Abstract
Rationale:
Malignant transformations of ovarian mature cystic teratomas (MCTs) occur rarely, especially in young women. Although it is extremely difficult to diagnose them, serum tumor marker level testing in combination with the use of imaging techniques may be useful in preoperative diagnosis.
Patient concerns:
We present the case of a 31-year-old Chinese woman with the malignant transformation of an ovarian MCT. The patient had a history of oophorocystectomy due to an MCT of the right ovary 6 years prior and a gemellary pregnancy owing to in vitro fertilization and embryo transfer. Her serum CA19-9 levels were persistently mildly elevated after the first surgery.
Diagnoses:
She was diagnosed with ovarian squamous carcinoma, arising from an MCT (International Federation of Gynecology and Obstetrics stage IA).
Interventions:
A right salpingo-oophorectomy and omentectomy were performed and the patient underwent chemotherapy.
Outcomes:
The patient was disease-free at the 6-month follow-up.
Lessons:
The malignant transformation of MCTs usually occurs in postmenopausal women with poor prognoses; it is very rarely observed in young women. Although the early detection and complete surgical resection of the tumor are crucial to survival, preoperative diagnosis of this malignancy is difficult. This case reiterates the fact that malignant transformation of MCTs can occur at any age. Rapid tumor growth along with persistently elevated tumor marker levels may be indicative of the malignant transformation of MCTs.
Keywords: diagnosis, malignant transformation, ovarian neoplasm, squamous cell carcinoma, teratoma
1. Introduction
Mature cystic ovarian teratoma is the most common germ cell tumor, accounting for approximately 20% of all ovarian neoplasms; it is predominantly observed in young women with a median age of 30 years.[1–5] It is usually a multiloculated cyst, comprising derivatives from the 3 germ layers, predominantly sebum, keratinous debris, adipose tissue and hair, and their combinations. Compared to benign MCTs, MCTs with malignant transformation are very rare, accounting for only 2% to 4% of all cases, and are usually observed in postmenopausal women with a mean age of 45 to 60 years.[1–4]
Clinically, it is usually asymptomatic and discovered accidentally during gynecologic investigations for other conditions or due to mass effect. The use of imaging techniques, such as ultrasonography of the pelvic region, computed tomography (CT), and magnetic resonance imaging (MRI) to detect calcified tissues, bone, and cartilage in tumors makes the preoperative diagnosis of MCTs easy. For patients with benign MCTs, ovarian cystectomy is curative. Compared to benign MCTs, the prognosis of MCTs with malignant transformation is poor, and the preoperative prediction and detection of these malignant MCTs are extremely difficult.[2,4] Moreover, the clinicopathologic characteristics, treatment, prognostic factors and the mechanism of malignant transformation are not yet well understood owing to the rarity of such tumors, especially in reproductive-age women. Therefore, we report a case of a young woman with the malignant transformation of a recurrent MCT and review the literature, with focus on the values and methods of early prediction or detection of the disease in clinical practice.
2. Case report
A 31-year-old Chinese woman—gravida 4, para 1+5—was presented to our gynecology clinic with pelvic mass. Six years prior to that visit, she had undergone oophorocystectomy for an MCT (Fig. 1A) of the right ovary at our hospital. In the first surgery, the tumor dimension was 3.2 cm×1.5 cm×1 cm and her serum CA19-9 level was 75.5 U/mL (<30.9 U/mL). Her serum CA19-9 level was persistently mildly elevated after the oophorocystectomy (57.4 U/mL, 72 U/mL, 79.4 U/mL, detected at the 25-month, 46-month, and 60-month after the surgery, respectively). Subsequently, she underwent in vitro fertilization and embryo transfer (IVF-ET) after 2 natural abortions and an ectopic pregnancy. During her gemellary pregnancy (owing to IVF-ET), a palpable mass on the right ovary was observed. However, we did not initiate treatment and one infant died due to a nuchal cord. One year after delivery, the patient underwent a routine physical examination and an abdominal plain CT revealed a right ovarian mass, with a dimension of 8.2 cm × 8 cm × 4.4 cm, containing calcification and lipo components; none of the findings were suggestive of malignancy. The tumor marker levels were as follows: CA 125: 95 U/mL (<35 U/mL), CA 19–9: 76 U/mL, and carcinoembryonic antigen: 12 ng/mL (<2.5 ng/mL). The tumor was diagnosed preoperatively as teratoma, based on the CT and laboratory findings.
