Abstract
Background:
Neoadjuvant chemotherapy (NAC) increases breast conservation rates in patients with resectable breast cancer at the associated cost of higher locoregional recurrence rates; however, the magnitude of the survival benefits of NAC for these patients remains undefined. Therefore, we aimed to clarify the survival benefit of NAC versus postoperative chemotherapy by conducting an updated meta-analysis of randomized clinical trials (RCTs).
Methods:
The authors searched the Cochrane Library, PubMed, Embase, Web of Science, Chinese biomedical literature database, and Chinese Scientific Journals full-text database from their inception to December 2016. The authors identified relevant RCTs that compared NAC with postoperative chemotherapy in the treatment of operable breast cancer. The main endpoints were overall survival (OS) and recurrence-free survival (RFS).
Results
: A total of 21 citations representing 16 unique studies were eligible. There were 787 deaths among 2794 patients assigned to NAC groups and 816 deaths among 2799 patients assigned to adjuvant chemotherapy groups. A meta-analysis of data indicated that there was no significant benefit in terms of OS ([hazard ratio [HR] = 1.03, 95% confidence interval [CI]: 0.94–1.13, P = .51) and RFS (HR = 1.01, 95% CI: 0.93–1.10, P = .80) between the NAC and postoperative chemotherapy groups. The pooled HR estimate for OS was not influenced by NAC cycles, the total number of chemotherapy cycles, administration of tamoxifen, administration of adjuvant chemotherapy, or type of NAC regimen. Subgroup analysis showed that the pooled HR estimate for RFS was influenced by anthracycline-containing regimens. Patients with a pathological complete response had superior survival outcomes compared with patients who had residual disease.
Conclusion:
The survival benefits for patients with operable breast cancer who received either NAC or adjuvant chemotherapy based on anthracycline regimens were comparable.
Keywords: breast cancer, meta-analysis, neoadjuvant chemotherapy, surgery
1. Introduction
Breast cancer is the most common cause of cancer-related death among women worldwide and the most frequently diagnosed type of cancer in women.[1] Neoadjuvant chemotherapy (NAC) is defined as chemotherapy administered before locoregional treatment, such as surgery and/or irradiation. It is well established that NAC plays an important role in downstaging tumors, eliminating micrometastases, and relieving tumor-related symptoms in patients with locally advanced breast cancer.[2] Currently, NAC is widely used in the management of breast cancer. NAC increases the rate of breast conserving surgery (BCS) if the breast cancer cell is sensitive to chemotherapy.[3] By contrast, there is a potential risk for tumor progression in patients with tumors resistant to chemotherapy. A meta-analysis in 2007 indicated that NAC increases the rate of BCS, yet at an associated higher risk of locoregional recurrences.[4] Recent studies have shown that a higher risk of local recurrences may eventually result in a decrease in the overall survival (OS) benefit.[5,6] NAC was initially only used for patients with locally advanced disease; however, at present, more patients present with early-stage breast cancer than in the past decades, and NAC has become common for operable breast cancer.[7] The 9-year follow-up results of the ABCSG-07 trial showed that NAC results in shorter recurrence-free survival (RFS), but not shorter OS, than does postoperative chemotherapy.[8] By contrast, the NSABP Protocol B-18 study showed equivalent OS and RFS rates for women who received NAC and those who underwent postoperative chemotherapy over 16 years of follow-up.[7] Furthermore, several studies have reported more recent long-term follow-up survival results. Given these circumstances, it is necessary to perform an updated meta-analysis to clarify the survival benefit of NAC versus postoperative chemotherapy in the treatment of operable breast cancer.
2. Methods
2.1. Search strategy and selection criteria
We searched the Cochrane Library, PubMed, Embase, Web of Science, Chinese biomedical literature database, and Chinese Scientific Journals full-text database from their inception to December 2016 to identify relevant randomized clinical trials (RCTs) that compare NAC with postoperative chemotherapy in the treatment of operable breast cancer. We used the following search terms: “breast cancer,” “breast carcinoma,” “neoadjuvant chemotherapy,” “primary chemotherapy,” and “preoperative chemotherapy.” We retrieved the bibliographies of eligible articles and previously published meta-analyses to identify additional relevant publications. Conference proceedings, including the American Society of Clinical Oncology and the European Society for Medical Oncology, were searched to identify any other trials. Literature searches were restricted to publications in English and Chinese. We included the study with the largest sample size and longest follow-up time when multiple articles of the same trial were published. Selection criteria for eligible trials were as follows: patients with operable breast cancer and no metastatic disease; RCTs that compared NAC followed by surgery with postoperative chemotherapy in the initial management of operable breast cancer; investigations of the survival benefits of NAC with any regimen; and RCTs published as a full-length article or an abstract. The present meta-analysis was based on previously published studies and no ethical approval or patient consent was required.
