Abstract
Background
Pesticides used in agriculture can be taken into worker homes and pose a potential risk for children and other family members. This study focused on identification of potential intervention points at the workplace.
Methods
Workers (N = 46) recruited from two tree fruit orchards in Washington State were administered a 63-item pesticide safety questionnaire. Dust was collected from commuter vehicles and worker homes and analyzed for four organophosphorus (OP) pesticides (azinphosmethyl, phosmet, chlorpyrifos, malathion).
Results
Geometric mean azinphosmethyl concentrations in dust for three worker groups (16 pesticide handlers, 15 green fruit thinners, 15 organic orchard workers) ranged from 0.027–1.5 μg/g, with levels in vehicle dust higher than in house dust, and levels in house dust from handlers’ homes higher than levels from tree fruit thinners’ homes. Vehicle and house dust concentrations of azinphosmethyl were highly associated (R2 = 0.44, P < 0.001). Significant differences were found across worker groups for availability of laundry facilities, work boot storage, frequency of hand washing, commuter vehicle use, parking location, and safety training.
Conclusions
These findings support a focus on intervention activities to reduce take home pesticide exposure closer to the source of contamination; specifically, the workplace and vehicles used to travel to the workplace. Am. J. Ind. Med. 56:1063–1071, 2013.
Keywords: pesticides, residential exposure, dust, para-occupational, take home exposure, workplace, vehicle, workers, applicators, thinners, pesticide handlers
INTRODUCTION
The potential for workers to bring occupational contaminants into the home has been a public health concern for well over a decade [NIOSH, 1995]. This take home exposure pathway, sometimes referred to as para-occupational exposure, has been studied most extensively for lead [Czachur et al., 1995; Sutton et al., 1995; Piacitelli et al., 1995; Piacitelli et al., 1997], and pesticides [Simcox et al., 1995; Loewenherz et al., 1997; Lu et al., 2000; McCauley et al., 2001, 2003, 2006; Curl et al., 2002; Quandt et al., 2006; Salvatore et al., 2009; Harnly et al., 2009].
Understanding take home pesticide exposure pathways and factors that contribute to agricultural worker family exposure is of particular importance due to the potential health effects of chronic exposure to pesticides. Prenatal and childhood exposure to organophosphorus (OP) pesticides may have significant consequences in neurobehavioral development in young children [Rohlman et al., 2005; Rauh et al., 2006; Marks et al., 2010; Bouchard et al., 2011]. Two studies have reported that parental occupations involving pesticides were associated with the development of childhood leukemia and other cancers [Daniels et al., 1997; Wigle et al., 2009].
Most take home pesticide exposure studies have been observational, with no systematic attempt made to reduce exposures. Those studies that have involved interventions have focused on personal and residential hygiene rather than workplace factors [McCauley et al., 2003, 2006; Rao et al., 2006; Strong et al., 2009]. The largest of such studies, conducted in the Yakima Valley of Washington State, did not find a change in exposure following a 2-year community-based intervention [Thompson et al., 2008], and reported only small improvements in 2 of 10 self-reported behaviors [Strong et al., 2009]. One exception is a recent study that reported positive findings for some worksite hygienic behaviors based on worker self-reports [Salvatore et al., 2009]. Two studies have documented a link between pesticide levels in farm worker commuter vehicles and elevated pesticide levels in the home, but these did not involve interventions [Lu et al., 2000; Curl et al., 2002].
The aim of this study was to identify take home pesticide exposure pathways and the occupational characteristics and behaviors associated with these pathways. The study focused on identification of potential intervention points at the workplace rather than interventions in worker residences. This approach is based on the industrial hygiene precept that the best approach to controlling exposures is at the source [Plog, 2001]. The guiding principle of the research is straightforward: workplace chemicals should stay in the workplace. Information from this study will be used to develop interventions to minimize take home pesticide exposure among farm workers and their families.
METHODS
The study was cross-sectional in design, focusing on agricultural workers at two eastern Washington State tree fruit orchards: one was a conventional orchard that used OP pesticides; the other was an orchard that followed organic production procedures [USDA, 2011]. Industrial hygiene walkthrough surveys documented the characteristics of facilities at each orchard. Workers were interviewed regarding use of facilities and hygienic behaviors. Commuter vehicle dust and house dust samples were collected for each worker. House dust was used as the outcome variable in determining the relative importance of factors affecting the take home exposure pathway.
