Abstract
α-Galacto-oligosaccharides have been reported to have beneficial health effects. The purpose of this study was to investigate the preventive effects of a newly synthesized α-galacto-oligosaccharide mixture (α-GOSg), as well as raffinose family oligosaccharides (RFOs), on dextran sulfate sodium (DSS)-induced colitis in mice. When administered in drinking water at 0.5% for 15 days, both α-GOSg and RFOs significantly decreased fecal hemoglobin content, partially prevented colon length shortening, reduced the severity of colon inflammation, and attenuated DSS-induced upregulation of cyclooxygenase-2. In addition, the activation of the inflammatory regulator nuclear factor-kappa B was slightly inhibited by α-GOSg. The results showed that the newly synthesized α-GOSg preparation has similar anti-inflammatory activities as RFOs in this colitis model. The anti-inflammatory activity of α-GOSg in humans remains to be investigated.
Keywords: colitis, anti-inflammatory, α-GOSg, cyclooxygenase-2
Graphical Abstract
1 Introduction
Galacto-oligosaccharides, comprised of a chain of galactose units with galactosidic linkages, are generally considered to be prebiotic (Torres, Gonçalves, Teixeira, & Rodrigues, 2010). A variety of beneficial health effects have been reported for this group of compounds, such as modulating immune activities, reducing serum cholesterol levels, improving mineral absorption and improving intestinal health (Dai et al., 2014; Rastall & Gibson, 2015; Vulevic, Juric, Tzortzis, & Gibson, 2013; Weaver et al., 2011). However, the mechanisms of action are not clearly understood.
Most of the galacto-oligosaccharides used in previous studies were β-galacto-oligosaccharides, which are typically synthesized by the enzymatic activity of β-galactosidase on lactose in a reaction known as transgalactosylation (Sangwan, Tomar, Singh, Singh, & Ali, 2011). For example, β-D-galactosidase from Bacillus circulans has been used to bio catalyze the formation of N-acetyl-oligosaccharides from lactose and N-acetylglucosamine (Li, Sun, Ye, & Zeng, 2010). A recent study suggested that galacto-oligosaccharides with β-(1→6) linkages had higher prebiotic index values than those of β-(1→4) linkages (Li, Wang, Sun, Ye, Hu, & Zeng, 2015). α-Galacto-oligosaccharides, derived from legumes, have been shown to modulate appetite, and decrease inflammation in overweight adults (Morel, Dai, Ni, Thomas, Parnet, & Fanca-Berthon, 2015). However, the biological activities of other galacto-oligosaccharides with α-glycosidic linkages still remain unclear.
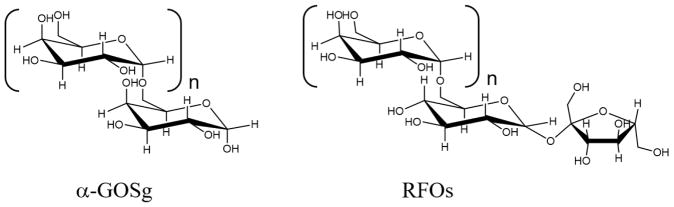
In this study, a newly synthesized α-galacto-oligosaccharide mixture (designated as α-GOSg), derived from galactose (Gal), was investigated together with raffinose family oligosaccharides (RFOs). The structures of the α-GOSg and RFOs are shown in Figure 1. RFOs, having structures with 1 to 3 galactose units linked to a sucrose molecule (known as raffinose, stachyose, verbascose, respectively), are typical α-galacto-oligosaccharides. They have been found in a variety of legumes and exist almost ubiquitously in the plant kingdom. In abundance, RFOs rank second only to sucrose as naturally occurring soluble carbohydrates (Kuo, Vanmiddlesworth, & Wolf, 1988). Some α-galacto-oligosaccharides are also known for their ability to stimulate the growth of beneficial bacteria, especially Bifidobacterium (Ehara et al., 2016).
Figure 1.
Chemical structures of α-GOSg and RFOs.
Colitis, also known as inflammatory bowel disease (IBD), usually shows clinical symptoms of abdominal pain, diarrhea, rectal bleeding, malaise and weight loss (Bouma & Strober, 2003). The incidence and prevalence of IBD are increasing around the world (Molodecky et al., 2012). Although the exact mechanisms of pathogenesis of IBD are still unclear, several studies have indicated that intestinal bacteria play a key role. For example, IBD usually occurs in intestinal regions where bacterial populations are most abundant (Jonkers, Penders, Masclee, & Pierik, 2012). In addition, the microflora composition of IBD patients usually differ from healthy controls (Sokol et al., 2006). Some clinical studies have investigated the effects of probiotics in IBD patients, and found that intake of probiotics attenuated inflammation-associated symptoms and delayed transition to epithelial dysplasia and cancer (Appleyard, Cruz, Isidro, Arthur, Jobin, & De Simone, 2011; Reiff & Kelly, 2010). One of the possible mechanisms is that the growth of commensal protective bacteria can decrease the colonization of disease-inducing bacteria in the colon. There is emerging interest in the general public and scientific communities on the possible use of probiotics in the treatment of colitis.
The administration of probiotics, however, is not the only way to modify the intestinal microflora. There is also a great interest in prebiotics, which have shown selective stimulatory effect on the growth of lactobacillus and bifidobacterium in the colon (Steed, Macfarlane, & Macfarlane, 2008). Changes in intestinal microbiota have been proposed as the basis for the colonic anti-inflammatory activity of prebiotics.
