Abstract
We report our preliminary results of a pilot clinical trial of late-stage breast cancer patients treated by laser immunotherapy (LIT), a local intervention using an 805-nm laser for non-invasive irradiation, indocyanine green for selective thermal effect, and immunoadjuvant (glycated chitosan) for immunological stimulation. Ten breast cancer patients were enrolled in this study; all the patients were considered to be out of other available treatment options. Preliminary data of toxicity tolerance was individually evaluated through physical exams and laboratory tests. Adverse reactions only occurred in the area of treatment due to photothermal injury and local administration of immunoadjuvant. No grade 3 or 4 side effects were observed. Treatment efficacy of LIT was also evaluated by physical examination and tomography. In 8 patients available for evaluation, the objective response rate was 62.5% and the clinical beneficial response rate was 75%. While the study is still ongoing, the initial outcomes of this clinical trial show that LIT is well tolerated and is of great promise in the treatment of metastatic breast cancer.
Keywords: Laser immunotherapy, local intervention, breast cancer, immunoadjuvant, antitumor immune responses
Introduction
Metastases of cancer are the major cause of treatment failure and deaths. Many approaches such as chemotherapy, radiation therapy, hormonal therapy, and other targeted therapies have been applied to manage metastases of cancer. However, so far, the effects of the current modalities on metastasis are severely limited. Recently, cancer immunotherapy has made major conceptual and technical advances.1 Although there is still a long way to go, cancer vaccines have been considered as the ultimate tool for treatment and prevention of cancer, activating and enhancing the patient’s own immune system to recognize and destroy the targeted tumor cells. Significant efforts have been devoted to develop vaccines that could teach the patients’ immune system to effectively fight cancer, in the same way that it fights an infection by germs.2 However, conventional immunotherapies so far have only achieved limited success,3,4 such as the cases of interleukin-2 and interferon-alfa 2b, the only currently approved immunotherapeutic agents for melanoma in the United States.5
Laser immunotherapy (LIT), proposed in 19976, provides a convenient and efficient way to generate in situ autologous whole-cells cancer vaccine, through a minimally invasive local intervention. The protocol consisted of three major components: (1) a near-infrared laser, (2) a light-absorbing agent, and (3) an immunoadjuvant. It is worth noting that the light-absorbing agent may be omitted if the tumor could directly absorb enough laser energy for the desired thermal effect. The therapy is based on two major local interactions: (1) a selective photothermal interaction, and (2) an active immunological stimulation.7,8 A novel immunoadjuvant, glycated chitosan (GC), was developed for immunological stimulation in the treatment solid tumors. Local administration of GC could directly activate immune cells and also enhance immune responses combining the released tumor antigens.9 Its non-toxic nature and immunological activity make GC a potential immunoadjuvant for treatment of metastatic tumors.
The pre-clinical studies have shown the specific antitumor effects of LIT.10 LIT could not only destroy the treated primary tumors but also eradicate untreated metastases at distant sites. The survival rate of the animals treated by LIT was much higher than that of the control group. Furthermore, these animals could resist repeated challenges with the tumors of same origin and also escalated tumor doses.11, 12 The promising results from these experiments indicate a bright future for the clinical application of LIT. The present study was designed to investigate the safety and treatment effect of LIT in the treatment of late-stage breast cancer patients.
Patients and Methods
Patient selection
Patients were eligible if they had histologically confirmed stage III or IV breast cancer according to the sixth edition of the AJCC cancer stage manual.13 Eligible patients needed to be over 18 years old, with a life expectancy 12 weeks and to be considered not as candidates for traditional cancer treatment modalities by their physicians. Patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of no more than 2. Patients were required to have adequate organ functions. Known HIV positive patients were excluded with additional exclusions for active autoimmune disease, or corticosteroid dependence.
The treatment protocol was developed according to the Declaration of Helsinki and Good Clinical Practice guidelines. All participants were required to comprehend and sign an informed consent form approved by the Institutional Review Board before treatment.
Laser immunotherapy
An 805-nm laser, provided by ImmunoPhotonics Inc. (Columbia, MO), was used in this study. The laser light is delivered by an optical fiber. A handle device is equipped at the end of the optical fiber, which makes the power density at the surface of the skin to be 1 W/cm2. Each targeted tumor was irradiated for 10 minutes.
