Abstract
Background
The implementation science literature has contributed important insights regarding the influence of formal policies and practices on healthcare innovation implementation, while informal implementation policies and practices have garnered little attention. The broader literature suggests informal implementation policies and practices could also influence innovation use.
Purpose
We used the Organizational Theory of Innovation Implementation to further understand the role of formal and informal implementation policies and practices as determinants of implementation effectiveness. We examined their role within the context of initiatives to increase palliative care consultation in inpatient oncology.
Methods
We used a case study design in two organizational settings within one academic medical center: medical and gynecologic oncology. We completed semi-structured interviews with medical (n=12) and gynecologic (n=10) oncology clinicians using questions based on organizational theory. Quantitative data assessed implementation effectiveness, defined as aggregated palliative care consult rates within oncology services from 2010–2016. Four palliative care clinicians were interviewed to gain additional implementation context insights.
Results
Medical oncology employed multiple formal policies and practices including training and clinician prompting to support palliative care consultation and a top-down approach, yet most clinicians were unaware of the policies and practices, contributing to a weak implementation climate. In contrast, gynecologic oncology employed one formal policy (written guideline of criteria for initiating a consult) but also relied on informal policies and practices such as spontaneous feedback and communication; they adopted a bottom-up approach, contributing to broader clinician awareness and strong implementation climate. Both services exhibited variable, increasing consult rates over time.
Practice Implications
Informal policies and practices may compensate or substitute for formal policies and practices under certain conditions (e.g., smaller healthcare organizations). Further research is needed to investigate the role of formal and informal policies and practices in shaping a strong and sustainable implementation climate, and subsequent effective innovation implementation.
Keywords: Implementation Policies and Practices, Innovation Implementation, Palliative Care, Oncology
Introduction
The implementation science literature has contributed to important insights regarding the influence of organizationally sanctioned formal policies and practices on effective implementation of healthcare innovations. In contrast, the role of informal implementation policies and practices, meaning those not officially sanctioned by organizational decision makers, has garnered relatively little attention. This is evident in compilations of implementation strategies (Powell et al, 2015), systematic reviews of implementation strategy effectiveness (Wolfdenden et al, 2016), and organizational theories commonly used in implementation science (Brewster et al., 2015; Helfrich, Weiner, McKinney, & Minasian, 2007; Teal, Bergmire, Johnston, & Weiner, 2012; Weiner, Haynes-Maslow, Kahwati, Kinsinger, & Campbell, 2012). For example, the Klein and Sorra (1996) Organizational Theory of Innovation Implementation features formal implementation policies and practices prominently as generators of a climate for implementation in which innovation use is expected, supported, and rewarded. In this theory, implementation policies and practices refers to “the array of innovation, implementation, organizational, and managerial policies, practices, and characteristics that may influence innovation use” (Klein & Sorra, 1996, p. 1059). Examples include formal training programs, guidelines, or protocols.
While the emphasis is on formal implementation policies and practices, the broader organizational science literature suggests that informal implementation policies and practices could also influence innovation use. Compared to formal implementation policies and practices, informal implementation policies and practices require less investment in resources and can readily be adapted to the organizations’ implementation needs. Informal implementation policies and practices may have other favorable characteristics, such as natural emergence from consensus among clinicians. However, less explicitly defined implementation policies and practices with no organizational mandate may have limited influence on innovation implementation.
Informal implementation policies and practices can be distinguished from other emergent organizational processes. For instance, coordination has been defined as a “process of interaction that integrates a collective set of interdependent tasks” and can involve informal activities such as spontaneous communication or “bottom-up” approaches (Okhuysen & Bechky, 2009). Likewise, culture has been conceptualized as a system of shared behavioral norms and underlying beliefs and values that shape the way of doing things in the organizations and represents the unwritten aspects of the organization (Verbeke, Volgering, & Hessels, 1998). Given these definitions, informal implementation policies and practices could include elements of coordination and culture, as well as other emergent processes not captured by these related constructs (e.g. on-the-job training).
This study sought to contribute to knowledge that can be used to minimize gaps in healthcare innovation implementation by furthering our understanding of the role of formal and informal implementation policies and practices as determinants of implementation effectiveness. We examined their role within the context of initiatives to increase palliative care consultation in two inpatient settings (medical oncology and gynecologic oncology) located at a single academic medical center.
