Abstract
Objectives
To evaluate the efficiency and safety of emergency department (ED) coronary computed tomography angiography (CTA) during a 3-year clinical experience.
Methods
Single-center registry of coronary CTA in consecutive ED patients with suspicion of acute coronary syndrome (ACS). The primary outcome was efficiency of coronary CTA defined as the length of hospitalization. Secondary endpoints of safety were defined as the rate of downstream testing, normalcy rates of invasive coronary angiography (ICA), absence of missed ACS, and major adverse cardiac events (MACE) during follow-up, and index radiation exposure.
Results
1022 consecutive patients were referred for clinical coronary CTA with suspicion of ACS. Overall, median time to discharge home was 10.5 (5.7–24.1) hours. Patient disposition was 42.7% direct discharge from the ED, 43.2% discharge from emergency unit, and 14.1% hospital admission. ACS rate during index hospitalization was 9.1%. 192 patients underwent additional diagnostic imaging and 77 underwent ICA. The positive predictive value of CTA compared to ICA was 78.9% (95%-CI 68.1–87.5%). Median CT radiation exposure was 4.0 (2.5–5.8) mSv. No ACS was missed; MACE at follow-up after negative CTA was 0.2%.
Conclusions
Coronary CTA in an experienced tertiary care setting allows for efficient and safe management of patients with suspicion for ACS.
Keywords: Coronary artery disease, Multidetector Computed Tomography, Emergency Service, Hospital, Length of Stay, Cardiovascular Diseases
Introduction
Coronary computed tomography angiography (CTA) has been rigorously tested in several recent randomized trials as a method of triage for appropriately selected patients with suspicion for acute coronary syndrome (ACS) [1–7]. A CTA-based strategy has consistently demonstrated dramatically reduced disposition times and similar or reduced costs versus the alternative standard of care, with excellent safety profiles [3; 4; 8]. The burden of proof to validate this relatively new modality has been heavy, given the advent of comparative effectiveness trials, continually heightened fiscal awareness, and the disproportionate costs of cardiac imaging without proof of commensurate health improvements [9; 10]. Thus, a thorough assessment of coronary CTA results in a representative real world scenario, outside the confines of a trial, is required [11].
Following the conclusion of a recent multicenter randomized controlled trial (RCT) [4], we convened a multidisciplinary task force representing all involved stakeholders (emergency medicine, internal medicine, cardiology, and radiology) and agreed to activate a clinical emergency department (ED) coronary CTA protocol at our hospital. We also established a registry to track all clinical ED coronary CTA patients. Our goals were to ensure maintenance of the excellent safety profile demonstrated by recent randomized trials in clinical practice, and to explore remaining open questions about the implementation of emergency department coronary CTA outside the confines of an RCT [12]. Whether or not a CTA paradigm works as well in clinical practice as it does in a RCT, and if secondary safety endpoints such as major adverse cardiac events (MACE) can be maintained in a clinical practice. We hypothesized coronary CTA would be an efficient and safe tool for the evaluation of patients with low-intermediate risk for ACS.
Materials and Methods
Study design
The current study is a single tertiary academic medical center registry evaluating the use of coronary CTA in consecutive ED patients with acute chest pain or other signs and symptoms suggestive of an ACS. This study of our clinical registry was approved by our institutional review board and informed consent was waived due to the retrospective design of the study. HIPAA (Health Insurance Portability and Accountability Act) compliance was maintained at all times. Patients were included if they underwent a coronary CTA as part of routine clinical care between October 01, 2012 and August 31, 2015 as part of an ED visit. Eligibility criteria were standard for coronary CTA at our institution, and did not restrict patients based on heart rate, rhythm, or body habitus. Recommended relative contraindications to scanning were prior revascularization or known CAD, ECG changes suggestive of myocardial ischemia, positive serum biomarker levels suggestive of high risk of MI, impaired renal function (eGFR<60 mL/min/1.73m2) and prior anaphylactoid reaction to iodinated contrast media. Patients with relative contraindications were permitted to be scanned only after cardiology consultation.
Coronary CTA Protocol
Scans were performed using a second- or third-generation dual-source CT scanner (SOMATOM Definition Flash or SOMATOM Force; Siemens Healthcare, Forchheim, Germany) using ECG synchronization under the supervision of a qualified technologist and subspecialty radiologist or cardiologist. Automatic tube potential selection software (CAREkV, Siemens Healthcare) and automatic tube current selection algorithm (CAREDose 4D, Siemens Healthcare) were utilized. Noncontrast calcium score and ventricular function analysis were obtained in the majority of patients. Breast displacement was performed in all females using the bed positioning strap as per previously published department policy [13]. Scan acquisitions were performed during a single breath-hold at end inspiration, and the scan range covered from the level of the carina to the diaphragm. A test bolus was used to determine contrast timing. Beta-blockers were administered in the CT suite according to site practice, which varied toward less use as the practice evolved. All heart rates and rhythms were accepted for scanning, provided patients were hemodynamically stable. Unless contraindicated, sublingual nitroglycerin was administered during the exam [14]. Scans were performed regardless of coronary calcium burden. Radiation dose was recorded as effective dose (mSv) and size-specific dose estimate (mGy) [15]. A more detailed description of the scan protocol is listed in the supplementary material.