Recruitment
Recruitment took place in mid-June 2003, and the study extended from late June through the end of July. The study was first described to workers in either a group setting or individually at the workplace. Interested workers provided their contact information and were telephoned at home to further explain the study and answer any questions. If the worker wanted to participate and met the participation requirements, an appointment was scheduled for a visit to the worker’s home for the interview and collection of dust samples. Eligibility requirements for the agricultural workers were as follows: 16 years or older; employed at the orchards; primary driver of a vehicle used to commute to work; carpeting present in common areas of the home. Participants were asked to refrain from vacuuming their homes for 3 days prior to the dust sampling and interview appointment. The University of Washington Institutional Review Board approved the study procedures, and all participants provided written informed consent. The 46 agricultural workers who agreed to participate were assigned to one of three worker groups: 16 pesticide handlers at the conventional orchard (i.e., workers who mixed, loaded, or applied pesticides), 15 green fruit thinners at the conventional orchard (i.e., workers who removed immature fruit from trees), and 15 workers at the organic orchard who served as a reference group (i.e., workers who conducted similar activities, but who did this work in the absence of contact with synthetic organic pesticides). All study participants were Latino males.
Pesticide Use
The study focused primarily on the OP pesticide azinphosmethyl (Guthion™) because of its relatively high toxicity and its widespread use in orchards to control the codling moth at the time of the study. We also measured residues of three other OP pesticides used commonly in tree fruit orchards in Washington and at the conventional orchard participating in this study: phosmet (Imidan™) to control codling moth; chlorpyrifos (Lorsban™) for leaf rollers; malathion for fruit fiies in cherries. Azinphosmethyl, phosmet, and chlorpyrifos were applied from the ground with airblast sprayers, whereas malathion was applied by helicopter. Malathion applications were not performed by any of the pesticide handlers in this study. Chlorpyrifos was applied in the second half of March, azinphosmethyl and phosmet were applied mid-May through July, and malathion was applied in late June and early July.
Geo-Mapping
A spatial analysis using geographic information system (GIS) methods was conducted to determine the distance of the residences of study participants to the closest orchards. Home addresses were obtained from participants. Based on middle-of-the-road field observations, points were placed into Esri ArcMap 9.0 (Redlands, CA) for measurement. Points locating the orchard field edges were also placed into Esri ArcMap 9.0 based on field observations. A measurement, in meters, from the middle-of-the-road-residence points to the nearest field edge was determined using the Central Feature distance tool in Esri ArcToolbox (Redlands, CA). The results were exported and sorted in Microsoft Excel for analysis.
Worksite Evaluation
An industrial hygienist conducted a worksite walk-through of both the central shop area and the orchard work locations to document the presence or absence of hygienic facilities, including changing areas, storage facilities for work clothes and personal protective equipment (PPE), laundry or washing facilities for work clothes and PPE, showers, hand wash stations, bathrooms, and drinking water.
Interviews
Bilingual and bicultural research team members administered a 63-item questionnaire to each study participant in the participant’s choice of Spanish or English. In addition to demographic information, questions covered six pesticide safety topic areas: workplace facilities, workplace behaviors, home laundry facilities, behaviors at home, commuter vehicle use, and pesticide safety training.
Dust Sampling and Analysis
Dust samples from the participant homes and vehicles were collected in late June and July 2003 at the same time that the questionnaire was administered. Trained field staff collected the dust with a high volume simplified small surface sampler (HVS4; CS3, Inc., Sandpoint, ID). The sampler was leak-checked prior to collecting each sample. After each sample was collected, loose material from inside the sampler was removed. The sampling apparatus was then rinsed three times with isopropanol and parts were air dried before reassembly for the next use.
House dust samples were collected from exposed high traffic carpeted common areas, typically the front entry and living room. A total of 1.5 m2 was sampled following a prescribed sampling procedure, and while maintaining a sampler pressure drop of approximately 30 cm water. If needed, additional 1.5 m2 areas were sampled until collection of approximately 10 g of bulk dust. Vehicle dust was collected from the driver’s foot well without moving FLoor mats or sampling from underneath. If necessary, the front passenger foot well was also sampled to obtain the 10 g sample. All dust samples were stored on ice at the field site, and at −20°C in the field laboratory until transported on ice to the UW laboratory and stored at −10°C until analysis. Field blank and field spike samples were treated in a manner similar to samples collected from homes and vehicles, and were transported, stored and analyzed with those samples. Blank samples were less than the limit of detection. No losses were observed in the field spikes.
