Abstract
Background
The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) study evaluated the effects of plasma and platelets on hemostasis and mortality after hemorrhage. The pulmonary consequences of resuscitation strategies that mimic whole blood, remain unknown.
Methods
A secondary analysis of the PROPPR study was performed. Injured patients predicted to receive a massive transfusion were randomized to 1:1:1 vs. 1:1:2 plasma-platelet-RBC ratios at 12 Level I North American trauma centers. Patients with survival >24 hours, an ICU stay, and a recorded PaO2/FiO2 (P/F) ratio were included. ARDS was defined as a P/F ratio <200, with bilateral pulmonary infiltrates, and adjudicated by investigators.
Results
454 patients were reviewed (230 received 1:1:1, 224 1:1:2). Age, sex, injury mechanism, and regional abbreviated injury scale (AIS) scores did not differ between cohorts. Tidal volume, PEEP, and lowest P/F ratio did not differ. No significant differences in ARDS rates (14.8 vs. 18.4%), ventilator-free (24 vs. 24) or ICU-free days (17.5 vs. 18), hospital length of stay (22 vs. 18 days), or 30-day mortality were found (28 vs. 28%). ARDS was associated with blunt injury (OR 3.61 [1.53-8.81] p<0.01) and increasing chest AIS (OR 1.40 [1.15-1.71] p<0.01). Each 500 mL of crystalloid infused during hours 0-6 was associated with a 9% increase in the rate of ARDS (OR 1.09 [1.04-1.14] p<0.01). Blood given at 0-6 or 7-24 hours were not risk factors for lung injury.
Conclusion
Acute crystalloid exposure, but not blood products, is a potentially modifiable risk factor for the prevention of ARDS following hemorrhage.
Keywords: massive hemorrhage, resuscitation, trauma, lung injury, damage control resuscitation
INTRODUCTION
Acute respiratory distress syndrome (ARDS) continues to be a significant driver of trauma patient morbidity and mortality 50 years after its first description.(1) In a recent, prospective cohort of approximately 30,000 critically ill patients, 10.4% fulfilled diagnostic criteria for ARDS.(2) In this predominantly medical population, mortality ranged from 34.9% to 46.1% depending on the Berlin classification for disease severity.(3) The incidence of ARDS in trauma-specific populations ranges from 3% to 40% depending on the severity of injury, definition applied, and the resuscitative needs of the patient; however, mortality rates parallel those described in the medical literature.(4–7) Despite clarity for defining ARDS and validation of clinical interventions, patients with underlying ARDS are often unrecognized and inappropriately managed. In a large, international, observational trial in which a computer algorithm defined the presence of ARDS by submitted data, clinical recognition of the disease ranged from 51% in mild to 78% in severe ARDS.(2) Furthermore, less than two-thirds of the ARDS population received lung protective mechanical ventilation including tidal volumes of 6 ml/kg and moderate positive end-expiratory pressure (PEEP), an accepted ARDS best practice.(2, 3, 8, 9)
Further confusion surrounds the safety of recent resuscitation practices in those with active hemorrhage in regards to negative pulmonary sequelae. When the strategy of damage control resuscitation (DCR) is employed, providers aim to resuscitate patients with hemorrhagic shock with blood component therapy in ratios that mimic whole blood and attempt to limit crystalloid fluids.(10) The goal of DCR is to reduce the coagulopathy of trauma by rapidly replenishing consumed coagulation factors and products while reducing the anticipated hemodilution of aggressive crystalloid use.(10) Previous literature has demonstrated that in patients who survive their initial injury, the amount of blood products transfused is independently associated with the development of ARDS, multi-organ failure, and death.(11–13) In contrast, secondary analysis of the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study demonstrated that increasing chest injury severity, age, and crystalloid exposure, but not blood, were risk factors for moderate to severe hypoxemia following injury with hemorrhage.(14) However, ARDS rates were excluded from this study.
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) study demonstrated that the resuscitation of severely bleeding, trauma patients with blood products (plasma, platelets, and RBCs) in a ratio of 1:1:1 (versus 1:1:2) achieved hemostasis faster and less deaths due to exsanguination, although there was no difference in 30-day mortality.(15) Although the rates of acute lung injury, ARDS, and number of ventilator-free days did not differ between the 1:1:1 and 1:1:2 groups, further pulmonary metrics were not discussed, nor were risks factors for ARDS evaluated in this large, multi-institutional trial.