2.2. Data extraction
Two investigators independently assessed potentially relevant articles for eligibility; data were extracted by the same 2 investigators. Discrepancies were discussed until a consensus was reached. The following data were extracted from each eligible trial: first author's name, year of publication, recruitment period, study location, stage, sample size, NAC regimen, NAC schedule, follow-up time, and study endpoints of OS and RFS in each arm. The primary outcome measure was OS; the secondary outcome measure was RFS. OS was defined as the time from randomization to death from any cause. RFS was measured from the time of random assignment to breast cancer progression or relapse.
2.3. Assessment of the risk of bias of included studies
We assessed the risk of bias of included studies according to the following criteria: sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective outcome reporting, and other possible sources of bias.[9]
2.4. Statistical methods
To assess the survival effects of NAC in operable breast cancer patients, hazard ratios (HRs) were used to compare trials. For meta-analyses of time-to-event outcomes such as OS and RFS, the most appropriate statistic is the HR. Where possible, the HRs and the 95% confidence intervals (CIs) were obtained directly from each published article. When the HRs were unavailable, we estimated the HR indirectly using Parmer et al's method by means of the following formula: ln (HR) = (O − E)/V,[10] where O refers to the observed number of events in each arm, E is the log rank of the expected number of events in each arm, and V is the variance of log (HR).[10] The I2 and P statistics were used to test statistical heterogeneity among included studies; an I2 value above 50% was considered to represent large heterogeneity across studies.[11] We used a fixed-effect model to combine HRs for OS and RFS when there was no significant heterogeneity among included studies (I2 < 50%); alternatively, a random-effects model was used. A funnel plot was used to investigate the presence of publication bias in the present meta-analysis. All statistical tests were 2-sided, and the significance level was set at 5%. Data were analyzed using Revman version 5.3 software provided by the Cochrane Collaboration.
3. Results
3.1. The characteristics of included studies
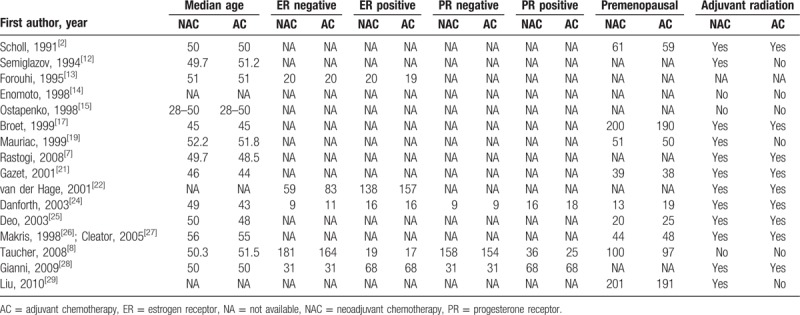
A total of 21 citations representing 16 unique studies were eligible. Figure 1 presents the Preferred Reporting Items for Systematic Reviews statement of the search results. One trial compared NAC plus radiotherapy with neoadjuvant radiotherapy[12]; the other trials were randomized comparisons of NAC versus surgery alone. Different combinations of NAC regimen, dosage, and cycle were tested in all the included studies; the main agents were fluorouracil, cyclophosphamide, and epirubicin compounds. Eligible patients received tamoxifen in 7 studies.[7,14,15,22,24,27,28] All trials were open-label without blinding procedures. Two studies had been published only in abstract form,[14,15] whereas the other studies were full-text publications. Five of the included studies extended their follow-up results,[7,17,19,21,27] and the survival data included in the present meta-analysis are from the updated final publications. All included studies reported follow-up times except for 1 trial[13]; median follow-up times ranged from 24 to 192 months. Three trials were conducted in France,[2,16,18] 3 in the UK,[13,21,26] 2 in the United States,[7,24] and 1 each in Russia,[12] Japan,[14] Lithuania,[15] Belgium,[22] India,[25] Austria,[8] Italy,[28] and China.[29] The characteristics of the included studies are described in Tables 1 and 2.
Figure 1.