Figure 1.
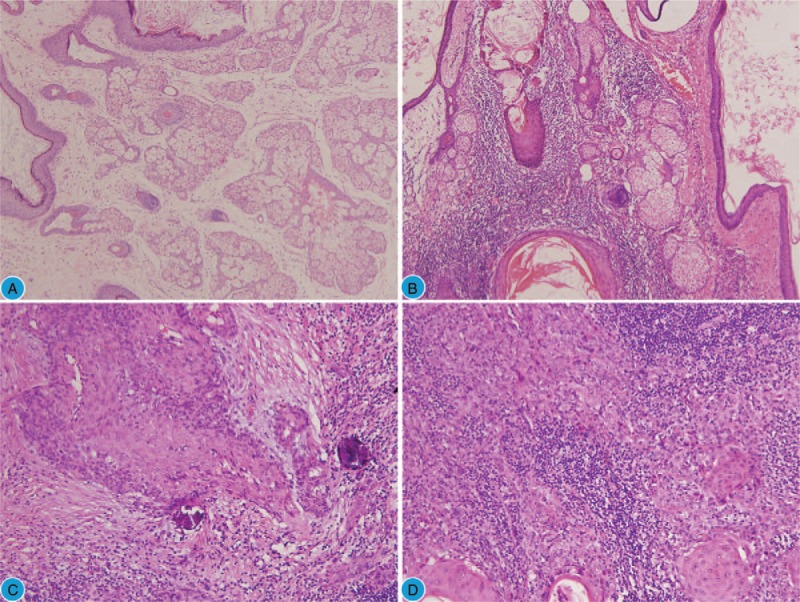
Morphological characteristics of the tumor. (A) The benign elements in the MCT during the first surgery (H&E, 100×). (B) The benign squamous epithelium and sebaceous gland in the recurrent MCT (H&E, 200×). (C) SCC transformed from the MCT (H&E, 200×) with hyperchromatic and pleomorphic nuclei. (D) Foci of invasive SCC (H&E, 200×). H&E = hematoxylin and eosin, MCT = mature cystic teratoma, SCC = squamous cell carcinoma.
An intraoperative frozen section of the left ovarian mass revealed benign MCT. However, the presence of squamous cell carcinoma (SCC) was observed in the solid region protruding inside the cyst wall in the postoperative samples. Microscopic examination revealed the transition from normal squamous epithelium to SCC (Fig. 1B–D) and ovarian squamous carcinoma arising from the MCT (International Federation of Gynecology and Obstetrics [FIGO], stage IA) was diagnosed finally. Immunohistochemical staining showed that the squamous carcinoma cells were positive for pancytokeratin (Fig. 2A) and P63 (Fig. 2B) and focal positive for p16 (cytoplasm and nuclei) (Fig. 2C), the proliferative index (ki67) was approximately 15% (Fig. 2D), and a human papillomavirus (HPV) test showed negative results. The cytology of the peritoneal fluid was negative for malignant cells.
Figure 2.
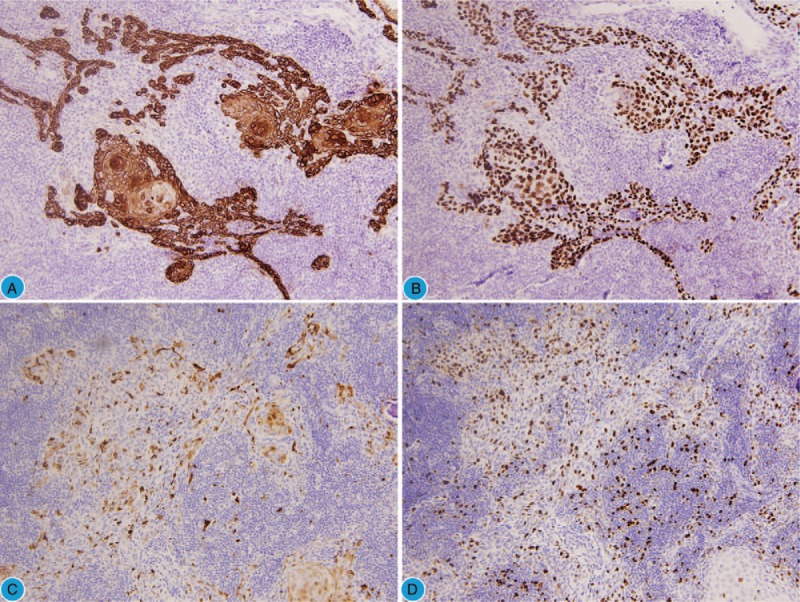
Immunohistochemistry results of the tumor. (A) Pancytokeratin diffuse positive (H&E, 200×). (B) p63 diffuse positive (H&E, 200×). (C) Focal positive for p16 (H&E, 200×). (D) The proliferative index (ki67) is approximately 15%.