Dust fines for each sample were obtained by sieving with a 150 micron sieve with a lid and catch pan (No. 100 USA Standard Testing Sieve, ASTME-11 specification; VWR, West Chester, PA) and shaken for 10 min in a sieve shaker (Model RX-24; WS Tyler, Inc., Mentor, OH). Dust fines were transferred from the pan to a clean polyethylene bottle, and stored in the freezer. Screens were cleaned with acetone and air-dried between each use. Dust was analyzed for the four OP pesticides used at the orchard study site. Procedures for sample extraction and gas chromatographic analysis were those described by Moate et al. [2002]. A pulsed flame photometric detector (PFPD) was used to increase sensitivity to low pesticide residue concentrations in the dust. The limit of detection was 0.030 μg/g and the limit of quantitation was 0.073 μg/g for all four pesticides.
Data Analysis
Pesticide residues in dust are reported as concentrations (μg/g). The data were right skewed and therefore log-transformed values were used for data analysis, with the geometric mean (GM) and geometric standard deviation (GSD) reported for each of the three worker groups. One-way ANOVA was used to assess differences between the GMs of the three worker groups. In those cases where significant differences were found the Tukey test for multiple comparisons was employed.
Scatterplots of log-vehicle dust and log-house dust pesticide concentrations were prepared. A linear regression model was used to predict pesticide concentrations in house dust from pesticide concentrations in vehicle dust. Comparisons of handler and thinner responses to questions regarding behaviors and workplace factors were made using Fischer’s Exact Test (two-sided).
RESULTS
All study participants were male Latinos, ranging in age from 21 to 61. Geospatial mapping indicated that 44 of the 46 study participants lived at a substantial distance from agricultural pesticide use: 32 workers (15 reference, 13 thinners, and 4 handlers) lived in cities or rural communities located at least 9 km distant from pesticide-treated farmland; 12 workers (10 handlers, 2 thinners) lived in a residential community approximately 1.5 km from orchards, with a set of hills separating the community from the orchards. Two workers (handlers) lived on orchard property, with their homes approximately 6 m from the nearest orchard.
GM pesticide concentrations in vehicle dust and house dust differed significantly across worker groups for all pesticides, except for malathion in house dust (Table I). Comparisons of worker groups with one another indicated that both thinners and handlers were significantly different from workers at the reference orchard in regard to azinphosmethyl, phosmet and chlorpyrifos concentrations in both vehicle and house dust, as well as malathion concentrations in vehicle dust for pesticide handlers.
TABLE I.
House and Commuter Vehicle Dust Pesticide Concentrations for Handlers, Thinners and Organic Orchard (Reference) Workers: Geometric Means (GM), 95% Confidence Intervals (CI), and Geometric Standard Deviations (GSD)