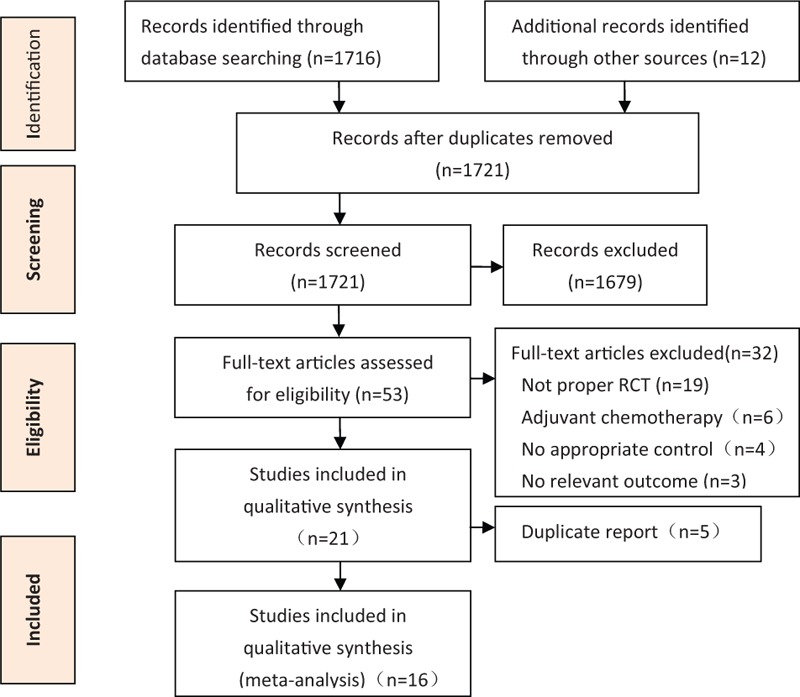
Preferred Reporting Items for Systematic Reviews statement of search results.
Table 1.
The characteristics of included studies.
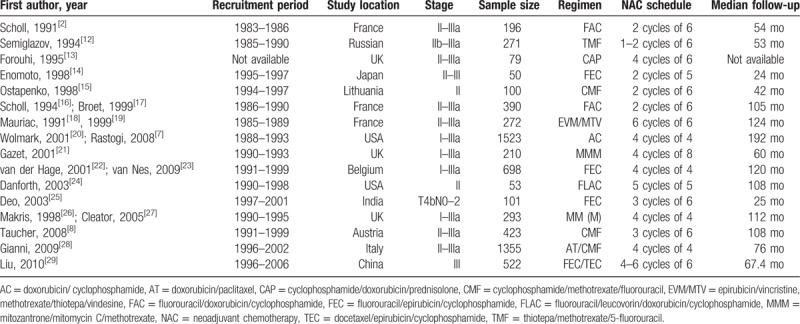
Table 2.
The characteristics of included studies continued.
3.2. Risk of bias in included studies
The risk of bias is summarized in Figs. 2 and 3. Inter-reviewer agreement for risk of bias assessment was excellent (κ = 0.91).
Figure 2.
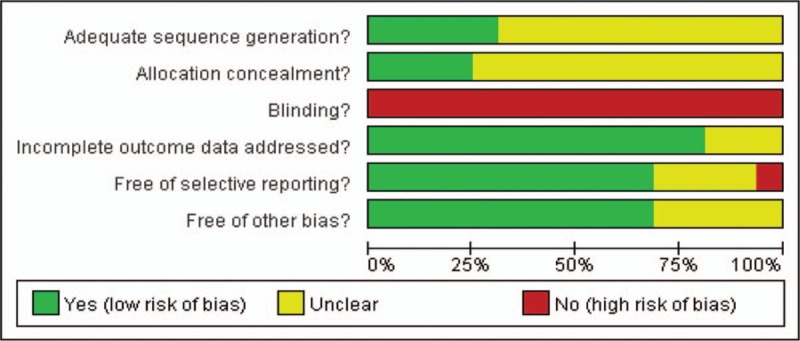
Risk of bias graph.
Figure 3.
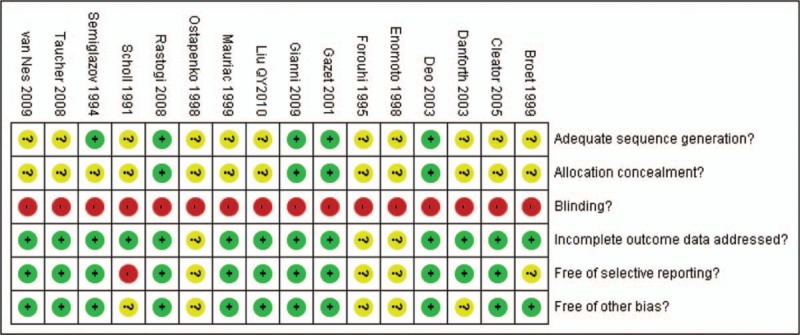
Risk of bias summary.