The aim of this secondary analysis is to clarify pulmonary outcomes in high-risk patients following severe injury with active hemorrhage after exposure to damage control resuscitation practices. Furthermore, we aim to delineate risk factors for ARDS development in this unique, resource-intense population. We hypothesize that pulmonary outcomes will not differ between PROPPR resuscitation cohorts and that crystalloid, not blood, will be implicated as an independent, modifiable factor for the development of ARDS.
MATERIALS AND METHODS
The study population consisted of severely injured patients predicted to receive a massive transfusion admitted to 12 twelve Level I North American trauma centers recruited for the PROPPR trial.(15) The PROPPR trial was performed under Exception From Informed Consent (EFIC) guidelines and approved by all of the institutional review boards of participating sites.(16) After excluding patients with a survival ≤ 24 hours, a lack of an intensive care unit (ICU) admission, and lack of a recorded PaO2 to FiO2 (P/F) ratio during days 1-7, a subset of the original 680 patients was analyzed. Demographic, injury mechanism and severity, blood and crystalloid exposure, and pulmonary characteristics of this subset were examined by ratio assignment (1:1:1 of plasma, platelets, RBCs vs. 1:1:2). Intravenous (crystalloid) fluids were defined as the sum of normal saline, lactated Ringer’s, and proprietary solutions (e.g. Normosol, Plasma-Lyte). Because of rare use, naturally or synthetically derived colloids (e.g. albumin, hetastarch) were excluded. Direct bedside data collection of all fluids and blood products infused, on an hourly basis, during the first 24 hours of care occurred during the trial. Mechanical ventilator tidal volume comparisons were made using predicted body weights (PBW, mL/kg).(17)
The primary outcome of interest was the development of acute respiratory distress syndrome (ARDS). ARDS was defined a priori to study implementation as a P/F ratio < 200 with bilateral pulmonary infiltrates on chest imaging (Berlin moderate to severe ARDS) as adjudicated by site principal investigators.(3) Secondary outcomes of interest included the timing of ARDS, the presence and timing of hypoxemia during days 1-7 as defined by a P/F ratio ≤ 300 mmHg without chest imaging requirements, the lowest P/F ratio on day 1 and days 1-7, the timing of ventilator liberation, and ventilator-free days within 30 days.(14, 18, 19) Ventilator-free days within 30 days are the number of days alive and without mechanical ventilation within the first 30 days of care. As such, those that die while receiving mechanical ventilation are assigned a value of zero ventilator-free days. We utilized the critical administration threshold (CAT) as a marker for a trauma subset at the highest risk of mortality from hemorrhage.(20) Being CAT positive represented having ≥ 3 units of RBCs transfused in a single hour during the first 24 hours of care.
The description of continuous variables used median values (with interquartile ranges) while proportions were used for categorical variables. Wilcoxon rank-sum, chi-square or Fisher’s exact test were applied as appropriate. To determine the timing of first ventilator liberation during days 0-7 and 0-30, Kaplan-Meier curves were generated. Reintubation metrics were not examined. Multivariable logistic regression with clinically meaningful variables, with adjustment by study site, was utilized to determine the odds of ARDS development (odds ratio [OR] with 95% confidence intervals [CI]). Significance was defined as p ≤ 0.05. Data analysis was performed using SAS version 9.4 (Cary, NC).
RESULTS
Of the 680 patients enrolled in the PROPPR trial, 454 were included in these analyses after the application of the exclusion criteria (1:1:1 n=230, 1:1:2 n=224). Patient demographics to include age, sex, actual and predicted body weight, and mechanism of injury were evenly balanced between blood ratio assignments (Table 1). Rates of emergency department (ED) systolic blood pressure < 90 mmHg and heart rate > 110 beats per minute as well as lactate and base deficit, Glasgow Coma Scale and respiratory rates were not different between groups. Injury severity as measured in totality by the Injury Severity Score (ISS) and individual head, chest, abdomen and extremity abbreviated injury scores (AIS) did not differ.
TABLE 1.