Indocyanine green (ICG), obtained from Akorn Inc. (Buffalo Grove, IL), is a water soluble, tricarbocyanine dye, which has be used in medical diagnostics, which has a peak spectral absorption at about 800 nm. A solution of 0.25% ICG was injected prior to laser irradiation. The formulation of glycated chitosan has been described previously.7 GC of 1% concentration was used for the treatment of breast cancer patients.
For breast cancer treatment, LIT was carried out with the following procedure: (1) Asepsis followed by local administration of anesthetic (lidocaine 2% with adrenaline); (2) Local injection of ICG; (3) Non-invasive laser irradiation; and (4) Local injection of 1% GC immediately after laser irradiation. GC was injected in the center of the tumor for immunological stimulation immediately after laser irradiation. The injection volumes of ICG and GC were determined according to the parameters given in Table 1. Maximum total dose of GC was 5 ml per treatment. Figure 1 shows the laser irradiation during the treatment of a breast cancer patient.
Table 1.
Dosage of indocyanine green (ICG) and glycated chitosan (GC) for local injection
| Longest axis of tumor* (cm) |
1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 |
|---|---|---|---|---|---|---|---|---|---|
| Volume of ICG (ml) | 0.2 | 0.5 | 2.0 | 2.3 | 4.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Volume of GC (ml) | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 |
In cases where multiple tumors are lumped together, each lesion was considered as an individual tumor; and in cases where the shape of the tumor does not approximate a sphere, the doses of ICG and GC were adjusted accordingly.
Fig. 1.
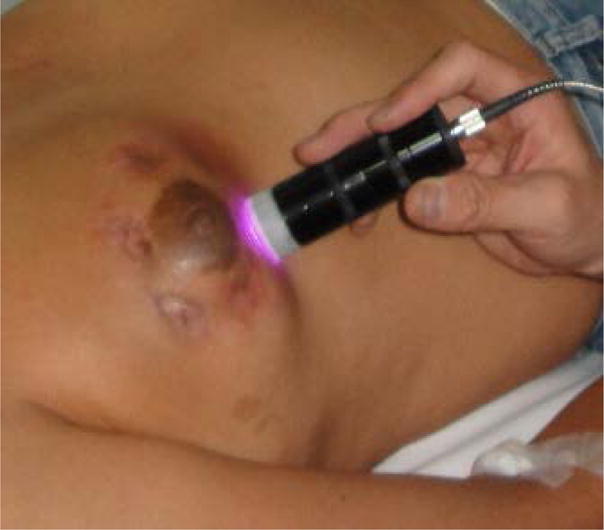
Laser irradiation for the treatment of a breast cancer patient.
For breast cancer treatment, the laser irradiation and administration of GC were applied with a time interval of 4 weeks. The time line for each cycle of LIT for is shown in Figure 2. Additional treatment cycles were carried out in the same treatment area or in different areas, if the response to the treatment was not complete. The duration between two treatment cycles was modified according to the local reaction of the patient treated by LIT.
Fig. 2. Treatment cycle of laser immunotherapy for breast cancer patients.
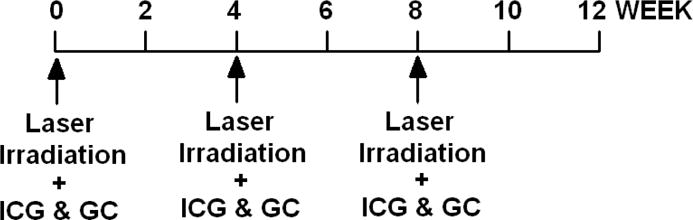
Laser irradiation, ICG and GC injection were performed every 4 weeks.
Assessment of safety
Local and systemic toxicity was graded according to National Cancer Institute Common Toxicity Criteria, version 3.0.14 Laboratory assessment and physical examinations were performed periodically. Adverse events were closely monitored and recorded throughout the study period.
Assessment of efficacy
Biopsies and medical imaging such as CT scan were used for the evaluation of the primary lesions and metastasis. The primary efficacy parameter was the best overall response by investigator’s assessment using the Response Evaluation Criteria in Solid Tumors (RECIST).15 Complete response (CR) was defined as disappearance of all target lesions, including local breast tumors treated by LIT, untreated breast tumors, as well as the metastases in different locations. Partial response (PR) was defined as a ⩾30% decrease from baseline in the sum of the longest diameter of target lesions. Progressive disease (PD) is defined as a ⩾20% increase in the sum of the longest diameter of target lesions or the appearance of 1 or more new lesions. Stable disease (SD) was defined as neither sufficient reduction to qualify for PR nor sufficient increase to qualify for PD. Reevaluation of response was processed 6 weeks after observation of a CR, PR, or SD for confirmation.
