Abstract
Background and aims:
Recent studies have shown that etomidate is associated with fewer serious adverse events than propofol and has a noninferior sedative effect. We investigated whether etomidate–midazolam is associated with fewer cardiopulmonary adverse events and has noninferior efficacy compared to propofol–midazolam for screening colonoscopy in the elderly.
Methods:
A prospective, single-center, double-blinded, randomized controlled trial was performed. Patients aged over 65 years who were scheduled to undergo screening colonoscopy were randomized to receive either etomidate or propofol based on midazolam. The primary outcome was all cardiopulmonary adverse events. The secondary outcomes were vital sign fluctuation (VSF), adverse events disturbing the procedure, and sedation-related outcomes.
Results:
The incidence of cardiopulmonary adverse events was higher in the propofol group (72.6%) than in the etomidate group (54.8%) (P = .040). VSF was detected in 17 (27.4%) and 31 (50.0%) patients in the etomidate and propofol groups, respectively (P = .010). The incidence rate of adverse events disturbing the procedure was significantly higher in the etomidate group (25.8%) than in the propofol group (8.1%) (P = .008). Moreover, the incidence rate of myoclonus was significantly higher in the etomidate group (16.1%) than in the propofol group (1.6%) (P = .004). There was no statistical significance between the 2 groups with respect to sedation times and sedation-related outcomes including patients’ and endoscopist's satisfaction. In the multivariate analysis, the etomidate group had significantly low odds ratio (OR) associated with VSF (OR: 0.407, confidence interval: 0.179–0.926, P = .032).
Conclusions:
We recommend using etomidate–midazolam in patients with high ASA score or vulnerable to risk factors; propofol–midazolam may be used as a guideline in patients with low ASA score.
Keywords: colonoscopy, elderly, etomidate, midazolam, sedation
1. Introduction
Endoscopic sedation is widely used to relieve patients’ anxiety and discomfort.[1–3] One of the most common sedatives is propofol owing to its convenience and fast effect.[4,5] However, propofol is associated with several serious adverse events including hypoxia, hypotension, arrhythmia, and respiratory depression.[6–8]
Etomidate is a nonbarbiturate sedative with rapid onset (5–15 seconds) and recovery (5–15 minutes).[9,10] Because it does not inhibit the sympathetic tone or myocardial function, it is used for critically ill patients or patients with cardiovascular diseases.[10,11] In addition, it can be used in patients with bronchospasms or those who cannot have hypotension because of intracranial problems.[12,13] It is also known that histamine release, apnea, and allergic reactions are less commonly associated with barbiturate or propofol use.[11] Recently, etomidate has been reported to be noninferior in stability and efficacy during endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) compared to propofol.[14,15] Cardiopulmonary adverse events such as hypotension, hypoxia, arrhythmias, and aspiration were more common in the elderly aged over 65 years than in young, healthy patients.[16,17] However, there were only a few studies on etomidate for screening endoscopy in the elderly.
In this study, we compared etomidate and propofol groups based on midazolam in the elderly for screening colonoscopy with respect to cardiopulmonary adverse events. Our aim was to evaluate the safety and efficacy of etomidate–midazolam for screening colonoscopy in the elderly.
2. Methods
2.1. Study design
A single-center, prospective, double-blinded, randomized controlled trial was performed from November 2017 to January 2018 at the Department of Gastroenterology and Digestive Endoscopy at Korea University Anam Hospital (Seoul, Korea). At the time of registration, subjects were randomly allocated to either group. They were randomized using a computer-generated list and were provided with written instructions. All patients provided written informed consent. This study was approved by the International Clinical Trials Registry Platform (KCT0002638).
2.2. Patients
All patients aged over 65 years old with American Society of Anesthesiologists (ASA) scores from I to III who were scheduled to undergo screening colonoscopy and/or gastroscopy were included in this study. Patients were excluded if they had a known or suspected history of adverse events with previous sedation; had known hypersensitivity to egg products, soy beans, etomidate, and propofol; had known adrenocortical insufficiency, or porphyria or received chronic corticoid therapy were pregnant or breastfeeding; desired to undergo endoscopy without sedation; and could not provide informed consent. If there was hemodynamic instability (systolic blood pressure [SBP] < 90 mm Hg or peripheral oxygen saturation [SpO2] of 90% on room air or <95% on 2 L/min oxygen) at baseline measurement before the procedure, the patient was withdrawn from the study.
2.3. Protocol
All procedures were performed by one experienced faculty level endoscopist (ESK). Two well-trained nurses who were trained in advanced cardiac life support and completed a training course operated by the Korean Society of Gastrointestinal Endoscopy participated in all procedures.