Patient characteristics by ratio cohort
| 1:1:1 (n=230) |
1:1:2 (n=224) |
P = | |
|---|---|---|---|
| Age, years | 34.5 (25-49) | 33 (24-50) | 0.65 |
| Male sex, # (%) | 179 (77.8) | 182 (81.3) | 0.37 |
| Actual body weight, kg | 80 (70-92.9) | 82 (70.2-95.3) | 0.15 |
| Predicted body weight+, kg | 70.8 (61.5-76.0) | 70.8 (64.2-77.7) | 0.32 |
| Blunt injury rate, # (%) | 139 (60.4) | 116 (51.8) | 0.06 |
| ED SBP < 90 mmHg, # (%) | 76 (33.3) | 66 (30.6) | 0.53 |
| ED HR > 110 beats per minute, # (%) | 131 (57.0) | 126 (56.5) | 0.92 |
| ED lactate, (mmol/L) | 5.9 (3.9-8.5) | 5.6 (3.5-8.8) | 0.99 |
| ED base deficit, (mmol/L) | −8 (−12 - −4) | −8 (−12 - −4.1) | 0.91 |
| ISS | 29 (19-41) | 28 (18-38) | 0.31 |
| Head AIS++ | 4 (2-5) | 3 (3-5) | 0.95 |
| Chest AIS++ | 3 (3-4) | 3 (3-4) | 0.80 |
| Abdomen AIS++ | 3 (3-4) | 3 (3-4) | 0.95 |
| Extremities AIS++ | 3 (2.5-4) | 3 (2-3) | 0.08 |
| Race, white, # (%) | 145 (63.0) | 149 (66.5) | 0.44 |
| ED GCS | 14 (3-15) | 14 (3-15) | 0.32 |
| ED respiratory rate, breaths per minute | 20 (17-26) | 21 (18-26) | 0.44 |
| Massive transfusion, # (%) | 108 (47.0) | 112 (50.0) | 0.52 |
| CAT+, # (%) | 193 (83.9) | 208 (92.9) | <0.01* |
Continuous values are presented as medians (interquartile range)
P ≤ 0.05
PBW: male= 50+0.91(centimeters of height-152.4); female = 45.5+0.91(centimeters of height-152.4)
medians represent non-zero values
kg, kilograms; ED, emergency department; SBP, systolic blood pressure; HR, heart rate; ISS, injury severity score; AIS, abbreviated injury scale; GCS, Glasgow coma scale; CAT+, critical admission threshold positive
Approximately 50% of the cohort received a massive transfusion defined as ≥10 units of RBC during the first 24 hours. Those randomized to 1:1:2 had a significantly higher rate of being critical admission threshold (CAT) positive (92.9 vs. 83.9%, P < 0.01). The exposure to component therapy as well as crystalloid was investigated during the pre, during, and post-randomization periods when products were delivered as 1:1:1 or 1:1:2 as well during the first 6 and 24 hours by similar assignment (Table Electronic 1). As expected, the 1:1:1 cohort was exposed to a higher ratio of plasma to RBCs, and overall units of plasma and platelets (one unit = six packs) during the randomization period. The 1:1:1 cohort also had significantly higher exposures to plasma and platelets units during the 0-6 and 0-24 hour intervals. Those in the 1:1:2 group, had a significantly higher ratio of plasma to RBCs post-randomization. This group also had more RBCs transfused during the randomization period. The 1:1:2 cohort had more post-randomization plasma and platelet needs. The exposure to crystalloid during the pre, during, and post-randomization periods as well as during the first 0-6 and 0-24 hour intervals did not differ by ratio category.
Overall, 75 patients developed ARDS (16.5%). ARDS occurrence did not differ by ratio assignment (14.8 vs. 18.3%, 1:1:1 vs. 1:1:2, Table 2). There was a trend towards ARDS occurring 1.5 days earlier in those receiving 1:1:2 (p=0.06). The rate of hypoxemia (81.3 vs. 76.8%) and day of occurrence during the first 7 days of care (both on day 1) did not differ by group. Furthermore, the lowest P/F ratio and highest PEEP applied on day 1 and during days 1-7 did not differ. The greatest tidal volume delivered by PBW on day 1 and on days 1-2 was unchanged by the groups. Ventilator-free days in the first 30 days of care (24 days free of mechanical ventilation) were nearly identical between the cohorts. This led to the same rate of successful ventilator liberation during the first 7 and 30 days of hospitalization regardless of blood ratio treatment (Figure 1 and 2).
TABLE 2.