| Pesticide | Pesticide concentration in dust (μg/g) | ANOVAd | Tukey test P-valuee (comparison of worker groups) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Handlers (n = 16)a | Thinners (=15)b | Reference (n = 15)c | P-value for occupation | Handlers vs. reference | Thinners vs. reference | Handlers vs. thinners | ||
| Azinphosmethyl | ||||||||
| Vehicle | GM 95% CI | 1.5 (1.04, 2.30) | 0.34 (0.16, 0.71) | 0.027 (0.015, 0.047) | <0.001 | <0.001 | <0.001 | 0.0011 |
| GSD | 2.0 | 3.6 | 2.8 | |||||
| House | GM 95% CI | 0.55 (0.37, 0.81) | 0.22 (0.10, 0.45) | 0.054 (0.024, 0.12) | <0.001 | <0.001 | 0.0089 | 0.086 |
| GSD | 2.0 | 3.8 | 3.9 | |||||
| Phosmet | ||||||||
| Vehicle | GM 95% CI | 1.6 (0.95, 2.7) | 0.62 (0.39, 0.99) | 0.098 (0.053, 0.18) | <0.001 | <0.001 | <0.001 | 0.031 |
| GSD | 2.5 | 2.3 | 3.0 | |||||
| House | GM 95% CI | 0.32 (0.16, 0.67) | 0.43 (0.31, 0.61) | 0.095 (0.052, 0.18) | <0.001 | 0.0087 | 0.0010 | 0.71 |
| GSD | 3.7 | 1.9 | 2.7 | |||||
| Chlorpyrifos | ||||||||
| Vehicle | GM 95% CI | 0.97 (0.45, 2.1) | 0.068 (0.039, 0.12) | 0.016 (0.014, 0.018) | <0.001 | <0.001 | <0.001 | <0.001 |
| GSD | 3.8 | 2.7 | 1.3 | |||||
| House | GM 95% CI | 0.21 (0.13, 0.35) | 0.081 (0.038, 0.17) | 0.026 (0.014, 0.050) | <0.001 | <0.001 | 0.031 | 0.062 |
| GSD | 2.5 | 3.8 | 2.9 | |||||
| Malathion | ||||||||
| Vehicle | GM 95% CI | 0.13 (0.053, 0.33) | 0.064 (0.023, 0.018) | 0.023 (0.016, 0.035) | 0.0083 | 0.0061 | 0.16 | 0.37 |
| GSD | 4.8 | 6.0 | 2.1 | |||||
| House | GM 95% CI | 0.048 (0.020, 0.11) | 0.029 (0.014, 0.058) | 0.029 (0.012, 0.073) | 0.55 | 0.63 | 0.99 | 0.59 |
| GSD | 4.8 | 3.5 | 4.6 | |||||
Sample size for handlers was 14 for vehicle dust (one vehicles old before sampling could take place, one vehicle was not sampled because it had nocarpetin footwell), and15for house dust (one sample with insufficient dust for analysis).
Sample size for thinners was14 for vehicle dust (one sample with insufficient dust for analysis).
Sample size for reference workers (organic orchard) was13 for house dust (two workers lived in same homes as other workers, so only one house dust sample was collected from each home and assigned to the first worker interviewed in the home. The second worker in the home did not have a house dust sample).
Test for statistical significance = 0.05.
Tukey test for multiple comparison of means; 95% family-wise confidence interval.
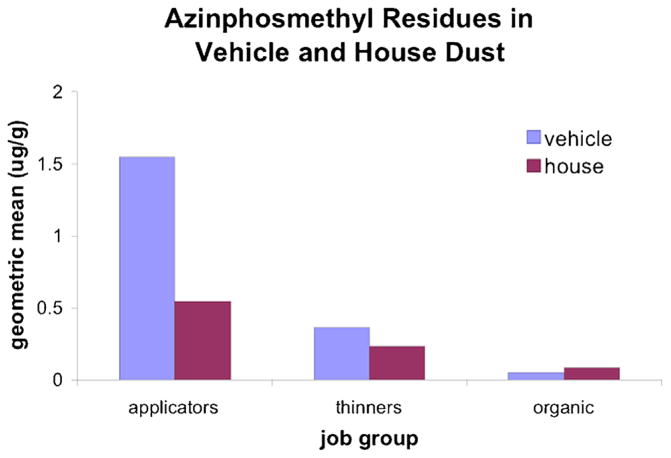
Vehicle dust concentrations of azinphosmethyl (P < = 0.0011), phosmet (P = 0.031) and chlorpyrifos (P < 0.001) were significantly higher for handlers when compared to thinners. House dust concentrations of azinphosmethyl were mariginally higher (P = 0.086) for handlers compared to thinners, as were concentrations of chlorpyrifos (P = 0.062). Figure 1 presents data for azinphosmethyl to illustrate the range of pesticide concentrations in vehicle and house dust across worker groups. Handlers were the most highly exposed group for azinphosmethyl in both the vehicle and the home, while thinners were subject to intermediate exposure, and workers in the reference orchard had minimal exposure.
FIGURE 1.
Azinphosmethyl concentration in vehicle and house dust (geometric mean, 95% confidence interval).