All patients were monitored by the endoscopist and 2 nurses for non-invasive blood pressure, SpO2, electrocardiographic recordings, and respiratory activity before and during endoscopy. They received nasal oxygen therapy at a rate of 2 L/min during the procedure according to the sedation guideline.[16,18] One nurse assisted with the procedure and another nurse checked and recorded the vital signs and patient status while injecting the sedative. Non-invasive blood pressure was automatically measured at every 5 minutes.
We used 70% of the usual dose considering the elderly.[16] In both groups, 0.035 mg/kg intravenous midazolam was initially administered. In the etomidate group, 0.07 mg/kg (0.035 mL/kg) bolus injection of etomidate (Etomidate Lipuro, 20 mg/10 mL/A, B. Braun Korea, Seoul, Korea) was administered. After that, titration with 0.035 mg/kg (0.018 mL/kg) etomidate was performed while assessing the patients’ consciousness if the Modified Observer's Assessment of Alertness/Sedation (MOAA/S) scale score was 0 to 2.[19,20] In the propofol group, 0.35 mg/kg (0.035 mL/kg) bolus injection of propofol (Freefol-MCT, 120 mg/12 mL, Daewon Pharm. Co., Ltd., Seoul, Korea) was initially administered. As with etomidate, if the MOAA/S scale score was > 3 to maintain appropriate sedation, additional injections were required after at least 60 s of observation. The MOAA/S scale score ranges from 0 to 5 (0 = general anesthesia and 5 = fully awake state),[19] and the adequate target range of the MOAA/S score is less than 3 (patient responds after his or her name is loudly or repeatedly called) during endoscopy.[14,19]
2.4. Assessment of patient safety and adverse events
To reduce deviation between endoscopists, one experienced faculty-level endoscopist performed all endoscopic procedures and used a conventional endoscope (Olympus 290, Olympus Corporation, Tokyo, Japan). In patients without glaucoma or benign prostate hyperplasia who underwent colonoscopy, 5 mg of cimetropium bromide (Bropium, 5 mg/1 mL, Bukwang Pharm Co., Ltd., Seoul, Korea) was administered at the start of colonoscopy to reduce colonic motor response. One nurse checked the baseline vital signs before the start of the procedure and injected sedatives after the endoscopist confirmed the Mallampati class.[21] When the patient had a MOAA/S scale score below 2, the vital signs were rechecked and, thereafter, were recorded every 5 minutes. Both the induction time and total procedure time were recorded by the endoscopist. The 2 nurses and endoscopist recorded the adverse events during the procedure, as well as the duration, onset time, and severity of myoclonus. After the procedure, the endoscopist recorded the physicians’ satisfaction, sedation level during the procedure, and any adverse events. After the patient was sent to the recovery room, the observer other than the nurses or endoscopist recorded the patients’ satisfaction, frequency of recall, abdominal pain, and post-procedural nausea or vomiting.
2.5. Study endpoint and definitions
The primary outcome was all cardiopulmonary adverse events including tachycardia, bradycardia, hypertension, transient hypotension, respiratory depression, oxygen desaturation, and fatal arrhythmia. The secondary outcomes were the following: vital signs fluctuation (VSF), which was defined as transient hypotension (SBP < 90 mm Hg or at least 20 mm Hg less than the baseline value, even at least once during the procedure) and oxygen desaturation (SpO2 < 90% on room air or < 95% on 2L/min oxygen) [22]; adverse events disturbing the procedure such as belching, severe coughing, needs for restraint, and myoclonus; and sedation-related outcomes including induction time, total procedure time, awake time, patients’ and endoscopist's satisfaction scores, and frequency of recall.
We defined a major adverse event as endotracheal intubation, permanent neurological impairment, and death.[14,15] Cardiovascular events included tachycardia (heart rate [HR] > 110 beats/min), bradycardia (HR < 50 beats/min), and fatal arrhythmia (sustained ventricular tachycardia or ventricular fibrillation). Respiratory depression was defined as the need for efforts to secure the airway by chin lift and jaw thrust
In addition, we defined induction time as the time interval from sedative injection to endoscope insertion. Total procedure time was defined as the time interval from endoscope insertion to endoscopic removal. Awake time was defined as the time interval from endoscope removal to full-recovery of the patient (Aldrete score of 10).[23] Moreover, total sedation time was defined as the time interval from administration of sedatives to full recovery of the patient.[14]
2.6. Statistical analysis
To calculate the sample size, cardiopulmonary adverse events according to the presence of etomidate should be first determined using the PASS 12 (NCSS software, Utah) program and their proportions was 28% using the results of Riphaus et al.[24] When the power was set at 80% to detect a moderate effect size (20% of absolute difference between the 2 groups) at an alpha level of 0.05, 57 patients per group were necessary, and the final sample size of 62 patients per group fulfilled the condition as 62 patients per group considering 10% drop-out rate.