Pulmonary outcomes by ratio cohort
| 1:1:1 (n=230) |
1:1:2 (n=224) |
P= | |
|---|---|---|---|
| ARDS, # (%) | 34 (14.8) | 41 (18.3) | 0.35 |
| Hospital day ARDS occurred during HD 1-7 | 3.5 (1-6) | 2 (1-4) | 0.06 |
| Hypoxemia (P/F ≤ 300 during HD 1-7), # (%) | 187 (81.3) | 172 (76.8) | 0.34 |
| Hospital day hypoxemia occurred during HD 1-7 | 1 (1-2) | 1 (1-2) | 0.94 |
| Lowest P/F ratio, HD 1 | 254 (172-373) | 258 (145-370) | 0.53 |
| Lowest P/F ratio, HD 1-7 | 173 (100-278) | 156 (89.5-283) | 0.40 |
| Highest PEEP, HD 1, (cm H2O) | 5 (5-8) | 5 (5-8) | 0.45 |
| Highest PEEP, HD 1-7, (cm H2O) | 7 (5-10) | 8 (5-10) | 0.44 |
| Highest tidal volume divided by patients predicted body weight, HD 1, (mL/Kg) | 7.8 (6.9-8.2) | 7.8 (6.9-8.2) | 0.91 |
| Highest tidal volume divided by patients predicted body weight, HD 1-2, (mL/Kg) | 7.9 (7.1-8.4) | 7.9 (7.1-8.6) | 0.63 |
| Ventilator days | 5 (2-13) | 4.5 (2-14) | 0.98 |
| Ventilator-free days in 30 | 24 (9-27) | 24 (9.5-28) | 0.90 |
| 7-day mortality, # (%) | 18 (7.8) | 14 (6.3) | 0.60 |
| 30-day mortality, # (%) | 28 (12.3) | 28 (12.5) | 0.91 |
Continuous values are presented as medians (interquartile range)
P ≤ 0.05
ARDS, acute respiratory distress syndrome; HD, hospital day; P/F, PaO2 to FiO2 ratio; PEEP, positive end-expiratory pressure
Figure 1.
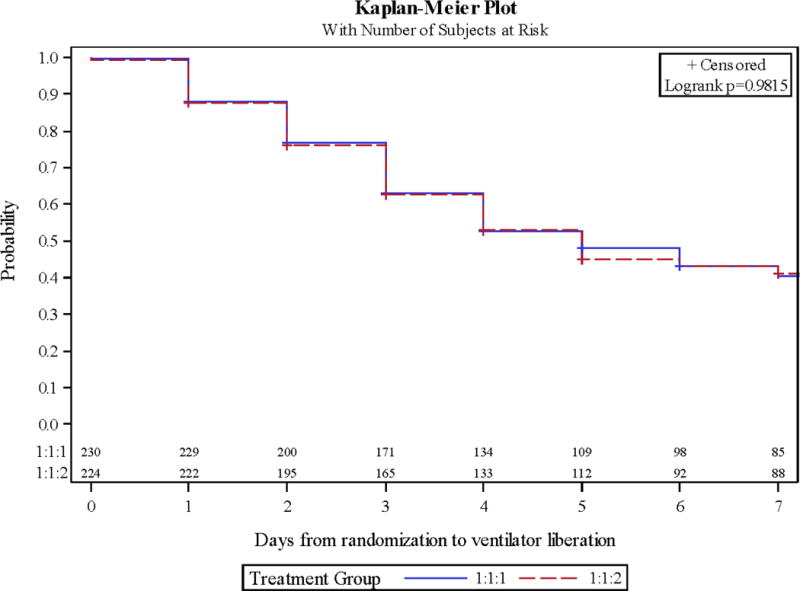
Kaplan-Meier curves of mechanical ventilation liberation during hospital days 0-7 by ratio cohort.
Figure 2.
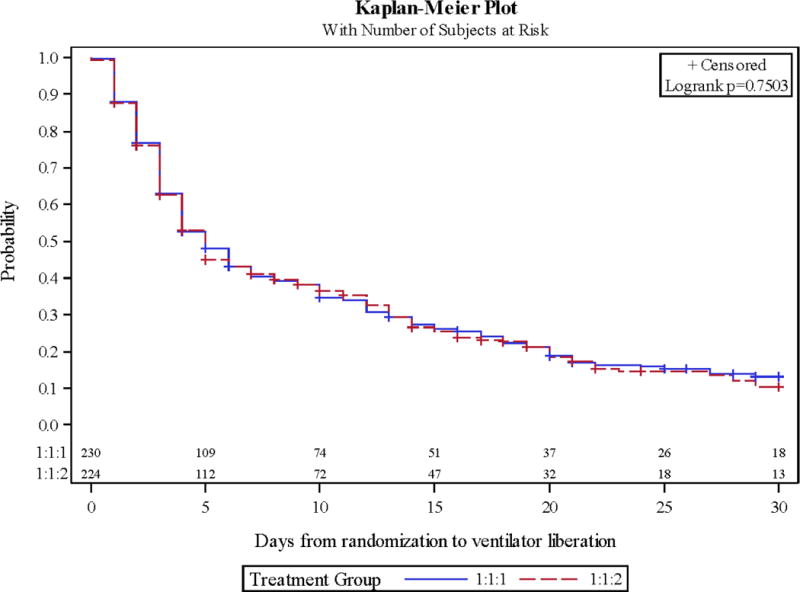
Kaplan-Meier curves of mechanical ventilation liberation during hospital days 0-30 by ratio cohort.
