Abstract
Introduction:
For patients with refractory secondary hyperparathyroidism (SHPT), parathyroidectomy (PTX) has received increasing attention. However, evidence-based medicine shows that there is still controversy regarding surgical methods, efficacy, and safety. We retrospectively analyzed the process of diagnosis and treatment in one patient with severe SHPT and long-term chronic renal failure (CRF), so as to further improve the therapeutic effect.
Case presentation:
A 61-year-old female with SHPT and CRF manifested as no urine for 18 years, underwent PTX 4 times since September 2010, with satisfactory final recovery. The first operation involved resection of 3 parathyroid glands in the normal position; the second operation involved removal of an ectopic parathyroid gland, combined with parathyroid gland autotransplantation; the third operation was performed to resect suspected recurrent parathyroid gland; the fourth operation involved partial excision of the autotransplanted parathyroid glands.
Conclusion:
Accurate preoperative localized diagnosis and optimal surgical approach play key roles in the prevention and treatment of SHPT; postoperative recurrence of SHPT caused by ectopic or autotransplanted parathyroid gland should receive more attention.
Keywords: chronic renal failure, parathyroid gland autotransplantation, parathyroid hormone, parathyroidectomy, secondary hyperparathyroidism
1. Introduction
Secondary hyperparathyroidism (SHPT) is a common complication in patients with hemodialysis with long-term chronic renal failure (CRF), and the incidence of SHPT increases with duration of hemodialysis. As an increase in parathyroid hormone (PTH) leads to disorders in calcium and phosphorus metabolism, patients with SHPT mainly present with bone pain, skin itch, multiple fractures, and even a significant increase in the incidence and mortality of cardiovascular events, which seriously affects patient quality of life and survival.[1] For patients with refractory SHPT, who have no response to therapies such as low phosphorus diet, phosphorus binders, calcium supplements and vitamin D, parathyroidectomy (PTX) has received increasing attention.[2] However, evidence-based medicine shows that there is still controversy regarding surgical methods, efficacy, and safety.[3] Based on our experience in the diagnosis and treatment of 1 patient with SHPT, we further explored the pathogenesis, clinical manifestations, diagnosis, and treatment of SHPT, to further improve the treatment of this disorder.
2. Case report
A 61-year-old female was admitted to Beijing Electric Power Hospital on May 17, 2016 with an 18-year history of no urine and a 2-year history of progressive aggravation of recurrent bone pain.
The study has been approved by the ethics committee of Beijing Electric Power Hospital, State Grid Corporation of China, Capital Medical University, China. The patient has provided informed consent for publication of the case.
Eighteen years ago, the patient developed CRF, mainly manifested as no urine, and underwent hemodialysis. During this 18-year period, she received hemodialysis 4 times a week. Six years ago, she was diagnosed with SHPT due to complaints of bone pain, skin itch, a 10-cm reduction in height, and a significant increase in serum PTH. Four operations were required. The first operation involved resection of 3 parathyroid glands (Fig. 1A) in the normal position on September 9, 2010. Pathologic analysis showed nodular parathyroid hyperplasia with interstitial fibrosis and calcification (Fig. 1B). However, there was no improvement in bone pain and skin itch, and no obvious decrease in PTH. Technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) parathyroid scintigraphy showed a single ectopic parathyroid gland in the superior mediastinum (Fig. 2A). The second operation was performed on December 29, 2010 for the ectopic parathyroid gland (diameter approximately 1.5 cm) in the deep side of the right acromioclavicular joint (Fig. 2B) was removed, and parathyroid gland autotransplantation was performed in the left pectoralis major muscle. Pathologic analysis of the resected specimen showed nodular hyperplasia of parathyroid tissue which was well differentiated (Fig. 2C). Postoperative PTH immediately returned to normal, and both bone pain and skin itch disappeared.
Figure 1.