After right salpingo-oophorectomy and omentectomy, the patient underwent a chemotherapy course. There were no signs of recurrence during the 6-month follow-up.
As this is a case report, ethical approval was not required. Informed patient consent was obtained for the publication of this report.
3. Discussion
MCTs are common ovarian neoplasms and are characterized by the presence of elements of the 3 germ layers. Teratomas rarely recur, and are most frequently observed in young or adolescent patients with immature components, especially immature neuroepithelium.[6,7] Malignant transformations of recurring MCTs very rarely occur in young women.
Malignant transformations of MCTs occur in <2% of all MCTs.[1,4,5] According to the tumor cell origin, the malignant differentiation of MCT can include adenocarcinoma, carcinoid tumor, sebaceous carcinoma, melanoma, small cell carcinoma, undifferentiated carcinoma, angiosarcoma, chondrosarcoma and, most commonly, SCC, which accounts for about 75% of such cases.[4,5,8–13]. Due to the rarity of malignant transformations and the complex components of MCTs, preoperatively distinguishing between these 2 conditions is extremely difficult, especially in young reproductive-age women with recurrent MCT.
In most patients, MCTs are asymptomatic and few patients have more than one symptom related to the tumor. Symptoms include the presence of a palpable abdominal mass, abdominal distention, lower abdominal pain, low back pain, weight loss, and cyst rupture or torsion.[4,10] Although MCTs can be easily diagnosed by imaging modalities such as gynecological sonography, plain radiography, CT and MRI, the malignant transformation of MCTs is difficult to predict or diagnose from imageological results. In the present case, the CT only revealed a right ovarian mass, which contained calcification and adipose components; there were no findings suggestive of malignancy. Few reports have focused on the diagnosis of malignant transformations of MCTs with these imaging modalities.[14–16] The presence of solid, friable, or variegated portions within the MCTs is an important feature of the diagnosis of malignant transformation.[14,15,17,18] Another attribute of MCTs is the identification of a soft tissue protuberance known as a Rokitansky nodule or dermoid plug. The angle formed between the inner cyst wall and the soft tissue components is reported to be a sensitive marker of malignancy.[14,15] MCTs that have undergone malignant transformation are usually larger (mean size of 15 cm) than benign MCTs (mean size of 6–9 cm); therefore, a tumor size ≥10 cm may be indicative of malignant transformation.[5,19] The smaller the tumor size, the more difficult the precise preoperative diagnosis of malignant transformation is.
Age may be a predictor of malignant transformation in MCTs. Previous studies have shown that the median age of patients with malignant transformation arising from MCTs ranged from 36 to 52 years; this tended to be higher than that of those with the benign form of the disease.[1,4,5] If MCTs are discovered in postmenopausal women, the likelihood of malignant transformation is very strong, as this occurs predominantly after the age of 40 years.[4,17] However, Araujo's cohort study showed that there is no relationship between age and the malignant transformation of MCTs.[5] In the present case, the patient was only 31 years old, reiterating the fact that young women too may experience the malignant transformation of MCTs.
Serum tumor marker levels may be high in patients with the malignant transformation of MCTs; this could be an indicator of malignant transformation. For instance, the SCC antigen and CA19-9 levels were found to be significantly higher in patients with SCC arising from MCTs than in those with MCTs alone.[4,17,20] Mori et al[17] stated that the use of single markers is not particularly effective and found that the use of SCC levels < 2.5 and age < 40 years has a 77% sensitivity and 96% specificity in predicting malignant transformation. In our case, the serum CA19-9 level was continuously mildly increasing after the first surgery, supporting previous findings which stated that tumor marker levels are important indicators of the malignant transformation of MCTs.