Continuous variables were expressed as mean ± standard deviation whereas discontinuous variables were expressed as counts and percentages. SPSS 24.0 for Windows (SPSS Inc., Chicago, IL) was used for data entry and statistical analyses. For the analyses between the 2 treatment groups, Student's t-test was used as appropriate to compare the continuous variables, and the chi-square or Fisher's exact test was used for categorical data. Binary logistic regression tests were used for multivariate analysis. P-values < .05 were considered statistically significant.
3. Results
3.1. Demographic and baseline characteristics
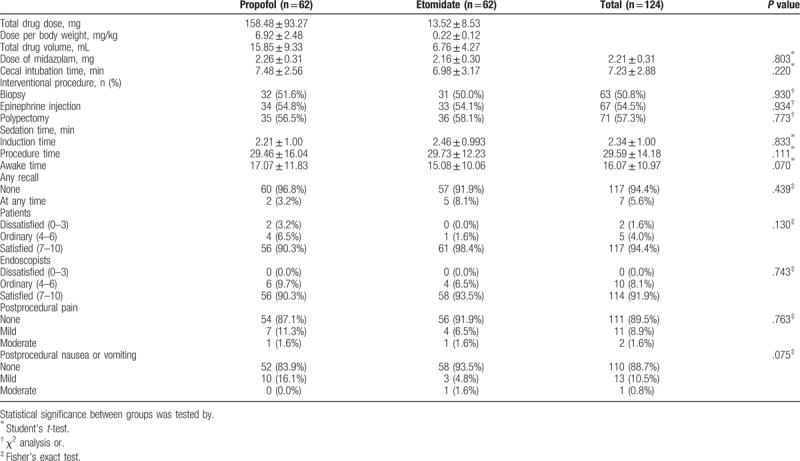
Initially, 130 patients aged over 65 years were assessed. Among them, six patients were excluded because of adrenocortical insufficiency (n = 1), desire to undergo endoscopy without sedation (n = 3), hypersensitivity to the drug (n = 1), or previous history of adverse events with sedation (n = 1) (Fig. 1). Finally, a total of 124 patients aged over 65 years were enrolled, analyzed, and randomly assigned to 2 groups: propofol group (n = 62) and etomidate group (n = 62). The baseline characteristics including age, sex, procedure type, body mass index, smoking and alcohol history, outpatient status, anticoagulant use, ASA score, modified Mallampati score, and underlying diseases were not significantly different in the 2 groups (Table 1). Additionally, there was no difference in interventional procedures such as polypectomy (P = .773), biopsy (P = .930), and epinephrine injection (P = .934). Sedation-related outcomes such as induction time (P = .833), total procedure time (P = .111) and awake time (P = .070) were not different (Table 2).
Figure 1.
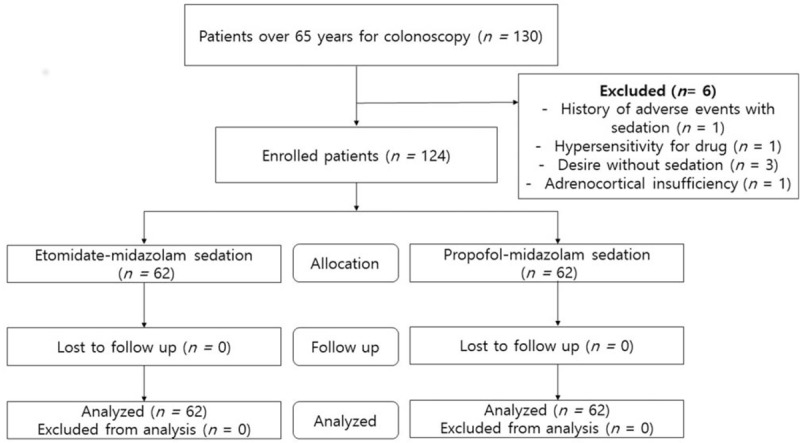
Assembly of patients. Flow chart showed the recruitment of the study patients.
Table 1.
Baseline characteristics.