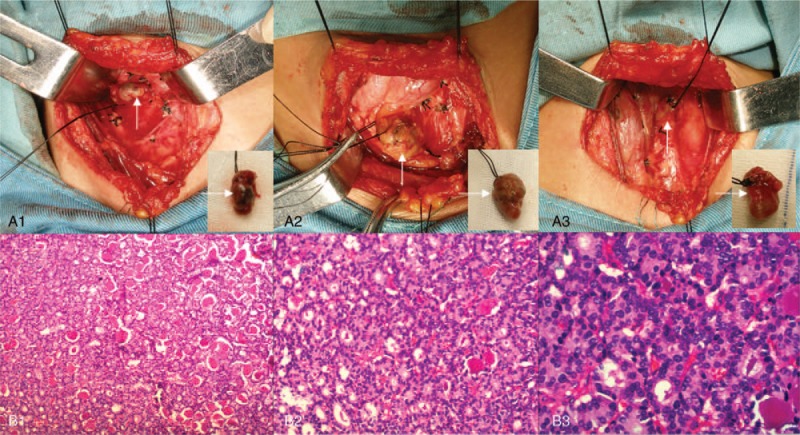
Parathyroidectomy and pathologic analysis. The first operation involved resection of 3 parathyroid glands (A1–3, arrows) in the normal position. Pathologic analysis showed nodular parathyroid hyperplasia with interstitial fibrosis and calcification (B1–3). Hematoxylin and eosin staining (magnification, ×100 for B1, ×200 for B2, ×400 for B3).
Figure 2.
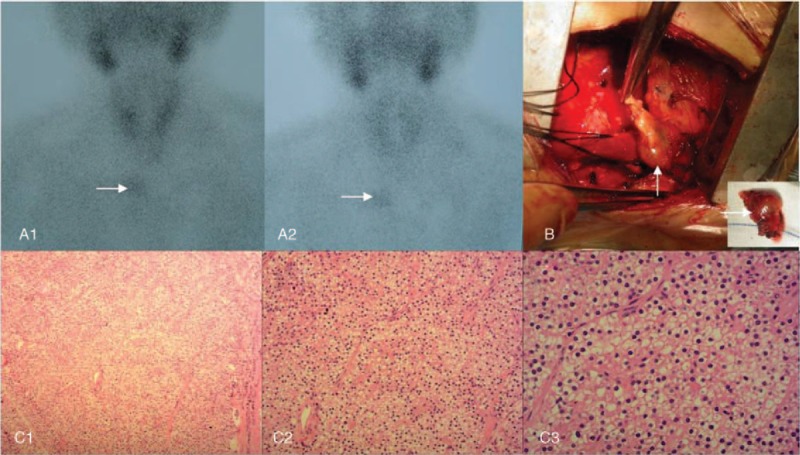
Ectopic parathyroidectomy and pathologic analysis. Before the second operation, technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) parathyroid scintigraphy showed a single ectopic parathyroid gland in the superior mediastinum (A1–2, arrows). Intraoperatively, the ectopic parathyroid gland in the deep side of the right acromioclavicular joint (B) was removed. Pathologic analysis of the resected specimen showed nodular hyperplasia of parathyroid tissue which was well differentiated (C1–3). Hematoxylin and eosin staining (magnification, ×100 for C1, ×200 for C2, ×400 for C3).
In October 2014, the patient was diagnosed with recurrent SHPT due to progressive aggravation of recurrent bone pain, skin itch, and a significant increase in PTH. Therapies such as low phosphorus diet, phosphorus binders, calcitriol, calcium supplements, and vitamin D were ineffective.
In March 2016, B-ultrasound revealed a 2.2 × 0.6 cm nodular mass (Fig. 3A) located on the outside inferior left lobe of the thyroid gland. A third operation was performed on March 23, 2016 to resect suspected recurrent parathyroid gland in the lateral side of the left common carotid artery. Intraoperatively, a nodular mass (with a diameter of approximately 2.5 cm) was visible (Fig. 3B) on the lateral side of the left common carotid artery. Pathologic examination showed reactive hyperplasia in the lymph node (Fig. 3C).
Figure 3.
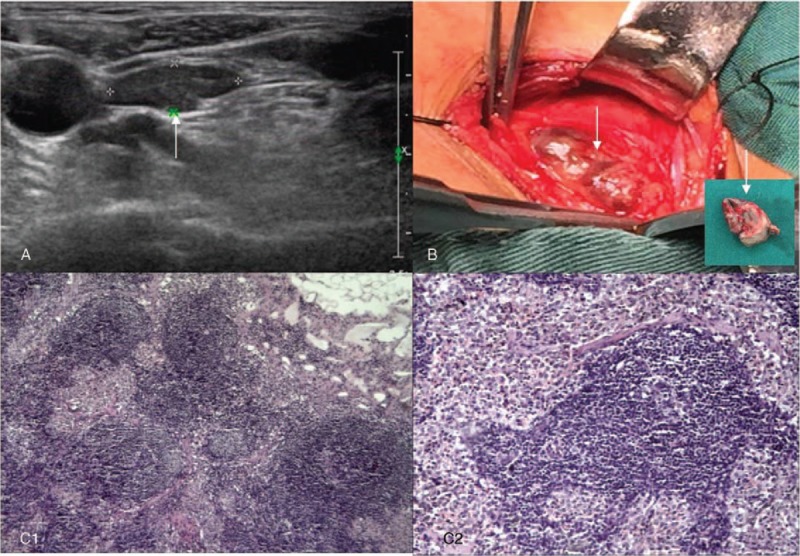
Excision of suspected recurrent parathyroid gland and pathologic analysis. Before the third operation, B-ultrasound revealed a 2.2×0.6 cm nodular mass (A, arrow) located on the outside inferior left lobe of the thyroid gland. Intraoperatively, the suspected recurrent parathyroid gland (B, arrow) in the lateral side of the left common carotid artery were resected. Pathologic examination showed reactive hyperplasia in the lymph node (C1–2). Hematoxylin and eosin staining (magnification, ×40 for C1, ×100 for C2).