Conceptual Framework
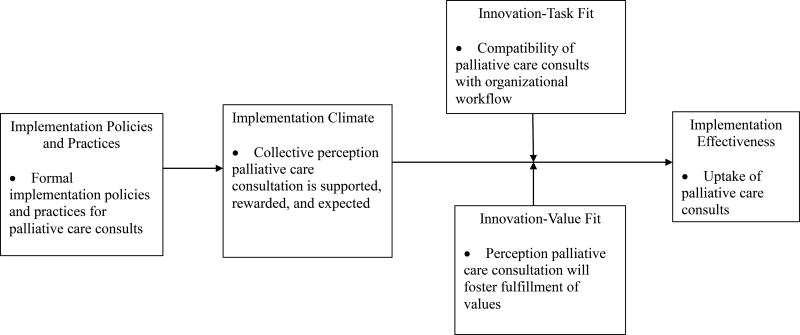
The Klein and Sorra Organizational Theory of Innovation Implementation was used to guide the development of our interview guide and interpret our results (Klein, Conn, & Sorra, 2001; Klein & Sorra, 1996). This theory is well suited for explaining implementation effectiveness for complex innovations, which are practices perceived as new by the users in an organization and require coordinated use of multiple organizational members to benefit the organization. We considered palliative care consults in inpatient oncology to be a complex innovation based on the following: (1) the integration of palliative care consults with cancer treatment is an expanding and evolving area of interest; (2) use of inpatient palliative care consults is complex, comprising multiple providers including physicians, nurses, pharmacists, chaplains, and social workers who coordinate care for inpatients receiving palliative care; and (3) implementation of inpatient palliative care consults requires extensive coordination between multidisciplinary palliative care teams and the oncology clinicians overseeing the care of a patient. Implementation is the action of putting the innovation (palliative care consults) to use. The Klein and Sorra Organizational Theory of Innovation Implementation (Klein, Conn, & Sorra, 2001; Klein & Sorra, 1996) posits that implementation effectiveness is a function of formal implementation policies and practices, a positive implementation climate, perception that the innovations’ use is congruent with the intended users’ values, and the extent to which the innovation fits with organizational workflow (Helfrich et al., 2007; Weiner et al., 2012). Figure 1 describes how we operationalized each construct within the context of this study.
Figure 1.
Organizational Theory of Innovation Implementation, adapted from Klein and Sorra (1996).
Organizations can employ a variety of implementation policies and practices to support the use of an innovation. According to the theory, implementation policies and practices are cumulative, compensatory, and equifinal (i.e., the more formal policies and practices that an organization uses to support the innovation use, the better) (Klein & Sorra, 1996; Teal et al., 2012). The collective influence of an organization’s implementation policies and practices shapes implementation climate for innovation use (Helfrich et al., 2007; Klein & Sorra, 1996; Teal et al., 2012). Climate refers to the shared perception among targeted organizational members of the “extent to which their use of a specific innovation is rewarded, supported, and expected within the organization” (Klein & Sorra, 1996). The more this shared sense is developed, the greater likelihood the innovation will be used consistently and with high quality.
A strong climate is necessary, but not sufficient, for effective innovation implementation. The association between climate and implementation effectiveness may be moderated by the innovation-values fit and innovation-task fit. Innovation-values fit is “the extent to which targeted users perceive that use of the innovation will foster (or, conversely, inhibit) the fulfillment of their values” (Klein & Sorra, 1996). Innovation task fit, which was not originally included in the theory, arose from Helfrich et al.’s (2007) and Weiner et al.’s (2012) prior research indicating the need to parse out the concept of innovation-value fit as encompassing not only normative values, but “the extent to which an innovation is compatible with work processes, task demands, and organizational capabilities” (Weiner et al., 2012, pg. 11). Even if the climate for innovation implementation is strong, a weak innovation-value fit or innovation-task fit will result in resistance and impede the organizations’ ability to effectively implement the innovation.