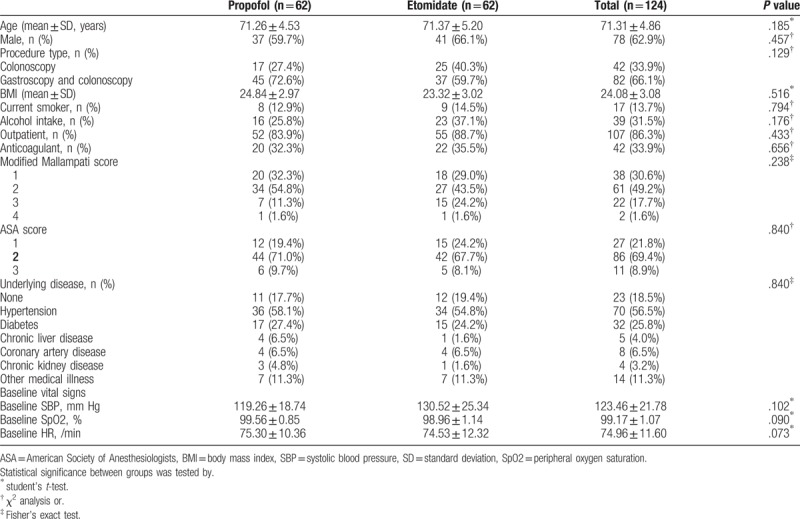
Table 2.
Procedure and sedation-related outcomes.
3.2. Safety-related outcomes
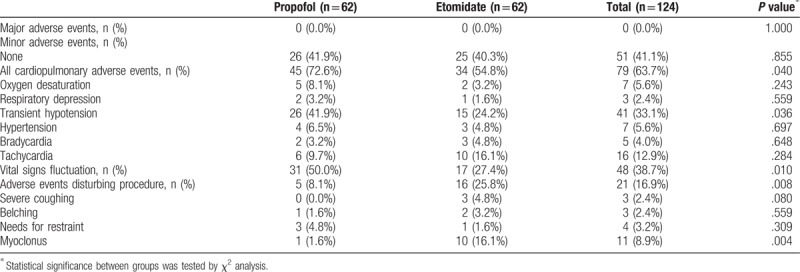
The incidence rate of all cardiopulmonary adverse events was significantly higher in the propofol group (72.6%) than in the etomidate group (54.8%) (P = .040) (Fig. 2). In both groups, there were no major adverse events including endotracheal intubation, permanent neurological impairment, and death. The incidence rate of VSF was significantly higher in the propofol group (50.0%) than in the etomidate group (27.4%) (P = .010). In addition, the incidence rate of transient hypotension was significantly higher in the propofol group (41.9%) than in the etomidate group (24.2%) (P = .036). In contrast, the incidence rate of adverse events disturbing the procedure was significantly higher in the etomidate group (25.8%) than in the propofol group (8.1%) (P = .008). The incidence rate of myoclonus was significantly higher in the etomidate group (16.1%) than in the propofol group (1.6%) (P = .004) (Table 3).
Figure 2.
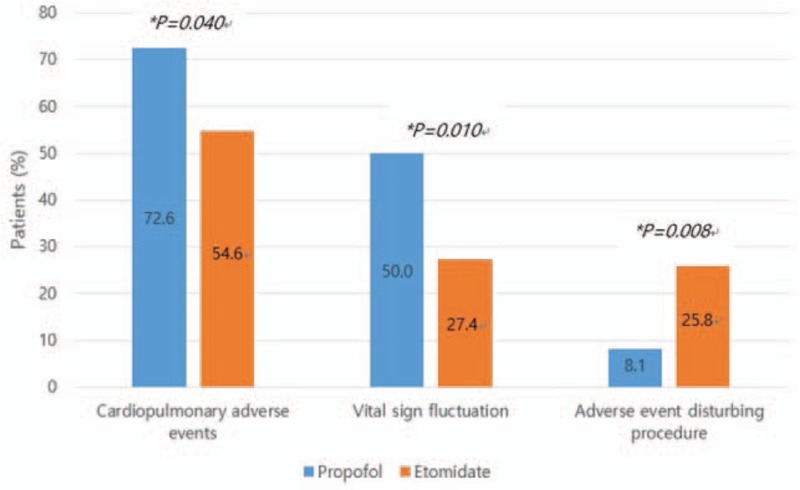
Assessment of adverse events. ∗Statistical significance between the 2 groups was tested by chi-square analysis for the excellent group.
Table 3.
Major and minor adverse events.
When vital sign changes over time were diagrammed on a scatter plot up to 35 minutes, there was a significant difference in change in median SBP with passing time between the 2 groups (P = .020) (Fig. 3A). Median HR significantly increased over time, but there was no significant difference between the 2 groups (Fig. 3B). Median SpO2 was sustained above 98% on 2 L/min oxygen during the procedure. There was no significant difference over time between the 2 groups (Fig. 3C).
Figure 3.