On admission, B-ultrasound revealed multiple hypoechoic nodules (Fig. 4A) in the left chest wall, and 99mTc-MIBI parathyroid scintigraphy showed increased uptake of soft tissue lesions in the left chest wall (Fig. 4B). The fourth operation on May 19, 2016 involved partial excision of the autotransplanted parathyroid glands. Intraoperatively, 4 markedly enlarged autotransplanted parathyroid glands (Fig. 4C) located on the left pectoralis major were resected. Pathologic analysis showed secondary hyperplasia of the parathyroid (Fig. 4D) in striated muscle tissue. PTH immediately decreased from a preoperative level of 1464 pg/mL to a postoperative level of 434.6 pg/mL. Following surgery, her bone pain, skin itch, and other symptoms significantly improved. During the 1.5-year follow-up period, the patient reported no obvious bone pain, skin itching, or other discomfort.
Figure 4.
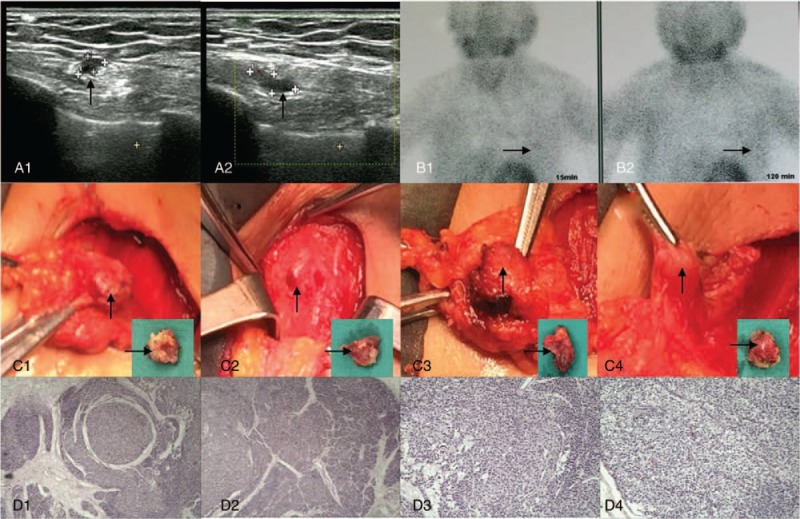
Partial excision of autotransplanted parathyroid gland and pathologic analysis. Before the fourth operation, B-ultrasound revealed multiple hypoechoic nodules (A1–2, arrows), and technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) parathyroid scintigraphy showed increased uptake of soft tissue lesions (B1–2, arrows). Intraoperatively, 4 markedly enlarged autotransplanted parathyroid glands (C1–4, arrows) were resected. Pathologic analysis showed secondary hyperplasia of the parathyroid (D1–4) in striated muscle tissue. Hematoxylin and eosin staining (magnification, ×40 for D1 and D2, ×100 for D3 and D4).