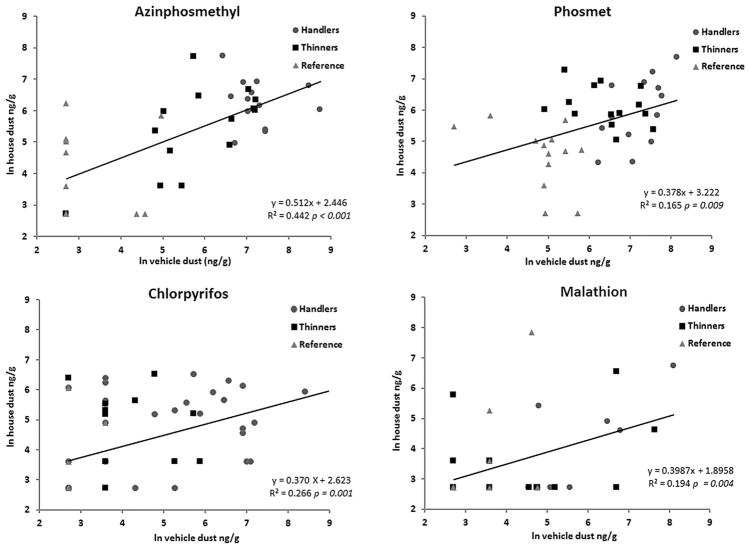
Pesticide concentration in vehicle dust was a significant predictor of pesticide concentration in house dust for all workers for each pesticide (Fig. 2), and was a significant predictor for thinners and handlers together for azinphosmethyl (P = 0.002) and malathion (P = 0.02). Only azinphos-methyl had a statistically significant R-squared value for handlers and thinners (R2 = 0.34).
FIGURE 2.
Regression analysis of pesticide concentration in vehicle dust as a predictor of pesticide concentration in house dust. Data are presented as natural logs of concentrations (ng/g). The sample size is 40 rather than 46 due to missing vehicle dust or house dust sample (see Table I footnotes).
Table II presents a comparison of responses from 16 handlers and 15 thinners to questions regarding workplace facilities, workplace behaviors, home laundry facilities, behaviors at home, commuter vehicle use, and pesticide safety training. Findings for handlers and thinners combined were as follows: (1) most workers did not have an area at the workplace for changing out of work clothes or for washing clothes, but did have access to hand washing facilities; (2) most workers washed with soap and water before leaving the workplace; (3) most did not change out of work clothes or boots before leaving the workplace; rather, they stored their work clothes and work boots at home; (4) more than two-thirds of workers did not have laundry facilities in their homes; (5) most wore both work clothes and work boots into their homes; (6) most workers parked their vehicles next to or in the fields and left windows open during work; (7) about half of the workers vacuumed their vehicles once a week, while the others did not vacuum regularly; and (8) most workers received pesticide safety training at the workplace and the training was delivered in a language that they understood well; however, about one-third of the workers had not received training in the past year.
TABLE II.
Self-Reported Access to Hygienic Facilities and Hygienic Practices Among Pesticide Handler (n = 16) and Green Fruit Thinners (n = 15)
| Category | Question | Responsea | Total | Handlers | Thinners | H vs. Tb (P-value) |
|---|---|---|---|---|---|---|
| Workplace facilities | Is there an area at your workplace where you can change into or out of your work clothes? | Yes | 8 | 6 | 2 | 0.22 |
| No | 23 | 10 | 13 | |||
| Are there laundry facilities available for you to wash your work clothes at your workplace? | Yes | 6 | 6 | 0 | 0.02 | |
| No | 24 | 10 | 14 | |||
| Is there an area at your workplace where you can wash? | Yes | 23 | 11 | 12 | 0.68 | |
| No | 8 | 5 | 3 | |||
| How often are soap and towels available for you to use? | Frequent | 26 | 14 | 12 | 1.00 | |
| Never | 4 | 2 | 2 | |||
| Workplace behavior | Do you usually change out of your work clothes before leaving the workplace? | >1/2 time | 3 | 3 | 0 | 0.23 |
| Never | 26 | 13 | 13 | |||
| Do you usually change out of your work boots before leaving the workplace? | Frequent | 4 | 3 | 1 | 0.61 | |
| Never | 25 | 13 | 12 | 0.10 | ||
| Where do you usually store your work clothes when you are not wearing them? | At work | 4 | 4 | 0 | ||
| Not at work | 27 | 12 | 15 | |||
| Where do you usually store your work boots when you are not wearing them? | At work | 5 | 5 | 0 | 0.04 | |
| Not a work | 26 | 11 | 15 | |||
| How often do you wash with soap and water before leaving the workplace? | Frequent | 18 | 14 | 4 | 0.002 | |
| Never | 12 | 2 | 10 | |||
| Home facilities | Do you have a washer and dryer available for you to use at home? | Yes | 9 | 2 | 7 | 0.05 |
| No | 22 | 14 | 8 | |||
| Home behavior | Do you usually wear your work clothes into your house when you come home from work? | Yes | 26 | 12 | 14 | 0.33 |
| No | 4 | 4 | 1 | |||
| Do you usually wear your work boots into your house when you come home from work? | Yes | 24 | 11 | 13 | 0.39 | |
| No | 7 | 5 | 2 | |||