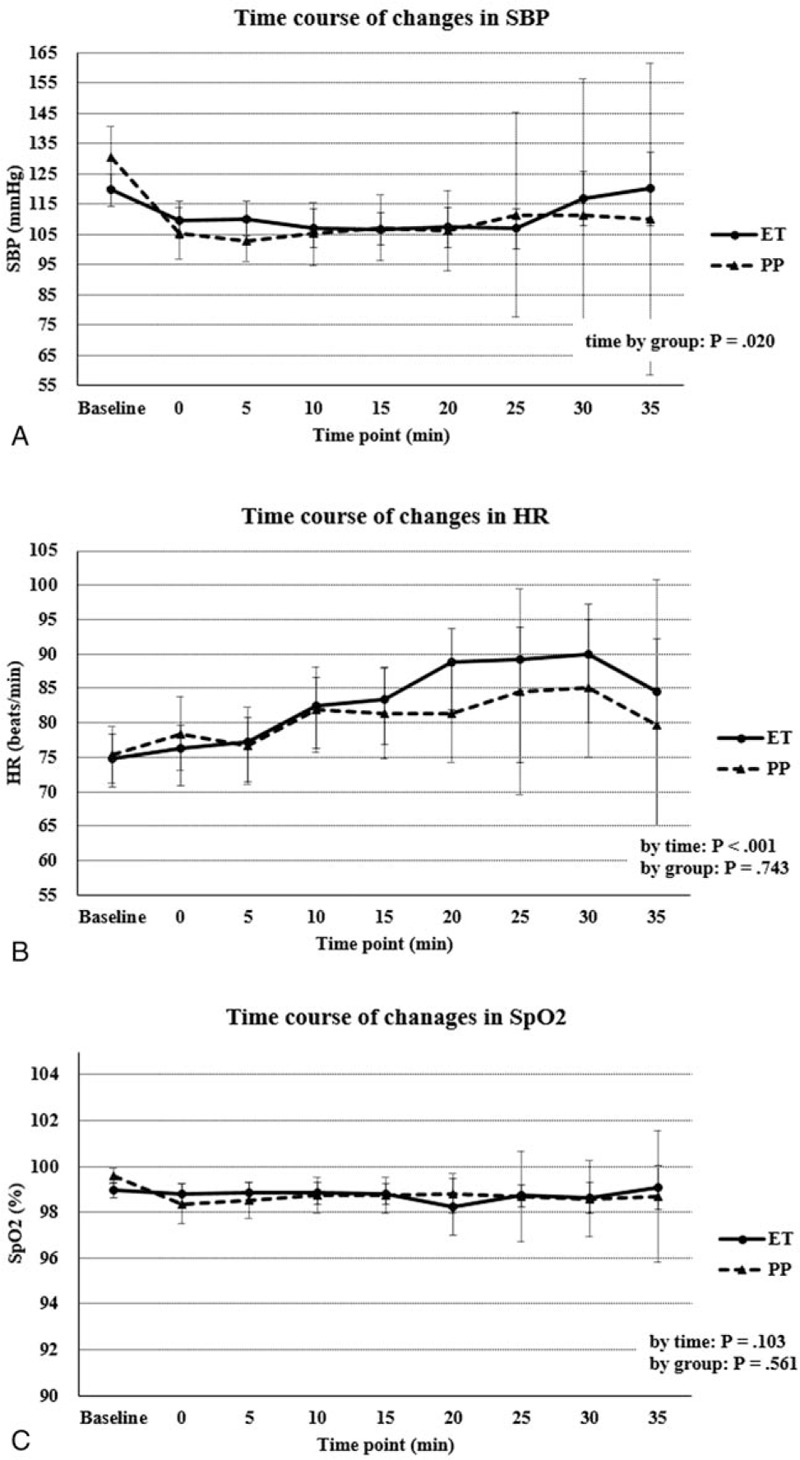
Time course of vital signs fluctuation. (A) Systolic blood pressure (mm Hg), (B) heart rate (beats/min), (C) peripheral oxygen saturation (as a percentage). SBP = systolic blood pressure, HR = heart rate, SpO2 = peripheral oxygen saturation.