Results
Enrolled patients
Ten breast cancer patients were enrolled from September 2009 to August 2010 in the Hospital Nacional Edgardo Rebagliati Martins, Lima, Peru. ECOG performance status of all patients was less than or equal to 1 at enrollment. Each patient received at least one LIT treatment. Two patients withdrew from this study.
The median age of the breast cancer patients was 52.5 years (36 to 85 years). Five patients had AJCC stage III and five patients had stage IV diseases. Three patients were diagnosed with triple-negative breast cancer patients. Three patients had prior surgery. Seven patients had received prior systemic chemotherapy for metastatic disease, six patients had received radiation therapy, and five patients received hormonal therapy. Three patients did not receive any treatment. The detailed patient information is shown in Table 2.
Table 2.
Clinical characteristics of breast cancer patients, safety and efficacy evaluation
| Patient No. | Age | AJCC stage | ER | PR | HER2/neu | Prior treatment
|
Best overall response | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Chemotherapy | Radiation therapy | Hormonal therapy | |||||||
| 1 | 71 | III | + | + | + | No | Yes | Yes | Yes | N/A |
| 2 | 47 | IV | + | + | − | No | Yes | No | Yes | CR |
| 3 | 43 | III | − | − | − | No | Yes | Yes | No | PD |
| 4 | 36 | III | + | + | − | No | Yes | Yes | No | N/A |
| 5 | 40 | IV | − | + | + | Yes | Yes | Yes | Yes | PR |
| 6 | 85 | IV | − | − | − | No | No | No | No | PR |
| 7 | 78 | III | N/A | N/A | N/A | No | No | No | No | PR |
| 8 | 58 | III | − | − | − | No | No | No | No | PD |
| 9 | 66 | IV | + | + | Yes | Yes | Yes | Yes | PR | |
| 10 | 39 | IV | − | + | − | Yes | Yes | Yes | Yes | SD |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; AJCC, American Joint Committee on Cancer; N/A, not available or not applicable; CR, complete response; PR, partial response; SD, Stable disease; PD, Progressive disease.
Safety of LIT
LIT only induced local reactions within the treatment area in breast cancer patients. Redness, pain, edema and ulceration of the treatment area were the common adverse events (AEs). The local thermal injuries usually recover with time, as shown in Figure 3. Each patient experienced various degrees of pain following the laser treatment. Pain reactions were noticeably stronger in patients that had received radiation therapy, but in most cases the pain had subsided significantly within 48 hours. No grade 3 or 4 adverse events were observed. In patients who had not received prior radiation therapy the swelling was minor. For the patients who have received prior radiation therapy, the swelling was substantial with much longer duration after LIT. Similarly, for the patients who had received radiation, the ulceration was much more severe and it took longer to heal than for the patients that had not received radiation therapy. The severity of these side effects was correlated with the previous irradiation therapy history of the treatment area.
Fig. 3.
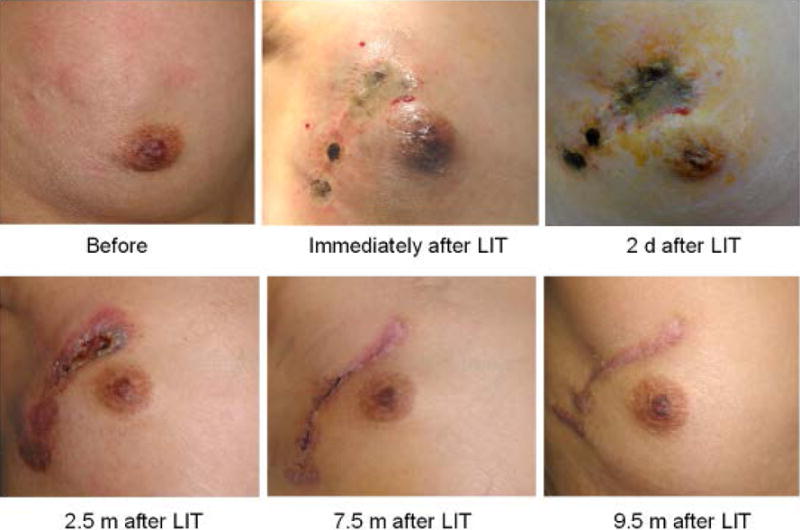
Photos of breast lesions of 47-year-old female patient with stage IV breast cancer treated by Laser immunotherapy (LIT).