In this study, we investigated the anti-inflammatory effects of a newly synthesized oligosaccharide preparation, α-GOSg, on a colitis model and compared the activities with those of RFOs. The oligosaccharides were administered in drinking water as a dose of 0.5%, which approximates (calculated based on energy consumption of mice and humans) the recommended amount of 5g of α-galacto-oligosaccharides for human consumption by the China Food and Drug Administration (Approval No. G20040234). Acute colitis was induced with dextran sulfate sodium (DSS) in mice on a high-fat Western-style diet (HFWD). This HFWD was modified from the Western-style diet designed by Newmark et al (Newmark et al., 2001), which mimics dietary risk factors of western populations for colon cancer, by increasing the total fat content from 40% to 60% of total calorie. The diet contains a higher level of fat and lower levels of calcium, vitamin D3, choline, folate, and fiber than the normal AIN (e.g. AIN76A and AIN93M) diet. We have used the HFWD to induce metabolic syndrome in mice in several studies, and the composition of the diet has been published (Chen et al., 2011). This HFWD diet was used in this study because it was readily available (being used in other projects in our laboratory) and because it was expected to promote the DSS-induced colitis.
2 Materials and methods
2.1. Chemicals and diets
DSS (MW=36,000–50,000) was purchased from MP Biomedicals, LLC (Solon, OH, USA). High-fat Western-style diet (HFWD; 60% energy as fat; reduced levels of calcium, vitaminD3, choline, folate, and fiber) was prepared by Research Diets Inc. (New Brunswick, NJ, USA). The preparation of RFOs used in this study was extracted from soybean (containing 81.2% of raffinose, stachyose and verbascose, 16.5% of sucrose and 2.3% of ash) and was purchased from Nanjing Duolong Bio-tech Co., Ltd (Nanjing, China). α-GOSg were enzymatically synthesized as described below. α-Galactosidase was purchased from AMANO International Trading Co., Ltd (Shanghai, China). The structures of α-GOSg and RFOs are shown in Figure 1.
2.2. Preparation of α-galacto-oligosaccharides
The synthesis of α-GOSg was carried out with galactose as the substrate and α-galactosidase as the catalyst. Galactose was dissolved in distilled water by autoclaving at 121°C for 20 min (96%, w/v), and then transferred to an incubator at a reaction temperature of 60°C. The reaction was started by the addition of α-galactosidase (35 U/g-galactose). After incubation for 24 h, the reaction mixture was heated to 100°C for 10 min to inactivate the enzyme. The solution was then put onto an activated carbon column (2.5 × 20 cm). The column was eluted with 4% ethanol to wash off galactose, and then eluted with 20% ethanol. The fractions containing the reaction products (α-GOSg) were collected, concentrated, and freeze-dried to yield a white powder. The α-GOSg contained 84% Gal2 (with 52% of Gal-α-1-6-Gal and 32% of other α-galactoside linked disaccharide) and 16% Gal3 (with 7% of Gal- α-1-6-Gal-α-1-6-Gal and 9% of other α-galactoside linked trisaccharides) based on HPLC analysis (Supplementary Figure 1). Results on the MS and NMR characterizations of different peaks are shown in Supplementary Figures 2 and 3.
2.3. Animal treatments
Male C57BL/6J mice (8 weeks old, 20–22g) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were carried out under protocol 02-027, approved by the Institutional Animal Care and Use Committee at Rutgers University (Piscataway, NJ, USA). Mice were maintained in standard shoe-box cages in a controlled room (temperature 24 to 25°C, humidity 70%–75%, and a lighting regimen of 12-hour light-dark cycles), with free access to food and water. Twenty mice were randomly allocated into four groups (5 per group): negative control (NC), DSS, DSS + α-GOSg, and DSS + RFOs. They were all fed the HFWD for 14 days. In the α-GOSg and RFOs treated groups, mice were given α-GOSg or RFOs in 0.5% drinking water for all 14 days. Colitis was induced by administration of 1.5% (w/v) DSS in drinking water from day 8 to day 12 for 5 days. When DSS and oligosaccharides were both present in drinking water, no turbidity of the solution was observed. NC group only received water. During the experiment, body weight, solid fecal weight, and food and water consumption were measured daily. Mice were sacrificed by CO2 asphyxiation 48 h after cessation of DSS treatment. Blood was collected by cardiac puncture; serum was prepared and stored at −80°C. Liver was quickly removed and stored at −80°C. The colon was removed, its length measured (from cecum to anus), rinsed with cold PBS and cut longitudinally into two halves: one half was stored in RNAlater solution (Ambion, Austin, TX, USA) for RNA extraction and the other was stored in 10% formalin for histological evaluation.
2.4. Measurement of fecal hemoglobin content
Fecal samples were collected daily and the samples collected on Days 10, 11, 12 and 13 were analyzed for hemoglobin content. In brief, 25 mg fecal samples were freeze-dried, ground into powder and mixed with 0.5 ml water. After boiling for 10 min, the mixtures were placed on ice. Then, 3 ml of 30% acetic acid were added into the mixtures. The mixtures were vortexed for 2 min, and then 4.5 ml ethyl acetate was added and mixed by gently shaking for 2 min. After centrifugation, the clear supernatant (0.5 ml) was collected and mixed immediately with 0.5 ml TMB working solution (0.6 mmol/L 3,3′,5,5′-tetramethylbenzidine in a mixture of acetic acid/dH2O/ethanol = 20/30/50) and 0.5 ml 3% H2O2. The absorbance at 660 nm was measured. Standard hemoglobin (Sigma, St. Louis, MO, USA) was used to prepare 125, 62.5, 31.25, 15.06, 7.53, and 3.76 μg/ml solution for generating standard curves. The fecal hemoglobin content was based on the standard curve. The measurements were done in triplicates.