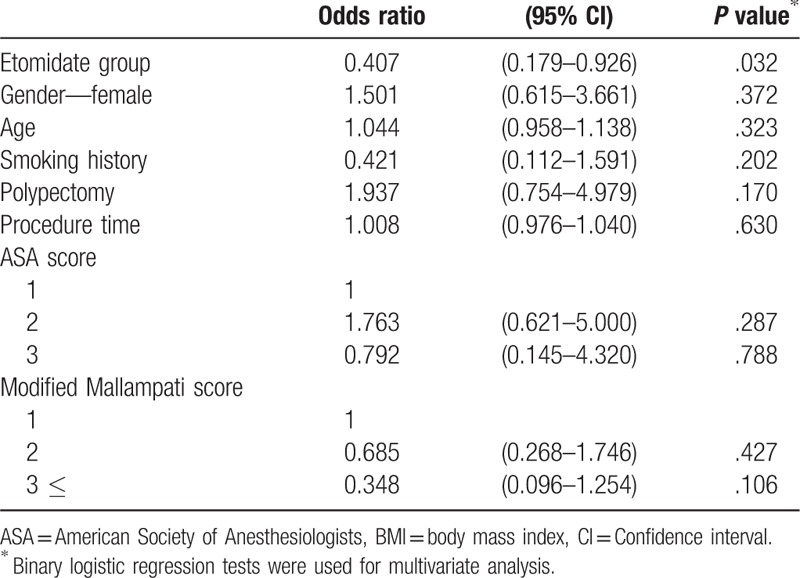
Multivariate analysis of VSF with logistic regression showed that the etomidate group had significantly low odds ratio (OR) associated with VSF (OR: 0.407, confidence interval: 0.179–0.926, P = .032) (Table 4).
Table 4.
Multivariate analysis for vital signs fluctuation.
3.3. Efficacy-related outcomes
Outcomes related to the efficacy of sedation including patients’ satisfaction, endoscopist's satisfaction, postprocedural abdominal pain, and postprocedural nausea or vomiting are shown in Table 2. There was no significant difference in patients’ satisfaction (P = .130) and endoscopist's satisfaction (P = .743) between the 2 groups. In addition, differences in patients’ postprocedural abdominal pain (P = .763) and nausea or vomiting (P = .075) between the 2 groups were not statistically significant. Moreover, the frequency of recall was not significantly different in both the groups (propofol group: 2/62 [3.2%], etomidate group: 5/62 [8.1%], P = .439).
4. Discussion
Recently, endoscopic sedation has been widely used.[4,18,25] Although propofol is commonly used as sedative for endoscopic procedures, respiratory depression is a major problem.[5,8,26] Etomidate has emerged as a new sedative that does not affect cardiopulmonary function.[27–30] Recent studies have demonstrated that etomidate for ERCP and EUS is associated with fewer serious adverse events, especially cardiopulmonary adverse events and that its sedative effect is noninferior in normal, healthy patients.[14,15]
Hemodynamic instability is more common during sedation in the elderly aged over 65 years than in young, healthy patients.[16,31,32] In addition, cardiopulmonary adverse events during sedation in the elderly can cause more fatal outcomes than in young patients.[32–34] A previous study demonstrated that etomidate-remifentanil was associated with more stable hemodynamic responses and fewer adverse events than propofol-remifentanil during gastroscopy in the elderly.[35] In addition, another study showed that a combination of etomidate and propofol improved hemodynamic stability and minimal respiratory depression during gastroscopy in the elderly.[36] However, there is no study on etomidate for colonoscopy in the elderly. For these reasons, we designed a comparison study in which etomidate can be used alternatively even in elderly patients during screening colonoscopy.
Similar to previous studies, we assumed that the etomidate group had fewer patients with oxygen desaturation and respiratory depression. The incidence of all cardiopulmonary adverse events and VSF was significantly higher in the propofol group. Our study has demonstrated that etomidate–midazolam is associated with fewer cardiopulmonary adverse events and its efficacy is noninferior to that of propofol–midazolam in the elderly similar to young patients. However, our experience indicates that patients on etomidate–midazolam more often move and have more adverse events disturbing the procedure than those on propofol–midazolam, which makes the procedure more difficult for the assistant or nurse than for the endoscopist. Therefore, propofol–midazolam may be used as a guideline in patients with low ASA score; however, we recommend using etomidate–midazolam in patients with high ASA score or vulnerable to risk factors, as shown by our study results.
In this study, we included not only healthy patients but also patients with an ASA III score and patients with Mallampati scores of 3 and 4. We demonstrated the sedative efficacy and safety of etomidate–midazolam for screening colonoscopy in the elderly and showed that there were more VSF and cardiopulmonary adverse events in the propofol group. This indicated that etomidate–midazolam was hemodynamically stable compared to propofol–midazolam. In addition, we showed that the etomidate group had lower OR associated with VSF than the propofol group in the multivariate analysis.