| Commuter vehicle use | Do you usually drive other people to work? | Yes | 14 | 3 | 11 | 0.004 |
| No | 17 | 13 | 4 | |||
| Where do you usually park your car at work? | Away from fields | 8 | 8 | 0 | 0.002 | |
| Next to/in fields | 23 | 8 | 15 | |||
| Do you usually leave your car windows closed or open while you are at work? | Closed | 8 | 5 | 3 | 0.68 | |
| Open | 18 | 9 | 9 | |||
| How often do you or someone else vacuum the inside of your car? | 1 time/week | 16 | 8 | 8 | 0.73 | |
| <1 time/month | 14 | 8 | 6 | |||
| Safety training | Has this employer or someone at your workplace trained you on how to work safely with pesticides? | Yes | 26 | 16 | 10 | 0.02 |
| No | 5 | 0 | 5 | |||
| Was the training offered in a language you understood well? | Yes | 22 | 16 | 6 | 0.34 | |
| No | 1 | 0 | 1 | |||
| When was the last time you had pesticide safety training? | <1 year ago | 19 | 15 | 4 | 0.001 | |
| >1 year ago/never | 9 | 1 | 8 |
Answers of don’t know or a no response were removed from the analysis for each question.
Comparison of handler and thinner responses using Fischer’s Exact Test (two-sided).
Several interesting differences emerged when handler and thinner responses were compared (Table II). Some of the handlers reported the availability of laundry facilities at work, but none of the thinners did; some of the handlers stored work boots at the workplace, but this was not the case for any of the thinners. There was a very significant contrast in washing with soap and water before leaving the workplace: nearly all handlers did so, while nearly all of the thinners did not. In regard to commuter vehicle use, most handlers drove to work alone, whereas most thinners drove others to work; half of the handlers parked away from the fields, but all of the thinners parked next to or in the fields. In regard to safety training, all handlers reported receiving training, but only two out of three thinners did so. Virtually all handlers had been trained within the year, but two-thirds of the thinners reported that training either did not occur or that it occurred more than a year ago.
DISCUSSION
Several important observations can be made with regard to the influence of occupational behaviors on the take home pesticide exposure pathway from these findings. Worker group (handlers > thinners) was found to be a significant predictor of pesticide concentrations in homes for three out of four of the pesticides tested, suggesting that an understanding of job tasks alone may serve as a reliable indicator of take home exposure potential. This finding is consistent with guidance provided by the U.S. Environmental Protection Agency in its Worker Protection Standard documentation (USEPA 2013). A recent study of Washington State agricultural workers found that mixing and loading activities were a significant risk factor for pesticide handlers [Hofmann et al., 2010].
In addition to work-to-home transmission, residential contamination with pesticides can be affected by pesticide drift, so distance between residence and pesticide-treated farmland is an important factor to consider. However, the evidence for home contamination due to proximity to agricultural spraying indicates that this effect is not demonstrable after about one-quarter mile, or 0.4 km [Simcox et al., 1995; Loewenherz et al., 1997; Lu et al., 2000]. Results from geo-coding found that all but two of the 46 workers in this study lived at least 1.5 km away from orchards, or more than three times the distance documented for this effect.
As in previous studies of this nature [Curl et al., 2002], there was a significant association between pesticide concentrations in vehicle dust and house dust for all of the pesticides tested when workers from the reference orchard were included, and for two of the pesticides when analysis was restricted to handlers and thinners at the primary orchard study site. These findings confirm that commuter vehicles play an important role in the transmission of workplace chemicals into the home.