3. Discussion
The SHPT is a compensatory response to CRF and metabolic disturbances in serum calcium, phosphorus, and vitamin D. In patients with CRF, glomerular filtration rate is decreased, resulting in phosphorus retention. Hyperphosphatemia inhibits the activity of vitamin D production in the kidney, causes vitamin D deficiency and reduces intestinal calcium absorption, leading to a decrease in serum calcium. Hypocalcemia plays a role in the continuous stimulation of the parathyroid gland, resulting in an increase in the secretion of PTH, which induces an increase in serum calcium, eventually leading to high serum calcium levels. In addition, patients with renal failure have a reduction in effective renal parenchyma, a decrease in the secretion of 1α-hydroxylase in renal proximal tubules, and inhibition of the translation of 25-vitamin D3 into 1, 25-hydroxy vitamin D3, which significantly improves the gene transcription of PTH, resulting in increased synthesis and secretion of PTH. Due to the increase in PTH, the sensitivity of the calcium receptor in parathyroid cells is decreased, and even if serum calcium is increased in the later stage of disease, more PTH is secreted, which aggravates hyperparathyroidism, resulting in a positive feedback loop, thereafter leading to the occurrence and progressive aggravation of SHPT.[4]
Patients with SHPT have no obvious symptoms in the early stage of the disease, but as the disease progresses, multi-system damage can occur: First, skeletal system: bone pain, joint pain, osteoporosis, osteitis fibrosa, pathologic fracture, acute peripheral arthritis, gout, pseudo spontaneous tendon rupture, and metastatic calcification. Patients with serious SHPT can even develop shrinking man syndrome with reduced body height. The height of patient 1 was reduced by 10 cm. Second, blood system: anemia, leukopenia, and platelet dysfunction. Third, mental and nervous system: peripheral neuropathy, muscle weakness, gait disturbance, irritability, inattention, depression, insomnia and dry mouth. Fourth, circulatory system: heart disease, decreased left ventricular ejection fraction, heart failure, hypertension, and arteriosclerosis. In addition, skin itch, skin calcification, tumor-like calcification of soft tissue, ulcers, and necrosis are also very common.
The patient in our study had anemia, osteoporosis, and bone pain, which affected daily activities and was accompanied by severe pruritus and insomnia. After surgery, these symptoms were alleviated and patient quality of life was significantly improved.
Common laboratory tests include the measurement of serum calcium, phosphorus, alkaline phosphatase, and PTH. Serum calcium is usually normal or decreased; however, if persistent hypercalcemia occurs, this can indicate that the parathyroid glands have formed an autocrine hyperplastic nodule or adenoma. Serum phosphorus is usually normal or increased. Alkaline phosphatase can be increased, and the degree of increase is directly proportional to the severity of hyperparathyroidism. PTH is an important index in the diagnosis of SHPT. In the early stage of CRF, when the glomerular filtration rate is less than 70 mL/min, serum PTH is increased slightly. A marked increase in PTH is seen in the later stage of CRF. In our study, preoperative PTH was significantly increased in the patient.
Commonly used imaging examinations are B-ultrasound and 99mTc-MIBI parathyroid scintigraphy.[5] The combination of these 2 methods can detect 64.6% of SHPT, which is significantly better than the detection rate of 51.2% for B-ultrasound and 53.5% for 99mTc-MIBI parathyroid scintigraphy alone. Accurate preoperative localization is the key to surgical success in SHPT[6,7] and the presence of ectopic parathyroid gland should be noted. An autopsy study of 503 patients found that 4 parathyroid glands accounted for 84%, and 5 or more accounted for 13%.[8] Extra parathyroid glands are often located in the thymus, which is a common cause of persistent SHPT after PTX. In our study, the patient had an ectopic parathyroid gland located in the superior mediastinum, which caused recurrence of SHPT after the first operation.
The treatment of SHPT includes: First, limiting the intake of dietary phosphorus, filter treatment to remove phosphorus, and the use of phosphate binders. Second, maintenance of appropriate calcium concentration by removing calcium and limiting calcium intake in the diet. Third, inhibition of PTH secretion by calcitriol and its analogues. Fourth, application of a calcium-sensing receptor (CaR) agonist,[9] such as cinacalcet hydrochloride,[10,11] a first-in-class calcimimetic, approved in both the United States and the European Union, which offers a new therapeutic approach in the treatment of SHPT. However, the safety and validity of its long-term use requires confirmation in further clinical trials.[12,13]
Surgical indications for SHPT are as follows[14,15]: serum PTH >800 pg/mL,[16,17] combined with serious clinical symptoms, such as skeletal deformities, fractures, and uremic arteriolopathy; persistent hypercalcemia and/or hyperphosphatemia, and ineffective therapy; drug resistance to active vitamin D; high-frequency Doppler ultrasound showing at least 1 enlarged parathyroid gland, with a maximum diameter of >1 cm and abundant blood flow; and normal liver function and blood coagulation. In our study, the patient had PTH >800 pg/mL and non-surgical treatment was ineffective, which is consistent with the above indications.