Our study has limitations. First, although the effect of a single injection of etomidate can last up to 72 hours,[37] studies on adrenal insufficiency have not been conducted. Second, we did not study oversedation and undersedation using bispectral index. Third, this randomized controlled trial was conducted at a single center and included a small number of patients. Multicenter randomized trials are required for further confirmation of the results. Fourth, although we intended to assess the efficacy and safety of etomidate–midazolam for colonoscopy, about 66% patients underwent both colonoscopy and gastroscopy in this study. The rate of patients who underwent both colonoscopy and gastroscopy was not significantly different between the 2 groups. However, the inclusion of patients who underwent both colonoscopy and gastroscopy may have led to bias.
In conclusion, we recommend using etomidate–midazolam in patients with high ASA score or vulnerable to risk factors; propofol–midazolam may be used as a guideline in patients with low ASA score.
Author contributions
Conceptualization: Hoon Jai Chun.
Data curation: Jung Min Lee, Geeho Min, Hoon Jai Chun.
Formal analysis: Jung Min Lee, Geeho Min, Eun Sun Kim.
Funding acquisition: Eun Sun Kim.
Investigation: Seung Han Kim, Bora Keum, Woojung Kim.
Methodology: Jae Min Lee, Seung Han Kim, Bora Keum, Hong Sik Lee, Beom Jae Lee, Seong Ji Choi, Woojung Kim.
Project administration: Jae Min Lee, Hyuk Soon Choi, Hoon Jai Chun, Seong Ji Choi.
Resources: Bora Keum, Beom Jae Lee.
Software: Jae Min Lee, Hyuk Soon Choi, Jong-Jae Park, Woojung Kim.
Supervision: Bora Keum, Yoon Tae Jeen, Hoon Jai Chun, Hong Sik Lee, Jong-Jae Park, Beom Jae Lee.
Validation: Yoon Tae Jeen, Hoon Jai Chun, Seong Ji Choi.
Visualization: Yoon Tae Jeen.
Writing – original draft: Jung Min Lee.
Writing – review & editing: Eun Sun Kim, Hong Sik Lee, Chang Duck Kim, Jong-Jae Park.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, ERCP = endoscopic retrograde cholangiopancreatography, EUS = endoscopic ultrasound, HR = heart rate, MOAA/S = Modified Observer's Assessment of Alertness/Sedation, OR = odds ratio, SBP = systolic blood pressure, SpO2 = peripheral oxygen saturation, VSF = vital signs fluctuation.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B5076677) and by Korea University.
The authors have no conflicts of interest to disclose.
References
- [1].Abraham NS, Wieczorek P, Huang J, et al. Assessing clinical generalizability in sedation studies of upper GI endoscopy. Gastrointest Endosc 2004;60:28–33. [DOI] [PubMed] [Google Scholar]
- [2].Igea F, Casellas JA, González-Huix F, et al. Sedation for gastrointestinal endoscopy. Endoscopy 2014;46:720–31. [DOI] [PubMed] [Google Scholar]
- [3].Warner ME, Warner MA. The value of sedation by anesthesia teams for complex endoscopy: perhaps not what you’d think. Anesth Analg 2014;119:222–3. [DOI] [PubMed] [Google Scholar]
- [4].Kang SH, Hyun JJ. Preparation and patient evaluation for safe gastrointestinal endoscopy. Clin Endosc 2013;46:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heuss LT, Hanhart A, Dell-Kuster S, et al. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: a randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest Endosc 2011;74:1207–14. [DOI] [PubMed] [Google Scholar]
- [6].Lichtenstein DR, Jagannath S. Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2008;68:815–26. [DOI] [PubMed] [Google Scholar]
- [7].Liu J, Liu R, Meng C, et al. Propofol decreases etomidate-related myoclonus in gastroscopy. Medicine (Baltimore) 2017;96:e7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang D, Wang S, Chen J, et al. Propofol combined with traditional sedative agents versus propofol- alone sedation for gastrointestinal endoscopy: a meta-analysis. Scand J Gastroenterol 2013;48:101–10. [DOI] [PubMed] [Google Scholar]
- [9].Song JC, Lu ZJ, Jiao YF, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci 2015;12:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shah SB, Chowdhury I, Bhargava AK, et al. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol 2015;31:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ebert TJ, Muzi M, Berens R, et al. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology 1992;76:725–33. [DOI] [PubMed] [Google Scholar]
- [12].Eames WO, Rooke GA, Wu RS, et al. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology 1996;84:1307–11. [DOI] [PubMed] [Google Scholar]
- [13].Oglesby AJ. Should etomidate be the induction agent of choice for rapid sequence intubation in the emergency department? Emerg Med J 2004;21:655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park CH, Park SW, Hyun B, et al. Efficacy and safety of etomidate-based sedation compared with propofol-based sedation during ERCP in low-risk patients: a double-blind, randomized, noninferiority trial. Gastrointest Endosc 2018;87:174–84. [DOI] [PubMed] [Google Scholar]
