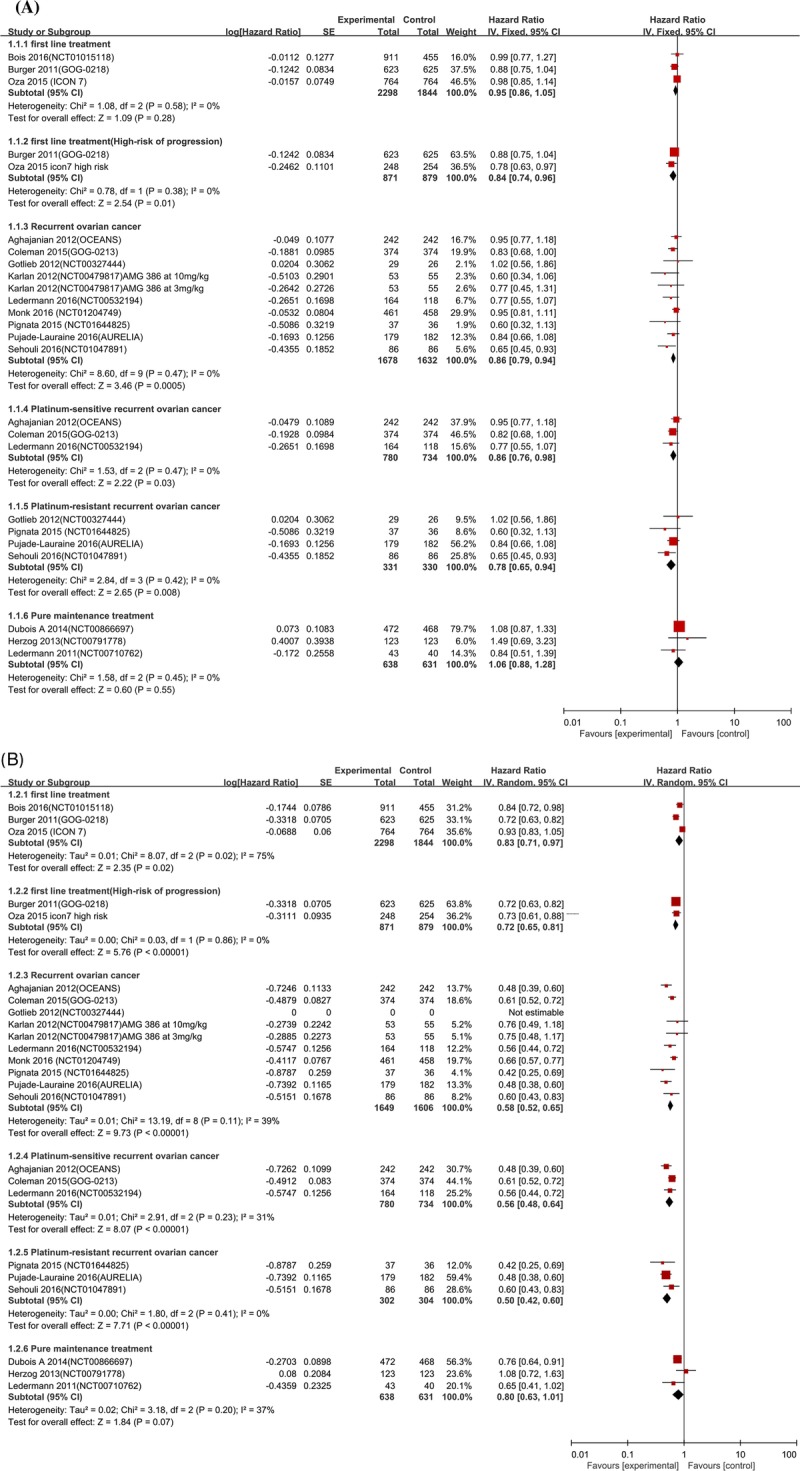
Three studies (n = 4142 participants) assessed the risk of death in patients with newly diagnosed ovarian cancer, pooling the data of these studies showed no significant difference in OS when participants were treated with angiogenesis inhibitors and chemotherapy combination treatment compared with chemotherapy alone (HR, 0.95; 95% CI, 0.86–1.05; I2 = 0%). In contrast, subgroup analysis suggested antiangiogenics-containing combination therapies had a significantly better OS in the patients with a high risk of progression from 2 studies with a total of 1750 participants (HR, 0.84; 95% CI, 0.74–0.96; I2 = 0%).