3.3. Results of the meta-analysis
3.3.1. Overall survival
OS data were available for a subset of 12 of the 16 trials with a total of 5593 patients. There were 787 deaths among 2794 patients assigned to NAC groups and 816 deaths among 2799 patients assigned to adjuvant chemotherapy groups. Meta-analysis indicated that there was no survival benefit between the NAC arm and the adjuvant chemotherapy arm in patients with operable breast cancer (HR = 1.03, 95% CI: 0.94–1.13, P = .51). This 0.03 relative increase in the risk of death was equivalent to an absolute detriment of 0.8% at 10 years decreasing the 10-year OS from 64% to 63.2%. There was no statistical heterogeneity among included studies (I2 = 0%, P = .89). HRs for OS in individual trials are shown in Fig. 4. The associated funnel plot showed a symmetrical distribution (Fig. 5).
Figure 4.
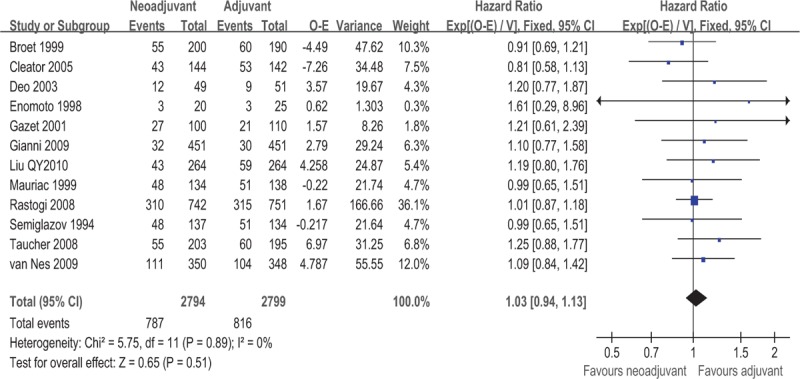
Forest plot of HRs comparing OS for patients NAC compared with surgery. HR = hazard ratio, NAC = neoadjuvant chemotherapy, OS = overall survival.
Figure 5.
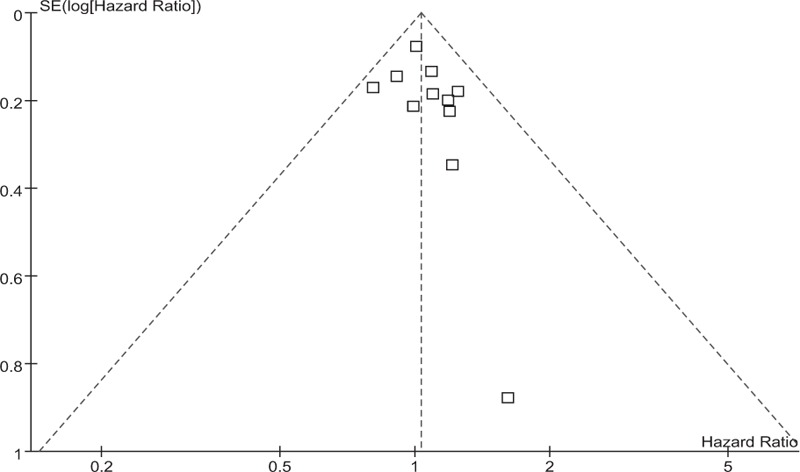
Funnel plot of the studies reporting on overall survival.
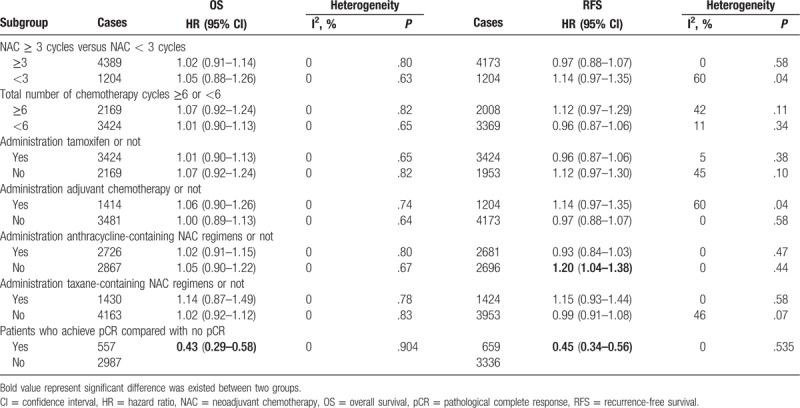
Subgroup analyses of OS according to NAC cycles (≥3 vs. <3), total number of chemotherapy cycles (≥6 vs. <6), administration of tamoxifen (yes vs. no), administration of adjuvant chemotherapy (yes vs. no), administration of anthracycline-containing regimens (yes vs. no), and administration of taxane-containing regimens (yes vs. no) were performed. There was no statistically significant difference between the pooled HR estimates in these subgroups (Table 2). Furthermore, patients with a pathological complete response (pCR) had a superior OS compared with patients without pCR (HR = 0.43, 95% CI: 0.29–0.58, P = .65); the results are shown in Table 3.