The mechanism of the malignant transformation of mature cystic teratomas remains unknown; however, the involvement of HPV has been suggested. Some studies showed that high-risk HPV infection may be a causal factor for the induction of the malignant transformation of MCTs to SCCs.[5,21,22] However, our patient was HPV-negative. Therefore, the possible role of HPV in the malignant transformation of MCTs must be further investigated.
Although the application of FIGO staging to SCCs in the case of MCTs does not clearly relate to the graduated outcome of the disease, patients with FIGO stages II to IV had a lower probability of long-term survival than patients with stage I disease.[18,23] Age, tumor size, clinical stage, histologic differentiation, capsular invasion, and the presence of vascular invasion can provide valuable information for the prediction of the survival of patients with SCCs arising from MCTs.[5,10,18] The early diagnosis of the malignant transformation before invasion or metastasis is important for treatment. A large ovarian mass suspected to be a mature teratoma should be more carefully managed in older patients. Recent studies showed that, while the mean tumor size was 10.8 cm, a tumor size ≥15.0 cm appeared to be strongly associated with aggressive disease and generally spread by direct invasion and peritoneal implantation rather than by metastasis to the regional lymph nodes.[4,18] SCCs of the ovary spread transmurally with extensive local invasion, which is different from that in the case of common ovarian tumors, and the overall 5-year survival rate is about 50%.[4,18]
In terms of treatment, multiple case series recommend surgery including total hysterectomy, bilateral salpingo-oophorectomy, omentectomy and pelvic-paraaortic lymph node dissection with further platinum-based agent chemotherapy.[4] The role of radiotherapy remains unclear. Owing to the limited number of cases for analysis, it was difficult to make definite conclusions with regards to the appropriate adjuvant chemotherapy for this disease.[4]
4. Conclusion
In conclusion, the malignant transformation of recurrent MCTs in young women is very rare and its preoperative diagnosis is difficult. Risk factors for the malignant transformation of MCTs include elevated tumor marker levels, age, tumor size, and postmenopausal status. Early detection and complete surgical resection of the tumor, along with an early FIGO stage, are crucial to patients’ survival.
Author contributions
Formal analysis: Xinge Feng.
Investigation: Xinge Feng.
Resources: Lian Xu.
Supervision: Lian Xu.
Writing – original draft: Xinge Feng.
Writing – review & editing: Lian Xu.
Footnotes
Abbreviations: CT = computed tomography, IVF-ET = in vitro fertilization and embryo transfer, MCT = mature cystic teratoma, MRI = magnetic resonance imaging, SCC = squamous cell carcinoma.
The authors have no conflicts of interest to disclose.
References
- [1].Choi EJ, Koo YJ, Jeon JH, et al. Clinical experience in ovarian squamous cell carcinoma arising from mature cystic teratoma: a rare entity. Obstet Gynecol Sci 2014;57:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Oranratanaphan S, Khemapech N. Characteristics and treatment outcomes of patients with malignant transformation arising from mature cystic teratoma of the ovary: experience at a single institution. Asian Pac J Cancer Prev 2013;14:4693–7. [DOI] [PubMed] [Google Scholar]
- [3].Park JY, Kim DY, Kim JH, et al. Malignant transformation of mature cystic teratoma of the ovary: experience at a single institution. Eur J Obstet Gynecol Reprod Biol 2008;141:173–8. [DOI] [PubMed] [Google Scholar]
- [4].Hackethal A, Brueggmann D, Bohlmann MK, et al. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol 2008;9:1173–80. [DOI] [PubMed] [Google Scholar]