Efficacy of LIT
All the enrolled patients received at least one LIT treatment. All the treatment area lesions of enrolled patients responded to LIT. All these 10 patients were still alive when the paper was written. Of the 8 breast cancer patients available for evaluation, complete response was observed in 1 patient, PR in 4 patients and SD in 1 patient, as shown in Table 2. Best overall response of these patients is summarized in Figure 4. In patients available for evaluation, the objective response rate (CR+PR) was 62.5%, and the clinical beneficial response rate (CR+PR+SD) was 75%. PD was observed in 2 patients.
Fig. 4. Evaluation of clinical response of breast cancer patients to laser immunotherapy.
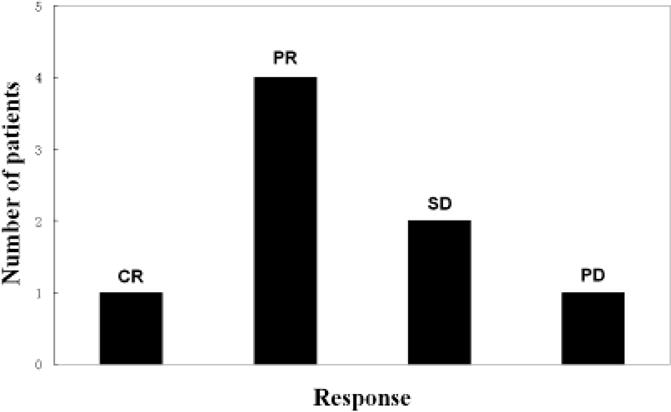
Abbreviations: CR, complete response; PR, partial response; SD, Stable disease; PD, Progressive disease.
All the local lesions irradiated by laser responded to LIT. In addition, metastases completely regressed in one patient and partially regressed or became stable in another five patients. The diameters of the metastases in lymph node, lung and liver in several patients decreased dramatically.
One breast cancer patient achieved complete response with all the pulmonary metastases disappeared. The patient was a 47-year-old female, who was diagnosed with stage IV breast cancer. She received prior chemotherapy with AC (doxorubicin/cyclophosphamide) for 4 cycles, paclitaxel for 3 cycles, capacitabine plus ixabepilone for 3 cycles, and hormonal therapy with tamoxifen. However, she was resistant to chemotherapy and hormonal therapy. This patient received 4 LIT treatments in total. Clinical observations before LIT showed that the sizes of the two tumors in the right breast of the patient were 6 × 4.5 cm and 2 × 2 cm. Pulmonary metastatic nodules were observed in bilateral lungs. Figure 5 shows the patient’s lung CT scans before, during, and after LIT. One of the small metastatic nodules was located in the left lung of the patient (indicated in arrow) before LIT (Figure 5-A). Two and a half months after the first LIT treatment, the lung metastases were still at the same level (Figure 5-B and 5-C). Five months after the first LIT treatment, CT scans showed that lung was normal without any metastasis (data not shown). Our one-year evaluation confirmed the absence of the lung metastases (Figure 5-D). This patient is completely tumor free at the time when this report is prepared.
Fig. 5. CT scans of pulmonary metastatic nodule in the left lung of 47-year-old female patient with stage IV breast cancer treated by laser immunotherapy (LIT).
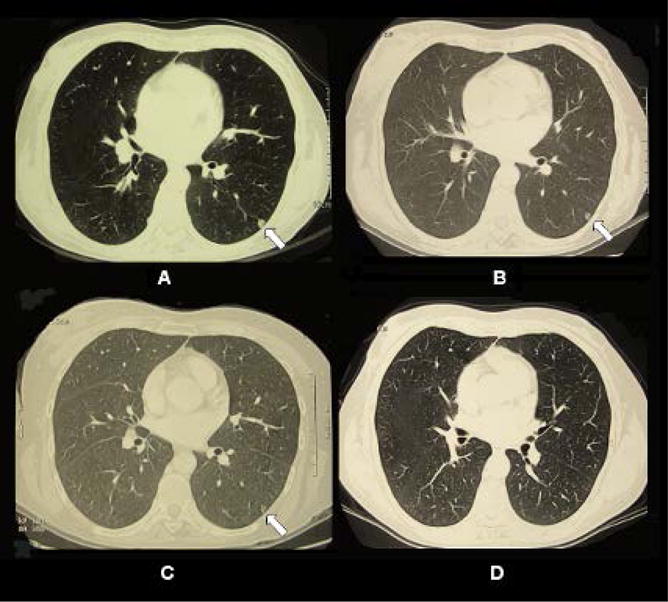
(A) CT scans of the patient taken before the first LIT treatment. A small metastatic nodule was located in the left lung of the patient (indicated in arrow). (B) CT scans taken 1 week after the first LIT treatment. (C) CT scans taken 2.5 months after the first LIT treatment. (D) CT scans taken 12 months after the first LIT treatment.