There are 4 options of surgical procedure for SHPT: total PTX (tPTX),[18] which involves the resection of parathyroid glands and suspected parathyroid tissue, and has the advantages of avoiding recurrence and the development of intractable postoperative hypocalcemia,[19,20] which necessitates replacement with long-term calcium supplements and vitamin D. Subtotal PTX (sPTX), which only preserves half of the smallest parathyroid gland in situ, and all other parathyroid glands are removed. This procedure has the advantage of a low incidence of postoperative hypocalcemia, and the disadvantage of SHPT recurrence caused by residual parathyroid tissue.[21] Near-total PTX (nPTX), which only preserves 1/4 of the smallest parathyroid gland in situ, and all other parathyroid glands are removed, with the same disadvantage of possible SHPT recurrence caused by residual parathyroid tissue. tPTX with autotransplantation (tPTX+AT),[22] which involves resection of all parathyroid glands, with the smallest and most “normal” one cut into flakes or powder, and then 60–100 mg transplanted into the forearm muscles (one side of the upper limb without arteriovenous fistula) or the sternocleidomastoid muscle, and possibly even subcutaneous tissue.[23] This method avoids postoperative hypocalcemia, and can effectively alleviate the symptoms, but also has limitations, as recurrence of SHPT may be induced by the autotransplanted parathyroid glands.[24,25] The patient in our study underwent parathyroid gland autotransplantation in the left pectoralis major muscle in the second operation, and was diagnosed with recurrent SHPT in the sixth postoperative year, and partial excision of the autotransplanted parathyroid glands was performed in the fourth operation. Due to the inability to identify and remove all of the transplanted parathyroid glands, serum PTH did not decrease to normal. However, following resection of the 4 markedly enlarged autotransplanted parathyroid glands, the serum PTH level decreased by 70.3% (from a preoperative level of 1464 pg/mL to a postoperative level of 434.6 pg/mL), and the patient's symptoms significantly improved.
This patient demonstrates that accurate preoperative localized diagnosis and optimal surgical approach play key roles in the prevention and treatment of SHPT; postoperative recurrence of SHPT caused by ectopic or autotransplanted parathyroid gland should receive more attention.
Author contributions
Conceptualization: Zongming Zhang.
Funding acquisition: Zongming Zhang.
Investigation: Mingwen Zhu, Fangcai Lin, Jieping Miao, Pei Wang, Chong Zhang, Hongwei Yu, Hai Deng, Zhuo Liu, Limin Liu, Baijiang Wan, Haiyan Yang, Mengmeng Song, Yue Zhao, Nan Jiang, Zichao Zhang, Zhenya Zhang, Lijie Pan.
Supervision: Zongming Zhang, Fangcai Lin.
Writing – original draft: Zongming Zhang.
Writing – review & editing: Zongming Zhang.
Footnotes
Abbreviations: CaR = calcium-sensing receptor, CRF = chronic renal failure, nPTX = near-total parathyroidectomy, PTH = parathyroid hormone, PTX = parathyroidectomy, SHPT = secondary hyperparathyroidism, sPTX = subtotal parathyroidectomy, 99mTc-MIBI = technetium-99m methoxyisobutylisonitrile, tPTX = total parathyroidectomy, tPTX+AT = tPTX with autotransplantation.
Supported by the Science and Technology Project of State Grid Corporation of China (SGHB0000AJJS1400182) and Beijing Municipal Science & Technology Commission (Grant No. Z171100000417056).
The authors have no conflicts of interest to disclose.