The investigation of risk factors for the development of ARDS was applied to the entire study group (Table 3). Clinically selected variables significant in the univariate analyses for the development of ARDS included age, blunt mechanism of injury, head, chest and extremity AIS, as well as intravenous crystalloid fluids (IVFs) given, by 500 mL aliquots, during hours 0-6. Multivariable logistic regression determined that blunt mechanism of injury (OR 3.61 [CI 1.53-8.51], P < 0.01), chest AIS (OR 1.40 [CI 1.15-1.71], P < 0.01), and IVFs provided during hours 0-6 (by 500 mL aliquots, OR 1.09 [CI 1.04-1.14], P <0.01) as significant risk factors for ARDS. The difference in IVFs provided during the first 6 hours of resuscitation between those with and without ARDS was a median of 12 (6.8-16.2) vs. 8 (4-13.2) 500 mL boluses (P < 0.0001), or approximately 2 liters (Figure 3).
TABLE 3.
Risk factors for the development of acute respiratory distress syndrome.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (years) | 1.02 (1.00-1.03) | 0.03* | 1.01 (0.99-1.03) | 0.52 |
| Male sex | 1.15 (0.61-2.16) | 0.67 | 1.25 (0.57-2.74) | 0.58 |
| Blunt mechanism of injury | 3.44 (1.91-6.20) | <0.01* | 3.61 (1.53-8.51) | <0.01* |
| ED SBP < 90 mmHg | 0.83 (0.48-1.45) | 0.52 | 0.97 (0.50-1.87) | 0.93 |
| ED HR > 110 BPM | 0.75 (0.45-1.23) | 0.25 | 0.62 (0.32-1.21) | 0.16 |
| Head AIS | 1.18 (1.04-1.34) | <0.01* | 1.00 (0.85-1.18) | 0.99 |
| Chest AIS | 1.45 (1.22-1.71) | <0.01* | 1.40 (1.15-1.71) | <0.01* |
| Abdomen AIS | 0.96 (0.83-1.10) | 0.53 | 0.85 (0.70-1.02) | 0.08 |
| Extremities AIS | 1.17 (1.00-1.37) | 0.05* | 1.01 (0.81-1.26) | 0.90 |
| RBC units given in hours 0-6 | 1.01 (0.99-1.04) | 0.31 | 1.00 (0.92-1.10) | 0.93 |
| RBC units given in hours 7-24 | 1.02 (0.98-1.07) | 0.30 | 0.98 (0.86-1.13) | 0.80 |
| Plasma units given in hours 0-6 | 1.01 (0.98-1.04) | 0.44 | 1.09 (0.95-1.25) | 0.23 |
| Plasma units given in hours 7-24 | 1.02 (0.98-1.07) | 0.35 | 1.05 (0.90-1.23) | 0.55 |
| Platelet units given in hours 0-6 | 1.00 (0.97-1.03) | 0.90 | 0.95 (0.85-1.06) | 0.34 |
| Platelet units given in hours 7-24 | 1.01 (0.98-1.05) | 0.41 | 0.96 (0.88-1.05) | 0.40 |
| IVFs given (by 500 mL units) in hours 0-6 | 1.09 (1.05-1.13) | <0.01* | 1.09 (1.04-1.14) | <0.01* |
| IVFs given (by 500 mL units) in hours 7-24 | 1.03 (0.98-1.07) | 0.23 | 1.02 (0.97-1.08) | 0.42 |
| Highest tidal volume divided by patients predicted body weight, HD 1-2, > 8 mL/Kg | 0.89 (0.52-1.52) | 0.66 | 1.02 (0.54-1.95) | 0.95 |
P ≤ 0.05
OR, odds ratio; CI, confidence interval; ED, emergency department; SBP, systolic blood pressure; HR, heart rate; AIS, abbreviated injury scale; RBC, red blood cell; IVFs, intravenous fluids; HD, hospital day
Figure 3.