2.5. Histological characterization
The formalin-fixed colon was used to prepare a paraffin embedded Swiss-Roll and sectioned serially at 4-μm thickness to contain the entire Swiss-Roll. The colon tissue slides were stained with hematoxylin and eosin (H&E) for histological characterization. Two H&E stained sections per colon were used for histological evaluation and all slides in the entire experiment were evaluated in a blind manner. The colitis severity was determined by the colitis score – the sum of the scores of four individual colitis parameters: inflammation severity (0, none; 1, minimal; 2, moderate; 3, severe), extent of inflammation (0, none; 1, mucosal; 2, mucosal and submucosal), crypt damage (0, none; 1, one-third of crypt damaged; 2, two-thirds of crypt damaged; 3, crypts lost, surface epithelium intact; 4, crypts lost, surface epithelium lost), and percent area involvement (0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%). This scoring system is commonly used for evaluating DSS-induced colitis in mice (Wen, Wang, Yu, Zhao, Zhang, Matin, et al., 2014).
2.6. RNA extraction and real-time quantitative reverse transcription PCR
Total RNA of colon was extracted from the samples stored in RNAlater solution using the RNeasy Mini Kit (Qiagen Inc., Valencia, USA). The concentration of RNA was determined by absorbance at 260 nm, and the quality was evaluated by the integrity of 8S and 18S RNA in agarose electrophoreses. The values of all RNA samples at absorbance 260/280 ranged between 1.8–2.0, and the RNA electrophoresis showed two bright bands. The PCR reaction was initiated by a 5 min activation of hot-start DNA polymerase at 95°C followed by 49 cycles of target cDNA amplification. The template was initially denatured at 95°C for 15 seconds followed by a 49-cycle program with 45 seconds annealing at 65°C and 10 seconds elongation at 72°C and acquisition of SYBR Green fluorescence. The cDNA was prepared using the SuperScript First-Strand Synthesis System for RT-PCR Kit (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Real-time PCR was then carried out by using Power SYBR® Green PCR Master Mix kit (ABI Co., Ltd., Foster City, CA, USA) on ABI ViiA™ 7 system (ABI). Primers used for PCR are listed in Table 1. All specific primers have only one amplification. GAPDH was used as the internal control and the expression of each target gene was normalized by GAPDH level.
Table 1.
Primer Sequences Used in Real-Time PCR (RTqPCR) Assays in Colonic Tissue
| Gene | Sequence (5′-3′) | Annealing temperature (°C) |
|---|---|---|
| TNF-α | F:CCCTCACACTCAGATCATCTTCT R:GCTACGACGTGGGCTACAG |
60 |
| IL-1α | F:CTGTGACTCATGGGATGATGATG R:CGGAGCCTGTAGTGCAGTTG |
60 |
| IL-6 | F:TAGTCCTTCCTACCCCAATTTCC R:TTGGTCCTTAGCCACTCCTTC |
60 |
| COX-2 | F:AGGTCATTGGTGGAGAGGTG R:CCTGCTTGAGTATGTCGCAC |
60 |
| MCP-1 | F:GAGGACAGATGTGGTGGGTTT R:AGGAGTCAACTCAGCTTTCTCTT |
60 |
| CSF-1 | F:GGCTTGGCTTGGGATGATTCT R:GAGGGTCTGGCAGGTACTC |
60 |
| GAPDH | F:AGGTCGGTGTGAACGGATTTG R:TGTAGACCATGTAGTTGAGGTCA |
60 |
2.7. ELISA analysis of colon protein level
Frozen colon tissue samples were weighted and added into ice-cold Cell Extraction Solution (the lysis buffer in mouse COX-2 ELISA kit; Abcam, Cambridge, MA, USA) at 0.1g/ml ratio. The tissue samples were then lysed using Omni Bead Raptor 24 (Omni International, Kennesaw, GA) at lower temperature. The supernatants were collected after the tissue lysis was centrifuged at 12,000 × g for 10 min at 4°C and stored at −20°C. Protein concentrations of these samples were determined by BCA protein assay kit (Thermo Scientific, Waltham, MA, USA). The levels of cyclooxygenase (COX-2) and colony stimulating factor (CSF-1) in these samples were determined using Mouse COX-2 and Mouse CSF-1 ELISA kits (Abcam) according to the manufacturer’s protocols. The values of COX-2 and CSF-1 were normalized by the protein concentration.
2.8. Immunohistochemical staining
Immunohistochemical staining was used to characterize the activation of nuclear factor-kappa B (NF-κB) in colon tissues. In brief, two tissue sections (~40 μm apart) were deparaffinated by xylene and rehydrated by a series of ethanol solutions. Then, tissue slides were boiled in antigen unmasking solution (DAKO, Copenhagen, Denmark) in a microwave oven for 6 min to unmask antigens. Endogenous peroxidase activity was quenched by 3% H2O2. Sections were then incubated with 10% normal serum for 1 h at room temperature, followed by incubation with the primary antibody (rabbit monoclonal anti-NF-κB p65, 1:2000; Abcam) overnight at 4°C. After washing, the tissue sections were incubated with the prediluted horseradish peroxidase-conjugated secondary antibody (1:200 in 10% normal serum) for 45 min at room temperature, followed by incubation with avidin-biotinylated enzyme complex (Vector Laboratories, Burlingame, CA, USA) for 50 min. Slides were developed with 3, 3′-diaminobenzidine solution (Vector Laboratories). Finally, slides were counterstained with hematoxylin and mounted with Permount (Sigma-Aldrich, St. Louis, MO, USA). For negative controls, 1% of non-immune serum in phosphate-buffered saline (PBS) replaced the primary antibodies. The immunohistochemistry staining of NF-κB p65 was quantified for the entire slide (cut from the Swiss Roll of the colon) using Aperio ScanScope GL system (Aperio, Vista, CA, USA). The results are presented as the percentage of the cells with positive stained nucleus.