Table 3.
Results of subgroup analysis.
3.3.2. Recurrence-free survival
Data on RFS were available for a subset of 11 of the 16 trials with a total of 5377 patients. Meta-analysis indicated that there was no significant difference in RFS between the 2 groups for patients with operable breast cancer (HR = 1.01, 95% CI: 0.93–1.10, P = .80). This 0.01 relative increase in the risk of death was equivalent to an absolute detriment of 0.4% at 10 years decreasing the 10-year RFS from 48% to 47.6%. There was moderate statistical heterogeneity among included studies (I2 = 40%, P = .08). The pooled estimates for RFS for the trials that compared NAC followed by surgery with surgery alone are shown in Fig. 6. The associated funnel plot showed a symmetrical distribution (Fig. 7).
Figure 6.
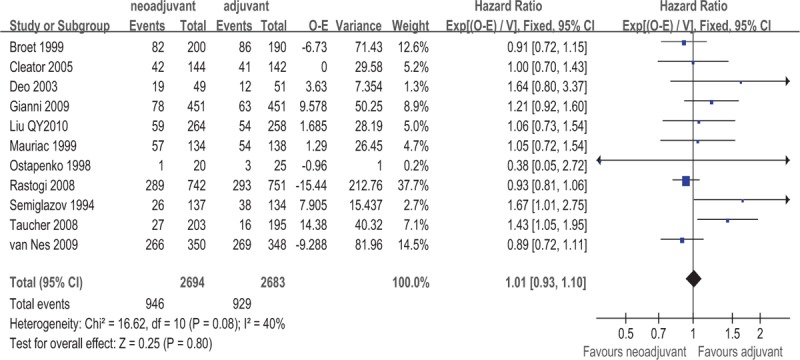
Forest plot of HRs comparing RFS for patients NAC compared with surgery. HR = hazard ratio, NAC = neoadjuvant chemotherapy, RFS = recurrence-free survival.
Figure 7.
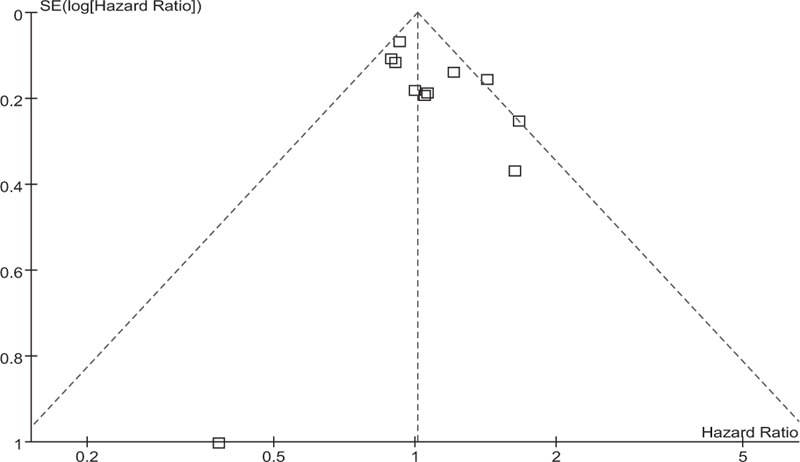
Funnel plot of the studies reporting on recurrence-free survival.
The subgroup analysis showed that RFS was decreased when the meta-analysis was restricted to studies of anthracycline-containing regimens (yes vs. no); a significant decrease in RFS was observed in the arms without anthracycline-containing regimens (HR = 1.20, 95% CI: 1.04–1.38, P = .01; Table 2). This 0.20 relative increase in the risk of death was equivalent to an absolute detriment of 6.5% at 10 years decreasing the 10-year RFS from 48% to 41.5%. The subgroup analysis showed that RFS was not improved even when the meta-analysis was restricted to trials on NAC cycle (≥3 vs. <3), total number of chemotherapy cycles (≥6 vs. <6), the administration of tamoxifen (yes vs. no), the administration of adjuvant chemotherapy (yes vs. no), or the administration of taxane-containing regimens (yes vs. no) (Table 3). Patients with a pCR had better RFS than patients without pCR (HR = 0.45, 95% CI: 0.34–0.56, P = .535); the results are shown in Table 3.