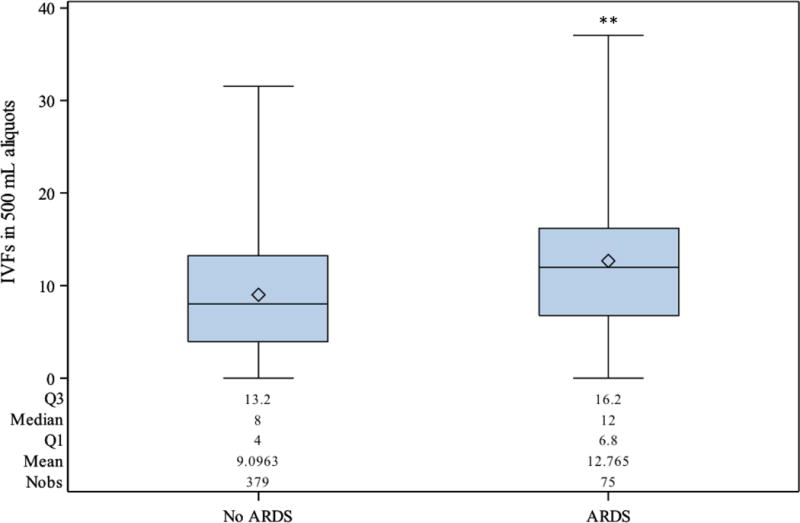
Intravenous fluids given during hours 0 to 6 for those without and with acute respiratory distress syndrome (ARDS) (P < 0.0001**).
DISCUSSION
In this study, we demonstrate that the rate of developing ARDS after utilization of damage control resuscitation in our high-risk population is relatively low at 16.5%. This rate did not differ by PROPPR resuscitation cohort. Other parameters of pulmonary morbidity to include day of ARDS diagnosis, rate and day of hypoxemia, tidal volume and PEEP application, and ventilator liberation did not differ between those in the 1:1:1 vs. 1:1:2 groups. The only potentially modifiable risk factor for the development of ARDS was crystalloid exposure during hours 0-6. A median of 2 liters difference during this time interval separated those with and without ARDS.
The safety profile of damage control resuscitation principles that provide significantly more plasma and platelets to patients while limiting crystalloid continue to support its application to those with active hemorrhage. More specifically, we failed to demonstrate a significant association of RBCs, plasma or platelets, given at 0-6 or 7-24 hours after admission, to the development of ARDS within a PROPPR cohort of approximately 450 patients. As expected, those in the 1:1:1 cohort received significantly more plasma and platelets at both intervals though had similar rates of ARDS, hypoxemia, and duration of mechanical ventilation compared to those in the 1:1:2 group.
Early work within trauma populations indicated that blood transfusions, to include RBCs, FFP and platelets, were independent risk factors for ARDS, multiple organ failure, and death.(12, 21, 22) Within this and recent works of our group, blood products fail to be associated with hypoxemia and ARDS.(14, 15) Initial concerns for the widespread application of DCR were rooted in reports of lung injury temporally related to the transfusion of blood products. Termed transfusion-related lung injury (TRALI), this neutrophil-mediated condition, occurring within six hours of transfusion, mimics the clinical picture of ARDS.(23) Both plasma and platelets have been implicated as having the largest risk for the development of TRALI in recipients who died of the disease (OR 12.5, OR 7.9 respectively, relative to RBCs transfused).(24) Nonetheless, previous works investigating TRALI failed to account for intravenous crystalloid exposures in a disease entity that continues to be exceedingly rare. In the United States, the reported incidence of TRALI is between 1/1,323 to 1/5,000 transfusions.(23)
Increasing evidence continues to highlight the strong association between intravenous crystalloid fluid exposures to the development of acute lung injury. “Crystalloid-related acute lung injury” (CRALI) appears to be a defined entity with the potential for mitigation in trauma patients. In PROMMTT, and now PROPPR, crystalloid has been identified as a modifiable risk factor for the development of lung injury. We found that even a relatively small volume of crystalloid fluid may have significant clinical effects, as each 500 mL aliquot of crystalloid provided during hours 0-6 after admission was associated with a 9% increased risk of ARDS. Within PROMMTT, each 500 mL unit was associated with a 6% increase risk of moderate to severe hypoxemia if given during hours 0-6 and a 5% increase if given during hours 7-24.(14)
Our findings support the previous seminal work of the Inflammation and the Host Response to Injury Large Scale Collaborative Program (Glue Grant). These investigators demonstrated that in those with a massive transfusion (≥ 10 units RBC, n = 452), an elevated crystalloid to RBC ratio at the end of the first 24 hours was independently associated with an increased risk ARDS (OR 2.2).(25) When investigating the impact of crystalloid on all patients (n = 1754), not just those with a massive transfusion, 24-hour exposure was significantly related to the development of acute lung injury/ARDS when controlling for age, admission Glasgow coma scale, injury and acute physiology severity, preexisting comorbidities, and colloid and blood products infused.(26)
Though the Glue grant focused on blunt injured patients only, investigators that included penetrating injuries have demonstrated comparable findings. Early work by Plurad et al, associated a fluid balance of ≥2 liters in the first 48 hours as a risk factor for the development of ARDS.(27) Cotton et al demonstrated that a significant reduction in crystalloid exposure at 24 hours (13.9 to 5.0 L) was associated with a similar trend for ARDS (4.6 to 0.9%) in a study population with a penetrating rate of 31% requiring damage control laparotomy.(28) In a 10-year review of DCR implementation in a study population receiving massive transfusions with a penetrating injury rate of 52%, Campion et al reported a significant decrease in crystalloid exposure and improving P/F ratios during the first 24 hours of care.(29)