Nine studies (n = 3310 participants) assessed the risk of death in the recurrent setting, pooling the data of these studies also found statistically significant lower risk of death in women who received antiangiogenics-containing combination therapies compared with those who received chemotherapy alone (HR, 086; 95% CI, 0.79–0.94; I2 = 0%).
In addition, further subgroup analysis showed angiogenesis inhibitors had significant survival benefits for both platinum-sensitive recurrent ovarian cancer from 3 trials with a total of 1514 participants (HR, 0.86; 95% CI, 0.76–0.98; I2 = 0%) and platinum-resistant recurrent ovarian cancer from 4 trials with a total of 661 participants (HR, 0.78; 95% CI, 0.65–0.94; I2 = 0%).
Conversely, no significant difference in the risk of death was observed in the pure maintenance antiangiogenics therapy who achieved a good response to before chemotherapy (HR, 1.06; 95% CI, 0.88–1.28; I2 = 0%) based on the results of 3 studies with a total of 1269 patients (Fig. 3a).
FIGURE 3.
Forest plots: A, OS and B, PFS.
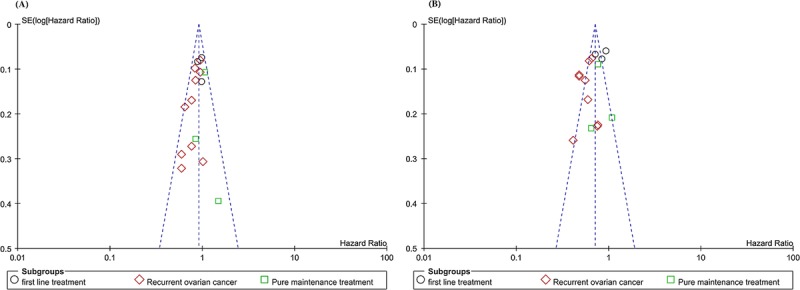
The funnel plot for OS revealed almost symmetry (Fig. 4a), and we further assessed publication bias on Egger test (P = 0.156), thus indicating no significant publication bias for OS.
FIGURE 4.
Forest plots: A, OS and B, PFS.
Progression-Free Survival
Angiogenesis inhibitors and chemotherapy combination treatment had significantly lower risks of disease progression compared with women with chemotherapy alone in both newly diagnosed setting (HR, 0.83; 95%CI, 0.71–0.97; I2 = 75%) and the recurrent setting (HR, 0.58; 95% CI, 0.52–0.65; I2 = 39%). Subgroup analysis for newly diagnosed patients with a high risk of progression indicated the PFS was significantly improved (HR, 0.72; 95% CI, 0.65–0.81; I2 = 0%). Moreover, further subgroup analysis comparing the benefit on PFS for platinum-sensitive recurrent ovarian cancer (HR, 0.56; 95% CI, 0.48–0.64; I2 = 31%) and platinum-resistant recurrent ovarian cancer (HR, 0.50; 95% CI, 0.42–0.60; I2 = 0%) both suggested significantly lower risks of disease progression. We detected no significant heterogeneity in both subgroups.
However, although pazopanib showed a significantly improved PFS (HR, 0.76; 95% CI, 0.64–0.91) from 1 trial,28 we found no significant improvement for PFS in the pure maintenance angiogenesis inhibitors therapy (HR, 0.80; 95% CI, 0.63–1.01; I2 = 37%), with no significant between-study heterogeneity (Fig. 3b).