Discussion
We report the preliminary clinical outcomes of the advanced breast cancer patients treated by LIT. Ten patients with advanced breast cancer, all of whom had either responded poorly to or could not be treated by conventional modalities, received at least one LIT treatment. The results show that LIT is well tolerated. It only induces local reactions including pain, edema, redness, and cellulites. No severe systemic adverse events were observed. CR was observed in 1 patient and PR in 4 patients. Two patients had PD and one patient had SD. In patients available for evaluation, the objective response rate was 62.5% and the clinical beneficial response rate was 75%.
Adverse reactions of the treatment were mainly related to the photothermal effect induced by the interaction between laser and ICG. Following each treatment, there were various degrees of swelling and skin blistering in each patient. In most cases the blisters led to ulceration, which were treated with topical antibiotics daily until wound closure. No severe systemic adverse events were observed. We also noticed that there was a distinct difference in adverse events depending on whether the patient had received prior radiation therapy, which was assumed to be due to the structure changes in the patients’ skin and mammary gland induced by radiation therapy.16,17
Among the 10 patients, three are diagnosed with triple-negative breast cancer. While two of them experienced progressive disease, we observed a partial response in one patient (No. 5 in Table 2). Although it is not conclusive, this observation indicates that LIT may be effective to different types of breast cancer patients, regardless of the status of estrogen receptor (ER), progesterone receptor (PR) or Her2/neu. Triple-negative breast cancer is characterized by its unique molecular profile, aggressive behavior, distinct patterns of metastasis, and lack of available targeted therapies.18 Recent studies show that patients with triple-negative breast cancer have a high incidence of visceral metastasis, including brain metastasis.19,20 Although only one of the three triple-negative breast cancer patients had a partial response to LIT, we hope this was due to the LIT treatment and more patients with such status could benefit from this unique approach. However, much work is needed in a larger population of patients with triple-negative breast cancer in order to confirm our findings.
LIT is a new approach using host immune system to fight cancer cells. The strategy of LIT is to directly destroy the tumors at the treatment site and to induce tumor-specific host immune responses, through which residual tumor cells and untreated metastases at remote sites can be eliminated. The LIT-treated tumors in patients serve as the sources of cancer antigens in situ and all the cancer antigens come from the patients’ own tumor cells, as in the case of autologous vaccination. In comparison with conventional immunotherapy and cancer vaccination approaches, it is the components from the whole tumor cells exposed by LIT that directly provide the targeted immunological stimulation in the host, without any in vitro or ex vivo processing.21 In fact, this approach allows the host immune system to select the desirable tumor antigens, resulting in an in situ autologous whole-cell cancer vaccination.
Photothermal interaction between 805nm laser and ICG can induce a high temperature increase in the target tissue, which creates a selective tissue destruction zone covering the target tumor mass. Although this thermal reaction usually does not result in complete destruction or total acute eradication of target tumors, it can cause tumor cells to swell and lyse, allowing the release of antigens with the increase of temperature.22, 23 These antigens include tumor-associated antigens, thermally induced heat shock proteins (HSPs), and a large number of self-antigens. Antigen presenting cells (APCs), particularly dendritic cells (DCs), can capture these antigens and migrate to lymph nodes.24 They present the antigens to T cells to induce an immune response that can be effective against specific tumor cells. As the immune systems of cancer patients are often compromised, tumor debris generated by laser photothermal therapy may not be sufficient to induce a potent anti-tumor response. Additional immunological stimulations are required to invoke the immune system to achieve an effective and protective immune response against residual tumor cells.
LIT can be combined with many other immunotherapy modalities to further improve the efficacy.25,26,27 LIT is particularly designed for the treatment of late-stage, cancer patients, who have failed conventional treatment modalities and face severely limited options. The clinical outcome of this study is promising, considering the severity of these late stage patients. The systemic effects in these cancer patients demonstrated the potential of LIT as an effective, local, and safe intervention for metastatic cancers. It can reduce the primary tumors and metastases of the patients with far fewer harsh side effects of the traditional treatments.