Our finding of direct pulmonary injury as independent factors for the development of ARDS continues to support historical literature. Hudson et al demonstrated in 1995 that pulmonary contusion, multiple fractures (two or more major long bones, an unstable pelvic fracture, or one major long bone and a major pelvic fracture), and multiple transfusions as presenting clinical risks for the development of ARDS in a trauma cohort.(4) When only one of these factors was present, multiple fractures were associated with the lowest risk of ARDS development (11.1%), while pulmonary contusion (21.8%) and multiple transfusions (25%) were higher. ARDS developed at a rate of 47% in those with multiple transfusions and a combination of pulmonary contusions or multiple fractures. Our group has used chest AIS score as a surrogate for direct thoracic injury. In this current study, increasing chest AIS has been shown to be an independent factor for the development of lung injury. Though not modifiable variables, these specific injury patterns should serve to alert clinicians to possible pulmonary decompensation so that early interventions, specifically the reduction of crystalloid, may occur.
We do recognize several limitations of this work. This study represents a secondary analysis of the PROPPR trial that was originally powered for the primary outcomes of 24-hour and 30-day all-cause mortality. As such, investigations of lung injury continue to represent a minority of complications accrued within this cohort. Nonetheless, both intervention groups within our subpopulation appear evenly matched for baseline characteristics and were accrued from multiple Level I, high-volume, trauma centers throughout North America. The definition of ARDS for this study represented the 1994 American-European Consensus Conference recommendations and not the 2012 Berlin definition thus excluding those with mild ARDS.(3, 8) Regardless, the adjudication of ARDS was made by site principal investigators familiar with the diagnostic criteria and after review of clinical as well as radiographic data. Unfortunately, the intensity of pulmonary care delivered and specific ventilatory settings and modes (invasive and non-invasive), beyond tidal volume by ideal body weight and PEEP, were not recorded. Future work that includes airway pressure, driving pressure, and breath waveform analyses of this unique trauma cohort is needed. Though crystalloid was implicated as a risk factor for ARDS, the unrecorded, precipitating events requiring crystalloid may be confounding variables associated with negative pulmonary outcomes. Future work will need to untangle the events requiring intravenous fluids and blood administration during the acute phases of resuscitation. Finally, the results of this trial may not be widely applicable as they represent mostly urban centers with mature infrastructure to deliver interventions consistent with damage control resuscitation and advance critical care techniques.
To conclude, acute crystalloid exposure, but not blood products, emerges as a modifiable risk factor for the prevention of ARDS following hemorrhage. Relatively small volumes of crystalloid fluids given during the acute period of resuscitation appear to be associated with the development of lung injury. We were unable to demonstrate negative pulmonary sequelae with the use of damage control resuscitation (DCR) strategies that emphasis the early transfusion of products in ratios that mimic whole blood, specifically the exposure of plasma and platelet in a population of injured patients with active hemorrhage.
Supplementary Material
Acknowledgments
Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group:
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee Cochair); Jeanette Podbielski, RN; and Angela M. Beeler, BS. Data Coordinating Center: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; and Michael O. Gonzalez, MS. Core Laboratory: Lisa Baer, MS; Yao-Wei WillaWang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; and David Clark, BS.
Resuscitation Outcomes Consortium: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; and Kellie Sheehan, BSN.
Systems Biology Committee: Alan Hubbard, PhD (Cochair); and Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD (Cochair); and Jeanne Callum, MD (Cochair).
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole,DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; and Rhonda Hobbs, MT (ASCP) SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; and Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees Cochair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; and Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD, MS; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Christopher N. Miller, MD; Helen Hancock, BS, MT(ASCP); and Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT (ASCP), SBB; and Cheryl Y. Matsushita, BS, MT (ASCP).
Shock, Trauma and Anesthesiology Research–Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr., DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; and Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer Baggett, RN; Lynda Waddle-Smith, BSN; and Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; and Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; and Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Jean-Francois Pittet, MD (Anesthesia Chair); Patrick L. Bosarge, MD; Albert T.Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS; and Marisa B. Marques, MD.