The funnel plot for PFS revealed almost symmetry (Fig. 4b), and we further assessed publication bias on Egger test (P = 0.185), thus indicating no significant publication bias for PFS.
Adverse Events
Supplementary Figure A http://links.lww.com/IGC/A709 presents 7 common adverse events that are potentially associated with angiogenesis inhibitors during treatment. Among this updated analysis, the risks of adverse events (AEs) were significantly increased as follows: gastrointestinal perforation (G ≥ 3; RR, 2.57; 95% CI, 1.66–3.97; I2 = 63%), hypertension (G ≥ 3; RR, 7.60; 95% CI, 2.79–20.70; I2 = 74%), arterial thromboembolism (RR, 2.27; 95% CI, 1.34–3.84; I2 = 0%), proteinuria (G ≥ 3; RR, 4.31; 95% CI, 2.15–8.64; I2 = 0%), and complication of wound healing (RR, 1.72; 95% CI, 1.12–2.63; I2 = 1%).We found no significant increased risks for either neutropenia (G ≥ 4; RR, 1.09; 95% CI, 0.93–1.28; I2 = 46%) or venous thromboembolism (RR, 1.08; 95% CI, 0.79–1.48; I2 = 26%).
DISCUSSION
This updated meta-analysis was derived from 3 new RCTs and final data to reassess the efficacy and safety of angiogenesis inhibitors and chemotherapy combination treatment in ovarian cancer. The conclusion is different from the previous meta-analysis, especially in the grouping of statistical analysis. Considering the clinical settings to use angiogenesis inhibitors may play a major role in the treatment benefit, we divided 15 trials into 3 groups.
For newly diagnosed ovarian cancer, the addition of angiogenesis inhibitors to chemotherapy was associated with a significant improvement on PFS with large heterogeneity, but there was no evidence of a benefit on OS. Considering the large heterogeneity, we performed further subgroup analysis in patients with a high risk of progression who were predefined in the ICON7 trial and matched all the recruited patients in the GOG-218 trial, the results of which showed bevacizumab-containing therapy had significant improvement in both PFS and OS, with no significant between-study heterogeneity. Hence, our analysis showed that bevacizumab plus chemotherapy, followed by maintenance bevacizumab therapy, could be considered a front-line treatment option for patients with high-risk features or high-postsurgical tumor burden, with evidence of both PFS and OS benefits for this subgroup. However, because the survival benefit of angiogenesis inhibitors in high-risk patients was concluded from subgroup analysis, the results should be noted as the consistency of patient characteristics and principle of randomization were not ensured.
Although women with advanced epithelial ovarian cancer responded to many available therapeutic agents, almost all die from recurrence, which makes the treatment of recurrent ovarian cancer important. In the present study, antiangiogenics-containing therapies significantly reduced the HR of progression by 42% and risk of death by 14%, compared with chemotherapy alone with no significant between-study heterogeneity. Further analysis of 2 subgroups (ie, platinum-sensitive recurrent ovarian cancer and platinum-resistant recurrent ovarian cancer) both showed improvement on PFS and OS, with no significant between-study heterogeneity. The results were encouraging among women with recurrent ovarian cancer no matter whether responded to previous platinum-containing chemotherapy or not, demonstrating that angiogenesis inhibitors combined with chemotherapy is a great treatment option for recurrent ovarian cancer. Among them, bevacizumab, a kind of antiangiogenics by binding VEGF, has demonstrated a significant clinical benefit from several trials, and on the basis of these trials, bevacizumab was approved for first-line and second-line treatment of patients with both platinum-sensitive and platinum-resistant ovarian cancer.26 However, its activity in patients whose disease relapses after first-line bevacizumab-containing therapy is still unknown. Hence, further studies addressing this issue need to be performed.