2.9. Statistical analysis
Statistical analysis was performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± SD. One-way ANOVA followed by Tukey’s post hoc test was used to compare multiple groups. Student t-test was used to determine the differences in two groups. Differences were considered statistically significant when P < 0.05.
3 Results
3.1. Food and water consumption
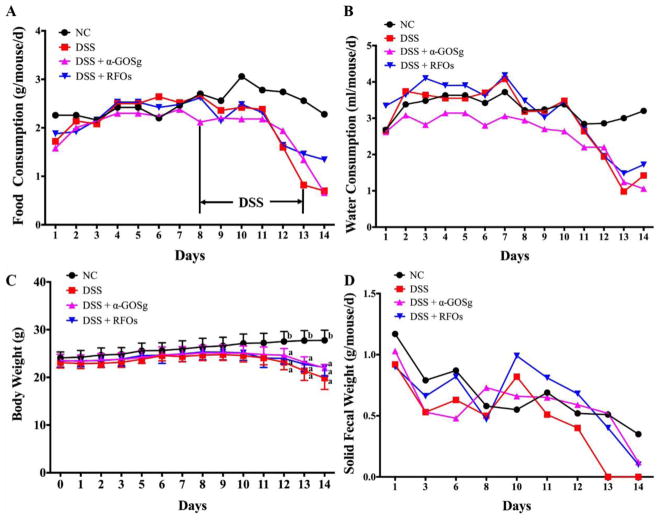
It has been reported that DSS treatment causes anorexia in rats (Mercier, Breuille, Mosoni, Obled, & Patureau Mirand, 2002). In the DSS-control mice, food intake was reduced during the five days of treatment with DSS and the three days of recovery (Figure 2A). During the five days of DSS treatment, the mean liquid consumption of each group (NC, DSS, DSS + α-GOSg or DSS + RFOs) were 3.11, 2.89, 2.54 and 2.92 ml/d/mouse, respectively (Figure 2B). However, significant difference among groups in the mean water consumption of days 8–12 was not shown by ANOVA. It was noted that α-GOSg significantly decreased water consumption before the DSS treatment, based on the average water consumption of different groups for the first 7 days.
Figure 2.
Food and water consumption and effects of treatments on body weight and solid fecal weight. Food consumption (A) and water consumption (B) were measured daily. The mean ± SD (n=7) of water consumption of the first 7 days for groups NC, DSS, DSS + α-GOSg and DSS + RFOs were 3.42 ± 0.24a,b, 3.56 ±0.44b, 2.95 ± 0.20a and 3.81 ± 0.30b ml/mouse/day, respectively. a,b represent significant differences among groups by ANOVA test (P < 0.05). Body weights were measured every day at 9:00 a.m. (C). Solid fecal samples were collected for 24 h daily and weighted at the time of sample collection (D). DSS treatment started at 9:00 a.m. on day 8 and ended at 9:00 a.m. on day 13. Body weights are shown as mean ± SD (n=5). Other parameters were calculated from the daily measurement for each cage (5 mice). * represent significant differences among DSS treated groups and NC group by ANOVA test (P < 0.05). The body weight started to show significant differences from day 12.
3.2. Body weight and fecal weight
Body weights of mice are shown in Figure 2C. The average body weight of each group was similar at the start. After treatment with DSS for three days, the body weight of the DSS-treated groups started to drop. At the end of the experiment, there was a significant difference between the NC and DSS groups (27.8 ± 2.1 g versus 19.9 ± 2.4, 22.0 ± 0.7, 22.1 ± 1.8 g, P < 0.05), but there was no significant difference among the three DSS-treated groups. Bloody stools were found from the mice of DSS-treated groups on the third day of DSS treatment. There was also much less solid fecal mass in the three DSS-treated groups (Figure 2D). After treatment with DSS for five days, there was severe diarrhea with bloody stools in mice in all three groups.
3.3. Effects on fecal hemoglobin levels
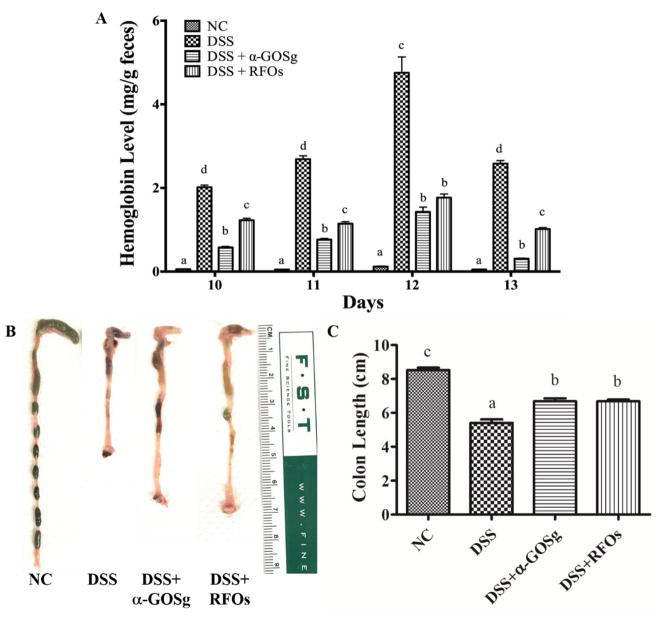
There was no fecal hemoglobin found in the NC group and the fecal hemoglobin contents in the DSS group were the highest among all groups (Figure 3A). The mean fecal hemoglobin contents on days 10, 11, 12 and 13 of the group treated with α-GOSg or RFOs were lower than the group treated with DSS alone. The mean ± SD (n=4) of the fecal hemoglobin levels of group NC, DSS, DSS + α-GOSg and DSS + RFOs were 0.07 ± 0.03a, 3.01 ± 1.20b, 0.77 ± 0.48a and 1.29 ± 0.33a, respectively (a,b represent significant differences among groups, P < 0.05 by ANOVA), suggesting a protective effect against intestinal bleeding.