Scan results
Coronary CTA readouts were performed by the daily covering board-certified radiologists or cardiologists on dedicated workstations (Aquarius versions 4.7–4.11, Terarecon Inc., San Mateo, CA, USA). The coronary artery tree was assessed for the presence of stenosis, which was graded as no plaque nor stenosis, mild narrowing (unlikely to cause hemodynamically significant narrowing, i.e. 1–49%), moderate stenosis (possibly but not definitely causing hemodynamically significant narrowing, i.e. 50–69%), severe stenosis (likely to cause hemodynamically significant narrowing, i.e. ≥70%) or occlusion [16]. A potentially significant stenosis was defined as ≥50%, and these cases were triaged as “positive”. Cases with wall motion abnormalities were also triaged as “positive” regardless of coronary artery findings. Thus CTA results were defined as “negative” if there was no or minimal stenosis on CTA and no wall motion abnormality. Clinical management was based on CTA results including coronary stenosis degree and LV function and communicated to ED caregivers; in patients with moderate stenosis functional testing was encouraged; in patients with severe stenosis or wall motion abnormality, admission and cardiology consult was recommended. Noncoronary findings (such as pulmonary emboli, esophageal masses, discogenic disease) were not considered for the purposes of this analysis.
CTA operation hours, location, and equipment
Because the hours and location of operation were gradually expanded as utilization increased, eventually including early evening, and mornings of weekends and holidays on a scanner located in the ED, we distinguished patients according to their time of scanning in relation to time of triage to elucidate effects on length of stay. For the present analysis, patients were considered to have “same day scans” if the date of triage matched the date of CTA scan, or “next day scans” if the date of triage was different than the date of CT scan (i.e. patient stayed in-house past midnight).
Baseline characteristics – index hospitalization
Patients’ baseline characteristics, disposition from the ED and details of all diagnostic testing were collected from electronic medical records. CTA characteristics including heart rate and rhythm during scan, tube potential, tube current-time product, radiation exposure, and the presence of artifacts were recorded.
Follow-up
Patient follow-up was done by electronic medical record review. Discharge diagnosis and MACE (cardiovascular death, myocardial infarction, unstable angina, or urgent coronary revascularization) at a minimum of 60 days were adjudicated based on medical records by an independent cardiologist (blinded) as previously described [4] Records were checked for physician visits (and specifically noted if a cardiologist visit), cardiac testing, revascularization, MACE after discharge.
Study endpoints
The primary outcome was efficiency of cardiac CTA defined as the length of hospitalization, from the time from triage until discharge. This end point was chosen because it encompasses many steps in the work up of chest pain [17]. Secondary endpoints of safety were defined as the rate of downstream testing, the positive predictive value of CTA (i.e. predictive value for ≥50% stenosis subsequent ICA), rate of missed ACS, MACE during follow-up, and radiation exposure.
Statistical analysis
All analyses were performed on a per-patient level, grouping patients by their worst stenosis severity. Continuous variables were expressed as mean (± standard deviation [SD]) and compared with Student’s t-test for independent samples. Non-normally distributed variables were expressed as median (P25–P75) and compared with Mann-Whitney U test. Quantile-quantile plots were generated to determine distribution of the variable. Categorical variables were expressed as frequencies and percentages and differences were assessed using the chi-square test. Kaplan-Meier curves were generated to visualize length of stay until discharge to home. P-values were two-sided, and a P-value of less than 0.05 was considered statistically significant. Statistical computations were performed using SPSS (version 22, IBM, Armonk, New York, USA).
Results
Baseline characteristics
We included 1022 consecutive patients in the ED coronary CTA registry. Three patients were excluded because they underwent only the noncontrast portions of the imaging exam. Detailed baseline patient characteristics are listed in Table 1. The average age of our cohort was 52.6±11.0 years and 57.6% were male. BMI was an average of 29.2±6.0 kg/m2. Their TIMI risk score was low for ACS, with 97.7% of patients having a score ≤ 2. During index hospitalization, 9.1% of patients (n=93) were diagnosed with ACS (non-ST-elevation myocardial infarction 14, unstable angina pectoris 79). The majority of all patients (85.2%, n=872) were ultimately deemed to have chest pain of non-cardiac origin.
Table 1.