References
- [1].Komaba H, Kakuta T, Fukagawa M. Management of secondary hyperparathyroidism: how and why? Clin Exp Nephrol 2017;21suppl 1:37–45. [DOI] [PubMed] [Google Scholar]
- [2].Ho LY, Wong PN, Sin HK, et al. Risk factors and clinical course of hungry bone syndrome after total parathyroidectomy in dialysis patients with secondary hyperparathyroidism. BMC Nephrol 2017;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015;10:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salam SN, Khwaja A, Wilkie ME. Pharmacological management of secondary hyperparathyroidism in patients with chronic kidney disease. Drugs 2016;76:841–52. [DOI] [PubMed] [Google Scholar]
- [5].Vulpio C, Bossola M, De Gaetano A, et al. Usefulness of the combination of ultrasonography and 99mTc-sestamibi scintigraphy in the preoperative evaluation of uremic secondary hyperparathyroidism. Head Neck 2010;32:1226–35. [DOI] [PubMed] [Google Scholar]
- [6].Gwiasda J, Kaltenborn A, Müller JA, et al. Ultrasound-based scores as predictors for nodular hyperplasia in patients with secondary hyperparathyroidism: a prospective validation study. Langenbecks Arch Surg 2017;402:295–301. [DOI] [PubMed] [Google Scholar]
- [7].LoPinto M, Rubio GA, Khan ZF, et al. Location of abnormal parathyroid glands: lessons from 810 parathyroidectomies. J Surg Res 2017;207:22–6. [DOI] [PubMed] [Google Scholar]
- [8].Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery 1984;95:14–21. [PubMed] [Google Scholar]
- [9].Chen RA, Goodman WG. Role of the calcium-sensing receptor in parathyroid gland physiology. Am J Physiol Renal Physiol 2004;286:1005–11. [DOI] [PubMed] [Google Scholar]
- [10].Torres PU. Cinacalcet HCl: a novel treatment for secondary hyperparathyroidism caused by chronic kidney disease. J Ren Nutr 2006;16:253–8. [DOI] [PubMed] [Google Scholar]
- [11].van der Plas WY, Engelsman AF, Özyilmaz A, et al. Impact of the introduction of calcimimetics on timing of parathyroidectomy in secondary and tertiary hyperparathyroidism. Ann Surg Oncol 2017;24:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gojaseni P, Pattarathitinan D, Chittinandana A. Efficacy of low-dose cinacalcet on alternate days for the treatment of secondary hyperparathyroidism in hemodialysis patients: a single-center study. Int J Nephrol Renovasc Dis 2017;10:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ballinger AE, Palmer SC, Nistor I, et al. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev 2014;CD006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tominaga Y, Matsuoka S, Uno N. Surgical and medical treatment of secondary hyperparathyroidism in patients on continuous dialysis. World J Surg 2009;33:2335–42. [DOI] [PubMed] [Google Scholar]
- [15].Ho LC, Hung SY, Wang HH, et al. Parathyroidectomy associates with reduced mortality in Taiwanese dialysis patients with hyperparathyroidism: evidence for the controversy of current guidelines. Sci Rep 2016;6:19150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma TL, Hung PH, Jong IC, et al. Parathyroidectomy is associated with reduced mortality in hemodialysis patients with secondary hyperparathyroidism. Biomed Res Int 2015;2015:639587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin HC, Chen CL, Lin HS, et al. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clin Endocrinol (Oxf) 2014;80:508–15. [DOI] [PubMed] [Google Scholar]
- [18].Puccini M, Carpi A, Cupisti A, et al. Total parathyroidectomy without autotransplantation for the treatment of secondary hyperparathyroidism associated with chronic kidney disease: clinical and laboratory long-term follow-up. Biomed Pharmacother 2010;64:359–62. [DOI] [PubMed] [Google Scholar]
- [19].Wetmore JB, Liu J, Do TP, et al. Changes in secondary hyperparathyroidism-related biochemical parameters and medication use following parathyroidectomy. Nephrol Dial Transplant 2016;31:103–11. [DOI] [PubMed] [Google Scholar]
- [20].Jia X, Wang R, Zhang C, et al. Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: a meta-analysis. Ther Apher Dial 2015;19:477–85. [DOI] [PubMed] [Google Scholar]
- [21].Konturek A, Barczyński M, Stopa M, et al. Subtotal parathyroidectomy for secondary renal hyperparathyroidism: a 20-year surgical outcome study. Langenbecks Arch Surg 2016;401:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Naranda J, Ekart R, Pečovnik-Balon B. Total parathyroidectomy with forearm autotransplantation as the treatment of choice for secondary hyperparathyroidism. J Int Med Res 2011;39:978–87. [DOI] [PubMed] [Google Scholar]
- [23].Conzo G, Della Pietra C, Tartaglia E, et al. Long-term function of parathyroid subcutaneous autoimplantation after presumed total parathyroidectomyin the treatment of secondary hyperparathyroidism. A clinical retrospective study. Int J Surg 2014;12suppl 1:S165–9. [DOI] [PubMed] [Google Scholar]
- [24].Kim BK, Lee J, Sun WY. Recurrent hyperparathyroidism due to proliferation of autotransplanted parathyroid tissue in a multiple endocrine neoplasia type 2A patient. Ann Surg Treat Res 2016;91:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Agha A, Loss M, Schlitt HJ, et al. Recurrence of secondary hyperparathyroidism in patients after total parathyroidectomy with autotransplantation: technical and therapeutic aspects. Eur Arch Otorhinolaryngol 2012;269:1519–25. [DOI] [PubMed] [Google Scholar]