Methods
Study Setting
The study was conducted in two distinct oncology services at University of North Carolina (UNC) Hospitals, an 804-bed acute care facility and National Cancer Institute Comprehensive Cancer Center. Gynecologic oncology is composed of teams of clinicians who provide care for patients with solid tumor gynecological cancers. Compared to medical oncology, gynecologic oncology is a much smaller service, composed of only eight attending clinicians who specialize in gynecologic oncology and a small tight-knit group of specialty and subspecialty residents. In contrast, medical oncology has approximately 26 attending clinicians who specialize in solid tumors and a large pool of specialty residents. The teams on both services include an attending and several house-staff clinicians (residents and medical students). The services are characterized by frequent rotation of attending clinicians and turnover of residents. For example, attending clinicians rotate every two weeks in medical oncology and every eight weeks in gynecologic oncology, while residents and students rotate monthly and subspecialty residents in gynecologic oncology rotate on a weekly basis. Each subspecialty resident in gynecologic oncology is assigned the primary responsibility for overall organization and delegation of patient care during the week they are on rotation. The medical center’s inpatient palliative care team is interdisciplinary, composed of an attending palliative physician, two nurse practitioners, a social worker, and a chaplain; it supports symptom management, goal setting, and decision-making for inpatients and is available to patients only by referral of the primary treating team in the oncology services.
Palliative Care Consult Implementation in Oncology Services
Starting in August 2014, gynecologic oncology began using a single formal implementation policy—a one-page written guideline describing the clinical criteria (e.g., unplanned admission for symptom management, frequent readmissions, malignant small bowel obstruction) for initiating a palliative care consult posted in the residents’ work area. Oncologists in gynecologic oncology developed the guideline internally, without input from palliative care service.
Starting in October 2015, medical oncology began using multiple formal implementation policies and practices, including chart review by a trained research assistant in the palliative care service to identify all cancer inpatients with Stage IV disease and uncontrolled symptoms, prompting for palliative care consultation, and monthly training for residents in palliative care skills of advanced care planning communication. Palliative care attending clinicians functioned as champions for promoting palliative care consultation and institutional funding was secured to support these formal implementation policies and practices. All implementation policies and practices were led by the palliative care service, with significant input throughout implementation from selected oncology clinicians.
Study design
This study used a two-case study design of palliative care consult implementation in the medical oncology and gynecologic oncology services. Case study methods use mixed-methods to provide an in-depth analysis of the organizational context and is well-suited for studying implementation of innovations (Yin, 2014). Specifically, we explored the organizational context for palliative care consult implementation with qualitative data from key-informant interviews (medical oncology, gynecologic oncology, and palliative care clinicians). Consistent with a mixed-methods approach, quantitative data on palliative care consult uptake were used to complement the qualitative findings and to gain a comprehensive understanding of palliative care consult implementation for each of the cases (Fetters, Curry, & Creswell, 2013). We defined uptake as completion of a palliative care consult (as opposed to making a referral). The University of North Carolina Institutional Review Board reviewed and approved this study.
Qualitative Data Collection
One investigator (LDD) gathered qualitative data through in-person interviews with inpatient medical oncology and gynecologic oncology clinicians (attendings, house-staff) from March to May 2016. Palliative care clinicians were also interviewed to gain additional insights on implementation context. Interview participants were recruited if they had provided patient care in the gynecologic oncology or medical oncology services after the formal implementation policies and practices were initiated. Participants in the oncology services were purposively sampled according to their clinical role and whether they were involved in developing the formal implementation policies and practices. Interview participants were recruited in-person and via e-mail and compensated with a $25 gift card for their time. Questions for the semi-structured interviews were developed using the Organizational Theory of Innovation Implementation as a guide. For example, participants were asked to describe training received in palliative care skills (implementation policies and practices), feedback received regarding patient referrals for palliative care consults (implementation policies and practices), incentives used by the oncology services to encourage clinicians to refer patients for palliative care consults (implementation policies and practices), barriers or disincentives to palliative care consultation (implementation climate), criteria used to decide whether to refer a patient for a palliative care consult (innovation-task fit), and whether or not palliative care consultation helped achieve clinicians’ priorities during the time they were rotating on the service (innovation-values fit). Participants were also asked whether there were any other major events or changes that occurred in the oncology services in the past year that may have impacted palliative care consult implementation. For the interviews with palliative care clinicians, questions were rephrased to obtain their perceptions of the oncology services’ palliative care consult implementation. A variety of probes were used to elicit thorough responses. All interviews were audio-recorded and transcribed verbatim.