Baseline Patient and Scan Characteristics
| Characteristics | Full cohort (n=1022) |
At least 60-day FU or event at index (n=550) |
Less than 60- day FU (n=472) |
P value |
|---|---|---|---|---|
| Age (years) | 52.6±11.0 | 53.9±11.7 | 51.1±10.0 | <0.001 |
| Male (%) | 589 (57.6) | 297 (54.0) | 292 (61.9) | 0.011 |
| Race | 0.289 | |||
| Caucasian | 714 (69.9) | 388 (71.9) | 326 (70.3) | |
| African-American | 101 (9.9) | 47 (8.7) | 54 (11.6) | |
| Asian | 41 (4.0) | 26 (4.8) | 15 (3.2) | |
| Other/unknown | 166 (16.2) | 89 (16.2) | 77 (16.3) | |
| Hispanic Ethnicity | 91 (8.9) | 56 (10.2) | 35 (7.4) | 0.122 |
| BMI (kg/m2) | 29.2±6.0 | 29.4±6.2 | 28.9±5.7 | 0.205 |
| Current smoker (%) | 223 (21.8) | 125 (22.7) | 98 (20.8) | 0.448 |
| Diabetes Mellitus (%) | 118 (11.5) | 76 (13.8) | 42 (8.9) | 0.014 |
| Dyslipidemia (%) | 288 (28.2) | 191 (34.7) | 97 (20.6) | <0.001 |
| Hypertension (%) | 410 (40.1) | 250 (45.5) | 160 (33.9) | <0.001 |
| Family history of CAD | 181 (17.7) | 107 (19.5) | 74 (15.7) | 0.115 |
| TIMI score | <0.001 | |||
| 0 | 622 (60.9) | 282 (51.3) | 340 (72.0) | |
| 1 | 279 (27.3) | 177 (32.2) | 102 (21.6) | |
| 2 | 98 (9.6) | 73 (13.3) | 25 (5.3) | |
| 3 | 19 (1.9) | 14 (2.5) | 5 (1.1) | |
| ≥4 | 4 (0.4) | 4 (0.7) | 0 (0.0) | |
| Heart rate (BPM) | 70.1±12.6 | 70.6±13.2 | 69.7±12.1 | 0.295 |
| KVp | 100 (80–120) | 100 (80–120) | 100 (80–100) | 0.030 |
| mAs | 285 (182–417) | 271 (175–419) | 295 (187–415) | 0.111 |
| Radiation dose (mSv) | 4.0 (2.5–5.8) | 3.8 (2.5–5.9) | 4.1 (2.6–5.8) | 0.111 |
| Size-specific dose estimate (mGy)* | 14.2 (10.1–22.8) | 16.1 (10.8–25.8) | 13.1 (8.7–19.6) | <0.001 |
| Prospective ECG-triggering | 847 (82.9) | 476 (86.5) | 371 (78.6) | <0.001 |
| Non-sinus rhythm | 13 (1.3) | 6 (1.2) | 7 (1.5) | 0.571 |
| CTA positive# | 193 (18.9) | 153 (27.8) | 40 (8.5) | <0.001 |
| Coronary calcium score strata** | <0.001 | |||
| 0 | 511 (50.0) | 236 (42.9) | 275 (58.3) | |
| 1–10 | 136 (13.3) | 76 (13.8) | 60 (12.7) | |
| 10–100 | 145 (14.2) | 79 (14.4) | 66 (14.0) | |
| 100–400 | 114 (11.2) | 68 (12.4) | 46 (9.7) | |
| >400 | 60 (5.9) | 53 (9.6) | 7 (1.5) |
Values are mean (SD), median (P25–P75) or n (%).
available in 796,
available in 967,
Stenosis degree >50% or abnormal function.
CTA characteristics
Coronary calcium score was performed in 967 patients and ventricular function was obtained in 858 patients. Metoprolol was administered in the CT suite to 202/1022 (19.8%) patients overall. Beta-blocker usage during scanning decreased steadily during the course of the registry (as site practice and dual-source triggering algorithms evolved and subsequently site protocols were implemented), from 100% (4/4) patients receiving metoprolol in October 2012, to 0% (0/52) in July 2015. Median radiation exposure was 4.0 (2.5–5.8) mSv. The effective dose increased with increasing BMI. Size-specific dose estimate for all patients was 14.2 (10.1–22.8) mGy. Patients with a BMI smaller than 30 kg/m2 had a median radiation exposure of 3.0 (2.1–4.2) mSv exposure. Detailed scan characteristics are listed in Table 1 and divided patients in whom 60-day follow-up was completed and those who did not (including those who experienced an event before 60-days). 511 (50.0%) of patents had a coronary calcium score of zero (Table 2). In patients with a zero coronary calcium score, we observed 6 (0.6%) cases of severe stenosis or occlusion. Coronary CTA ruled out the presence of more than mild coronary artery stenosis (<50% stenosis degree) in 83.8% of patients. Left ventricular wall motion abnormalities were observed in 61 patients, resulting in a scan result being upgraded from negative to positive in 22 total patients (39 patients were already classified as positive because of ≥50% worst stenosis degree).