Questionnaire results indicated the absence of changing and laundry facilities at the workplace. If workers have no place to store or clean contaminated work clothing and boots, then they have little choice but to wear them home. Isolation of work clothing and boots could be an effective intervention. If commuter vehicles are considered to be vectors of work-to-home pesticide transmission, then special attention should be paid to practices associated with these vehicles. Parking near or in fields likely increases pesticide concentrations in vehicles, as does leaving windows open. Vacuuming the commuter vehicle on a regular basis would likely reduce pesticide loads and thereby reduce the potential for take home exposure. Finally, frequency of pesticide safety training almost certainly has an impact on hygienic behavior and the take home exposure pathway.
This study had several limitations, the primary ones being a small sample size and restriction of the study to two orchards. The small sample size limited our ability to attribute elevated or reduced vehicle and house dust pesticide levels with specific worker characteristics. A study of only two orchards indicates that caution should be taken in generalizing these findings to other Northwest orchards, although a review of previous studies suggests that conditions and practices at these orchard sites were representative of industry practices. Previous studies have shown that workers bring pesticides home through vehicles [Curl et al., 2002] and by wearing work clothing and boots home [Simcox et al., 1995; Lu et al., 2000; McCauley et al., 2003]. Salvatore et al. [2008, 2009] found that when gloves, soap and warm water, and clean clothes were provided as part of an educational intervention, workers reported improved behaviors at the midday break and before going home. However, these self-reports were not validated with field observations.
In conclusion, the findings in this study are supportive of moving intervention activities closer to the source of contamination (i.e., the workplace) in order to reduce take home pesticide exposure, rather than focusing on community or home interventions that appear to be less effective in most cases. Commuter vehicles are an important component of the take home exposure pathway. The provision of changing areas, storage facilities, and proper pesticide safety training—all interventions at the worksite—will likely reduce pesticide exposures for both workers and their families.
Acknowledgments
Contract grant sponsor: National Institute for Occupational Safety and Health; Contract grant number: 2 U50 OH07544.
Contributors to this work included Adrian Negrete, Karen M. Powers, Dr. Paul Sampson, Sarah Weppner, Gwen Wist, Hilary Zetlen.
Footnotes
Disclosure Statement: The authors report no conflicts of interests.
References
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorous pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachur M, Stanbury M, Gerwel B, Gochfeld M, Rhoads GG, Wartenberg D. A pilot study of take-home lead exposure in New Jersey. Am J Ind Med. 1995;28:283–289. doi: 10.1002/ajim.4700280213. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Olshan AF, Savitz DA. Pesticides and childhood cancers. Environ Health Perspect. 1997;105:1068–1077. doi: 10.1289/ehp.971051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Barr D, Needham L. Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly ME, Bradman A, Nishioka M, McKone TE, Smith D, McLaughlin R, Kavanagh-Baird G, Castorina R, Eskenazi B. Pesticides in dust from homes in agricultural areas. Environ Sci Technol. 2009;43:8767–8774. doi: 10.1021/es9020958. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Keifer MC, De Roos AJ, Fenske RA, Furlong CE, van Belle G, Checkoway H. Occupational determinants of serum cholinesterase inhibition among organophosphate-exposed agricultural pesticide handlers in Washington State. Occup Environ Med. 2010;67:375–386. doi: 10.1136/oem.2009.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenherz C, Fenske RA, Simcox NJ, Bellamy G, Kalman D. Biological monitoring of organophosphorous pesticide exposure among children of agricultural workers in central Washington State. Environ Health Perspect. 1997;105:1344–1353. doi: 10.1289/ehp.971051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposures of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways. Environ Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: The CHAMACOS study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley LA, Lasarev MR, Higgins G, Rothlein J, Muniz J, Ebbert C, Phillips J. Work characteristics and pesticide exposures among migrant agricultural families: A community-based research approach. Environ Health Perspect. 2001;109:533–538. doi: 10.1289/ehp.01109533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley LA, Michaels S, Rothlein J, Muniz J, Lasarev M, Ebbert C. Pesticide exposure and self reported home hygiene: Practices in agricultural families. AAOHN J. 2003;51:113–119. [PubMed] [Google Scholar]