Quantitative Data Collection
We obtained quantitative data on palliative care consult uptake from January 2010 to June 2016 using data from the UNC Palliative Care Clinical Research database. This database includes data abstracted from medical charts for all patients who receive palliative care consultation, including dates of service and oncology service line in which the palliative care consult was initiated. These data were then linked to all hospital stays with an admission and/or discharge from the medical oncology or gynecologic oncology service with a solid tumor diagnosis based on International Classification of Diseases 9 and 10 diagnosis codes documented during the hospital stay using data from the Carolina Data Warehouse for Health (a central data repository containing clinical, research, and administrative data from the institution electronic health record system). If multiple palliative care consults occurred during a hospital stay, only the first consult was included in the dataset.
Qualitative Analysis
Using codes identified deductively based on the conceptual framework (Figure 1), qualitative data were coded and analyzed using Atlas.ti (version 7.0). Two members of the research team (LDD, ASC) independently coded all interview transcripts (nearly 300 pages from all three inpatient service lines) using a common codebook and reconciled codes after completion of independent coding. Within each service, we assessed the degree to which each construct appeared in the data (salience) by counting the text segments (i.e., a sentence or paragraph that encompassed one or more constructs of interest) assigned to the construct’s code, the degree to which the construct positively or negatively affected implementation (valence), and the degree to which relationships among the constructs were supported in the conceptual framework. We also conducted a cross-case synthesis (Yin, 2014) to explore whether organizational determinants of palliative care consult implementation varied across the service lines. We then analyzed the data for key themes and patterns by each construct.
Quantitative Analysis
Palliative care consult uptake was derived from aggregated palliative care consult rates within the gynecologic oncology and medical oncology services. We calculated monthly and annual rates by dividing the number of encounters that involved a palliative care consult (numerator) by the total number of encounters eligible for a consult (denominator), which was defined as all hospital admissions within each of the services. We provided visual representation of annual and monthly trends in palliative care consult uptake in the form of graphs. A scatterplot of the monthly rates was overlaid with a fractional polynomial prediction plot to provide a flexible summary of the relationship.
Results
We analyzed data from interviews (N=26) representing three services: 12 from medical oncology, 10 from gynecologic oncology, and 4 from palliative care (Table 1). Roles represented across the interviews included attending clinicians (N=13), specialty residents (N=6), subspecialty residents (N=3), medical students (N=2), and staff (N=2). Interviews ranged from approximately 15 to 45 minutes (mean=28 minutes).
Table 1.
Number and Characteristics of Key-Informant Interview Participants, by Service
| Service | Medical Oncology | Gynecologic Oncology | Palliative Care |
|---|---|---|---|
|
| |||
| Number | 12 | 10 | 4 |
|
| |||
| Role | 7 Attending Clinicians | 4 Attending Clinicians | 2 Attending Clinicians |
| 3 Specialty Residents | 3 Subspecialty Residents | ||
| 2 Medical Students | 3 Specialty Residents | 2 Staff | |
|
| |||
| Gender | 5 Males | 2 Males | 3 Female |
| 7 Females | 8 Females | 1 Male | |
Results are summarized in Table 2. Briefly, medical oncology employed multiple formal implementation policies and practices to support palliative care consultation, yet most clinicians were unaware of the implementation policies and practices, contributing to a weak implementation climate. In contrast, gynecologic oncology employed one formal implementation policies and practices but also relied on multiple informal implementation policies and practices, which contributed to broader clinician awareness and a strong implementation climate. Both services exhibited temporal increases in consult uptake. Palliative care clinician interviews generally corroborated the findings in the oncology services.
Table 2.