Table 2.
CTA results
| Characteristics | Full cohort (n=1022) |
Males (n=589) |
Females (n=433) |
P value |
|---|---|---|---|---|
| Stenosis degree | <0.001 | |||
| No stenosis | 486 (47.6) | 236 (40.1) | 250 (57.6) | |
| Mild | 364 (35.6) | 228 (38.7) | 136 (31.4) | |
| Moderate | 72 (7.0) | 52 (8.8) | 20 (4.6) | |
| Severe | 77 (7.5) | 56 (9.5) | 21 (4.8) | |
| Occluded | 22 (2.2) | 17 (2.9) | 5 (1.2) | |
| Non-diagnostic | 1 (0.2) | 0 (0.0) | 1 (0.2) | |
| Wall motion abnormality* | 61 (6.0) | 39 (6.6) | 22 (5.1) | 0.304 |
Assessed in 804
Time to discharge home
Median time to discharge home was 10.5 hours (Figure 1a). When evaluating time to discharge by stenosis degree, we observed a clear increase in time with increasing stenosis severity (Figure 1b). Among patients with “negative” scans, median time to discharge home was 7.9 hours (all triage times, p<0.001 compared to full cohort), or 6.1 hours (same day scans, p<0.001 compared to full cohort), and 20.7 hours (next day scans, p<0.001 compared to full cohort) (Table 3).
Figure 1. Length of stay in hospital and proportion of patients discharged.
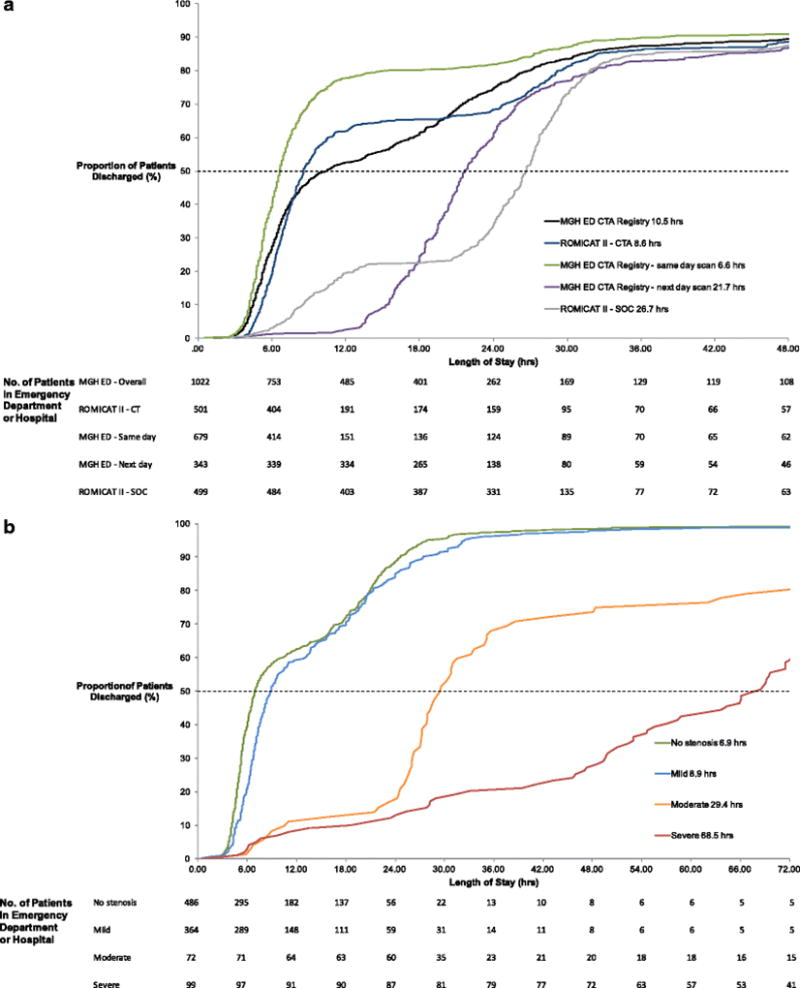
Trial comparison (a) and per stenosis degree (b).
Table 3.