4. Discussion
NAC for the management of inoperable or locally advanced breast cancer only began in the 1970s.[25] During the past 20 years, numerous RCTs have studied the effect of NAC in the treatment of early-stage or operable breast cancer; however, whether these patients gain survival benefits and obtain improved prognoses remains controversial. The earlier meta-analysis indicated that NAC improved absolute breast conservation rates by 16.6% compared to postoperative chemotherapy, although at the associated cost of higher numbers of locoregional recurrences. Nevertheless, NAC was associated with fewer adverse effects.[4] However, it is still unclear whether higher locoregional recurrence rates would translate into a deterioration in survival. The objective of the present meta-analysis was to estimate the magnitude of OS and RFS benefits of NAC in patients with operable breast cancer based on time-to-event data. We identified 16 studies that assessed the effectiveness of NAC on clinical outcomes. These data were sufficient to provide reliable evidence regarding the application of NAC in the treatment of breast cancer. The present meta-analysis yielded several main findings. First, OS and RFS for patients with operable breast cancer who received either NAC or adjuvant chemotherapy were comparable. Second, anthracycline-containing regimens were superior to other regimens in terms of RFS; therefore, anthracycline-containing regimens should still be the first choice for NAC in patients with operable breast cancer. Third, OS reduction was not influenced by the number of NAC cycles, total number of chemotherapy cycles, administration of tamoxifen, administration of adjuvant chemotherapy, administration of anthracycline-containing regimens, and administration of taxane-containing regimens. Fourth, patients with a pCR achieved better OS and RFS than those with residual disease.
To our knowledge, there is the potential risk for tumor progression or delay to surgery during the NAC period in patients with tumors resistant to the NAC regimen.[4] Previous studies have demonstrated that tumor response rate may be an important predictor of survival benefit as a surrogate endpoint in trials of NAC for the treatment of breast cancer.[28] A Cochrane Systematic Review also demonstrated that patients with pCR were associated with superior survival benefits compared with those who had residual disease at pathological examination (HR = 0.48; 95% CI: 0.33–0.69).[30] Recently, the use of magnetic resonance imaging (MRI) to evaluate tumor response rates has shown benefits in predicting RFS after NAC in estrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative tumors; a radiological complete response at MRI after NAC is associated with a better prognosis.[31] Moreover, it is worth emphasizing that pCR can only be confirmed with a histopathological examination, but not with radiological or clinical examinations. Who will gain the largest survival benefit from NAC for breast cancer remains unclear; therefore, the identification of molecular marker expression in patients with breast cancer would be useful in predicting the response rate to NAC and individualizing patient treatment. A number of studies have been dedicated to the identification of predictive factors for NAC. Several studies have indicated that molecular markers such as the Ki-67 antigen, tumor suppressor p53, epidermal growth factor receptor, Bcl-2, ER/progesterone receptor status, and nm23-H1 may predict the response to NAC in patients with breast cancer.[32–34] Therefore, during the planning of an NAC treatment strategy, the choice of optimal treatment should be based not only on the disease stage but also on its biological characteristics that may predict cancer responsiveness to treatment. During the 2015 St. Gallen International Expert Consensus Conference on neoadjuvant therapy for early breast cancer, the panel strongly endorsed the use of NAC for stage II or III cancer and HER2-positive or triple-negative breast cancer as the preferred initial treatment approach. For HER2-positive cancers, the panel endorsed dual anti-HER2 neoadjuvant therapy with pertuzumab, or trastuzumab with chemotherapy. For triple-negative cancers, the panel recommended similar approaches to those that would be used in adjuvant therapy.[35] However, the design of the included studies was not based on molecular classification; therefore, it was not possible to carry out subgroup analysis according to cellular–molecular phenotype in the present meta-analysis.