Summary of findings by Organizational Theory of Innovation Implementation constructs
| Service | IPPs | Implementation Climate |
Innovation- Task Fit |
Innovation- Values Fit |
Implementation Effectiveness |
|---|---|---|---|---|---|
| Gynecologic Oncology |
Formal IPP:
|
Strong climate for PC consult implementation
|
Strong innovation-task fit for PC consults | Strong innovation-values fit for PC consults | Increase in consult uptake |
| Medical Oncology |
Formal IPPs:
|
Weak climate for PC consult implementation
|
Moderate innovation-task fit for PC consults
|
Strong innovation-values fit for PC consults | Increase in consult uptake |
PC: Palliative care; IPPs: Implementation Policies and Practices
(*) = finding was same across both services; (+) = positively associated with implementation; (−) = negatively associated with implementation; (+/−) = positively and negatively associated with implementation
Implementation Effectiveness
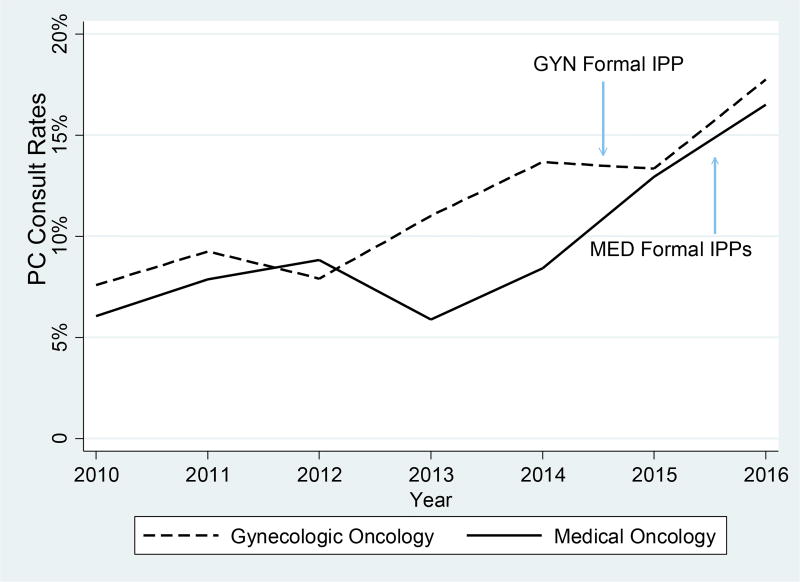
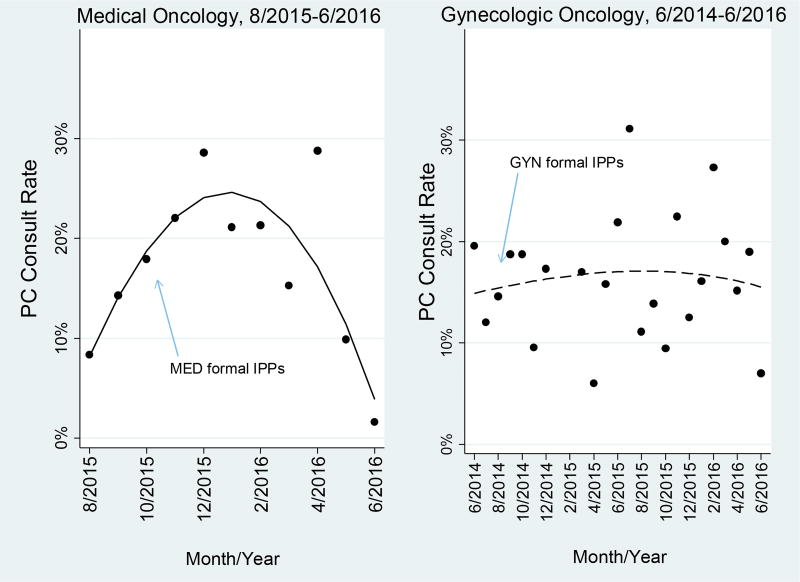
Gynecologic oncology and medical oncology services exhibited variable but increasing aggregated rates of consults over time. Both services had similar annual rates of consults in 2010, exhibited a decrease between 2011–2012 and 2012–2013, and then increased after 2013 (Figure 2). Starting in mid-2014, both services met or exceeded the national goal of providing consults to approximately 10% of all hospital admissions (although no benchmark currently exists specific to inpatient oncology) (Center to Advance Palliative Care, 2014). Although at first glance these trends appear to be a part of a broader trajectory, Figure 3 indicates gynecology oncology experienced a slight increase in monthly consult rates in August 2014 after initiation of the single formal implementation policies and practices and maintained roughly similar rates throughout the remainder of the study. In contrast, medical oncology experienced a strong upward spike in monthly consult rates in October 2015 following initiation of multiple formal implementation policies and practices but immediately afterward exhibited a sharp decline. Below, we explain that these trends in the services might be attributed to not only the initiation of formal implementation policies and practices but also informal implementation policies and practices, implementation climate, innovation-task fit, and innovation-values fit for palliative care consultation.
Figure 2.
Annual uptake of palliative care consults, 2010–2016. The blue arrows indicate initiation of the formal implementation policies and practices in the oncology services.
GYN: gynecologic oncology
MED: medical oncology
PC: palliative care
IPP: implementation policies and practices
Figure 3.