Primary effectiveness end point
| Characteristics | All | Same day scan | Next day scan |
|---|---|---|---|
| Length of stay - hours | |||
| All patients | n=1022 | n=679 | n=343 |
| Median (Q1–Q3) | 10.5 (5.7–24.1) | 6.6 (5.1–10.5) | 21.7 (18.2–28.5) |
|
| |||
| CTA positive | n=193 | n=129 | n=64 |
| Median (Q1–Q3) | 46.0 (25.6–75.6) | 31.5 (23.5–72.6) | 64.1 (32.5–86.2) |
| CTA negative | n=828 | n=549 | n=279 |
| Median (Q1–Q3) | 7.9 (5.3–19.4) | 6.1 (4.8–7.9) | 20.7 (17.6–24.4) |
|
| |||
| Males | n=589 | n=404 | n=185 |
| Median (Q1–Q3) | 10.6 (5.9–24.4) | 6.9 (5.2–13.5) | 20.8 (17.6–26.7) |
| Females | n=433 | n=275 | n=158 |
| Median (Q1–Q3) | 10.0 (5.6–23.5) | 6.4 (4.9–9.2) | 22.9 (19.4–32.8) |
|
| |||
| Final diagnosis ACS | n=93 | n=62 | n=31 |
| Median (Q1–Q3) | 66.1 (43.1–97.1) | 55.2 (27.8–80.5) | 79.5 (58.4–115.5) |
| Final diagnosis not ACS | n=929 | n=618 | n=312 |
| Median (Q1–Q3) | 8.8 (5.5–21.2) | 6.4 (4.9–8.9) | 21.2 (18.0–25.7) |
Patient management
42.7% of patients were directly discharged to home from the ED, 43.2% were discharged home from the observation unit, and 14.1% were admitted to the hospital. 192 (18.8%) patients underwent additional testing (i.e. SPECT, ETT, ultrasound, ICA) for exclusion of ACS at time of index visit — 58 (6.8%) out of 850 patients with no or mild stenosis, 53 (73.6%) out of 72 patients with moderate stenosis, and 81 (81.8%) out of 99 patients with severe stenosis or occlusion). Among the 77 (7.5%) patients who underwent invasive coronary angiography, 76 were interpreted as having significant stenosis on CTA. In these patients, a positive CTA was confirmed by invasive coronary angiography in 60 (78.9%). Patients with relative contraindications, patient management and presence of ACS is displayed in eTable and Figure 2.
Figure 2. Flow-chart showing patient diagnostic test results up to follow-up.
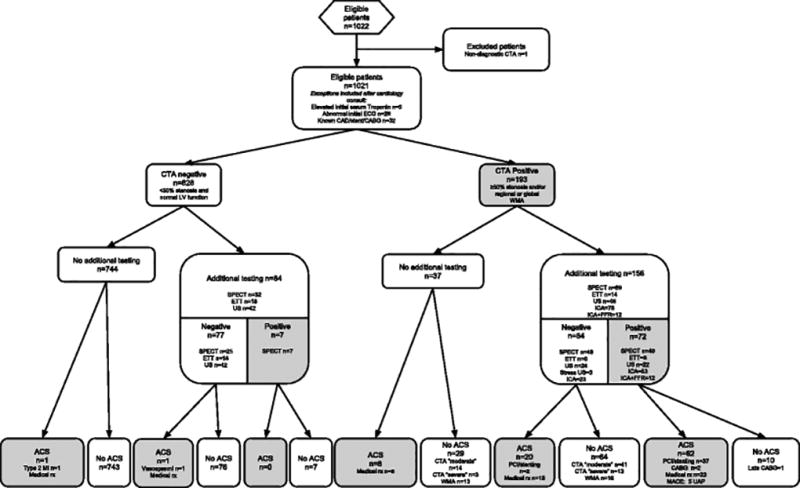
Patient was classified as positive if any additional test (either during index visit or within 60-days after discharge) was regarded positive.
Follow-up
Median follow-up time in those without an event at index hospitalization was 106 days, and 60-day follow-up was ascertained in 550 patients (53.8%). The cohort in whom a minimum of 60-day follow-up could not be obtained had lower risk baseline characteristics in all metrics, with statistically significant differences in dyslipidemia, hypertension, TIMI score, calcium score and also a positive CTA (Table 1). 130 (12.7%) patients were seen by a cardiologist. In the 5 (0.5%) patients who experienced a MACE (all unstable angina pectoris), coronary CTA was positive (severe stenosis) in all cases. 3 (0.3%) patients died, 2 of non-cardiac causes and one due to cardiovascular but non-coronary disease (heart failure). Two cases of ACS were missed by CTA (one vasospasm and one Type 2 demand MI; both were medically managed) thus yielding event rate of 0.24% (95% CI −0.09–0.58) after negative CTA. There were no post-discharge missed MACE or revascularization in the cohort with negative scans and a small number of late elective revascularizations (2 PCI and 1 CABG) in the cohort of moderate and severe/occluded stenoses, as depicted in eFigure. Kaplan-Meier curves reveiled a clear decrease in survival with worsening stenosis degree and with abnormal ventricular function (Figure 3).