- [5].Araujo IB, Pinheiro MVC, Zanvettor PH, et al. High frequency of malignant transformation of ovarian mature teratoma into squamous cell carcinoma in young patients in Northeast Brazil. Int J Gynecol Pathol 2016;35:176–84. [DOI] [PubMed] [Google Scholar]
- [6].Ulbright TM. Gonadal teratomas: a review and speculation. Adv Anat Pathol 2004;11:10–23. [DOI] [PubMed] [Google Scholar]
- [7].Papadias K, Kairi-Vassilatou E, Kontogiani-Katsaros K, et al. Teratomas of the ovary: a clinico-pathological evaluation of 87 patients from one institution during a 10-year period. Eur J Gynaecol Oncol 2005;26:446–8. [PubMed] [Google Scholar]
- [8].Sumi T, Ishiko O, Maeda K, et al. Adenocarcinoma arising from respiratory ciliated epithelium in mature ovarian cystic teratoma. Arch Gynecol Obstet 2002;267:107–9. [DOI] [PubMed] [Google Scholar]
- [9].Kudva R, Ayachit GS, Ayachit A. Malignant melanoma arising in an ovarian mature cystic teratoma - a rare entity. J Clin Diagn Res 2015;9:ED14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. Int J Gynecol Cancer 2006;16:140–4. [DOI] [PubMed] [Google Scholar]
- [11].Yasunaga M, Saito T, Eto T, et al. Dedifferentiated chondrosarcoma arising in a mature cystic teratoma of the ovary: a case report and review of the literature. Int J Gynecol Pathol 2011;30:391–4. [DOI] [PubMed] [Google Scholar]
- [12].Contreras AL, Malpica A. Angiosarcoma arising in mature cystic teratoma of the ovary: a case report and review of the literature. Int J Gynecol Pathol 2009;28:453–7. [DOI] [PubMed] [Google Scholar]
- [13].Kita N, Satoh T, Onuki-Tanabe M, et al. Undifferentiated carcinoma with osteoclast-like multinucleated giant cells arising in an ovarian mature cystic teratoma. Gynecol Obstet Invest 2003;56:184–7. [DOI] [PubMed] [Google Scholar]
- [14].Park SB, Kim JK, Kim KR, et al. Preoperative diagnosis of mature cystic teratoma with malignant transformation: analysis of imaging findings and clinical and laboratory data. Arch Gynecol Obstet 2007;275:25–31. [DOI] [PubMed] [Google Scholar]
- [15].Park SB, Cho KS, Kim JK. CT findings of mature cystic teratoma with malignant transformation: comparison with mature cystic teratoma. Clin Imaging 2011;35:294–300. [DOI] [PubMed] [Google Scholar]
- [16].Lai PF, Hsieh SC, Chien JC, et al. Malignant transformation of an ovarian mature cystic teratoma: computed tomography findings. Arch Gynecol Obstet 2005;271:355–7. [DOI] [PubMed] [Google Scholar]
- [17].Mori Y, Nishii H, Takabe K, et al. Preoperative diagnosis of malignant transformation arising from mature cystic teratoma of the ovary. Gynecol Oncol 2003;90:338–41. [DOI] [PubMed] [Google Scholar]
- [18].Dos Santos L, Mok E, Iasonos A, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol 2007;105:321–4. [DOI] [PubMed] [Google Scholar]
- [19].Mekaru K, Kamiyama S, Masamoto H, et al. Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy: a case report. Arch Gynecol Obstet 2008;278:287–90. [DOI] [PubMed] [Google Scholar]
- [20].Arioz DT, Tokyol C, Sahin FK, et al. Squamous cell carcinoma arising in a mature cystic teratoma of the ovary in young patient with elevated carbohydrate antigen 19-9. Eur J Gynaecol Oncol 2008;29:282–4. [PubMed] [Google Scholar]
- [21].Liu LC, Huang RL, Lin YC, et al. Squamous cell carcinoma arising from an ovarian teratoma related to human papillomavirus infection: using a PCR-based reverse-blot assay. Taiwan J Obstet Gynecol 2011;50:543–5. [DOI] [PubMed] [Google Scholar]
- [22].Chiang AJ, Chen DR, Cheng JT, et al. Detection of human papillomavirus in squamous cell carcinoma arising from dermoid cysts. Taiwan J Obstet Gynecol 2015;54:559–66. [DOI] [PubMed] [Google Scholar]
- [23].Korkontzelos I, Stamatopoulos C, Antoniou N, et al. Malignant transformation of ovarian mature cystic teratoma in a postmenopausal woman presented as acute abdomen. Arch Gynecol Obstet 2010;281:177–9. [DOI] [PubMed] [Google Scholar]