Maintenance therapy has been one proposed strategy to improve outcomes, and incorporation of angiogenesis inhibitors had also been of interest. Recently, a number of clinical trials took combined strategies, using angiogenesis inhibitors in the maintenance setting. In the present study, we mainly analyzed the maintenance antiangiogenics monotherapy in the trials, which recruited patients who responded to previous chemotherapy (ie, a Partial Response or Complete Response according to the RECIST criteria in patients with measurable disease). In the trial,25 BIBF 1120 was not given to treat recurrent disease but to prolong the progression-free interval. It was evaluated after the completion of chemotherapy for relapsed ovarian cancer. The other 2 trials28,29 were designed to compare pazopanib or sorafenib to placebo as maintenance treatment after first-line therapy with systemic chemotherapy, and pazopanib showed a significant better PFS in the maintenance setting. However, pooled analysis of the 3 studies suggested no significant improvement in either PFS or OS. The lack of statistical significance may be because of lack of statistical power. In addition, more patients in the experience arm required dose modifications and discontinued treatment because of severe AEs, such as severe liver-related toxicity, severe gastrointestinal events, resulting in reduced dose of the planned dose. As a group, both short-term and longer-term adverse effects, the negative impact on quality of life associated with frequent visits to a physician or clinic and the cost may resulting in no significantly clinical benefit. Hence, further study should be performed to select patients who can really benefit from long-term maintenance treatment, particularly those who are at high risk of progression.
Adverse events were more common in the angiogenesis inhibitors-containing arm compared with the control arm, several significantly so (severe gastrointestinal events, severe hypertension, severe proteinuria, arterial thromboembolism, and complication of wound healing). It is necessary to monitor and manage these adverse events during the antiangiogenics therapy to minimize the risks. If severe adverse events such as gastrointestinal events can be controlled, antiangiogenics can be used safely.
This updated meta-analysis included 15 RCTs with 8721 patients, whereas the previous publication contained 12 RCTs with 7775 patients. One additional trial, NCT00327444,15 to our knowledge, was the first phase 2 study to show the effectiveness of VEGF blockade (aflibercept) in the reduction of malignant ascites for advanced chemoresistant ovarian cancer and recurrent symptomatic malignant ascites. The other 2 additional trials had final results published in abstract form from conference proceedings. Moreover, the most recent meta-analysis divided 12 trials into 3 groups: the bevacizumb group, the VEGFRIs group, the trebananib group. Improvement on PFS was seen in all groups and only the trebananib group demonstrated a significant prolongation on OS. However, to assess the role of clinical setting to use angiogenesis inhibitors in the treatment benefit, we divided 15 trials into 3 groups: first-line setting, the recurrent setting, and pure maintenance setting. Our results indicated that combination treatment with angiogenesis inhibitors and chemotherapy improved PFS and OS in the recurrent setting and high-risk progression subgroup, with no statistically significant improvement in OS for newly diagnosed ovarian cancer. We detected no significant improvement for either PFS or OS in pure maintenance setting.
A limitation of this analysis is clinical heterogeneity across the studies, including the different chemotherapy regimens, the tumor stages, and the length of follow-up. Secondly, there are 2 trials, the data of which have thus far been published only as conference abstracts, and they must be judged as being at high risk of bias until further details are known. Thirdly, although most of the included studies were published in high-impact journals, there were study features that carry potential risk of bias such as pharmaceutical industry funding and open-label design. Fourthly, there are differences in angiogenesis inhibitors (which include VEGF blockade, VEGF-R tyrosine kinase inhibitors, angiopoietin inhibitor) that might dictate an optimal choice for combination with chemotherapy or other biological agents. Finally, issues such as the optimize duration and timing of treatment, the potential tumor or host biologic factors to identify, which patients will benefit most (and perhaps more importantly, those who are not likely to respond), have not been established.