Figure 3.
Effects of different treatments on fecal hemoglobin content and colon length. A. Hemoglobin levels were determined in solid fecal samples collected on different days. D10, D11 and D12 were the third, fourth and fifth day of DSS treatment and D13 was the first day after stopping DSS. The value is the mean of a triplicated determination of the one fecal sample collected daily from each cage. For days 10, 11, 12 and 13, the mean ± SD (n=4) of the fecal hemoglobin levels of groups NC, DSS, DSS + α-GOSg and DSS + RFOs were 0.07 ± 0.03a, 3.01 ± 1.20b, 0.77 ± 0.48a and 1.29 ± 0.33a, respectively (a,b represent significant differences among groups, P < 0.05 by ANOVA followed by Tukey’s post hoc test). B. photos of colon length (one representative colon from each group). C. Colon length of each group, the length of each group (mean ± SD, n=5). a,b,c represent significant differences among different groups by ANOVA test (P < 0.05).
3.4. Colon length shortening
Visually, the colons of the NC group were normal with fecal pellets inside, but there were little or no pellets inside of the colons of the DSS group, due to diarrhea (Figure 3B). In the two groups treated with α-GOSg or RFOs, there were shaped fecal pellets in the colon. The colon length from the cecum to the anus of each mouse was measured. Compared to the NC group, the colon of the DSS group was markedly shortened (5.40 ± 0.47 versus 8.52 ± 0.31 cm, P < 0.05). Treatment with α-GOSg and RFOs partially, but significantly, prevented the shortening (P < 0.05), suggesting a protective effect.
3.5. Effects on histopathology and colonic expression of inflammatory factors
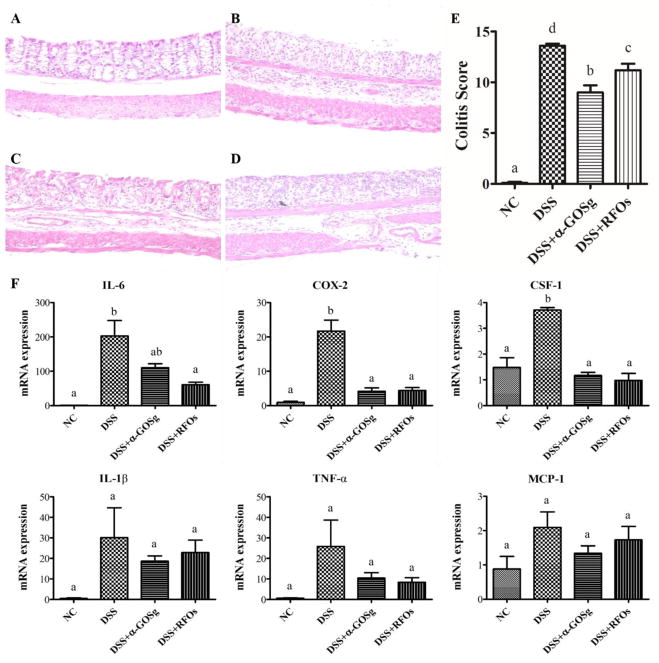
Histopathological changes in colitis were analyzed and scored in colon tissues collected three days after termination of DSS treatment. Representative H&E stained histological sections and histopathology scores are shown in Figure 4. The colon of NC mice showed intact epithelium, well-organized crypt, no neutrophil infiltration in mucosa and submucosa, and no ulceration or erosion. In contrast, the colon tissues from the DSS group showed severe inflammatory lesions, characterized by complete loss of crypts, surface erosion and ulceration, and acute transmural inflammatory infiltrates. For mice treated with oligosaccharides, there was a significant reduction in inflammation and injury. Colon tissues from the DSS + α-GOSg group showed mild inflammation and colon mucosa that had tightly packed glands with a normal amount of goblet cells. In the DSS + RFOs group, colon tissue had moderately severe inflammation in mucosa and two-thirds of the crypts were damaged, but the surface epithelium remained intact. Based on the colitis score, both α-GOSg and RFOs treatments significantly decreased (P < 0.05) the severity of inflammation and prevented damage of the crypts, with α-GOSg showing a stronger effect. Colon inflammation was also assessed by the levels of pro-inflammatory genes (TNF-α, IL-1β, IL-6, COX-2, MCP-1 and CSF-1) determined by qPCR (Figure 4). Mice from the DSS group showed upregulation of these pro-inflammatory genes. Treatment with α-GOSg and RFOs significantly suppressed the DSS-induced upregulation of COX-2 and CSF-1. IL-6 was only reduced by RFOs. The DSS-induced upregulation of IL-1β, MCP-1 and TNF-α appeared to be reduced by α-GOSg and RFOs, but the reduction was not statistically significant.
Figure 4.
Effects of treatments on histopathological changes and colonic expression of inflammatory factors (IL-6, COX-2, CSF-1, IL-1β, TNF-α, MCP-1). Histological sections of colonic tissue stained with hematoxylin and eosin are shown: (A) NC group; (B) DSS group; (C) DSS + α-GOSg group; (D) DSS + RFOs; (E) colitis score of each group (mean ± SD, n=10); (F) the data of gene expression expressed as mean ± SD (n=5). a–d represent significant differences among different groups by ANOVA test (P < 0.05).