Several studies have shown that different NAC regimens have distinct effects on the response rate of patients with breast cancer.[28] In the present meta-analysis, the NAC regimens were mostly fluorouracil, cyclophosphamide, and anthracycline regimens. The optimum NAC treatment regimen was not established, because included trials used different drugs, doses, and cycles of chemotherapy. Preliminary data from a trial have shown that incorporating paclitaxel into anthracycline-based NAC resulted in a significant improvement in RFS (HR = 0.73; 95% CI: 0.57–0.97) and distant RFS (HR 0.70; 95% CI: 0.51–0.96).[28] Furthermore, another meta-analysis demonstrated that the addition of taxane to an anthracycline-based regimen as adjuvant chemotherapy significantly improved both disease-free survival (HR = 0.83; 95% CI: 0.79–0.91) and OS (HR = 0.85; 95% CI: 0.79–0.87) for patients with high-risk early-stage breast cancer, irrespective of the type of taxane, ER expression, the number of axillary metastases, patient's age, and menopausal status.[36] A recent network meta-analysis identified the most effective adjuvant chemotherapy regimen for early-stage breast cancer in terms of OS by comparing regimens listed in the National Comprehensive Cancer Network guidelines, which showed that sequential anthracycline–cyclophosphamide and taxane is likely to be the most effective regimen regardless of hormone receptor status.[37] Recently, a meta-analysis based on 5 RCTs indicated that NAC in combination with dual HER2-targeted therapy with lapatinib and trastuzumab significantly improved pCR rates in patients with HER2-positive breast cancer, regardless of the pCR definition or hormone receptor status.[38] However, the administration of lapatinib is associated with a higher risk of adverse events,[39] and thus adding anti-HER2 drugs to NAC is valuable. Therefore, future trials should identify the optimum NAC regimen and aim to minimize treatment toxicities and the effect of treatment on patients’ quality of life.
The pCR rate was only 2% for patients with lobular cancer, whereas it was 12% for patients with ductal cancer, confirming that breast cancer subtype may be a predictive factor for achieving pCR to NAC.[40] Straver et al[41] also suggested that sensitivity to NAC depends on breast cancer subtype; however, the predictive value of the histological breast cancer subtype was emphasized. It is well-known that breast cancer is a disease with clinical and biological heterogeneity; therefore, different molecular subgroups display distinct clinical behavior, which results in significant differences in survival outcomes. Our findings highlight the need to differentiate and analyze breast cancer subtypes separately in future trials. Thus, future trials should focus on the identification and selection of patients who will be most likely to benefit from specific treatment options.
Although recurrence rates of NAC and adjuvant chemotherapy are significantly different, they have comparable survival outcomes in terms of OS and RFS. Concerns may be raised as to why increases in locoregional recurrence rates did not translate to decreases in survival outcomes. It is possible that NAC increases the breast conservation rate compared with adjuvant chemotherapy.[4] It is well known that BCS is associated with a higher locoregional recurrence rate than mastectomy, although evidence is increasingly demonstrating that BCS after systemic treatment can be equivalent to mastectomy in terms of long-term OS.[42] Furthermore, adjuvant chemotherapy, radiotherapy, hormonal therapy, and molecular targeted therapy are important in the treatment of breast cancer. Multidisciplinary systemic treatment plays a crucial role in treating patients with breast cancer. In conclusion, the available evidence for operable breast cancer points to similar OS and RFS rates between NAC followed by surgery versus surgery followed by adjuvant chemotherapy.
Author contributions
Conceptualization: Yan Chen, Xiu-E Shi, Ke-Hu Yang.
Data curation: Jin-Hui Tian.
Formal analysis: Xu-Juan Yang.
Investigation: Xu-Juan Yang, Yong-Feng Wang.
Methodology: Yan Chen, Ke-Hu Yang.
Project administration: Yan Chen, Xiu-E Shi.
Resources: Yong-Feng Wang.
Software: Jin-Hui Tian.
Supervision: Yong-Feng Wang, Ke-Hu Yang.
Validation: Jin-Hui Tian, Yong-Feng Wang.
Visualization: Yong-Feng Wang.
Writing – original draft: Yan Chen, Xiu-E Shi.
Writing – review and editing: Yan Chen, Xiu-E Shi, Yong-Feng Wang, Ke-Hu Yang.
Footnotes
Abbreviations: BCS = breast conserving surgery, CI = confidence interval, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, MRI = magnetic resonance imaging, NAC = neoadjuvant chemotherapy, OS = overall survival, pCR = pathological complete response, RCTs = randomized clinical trials, RFS = recurrence-free survival.
Yan Chen and Xiu-E Shi have equally contribution to this work.