Figure 3. Kaplan-Meier curves for index diagnosis of ACS and MACE at follow-up.
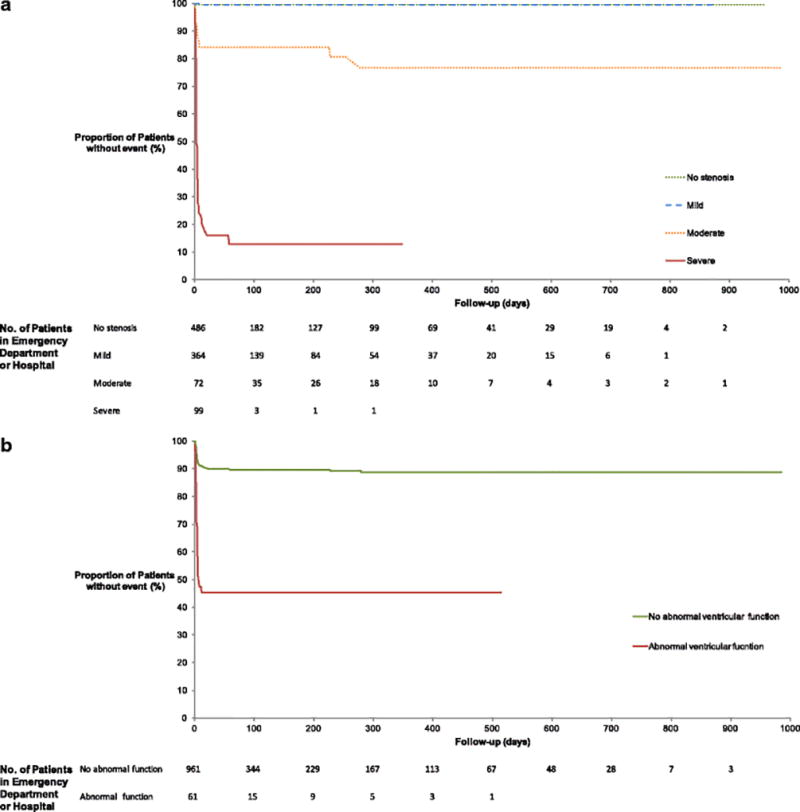
Stenosis degree (a) and abnormal ventricular function (b).
Discussion
We report the results of a 3 year CTA registry with over 1000 patients in a large tertiary care hospital. Our data demonstrate that integration of coronary CTA into the work up of patients with suspected ACS can be achieved with very short median time to discharge home (6 to 10 hours for various subgroups) and that only patients with a reasonable risk for ACS are referred for CTA (ACS rate: 9.1% (n=93/1022) and prevalence of obstructive CAD 9.7%). Moreover, coronary CTA in a robust program led relatively few patients to undergo additional diagnostic imaging (18.8%) and ICA (7.6%) while being safe (median CT radiation exposure: 4.0 (2.5–5.8) mSv, only two cases of ACS after negative CTA, with none clinically missed.
Our data represent the largest contemporary coronary CTA registry for acute chest pain in ED patients and suggests that with growing experience, efficiency and safety can be increased as compared to randomized trials performed in the early stage of implementation of CTA in the ED [18]. Important for comparability, we demonstrate that the referral mix parallels that of preceding trials, with a low TIMI score (97.7% with TIMI≤2) but a sizable number of patients diagnosed with ACS during index hospitalization (9.1%, n=93) emphasizing the incremental information that is provided by coronary CTA.
Overall, our data is concordant with the results of randomized trials showing that CTA is safe and efficient technique. Moreover, we demonstrate that a collaborative effort of radiology, cardiology, internal medicine and emergency medicine in combination with clinical and CT expertise using newest CT technology cardiac CT may be more efficient than previously shown despite the more flexible referral hours and patient eligibility criteria as compared to RCTs. Compared to the previously published RCTs [4; 5] we observed a comparable median length of stay and if the scan was perfomed on the day of triage, our times were substantially shorter (6.6 hours vs. 8.6 and 18.0 hours). In addition, the positive predictive value of 79% for CTA compared to ICA in our registry was better compared that of previous RCT’s (54%–71%) [3; 5]. A notable finding of our analysis was the median radiation exposure of 4.0 (2.5–5.8) mSv, which is considerable lower than in prior RCT’s (>10 mSv) [3; 4]; this was achieved using an updated, modern cardiac CT protocol. Our comprehensive dual-source CT protocol included calcium scoring and ventricular functional assessment, did not require medications for heart rate control, and was applied to an overweight population (average BMI 29.2±6.0 kg/m2) [19]. Our practice model allows for efficiencies in acquisition by not requiring pre-scan heart rate control, allowing compensation for arrhythmia, while automatically provide appropriate radiation exposures. These technologies were not uniformly available at all sites during prior trials.