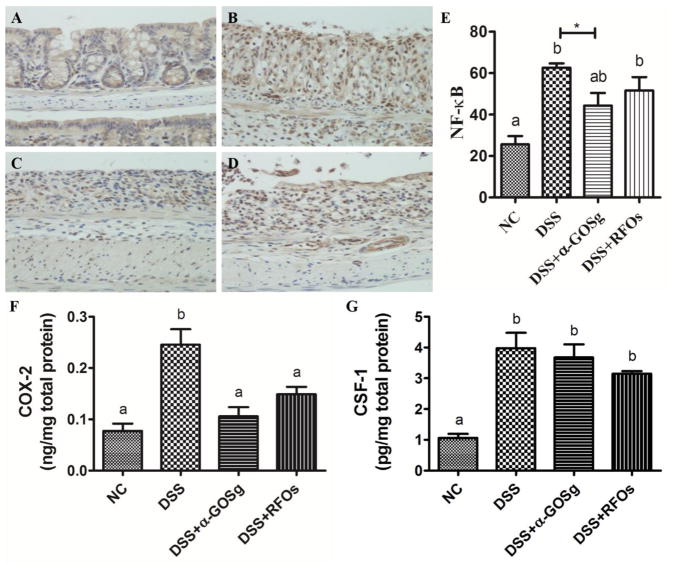
3.6. Effects on nuclear levels of NF-κB p65 and protein expression of COX-2 and CSF-1
The activation of NF-κB was assessed by the nuclear levels of NF-κB p65 as determined by immunohistochemistry. Most of the positive NF-κB p65 staining was observed in cytoplasm in the NC group. In the DSS group, a significantly higher level of positive NF-κB p65 was found in the nucleus (Figure 5). α-GOSg and RFOs treatments appeared to reduce the DSS-induced nuclear accumulation of NF-κB p65. However, a significant difference was only observed in the DSS + α-GOSg group by t-test (P < 0.05). Colonic protein levels of COX-2 and CSF-1 were determined by ELISA (Figure 5F,G). The DSS-induced overexpression of COX-2 was significantly decreased by α-GOSg and RFOs (P < 0.05), which is consistent with results on gene expression. The slight decrease of CSF-1 protein level by α-GOSg and RFOs was not statistically significant.
Figure 5.
Effects of treatments on levels of NF-κB p65 by immunohistochemical analysis (A–E) as well as COX-2 (F) and CSF-1 (G) expression determined by ELISA in DSS-treated mice. For immunohistochemical staining: (A) NC group; (B) DSS group; (C) DSS + α-GOSg group; (D) DSS + RFOs; (E) Positive staining was scored (using Aperio ScanScope GL system and expressed as a percentage of positive stained cells/total cells (mean ± SD, n=10). The protein levels of COX-2 and CSF-1 determined by ELISA were expressed as mean ± SD (n=10). a–b represent significant differences between different groups by ANOVA test (P < 0.05). * represent significant differences between DSS group and DSS + α-GOSg by student’s t-test (P < 0.05). ANOVA showed no significant difference among DSS treated groups.
4 Discussion
In this study, acute mucosal injury was induced by administration of 1.5% DSS in drinking water in mice on the HFWD. Symptoms of diarrhea, body weight loss, hematochezia, and colon length shortening were observed in DSS-treated mice. Colon histopathology of mice from the DSS group showed crypt abscess, mucosal erosion and mucosal inflammatory cell infiltration. The results were consistent with other reports (Park et al., 2015; Sann, Erichsen, Hessmann, Pahl, & Hoffmeyer, 2013). All these changes were ameliorated by α-GOSg and RFOs, reflecting alleviation of colitis. In addition, α-GOSg and RFOs also significantly suppressed the DSS-induced expression of some pro-inflammatory factors: COX-2, IL-6 or CSF-1.
In our work, a semi-synthetic modified AIN type diet was used and colitis was induced by a rather low concentration of DSS (1.5%); whereas in many previous reports much higher concentrations (2 – 3%) of DSS were used (Kverka, Rossmann et al., 2011; Okayasu, Hatakeyama, Yamada, Ohkusa, Inagaki, & Nakaya, 1990). This difference could be due to the fact that the latter studies used “chow diets”, which contained phytochemicals that induce aryl hydrocarbon receptor and prevent colonic inflammation (Li et al., 2011). In theory, the high-fat and low-calcium/vitamin D/folate/choline status of the HFWD should contribute to colon inflammation (Dolan & Chang, 2017; Newmark et al., 2001). In this experiment, it took five days of 1.5% DSS treatment to produce colitis. In other studies in our laboratory with C57BL/6J mice on the AIN93 M diet (10% calories from fat), it took 10 days to produce less severe colitis by 1.5% DSS (Yang, C.S. et al., unpublished results). However, the possible synergistic effect of DSS and HFWD in the induction of colitis needs to be substantiated by direct comparisons in the same experiment.
In the present work, we demonstrate the anti-inflammatory effects of α-GOSg and RFOs. α-GOSg was more effective than RFOs in decreasing the histological score of colitis and fecal hemoglobin levels. However, α-GOSg also contained more oligosaccharides than RFOs (containing 14.5% sucrose). In structure, α-GOSg are only composed of galactose, whereas RFOs have a sucrose molecule as a terminal. It remains to be investigated whether this structural difference translates to difference in biological activity. Similar beneficial effects of other oligosaccharides in attenuating colitis have also been reported. In a DSS-induced colitis model, administration of fructo-oligosaccharides reduced disease activity index, colonic crypt loss and histological damage in mice (Winkler, Butler, & Symonds, 2007). However, in another experiment, fructo-oligosaccharides did not reduce DSS-induced colitis, whereas resistant starch did (Moreau et al., 2003). A reduction of inflammation in the DSS model was also shown with goat milk oligosaccharides (Lara-Villoslada et al., 2006).