- [15].Kim MG, Park SW, Kim JH, et al. Etomidate versus propofol sedation for complex upper endoscopic procedures: a prospective double-blinded randomized controlled trial. Gastrointest Endosc 2017;86:452–61. [DOI] [PubMed] [Google Scholar]
- [16].Qureshi WA, Zuckerman MJ, Adler DG, et al. ASGE guideline: modifications in endoscopic practice for the elderly. Gastrointest Endosc 2006;63:566–9. [DOI] [PubMed] [Google Scholar]
- [17].Jafri SM, Monkemuller K, Lukens FJ. Endoscopy in the elderly: a review of the efficacy and safety of colonoscopy, esophagogastroduodenoscopy, and endoscopic retrograde cholangiopancreatography. J Clin Gastroenterol 2010;44:161–6. [DOI] [PubMed] [Google Scholar]
- [18].Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology 2007;133:675–701. [DOI] [PubMed] [Google Scholar]
- [19].Sachar H, Pichetshote N, Nandigam K, et al. Continued midazolam versus diphenhydramine in difficult-to-sedate patients: a randomized double-blind trial. Gastrointest Endosc 2017;Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol 1990;10:244–51. [PubMed] [Google Scholar]
- [21].Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep 2006;29:903–8. [DOI] [PubMed] [Google Scholar]
- [22].Banno S, Kato M, Sunata Y, et al. Risk factor for vital signs fluctuation during colonoscopy under conscious sedation consisting of midazolam and meperidine. Dig Dis 2018;36:113–7. [DOI] [PubMed] [Google Scholar]
- [23].Ead H. From Aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006;21:259–67. [DOI] [PubMed] [Google Scholar]
- [24].Riphaus A, Geist C, Schrader K, et al. Intermittent manually controlled versus continuous infusion of propofol for deep sedation during interventional endoscopy: a prospective randomized trial. Scand J Gastroenterol 2012;47:1078–85. [DOI] [PubMed] [Google Scholar]
- [25].Sachdeva A, Bhalla A, Sood A, et al. The effect of sedation during upper gastrointestinal endoscopy. Saudi J Gastroenterol 2010;16:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang D, Chen C, Chen J, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: a meta-analysis. PLoS One 2013;8:e53311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Upchurch CP, Grijalva CG, Russ S, et al. Comparison of etomidate and ketamine for induction during rapid sequence intubation of adult trauma patients. Ann Emerg Med 2017;69:24–33. e2 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vargo JJ. Etomidate: coming to an endoscopy unit near you? Gastrointest Endosc 2017;86:462–3. [DOI] [PubMed] [Google Scholar]
- [29].Ye L, Xiao X, Zhu L. The comparison of etomidate and propofol anesthesia in patients undergoing gastrointestinal endoscopy: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech 2017;27:1–7. [DOI] [PubMed] [Google Scholar]
- [30].Yates AM, Wolfson AB, Shum L, et al. A descriptive study of myoclonus associated with etomidate procedural sedation in the ED. Am J Emerg Med 2013;31:852–4. [DOI] [PubMed] [Google Scholar]
- [31].Kim SG. The elderly also deserves to undergo therapeutic endoscopy safely under sedation with propofol by gastroenterologists. Gut Liver 2015;9:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nonaka M, Gotoda T, Kusano C, et al. Safety of gastroenterologist-guided sedation with propofol for upper gastrointestinal therapeutic endoscopy in elderly patients compared with younger patients. Gut Liver 2015;9:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buri L, Zullo A, Hassan C, et al. Upper GI endoscopy in elderly patients: predictive factors of relevant endoscopic findings. Intern Emerg Med 2013;8:141–6. [DOI] [PubMed] [Google Scholar]
- [34].Xu CX, Chen X, Jia Y, et al. Stepwise sedation for elderly patients with mild/moderate COPD during upper gastrointestinal endoscopy. World J Gastroenterol 2013;19:4791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shen XC, Ao X, Cao Y, et al. Etomidate-remifentanil is more suitable for monitored anesthesia care during gastroscopy in older patients than propofol-remifentanil. Med Sci Monit 2015;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meng QT, Cao C, Liu HM, et al. Safety and efficacy of etomidate and propofol anesthesia in elderly patients undergoing gastroscopy: a double-blind randomized clinical study. Exp Ther Med 2016;12:1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ruth WJ, Burton JH, Bock AJ. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med 2001;8:13–8. [DOI] [PubMed] [Google Scholar]