This study was supported by the Natural Science Foundation of Gansu Province (No. 1606RJYA265).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Scholl SM, Asselain B, Palangie T, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991;27:1668–71. [DOI] [PubMed] [Google Scholar]
- [3].Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672–85. [DOI] [PubMed] [Google Scholar]
- [4].Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007;94:1189–200. [DOI] [PubMed] [Google Scholar]
- [5].Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–55. [DOI] [PubMed] [Google Scholar]
- [6].Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8. [DOI] [PubMed] [Google Scholar]
- [7].Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- [8].Taucher S, Steger GG, Jakesz R, et al. The potential risk of neoadjuvant chemotherapy in breast cancer patients—results from a prospective randomized trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-07). Breast Cancer Res Treat 2008;112:309–16. [DOI] [PubMed] [Google Scholar]
- [9].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [10].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [11].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [12].Semiglazov VF, Topuzov EE, Bavli JL, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol 1994;5:591–5. [DOI] [PubMed] [Google Scholar]
- [13].Forouhi P, Dixon JM, Leonard RC, et al. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br J Surg 1995;82:79–82. [DOI] [PubMed] [Google Scholar]
- [14].Enomoto K, Ikeda T, Matsui A, et al. P73 neoadjuvant therapy in stage II with T ≥ 4CM and stage III breast cancer. Eur J Cancer 1998;34:S33. [Google Scholar]
- [15].Ostapenko V, Pipiriené T, Valuckas K. P78 primary chemotherapy in conservative treatment of stage II breast cancer patients. Eur J Cancer 1998;34:S34. [Google Scholar]
- [16].Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 1994;30:645–52. [DOI] [PubMed] [Google Scholar]
- [17].Broet P, Scholl SM, de la Rochefordiere A, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58:151–6. [DOI] [PubMed] [Google Scholar]
- [18].Mauriac L, Durand M, Avril A, et al. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm. Results of a randomized trial in a single centre. Ann Oncol 1991;2:347–54. [DOI] [PubMed] [Google Scholar]
- [19].Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 1999;10:47–52. [DOI] [PubMed] [Google Scholar]
- [20].Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;30:96–102. [DOI] [PubMed] [Google Scholar]
- [21].Gazet JC, Ford HT, Gray R, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol 2001;12:685–91. [DOI] [PubMed] [Google Scholar]
- [22].van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224–37. [DOI] [PubMed] [Google Scholar]
- [23].van Nes JG, Putter H, Julien JP, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat 2009;115:101–13. [DOI] [PubMed] [Google Scholar]
- [24].Danforth DN, Jr, Cowan K, Altemus R, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Ann Surg Oncol 2003;10:635–44. [DOI] [PubMed] [Google Scholar]
- [25].Deo SV, Bhutani M, Shukla NK, et al. Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). J Surg Oncol 2003;84:192–7. [DOI] [PubMed] [Google Scholar]
- [26].Makris A, Powles TJ, Ashley SE, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998;9:1179–84. [DOI] [PubMed] [Google Scholar]
- [27].Cleator SJ, Makris A, Ashley SE, et al. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol 2005;16:267–72. [DOI] [PubMed] [Google Scholar]
- [28].Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol 2009;27:2474–81. [DOI] [PubMed] [Google Scholar]
- [29].Liu Q-Y, Huang X-Y, Li S-Y, et al. Effects of neoadjuvant chemotherapy for locally advanced breast cancer. Chin J Oncol Prevent Treat 2010;2:85–8. [Google Scholar]
- [30].Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007;2:CD005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Loo CE, Rigter LS, Pengel KE, et al. Survival is associated with complete response on MRI after neoadjuvant chemotherapy in ER-positive HER2-negative breast cancer. Breast Cancer Res 2016;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li XR, Liu M, Zhang YJ, et al. ER, PgR, HER-2, Ki-67, topoisomerase IIalpha, and nm23-H1 proteins expression as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol 2011;28:48–54. [DOI] [PubMed] [Google Scholar]
- [33].Chen MB, Zhu YQ, Xu JY, et al. Value of TP53 status for predicting response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. PLoS ONE 2012;7:e39655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang D, Chen MB, Wang LQ, et al. Bcl-2 expression predicts sensitivity to chemotherapy in breast cancer: a systematic review and meta-analysis. J Exp Clin Cancer Res 2013;32:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 2008;26:44–53. [DOI] [PubMed] [Google Scholar]
- [37].Fujii T, Le Du F, Xiao L, et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol 2015;1:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hicks M, Macrae ER, Abdel-Rasoul M, et al. Neoadjuvant dual HER2-targeted therapy with lapatinib and trastuzumab improves pathologic complete response in patients with early stage HER2-positive breast cancer: a meta-analysis of randomized prospective clinical trials. Oncologist 2015;20:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun J, Chen C, Yao X, et al. Lapatinib combined with neoadjuvant paclitaxel-trastuzumab-based chemotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a meta-analysis of randomized controlled trials. Oncol Lett 2015;9:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Debska-Szmich S, Krakowska M, Czernek U, et al. The role of preoperative systemic treatment in patients with breast cancer. Contemp Oncol (Pozn) 2016;20:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Straver ME, Rutgers EJ, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol 2010;17:2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412–9. [DOI] [PubMed] [Google Scholar]