Many pro-inflammatory cytokines are transcriptionally regulated by NF-κB, and their increased expression has been implicated in the pathogenesis of IBD (Lee et al., 2006). NF-κB can be activated by a variety of inducers (e.g., cytokines), which activate the IκB kinase complex (IKK). Once activated, the NF-κB complex translocates to the nucleus to activate many pro-inflammatory cytokines and enzymes (Lawrence, 2009). In IBD, NF-κB promotes the expression of many pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, as well as pro-inflammatory mediators, such as COX-2 and nitric oxide synthase (iNOS) (Tak & Firestein, 2001). In the present study, the activation of NF-κB and related gene expression by DSS was clearly demonstrated. Of these downstream factors, however, only COX-2 expression was significantly decreased at the mRNA and protein levels by α-GOSg and RFOs. COX-2, an enzyme that catalyzes the transformation of arachidonic acid to pro-inflammatory PGE2, plays an important role in the inflammatory process. The NF-κB p65 level was only slightly suppressed by α-GOSg (p < 0.05 by t-test only), but not RFOs. CSF-1 is a hematopoietic growth factor involved in inflammation and the proliferation, differentiation and survival of monocytes, macrophages and bone marrow progenitor cells (Marshall, Cameron, Lightwood, & Lawson, 2007). However, CSF-1 expression was decreased only at the mRNA level. The molecular mechanisms for the suppression of CSF-1 by α-GOSg and RFOs are not known. It could be a consequence of the anti-inflammatory action of these compounds.
The precise molecular mechanisms of the presently observed anti-inflammatory actions of α-GOSg and RFOs are unclear. One possibility is that α-GOSg and RFOs directly interact with epithelial or immune cells. Pretreatment of rats with goat milk oligosaccharides, lactulose-oligosaccharides or fructose-oligosaccharides has been shown to significantly ameliorate intestinal inflammatory process (Koleva, Valcheva, Sun, Ganzle, & Dieleman, 2012; Lara-Villoslada et al., 2006). Human milk oligosaccharides and bovine colostrum oligosaccharides have shown effects on gene expression of colonic epithelial cells (Lane, O’Callaghan, Carrington, & Hickey, 2013). Chitosan oligosaccharides have shown to suppress inducible iNOS and nuclear translocation of NF-κB p65 in RAW264.7 cells (Kim et al., 2014).
Another possibility is through their prebiotic activities. In a similar study with HFWD on fecal microbiota, after 13 weeks of feeding, the abundance of bifidobacteria was lower (as compared to mice fed a low-fat diet), and it was significantly increased in mice that also received α-GOSg, but not RFOs (Dai et al., unpublished data). On the other hand, the abundance of the Clostridium leptum group was decreased by either α-GOSg or RFOs. The dietary treatments did not significantly affect the abundance of Bacteroids, Lactobacilli and Eubacterium rectale/Clostridium coccoides group (Dai et al., unpublished data). In other studies, the protective effect of oligosaccharides was associated with increased production of organic acids and luminal microbiota balance (Algieri et al., 2014). The metabolic products of gut microbial may lead to the translocation of NF-κB into the nucleus to activate the expression of cytokines, chemokines and other effectors, which play an important role in supporting epithelial function (Wells, Rossi, Meijerink, & van Baarlen, 2011). The enhanced abundance of bifidobacterium and lactobacillus by galacto-oligosaccharides and RFOs in the colon have also been demonstrated (Sangwan, Tomar, Singh, Singh, & Ali, 2011; Vulevic, Juric, Tzortzis, & Gibson, 2013). In the present short-term experiment, it is unclear whether and when the changes in microbiota play a significant role.
5 Conclusion
In conclusion, we demonstrated that oral administration of α-GOSg and RFOs exerted significant protective effects against DSS-induced colitis. The beneficial effects of α-GOSg and RFOs are associated with the suppression of COX-2 expression and possibly other NF-κB related pro-inflammatory genes. The newly synthesized α-GOSg has comparable activation to RFOs. More studies are warranted to further investigate the potential beneficial health effects of α-GOSg in humans.
Supplementary Material
Highlights.
Newly synthesized α-galacto-oligosaccharides (α-GOSg) alleviates colitis in mice.
α-GOSg has comparable activities than raffinose family oligosaccharides.