Additionally, we showed similar or higher rates of downstream testing (18.8%) versus prior studies and RCTs (Baptist Health 11.9%; ACRIN-PA 14% stress testing only; MonashHeart 17%;). Our rate of moderate or greater stenosis by CT was 16.7%, which is comparable to the published results of ROMICAT-II [4] ACRIN-PA [5], MonashHEART [20], and Baptist Health [21], of 15%, 16%, 17%, and 12%, respectively. Our safety data showed an event rate of 0.24% (95% CI −0.09–0.58) after negative CTA, which is consistent with the results and within the pre-specified safety thresholds by prior trials [5; 21; 22], although these events were not missed clinically and treated appropriately.
In clinical practice, the complex inclusion and exclusion criteria of a trial can sometimes be ignored, and “indication creep” is a known phenomenon throughout medicine [23]. This can be a driver for contrarian views of imaging. Additionally, the Hawthorne effect can lead to better outcomes in both arms of a RCT simply via the investigators’ awareness of the trial [24]. Though our registry is not a trial, our work was borne out of a careful multidisciplinary task force and the intention to follow-up and potentially publish our results was well known to all stakeholders involved. We enjoyed the advantage of institutional participation and leadership in earlier trials, and thus built upon the prior experience and scientific knowledge base surrounding coronary CTA for acute chest pain. As performance of a test shifts beyond the confines of a trial, a regression toward the mean is also possible, as more practitioners perform, interpret, and act upon exams. Despite all of this, our “real world” clinical use of coronary CTA in the ED was associated with favorable results. Wider integration of coronary CTA in ED settings may allow for faster patient disposition, less utilization of downstream testing and more accurate selection of patients who benefit from invasive procedures.
Some limitations warrant additional consideration to place our results into the proper context. We report results from a single center study based on a highly experienced team using high-end CT systems for coronary CTA (which allowed advantages, for instance, the elimination of beta-blockade from this protocol with maintenance of high accuracy and low radiation dose). Hence, results may not be immediately generalizable to other hospital settings or other equipment. However, they demonstrate what can be achieved in specialized settings. However, they demonstrate what can be achieved. Our follow up rates were limited to a subset of patients (median follow-up time of 106 days), in part due to the limitations of our clinical registry, which does not allow for contacting patients outside of their clinical care. However, the risk class and scan results of the cohort for whom the detailed 60-day follow-up was identified was actually higher than in those in whom follow-up was not available, and in the large cohort of normal or mild stenoses, event rates were very low even at longer-term follow-up (Figure 2). While this strengthens our argument for the accuracy of a CTA strategy, it does tend toward a confirmation bias that could only be mitigated in a prospective, randomized trial setting. Further, TIMI score was used for pre-CTA risk classification. A score that was originally validated in patients with confirmed ACS [25], nevertheless it has been demonstrated to be also usefull for risk classification in ED patients suspected to have ACS [26; 27], to ensure high risk patients were not routinely included in the CTA pathway. Finally, our clinical services did not have access to high-sensitivity troponin assays [28] (not currently approved for clinical use in the United States); improved biomarkers might eventually reduce the need for imaging in some patients [29].
Conclusion
Our registry demonstrates that in experienced hands, carefully practiced coronary CTA imaging in ED patients with chest pain can be performed in accordance with, and improvement upon, the results of published trials. Notably, using advanced CT systems not requiring pre-scan heart rate control and with the aid of automatic tube potential and current selection software, CTA radiation dose was decreased by more than 50% versus published trials, and the positive predictive value of CTA was increased compared to recent randomized trials, while patients triaged after operating hours (i.e. “next day” scans) were still discharged several hours faster than the standard-of-care arm, routine-hours-only patients in recent trials.
Supplementary Material
Key points.
-
-
ED Coronary CTA using advanced systems is associated with low radiation exposure.
-
-
Negative coronary CTA is associated with low rates of MACE.
-
-
CTA in ED patients enables for short median time to discharge home.
-
-
CTA strategy is characterized by few downstream tests including unnecessary ICA.
Acknowledgments
The scientific guarantor of this publication is Brian B. Ghoshhajra. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained.
Written informed consent was waived by the Institutional Review Board. Methodology: registry, observational, performed at one institution.