- McCauley LA, Travers R, Lasarev M, Muniz J, Nailon R. Effectiveness of cleaning practices in removing pesticides from home environments. J Agromed. 2006;11:81–88. doi: 10.1300/J096v11n02_11. [DOI] [PubMed] [Google Scholar]
- Moate T, Furia M, Curl C, Muniz JF, Yu J, Fenske RA. Size exclusion of chromatographic cleanup for the determination of organophosphorus pesticide residues in household and vehicle dust. J AOAC Int. 2002;85:36–43. [PubMed] [Google Scholar]
- NIOSH. Report to Congress on Workers’ Home Contamination Study Conducted Under the Workers’ Family Protection Act (29 USC 671a) National Institute for Occupational Safety and Health. US Department of Health and Human Services; 1995. Sep, [Google Scholar]
- Piacitelli GM, Whelan EA, Ewers LM, Sieber WK. Lead contamination in automobiles of lead-exposed bridgeworkers. Appl Occup Environ Hyg. 1995;10:849–855. [Google Scholar]
- Piacitelli GM, Whelan EA, Sieber WK, Gerwell B. Elevated lead contamination in homes of construction workers. AIHA J. 1997;58:447–454. doi: 10.1080/15428119791012694. [DOI] [PubMed] [Google Scholar]
- Plog BA, Quinlan PJ. Fundamentals of Industrial Hygiene. 6. Itasca, IL: National Safety Council; 2012. [Google Scholar]
- Quandt SA, Hernandez-Valero MA, Grzywacz JG, Hovey JD, Gonzales M, Arcury TA. Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environ Health Perspect. 2006;114:943–952. doi: 10.1289/ehp.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Gentry AL, Quandt SA, Davis SW, Snively BM, Arcury TA. Pesticide safety behaviors in latino farmworker family households. Am J Ind Med. 2006;49:271–280. doi: 10.1002/ajim.20277. [DOI] [PubMed] [Google Scholar]
- Rauh Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:845–859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Arcury TA, Quandt SA, Lasarev M, Rothlein J, Travers R, Tamulinas A, Scherer J, Early J, Marín A, Phillips J, McCauley L. Neurobehavioral performance in preschool children from agricultural and non-agricultural communities in Oregon and North Carolina. Neurotoxicol. 2005;26:589–598. doi: 10.1016/j.neuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Salvatore AL, Bradman A, Castorina R, Camacho J, López J, Barr DB, Snyder J, Jewell NP. Occupational behaviors and farmworkers’ pesticide exposure: Findings from a study in Monterey County, California. Am J Ind Med. 2008;51:782–794. doi: 10.1002/ajim.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore AL, Chevrier J, Bradman A, Camacho J, López J, Kavanagh-Baird G, Minkler M, Eskenazi B. A community-based participatory worksite intervention to reduce pesticide exposures to farmworkers and their families. Am J Public Health. 2009;99(Suppl 3):S578–S581. doi: 10.2105/AJPH.2008.149146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ Health Perspect. 1995;103:1126–1134. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong LL, Thompson B, Koepsell TD, Meischke H, Coronado GD. Reducing the take-home pathway of pesticide exposure: Behavioral outcomes from the Para Niños Saludables study. J Occup Environ Med. 2009;51:922–933. doi: 10.1097/JOM.0b013e3181ad4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton PM, Athanasoulis M, Flessel P, Guirguis G, Haan M, Schlag R, Goldman LR. Lead levels in the household environment of children in three high-risk communities in California. Environ Res. 1995;68:45–57. doi: 10.1006/enrs.1995.1007. [DOI] [PubMed] [Google Scholar]
- Thompson B, Coronado GD, Vigoren EM, Griffith WC, Fenske RA, Kissel JC, Shirai JH, Faustman EM. Para niños saludables: A community intervention trial to reduce organophosphate pesticide exposure in children of farmworkers. Environ Health Perspect. 2008;116:687–694. doi: 10.1289/ehp.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. National Organic Program. U.S. Department of Agriculture; 2011. [accessed August 17, 2011]. http://www.ams.usda.gov/AMSv1.0/nop. [Google Scholar]
- USEPA. Worker Protection Standard for Agricultural Pesticides. U.S. Environmental Protection Agency; 2013. [accessed April 10, 2013]. http://www.epa.gov/agriculture/twor.html. [Google Scholar]
- Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117:1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]