Anti-inflammatory effects of α-GOSg are associated with suppression of COX-2.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (CA120915 and shared facilities funded by CA72720 and ES05022), the John L. Colaizzi Chair Endowment fund, Grants-in-Aid for scientific research from the National Natural Science Foundation of China (31171750), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors thank the personnel of Laboratory Animal Service for taking care of our research mice, and thank Ms. Vi P. Dan for assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algieri F, Rodriguez-Nogales A, Garrido-Mesa N, Vezza T, Garrido-Mesa J, Utrilla MP, Montilla A, Cardelle-Cobas A, Olano A, Corzo N, Guerra-Hernandez E, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. Intestinal anti-inflammatory effects of oligosaccharides derived from lactulose in the trinitrobenzenesulfonic acid model of rat colitis. Journal of Agricultural & Food Chemistry. 2014;62(19):4285–4297. doi: 10.1021/jf500678p. [DOI] [PubMed] [Google Scholar]
- Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;301(6):G1004–1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. Journal of Agricultural and Food Chemistry. 2011;59(21):11862–11871. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Su D, Zhang Y, Sun Y, Hu B, Ye H, Jabbar S, Zeng X. Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus) Journal of Agricultural and Food Chemistry. 2014;62(44):10727–10735. doi: 10.1021/jf503510h. [DOI] [PubMed] [Google Scholar]
- Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Molecular Nutrition & Food Research. 2017;61(1) doi: 10.1002/mnfr.201600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T, Izumi H, Tsuda M, Nakazato Y, Iwamoto H, Namba K, Takeda Y. Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice. British Journal of Nutrition. 2016;116(2):270–278. doi: 10.1017/S0007114516001987. [DOI] [PubMed] [Google Scholar]
- Jonkers D, Penders J, Masclee A, Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72(6):803–823. doi: 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim YS, Hwang JW, Han YK, Lee JS, Kim SK, Jeon YJ, Moon SH, Jeon BT, Bahk YY, Park PJ. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-kappaB inactivation. Molecules. 2014;19(11):18232–18247. doi: 10.3390/molecules191118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva PT, Valcheva RS, Sun X, Ganzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. British Journal of Nutrition. 2012;108(9):1633–1643. doi: 10.1017/S0007114511007203. [DOI] [PubMed] [Google Scholar]
- Kuo TM, Vanmiddlesworth JF, Wolf WJ. Content of Raffinose Oligosaccharides and Sucrose in Various Plant Seeds. Journal of Agricultural and Food Chemistry. 1988;36(1):32–36. [Google Scholar]
- Kverka M, Rossmann P, Tlaskalova-Hogenova H, Klimesova K, Jharap B, de Boer NK, Vos RM, van Bodegraven AA, Lukas M, Mulder CJ. Safety and efficacy of the immunosuppressive agent 6-tioguanine in murine model of acute and chronic colitis. BMC Gastroenterol. 2011;11:47. doi: 10.1186/1471-230X-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JA, O’Callaghan J, Carrington SD, Hickey RM. Transcriptional response of HT-29 intestinal epithelial cells to human and bovine milk oligosaccharides. British Journal of Nutrition. 2013;110(12):2127–2137. doi: 10.1017/S0007114513001591. [DOI] [PubMed] [Google Scholar]
- Lara-Villoslada F, Debras E, Nieto A, Concha A, Gálvez J, López-Huertas E, Boza J, Obled C, Xaus J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clinical Nutrition. 2006;25(3):477–488. doi: 10.1016/j.clnu.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nature Cell Biology. 2006;8(12):1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Li W, Sun Y, Ye H, Zeng X. Synthesis of oligosaccharides with lactose and N-acetylglucosamine as substrates by using β-d-galactosidase from Bacillus circulans. European Food Research and Technology. 2010;231(1):55–63. [Google Scholar]
- Li W, Wang K, Sun Y, Ye H, Hu B, Zeng X. Influences of structures of galactooligosaccharides and fructooligosaccharides on the fermentation in vitro by human intestinal microbiota. Journal of Functional Foods. 2015;13:158–168. [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Marshall D, Cameron J, Lightwood D, Lawson AD. Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflammatory Bowel Disease. 2007;13(2):219–224. doi: 10.1002/ibd.20055. [DOI] [PubMed] [Google Scholar]
- Mercier S, Breuille D, Mosoni L, Obled C, Patureau Mirand P. Chronic inflammation alters protein metabolism in several organs of adult rats. Journal of Nutrition. 2002;132(7):1921–1928. doi: 10.1093/jn/132.7.1921. [DOI] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Champ MM. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. British Journal of Nutrition. 2003;90(1):75–85. doi: 10.1079/bjn2003867. [DOI] [PubMed] [Google Scholar]
- Morel FB, Dai Q, Ni J, Thomas D, Parnet P, Fanca-Berthon P. alpha-Galacto-oligosaccharides dose-dependently reduce appetite and decrease inflammation in overweight adults. The Journal of Nutrition. 2015;145(9):2052–2059. doi: 10.3945/jn.114.204909. [DOI] [PubMed] [Google Scholar]
- Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22(11):1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Park YH, Kim N, Shim YK, Choi YJ, Nam RH, Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, Yoon H, Lee HS, Lee DH. Adequate Dextran Sodium Sulfate-induced Colitis Model in Mice and Effective Outcome Measurement Method. Journal of Cancer Prevention. 2015;20(4):260–267. doi: 10.15430/JCP.2015.20.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Current Opinion in Biotechnology. 2015;32:42–46. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Reiff C, Kelly D. The role of probiotics in inflammatory bowel disease. International Journal of Medical Microbiology. 2010;300(1):25–33. doi: 10.1016/j.ijmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Sangwan V, Tomar SK, Singh RR, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. Journal of Food Science. 2011;76(4):R103–111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Science. 2013;92(12):708–718. doi: 10.1016/j.lfs.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Dore J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflammatory Bowel Diseases. 2006;12(2):106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- Steed H, Macfarlane GT, Macfarlane S. Prebiotics, synbiotics and inflammatory bowel disease. Molecular Nutrition & Food Research. 2008;52(8):898–905. doi: 10.1002/mnfr.200700139. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. Journal of Clinical Investigation. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DPM, Gonçalves MdPF, Teixeira JA, Rodrigues LR. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Comprehensive Reviews in Food Science and Food Safety. 2010;9(5):438–454. doi: 10.1111/j.1541-4337.2010.00119.x. [DOI] [PubMed] [Google Scholar]
- Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of Nutrition. 2013;143(3):324–331. doi: 10.3945/jn.112.166132. [DOI] [PubMed] [Google Scholar]
- Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD, McCabe GP, Duignan S, Schoterman MH, van den Heuvel EG. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. Journal of Agricultural and Food Chemistry. 2011;59(12):6501–6510. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proceedings of The National Academy of Sciences of The United States Of America. 2011;108:4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XD, Wang CZ, Yu C, Zhao L, Zhang Z, Matin A, Wang Y, Li P, Xiao SY, Du W, He TC, Yuan CS. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytotherapy Research. 2014;28(6):892–898. doi: 10.1002/ptr.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Butler R, Symonds E. Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis. Digestive diseases and sciences. 2007;52(1):52–58. doi: 10.1007/s10620-006-9224-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.