References
- 1.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 2.Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762–1768. doi: 10.1161/CIRCULATIONAHA.106.618389. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 6.Sandfort V, Lima JA, Bluemke DA. Noninvasive Imaging of Atherosclerotic Plaque Progression: Status of Coronary Computed Tomography Angiography. Circ Cardiovasc Imaging. 2015;8:e003316. doi: 10.1161/CIRCIMAGING.115.003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.investigators S-H. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 8.Chang AM, Shofer FS, Weiner MG, et al. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med. 2008;15:649–655. doi: 10.1111/j.1553-2712.2008.00159.x. [DOI] [PubMed] [Google Scholar]
- 9.Beinfeld MT, Gazelle GS. Diagnostic imaging costs: are they driving up the costs of hospital care? Radiology. 2005;235:934–939. doi: 10.1148/radiol.2353040473. [DOI] [PubMed] [Google Scholar]
- 10.Cook TS, Galperin-Aizenberg M, Litt HI. Coronary and cardiac computed tomography in the emergency room: current status and future directions. J Thorac Imaging. 2013;28:204–216. doi: 10.1097/RTI.0b013e3182956bbf. [DOI] [PubMed] [Google Scholar]
- 11.Nosek BA, Spies JR, Motyl M. Scientific Utopia: II. Restructuring Incentives and Practices to Promote Truth Over Publishability. Perspect Psychol Sci. 2012;7:615–631. doi: 10.1177/1745691612459058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreyer NA, Garner S. Registries for robust evidence. JAMA. 2009;302:790–791. doi: 10.1001/jama.2009.1092. [DOI] [PubMed] [Google Scholar]
- 13.Vadvala H, Kim P, Mayrhofer T, et al. Coronary CTA using scout-based automated tube potential and current selection algorithm, with breast displacement results in lower radiation exposure in females compared to males. Cardiovasc Diagn Ther. 2014;4:470–479. doi: 10.3978/j.issn.2223-3652.2014.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takx RA, Sucha D, Park J, Leiner T, Hoffmann U. Sublingual Nitroglycerin Administration in Coronary Computed Tomography Angiography: a Systematic Review. Eur Radiol. 2015;25:3536–3542. doi: 10.1007/s00330-015-3791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brink JA, Morin RL. Size-specific dose estimation for CT: how should it be used and what does it mean? Radiology. 2012;265:666–668. doi: 10.1148/radiol.12121919. [DOI] [PubMed] [Google Scholar]
- 16.Raff GL, Chinnaiyan KM, Cury RC, et al. SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:254–271. doi: 10.1016/j.jcct.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Lee CI, Enzmann DR. Measuring radiology's value in time saved. J Am Coll Radiol. 2012;9:713–717. doi: 10.1016/j.jacr.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Nieman K, Hoffmann U. Cardiac computed tomography in patients with acute chest pain. Eur Heart J. 2015;36:906–914. doi: 10.1093/eurheartj/ehv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangold S, Wichmann JL, Schoepf UJ, et al. Coronary CT angiography in obese patients using 3rd generation dual-source CT: effect of body mass index on image quality. Eur Radiol. 2015 doi: 10.1007/s00330-015-4161-x. [DOI] [PubMed] [Google Scholar]
- 20.Nasis A, Meredith IT, Sud PS, Cameron JD, Troupis JM, Seneviratne SK. Long-term outcome after CT angiography in patients with possible acute coronary syndrome. Radiology. 2014;272:674–682. doi: 10.1148/radiol.14132680. [DOI] [PubMed] [Google Scholar]
- 21.Cury RC, Feuchtner GM, Batlle JC, et al. Triage of patients presenting with chest pain to the emergency department: implementation of coronary CT angiography in a large urban health care system. AJR Am J Roentgenol. 2013;200:57–65. doi: 10.2214/AJR.12.8808. [DOI] [PubMed] [Google Scholar]
- 22.Poon M, Cortegiano M, Abramowicz AJ, et al. Associations between routine coronary computed tomographic angiography and reduced unnecessary hospital admissions, length of stay, recidivism rates, and invasive coronary angiography in the emergency department triage of chest pain. J Am Coll Cardiol. 2013;62:543–552. doi: 10.1016/j.jacc.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Djulbegovic B, Paul A. From efficacy to effectiveness in the face of uncertainty: indication creep and prevention creep. JAMA. 2011;305:2005–2006. doi: 10.1001/jama.2011.650. [DOI] [PubMed] [Google Scholar]
- 24.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 26.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–18. doi: 10.1197/j.aem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction Risk Score in the emergency department chest pain population. Ann Emerg Med. 2006;48:252–259. doi: 10.1016/j.annemergmed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Than M, Aldous S, Lord SJ, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174:51–58. doi: 10.1001/jamainternmed.2013.11362. [DOI] [PubMed] [Google Scholar]
- 29.Januzzi JL, Sharma U, Zakroysky P, et al. Sensitive troponin assays in patients with suspected acute coronary syndrome: Results from the multicenter rule out myocardial infarction using computer assisted tomography II trial. Am Heart J. 2015;169:572–578 e571. doi: 10.1016/j.ahj.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