CONCLUSIONS
Together, although there are significant differences of increased risks of adverse events with antiangiogenics therapy, findings from our meta-analysis are relatively promising. Our findings clearly lend support to the use of angiogenesis inhibitors in combination with chemotherapy in the clinical management of patients with newly diagnosed (especially for high-risk patients) or recurrent ovarian cancer. However, no statistically significant clinical benefit was identified in the pure maintenance settings.
Supplementary Material
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.Holmes D. Ovarian cancer: beyond resistance. . 2015;527:S217 doi:10.1038/527S217a. [DOI] [PubMed] [Google Scholar]
- 2.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. . 2017;18:75–87. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. . 2011;365:2473–2483. [DOI] [PubMed] [Google Scholar]
- 4.Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. . 2014;384:1376–1388. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Martin A, Bover I, Del Campo JM, et al. SEOM guideline in ovarian cancer 2014. . 2014;16:1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. . 2013;14:1020–1026. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E. How to approach patients in relapse. . 2012;23(suppl 10):x128–x131. [DOI] [PubMed] [Google Scholar]
- 8.Kaimal R, Aljumaily R, Tressel SL, et al. Selective blockade of matrix metalloprotease-14 with a monoclonal antibody abrogates invasion, angiogenesis, and tumor growth in ovarian cancer. . 2013;73:2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldridge L, Moldobaeva A, Zhong Q, et al. Bronchial artery angiogenesis drives lung tumor growth. . 2016;76:5962–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. . 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 11.Frumovitz M, Sood AK. Vascular endothelial growth factor (VEGF) pathway as a therapeutic target in gynecologic malignancies. . 2007;104:768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall M, Gourley C, McNeish I, et al. Targeted anti-vascular therapies for ovarian cancer: current evidence. . 2013;108:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma--mechanistics, review of phase III randomized clinical trials, and regulatory implications. . 2014;132:496–505. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zhu S, Hong C, et al. Angiogenesis inhibitors for patients with ovarian cancer: a meta-analysis of 12 randomized controlled trials. . 2016;32:555–562. [DOI] [PubMed] [Google Scholar]
- 15.Gotlieb WH, Amant F, Advani S, et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. . 2012;13:154–162. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RL, Brady MF, Herzog TJ, et al. A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinumsensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer (Gynecologic Oncology Group 0213). . 2015;137:3–4. [Google Scholar]
- 17.Sehouli J, Hilpert F, Mahner S, et al. Topotecan (T) ± sorafenib (S) in platinumresistant ovarian cancer (PROC): a doubleblind placebocontrolled randomized NOGGO-AGO intergroup Trial-TRIAS. . 2016;34. [Google Scholar]
- 18.Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. . 2012;30:362–371. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. . 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 20.Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. . 2014;384:2228–2234. [DOI] [PubMed] [Google Scholar]
- 21.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. . 2012;30:2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. . 2015;16:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. . 2014;32:1302–1308. [DOI] [PubMed] [Google Scholar]
- 24.du Bois A, Kristensen G, Ray-Coquard I, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. . 2016;17:78–89. [DOI] [PubMed] [Google Scholar]
- 25.Ledermann JA, Hackshaw A, Kaye S, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. . 2011;29:3798–3804. [DOI] [PubMed] [Google Scholar]
- 26.Monk BJ, Poveda A, Vergote I, et al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): long-term survival, impact of ascites, and progression-free survival-2. . 2016;143:27–34. [DOI] [PubMed] [Google Scholar]
- 27.Pignata S, Lorusso D, Scambia G, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. . 2015;16:561–568. [DOI] [PubMed] [Google Scholar]
- 28.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. . 2014;32:3374–3382. [DOI] [PubMed] [Google Scholar]
- 29.Herzog TJ, Scambia G, Kim BG, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. . 2013;130:25–30. [DOI] [PubMed] [Google Scholar]
- 30.Ledermann JA, Embleton AC, Raja F, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. . 2016;387:1066–1074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.