Acknowledgments
This research is supported in part by grants from American Cancer Society (IRG-05-066-01 ACS Institutional Research Seed Grant), from the US National Institutes of Health (P20RR016478 from the INBRE Program of the National Center for Research Resources) and from National Natural Science Foundation of China (No. 81000994).
References
- 1.Finn OJ. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann TA. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 3.Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, Mulligan R, Nelson WG. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 4.Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, Nguyen M, Hege K, Simons JW. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 5.Komenaka I, Hoerig H, Kaufman L. Clin Dermatol. 2004;22:251–265. doi: 10.1016/j.clindermatol.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen WR, Adams RL, Carubelli R, Nordquist RE. Cancer Lett. 1997;115:25–30. doi: 10.1016/s0304-3835(97)04707-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen WR, Carubelli R, Liu H, Nordquist RE. Mol Biotechnol. 2003;25:37–43. doi: 10.1385/MB:25:1:37. [DOI] [PubMed] [Google Scholar]
- 8.Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE. Cancer Res. 2002;62:4295–4299. [PubMed] [Google Scholar]
- 9.Song S, Zhou F, Nordquist RE, Carubelli R, Liu H, Chen WR. Immunopharmacol Immunotoxicol. 2009;31:202–208. doi: 10.1080/08923970802629593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WR, Jeong SW, Lucroy MD, Wolf RF, Howard EW, Liu H, Nordquist RE. Int J Cancer. 2003;107:1053–1057. doi: 10.1002/ijc.11501. [DOI] [PubMed] [Google Scholar]
- 11.Chen WR, Zhu WG, Dynlacht JR, Liu H, Nordquist RE. Int J Cancer. 1999;81:808–812. doi: 10.1002/(sici)1097-0215(19990531)81:5<808::aid-ijc23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Chen WR, Singhal AK, Liu H, Nordquist RE. Cancer Res. 2001;61:459–461. [PubMed] [Google Scholar]
- 13.Singletary SE, Connolly JL. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis. Common terminology criteria for adverse events v3.0 (CTCAE) Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf [accessed Sep 28, 2009]
- 15.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Martin S, Mannino M, Rostom A, Tait D, Donovan E, Eagle S, Haviland J, Yarnold J. Clin Oncol (R Coll Radiol) 2008;20:502–505. doi: 10.1016/j.clon.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Keskikuru R, Jukkola A, Nuutinen J, Kataja V, Risteli J, Autio P, Lahtinen T. Radiother Oncol. 2004;70:243–248. doi: 10.1016/j.radonc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Viale G, Bottiglieri L. Eur J Cancer. 2009;45 Suppl 1:5–10. doi: 10.1016/S0959-8049(09)70011-5. [DOI] [PubMed] [Google Scholar]
- 19.Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, Kwon Y, Kim EA, Ko KL, Shin KH, Lee KS, Park IH, Lee S, Kim SW, Kang HS, Ro J. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 20.Perez EA, Moreno-Aspitia A, Thompson E Aubrey, Andorfer CA. Breast Cancer Res Treat. 2010;120:285–291. doi: 10.1007/s10549-010-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäger E, Jäger D, Knuth A. Int J Cancer. 2003;106:817–820. doi: 10.1002/ijc.11292. [DOI] [PubMed] [Google Scholar]
- 22.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HG, Mehta K, Cohen P, Guha C. Cancer Lett. 2008;271:191–204. doi: 10.1016/j.canlet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Milani V, Noessner E. Cancer Immunol Immunother. 2006;55:312–319. doi: 10.1007/s00262-005-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koido S, Hara E, Homma S, Fujise K, Gong J, Tajiri H. Arch Immunol Ther Exp (Warsz) 2007;55:281–287. doi: 10.1007/s00005-007-0034-6. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhaya A, Mendecki J, Dong X, Liu L, Kalnicki S, Garg M, Alfieri A, Guha C. Cancer Res. 2007;67:7798–7806. doi: 10.1158/0008-5472.CAN-07-0203. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Zhu J, Sheng X, Wang X, Qu L, Han Y, Liu Y, Zhang H, Huo L, Zhang S, Lin B, Yang Z. Int J Cancer. 2007;120:2418–2425. doi: 10.1002/ijc.22551. [DOI] [PubMed] [Google Scholar]