Oregon Health and Science University: Martin A. Schreiber, MD; Jennifer M. Watters, MD; Samantha J. Underwood, MS; TahneeGroat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD; Richard M. Scanlan, MD; and Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; and Connie Colavecchia, BSc, MLT.
Disclosure of Funding:
This work was supported with grant U01HL077863 from the US National Heart, Lung, and Blood Institute and funding from the US Department of Defense, the Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research-Institute of Circulatory and Respiratory Health (grant CRR-120612).
Footnotes
Conflict of Interest:
Mr. Branson reports relationships with the following companies: Mallinckrodt
Ventec Life Systems, Meiji Pharmaceuticals, Bayer, MedPace, Medtronic, and Ciel Medical
Presentations:
This study was presented at the 75th annual meeting of the American Association for the Surgery of Trauma, September 14-17, 2016, in Waikoloa, Hawaii
Disclaimer:
The opinions or conclusions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of any sponsor. This article has been reviewed by the PROPPR Publication Committee for scientific content and consistency of data interpretation with previous PROPPR publications.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 5.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68:1. [PubMed] [Google Scholar]
- 6.Zielinski MD, Jenkins D, Cotton BA, Inaba K, Vercruysse G, Coimbra R, Brown CV, Alley DE, DuBose J, Scalea TM, et al. Adult respiratory distress syndrome risk factors for injured patients undergoing damage-control laparotomy: AAST multicenter post hoc analysis. J Trauma Acute Care Surg. 2014;77:886–891. doi: 10.1097/TA.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 7.Park PK, Cannon JW, Ye W, Blackbourne LH, Holcomb JB, Beninati W, Napolitano LM. Incidence, risk factors, and mortality associated with acute respiratory distress syndrome in combat casualty care. J Trauma Acute Care Surg. 2016;81:S156. doi: 10.1097/TA.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 11.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717–723. [PubMed] [Google Scholar]
- 12.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, Cuschieri J, Maier RV, Billiar TR, Peitzman AB, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:30. doi: 10.1097/TA.0b013e3181ad5957. [DOI] [PubMed] [Google Scholar]
- 13.Brakenridge SC, Phelan HA, Henley SS, Golden RM, Kashner TM, Eastman AE, Sperry JL, Harbrecht BG, Moore EE, Cuschieri J, et al. Early blood product and crystalloid volume resuscitation: risk association with multiple organ dysfunction after severe blunt traumatic injury. J Trauma. 2011;71:299–305. doi: 10.1097/TA.0b013e318224d328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson BR, Cotton BA, Pritts TA, Branson R, Holcomb JB, Muskat P, Fox EE, Wade CE, del Junco DJ, Bulger EM, et al. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. J Trauma Acute Care Surg. 2013;75:61. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniuk S, Tilley BC, del Junco DJ, Fox EE, van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation. Injury. 2014;45:1287–1295. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.Collier B, Vieau C, Lockhart E, Bradburn E, Hamill M, Love K, Reed C, Baker C. Provider Bias Impacts Tidal Volume Selection and Ventilator Days in Trauma Patients. J Am Coll Surg. 2016;222:527–532. doi: 10.1016/j.jamcollsurg.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld DA, Bernard GR, ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74:2. doi: 10.1097/TA.0b013e31827a3639. [DOI] [PubMed] [Google Scholar]
- 21.Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59:4. doi: 10.1097/01.ta.0000171459.21450.dc. [DOI] [PubMed] [Google Scholar]
- 22.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma: packed red blood cells the answer? J Trauma. 2008;65:1. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 23.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Eder AF, Herron R, Strupp A, Dy B, Notari EP, Chambers LA, Dodd RY, Benjamin RJ. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 25.Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, Billiar TR, Peitzman AB, Moore EE, Cohen MJ, et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: when a little goes a long way. J Trauma Acute Care Surg. 2012;72:892–898. doi: 10.1097/TA.0b013e31823d84a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasotakis G, Sideris A, Yang Y, de Moya M, Alam H, King DR, Tompkins R, Velmahos G, Inflammation and Host Response to Injury Investigators Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74:2. doi: 10.1097/TA.0b013e3182826e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, Demetriades D, Rhee P. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. J Trauma. 2007;63:7. doi: 10.1097/TA.0b013e318068b1ed. discussion 8. [DOI] [PubMed] [Google Scholar]
- 28.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campion EM, Pritts TA, Dorlac WC, Nguyen AQ, Fraley SM, Hanseman D, Robinson BR. Implementation of a military-derived damage-control resuscitation strategy in a civilian trauma center decreases acute hypoxia in massively transfused patients. J Trauma Acute Care Surg. 2013;75:221. doi: 10.1097/TA.0b013e318299d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