Monthly uptake of palliative care consults during implementation. The blue arrows indicate initiation of the formal implementation policies and practices the oncology services.
*graphs start two months prior to initiation of formal IPPs; dots represent monthly consult rates
PC: palliative care
IPP: implementation policies and practices
Implementation Policies and Practices
Medical oncology employed multiple formal implementation policies and practices to support palliative care consultation while gynecologic oncology employed one formal implementation policy; however, compared to medical oncology, gynecologic oncology was more apt to use informal implementation policies and practices. For example, several participants reported frequent spontaneous communication and feedback between gynecologic oncology and the palliative care service. Participants mentioned they were particularly incentivized to use the palliative care service because of its quickness to respond and strong presence in gynecologic oncology. In addition, in the absence of a formal training, most palliative care skills were learned on-the-job through informal interactions with the palliative care service. One resident stated,
It’s a constant dialogue. I don’t know if it’s truly feedback, but the nurse practitioner or the resident, whoever’s here, there’s almost always one of us kind of up here on the floor, whoever’s on the OR [operating room], and they [palliative care] come by and see our patients, and they sit in our workroom with us, and we talk about the patients, and they kind of tell us their thoughts, and they ask us clarifying questions.
Further, champions in gynecologic oncology were also more emergent and informal as opposed to appointed. All interview participants identified at least one attending clinician whom they considered to be a champion for palliative care consults, with one participant identifying the fellows and residents as emergent champions because they “do a good job at remembering to call palliative care.” Several participants also discussed how the formal implementation policies and practices (written guideline) was developed by subspecialty residents in the service by adopting an informal bottom-up approach, which was in contrast to the formal top-down approach to implementation observed in medical oncology.
Despite multiple formal implementation policies and practices in medical oncology, only 5 of 12 interview participants (all attending clinicians) were aware of the implementation policies and practices. Moreover, these participants had only a vague understanding about what the policies and practices entailed. As one attending clinician commented, “So I don’t know what the automatic trigger is, but I know that a lot of our patients had palliative care consults and it was very useful.” Participants in medical oncology interviews also discussed the need for more formal implementation policies and practices, including feedback mechanisms, training, and specific clinical criteria for initiating palliative care consults. As one resident commented, “So I guess kind of the issue is palliative care kind of consults so they’ll come in and they’ll see a patient and they’ll give their recs. It’s so separate that there’s not really usually an opportunity for feedback in either direction.” In contrast, several interview participants in gynecologic oncology were aware of their single formal implementation policy, a written guideline describing the clinical criteria or initiating a consult and spoke about it in detail; identifying specific clinical criteria that would oftentimes trigger a consult, such as frequent admissions or presence of recurrent disease.
Implementation Climate
Medical oncology employed multiple formal implementation policies and practices but most interview participants were unaware of the policies and practices, which contributed to a weak implementation climate. For example, few in medical oncology reported using palliative care consults was an expectation on the service. Similarly, medical oncology participants’ comments indicated palliative care consultation was not always strongly supported, mentioning many barriers including limited availability of palliative care resources and increasing complexity of care as possible disincentives to their use. Further, consistent with the lack of awareness of the implementation policies and practices in medical oncology, participants’ clarity about when to use consults and whether they had the skills and tools to play their part in making referrals was also absent on this service.
In contrast, gynecologic oncology employed only one formal implementation policy, instead relying on multiple informal implementation policies and practices that contributed to broader clinician awareness and a strong implementation climate. For example, although referral is ultimately up to the individual clinician, gynecologic oncology participants generally reported consultation was expected. Likewise, participants’ comments indicated that consultation was supported in their work, citing few barriers or disincentives. Participants indicated clarity about when to use consults was strong and mentioned the formal implementation policy (written guideline) contributed to this clarity. Also in contrast to medical oncology, gynecologic oncology participants generally reported having the skills and tools to play their part in referring patients for consults, although some discussed needing more training and feedback from the palliative care service in this area.
Across both services, none reported receiving any specific recognition or rewards for palliative care consultation. Most participants mentioned this was not needed; better patient care was identified as the primary reward for consultation. However, almost all felt supported when it came to the logistics surrounding consultation (i.e., use of electronic health record system for referrals, paging process, talking on rounds). Many participants discussed how the electronic health record system made it easier to make palliative care consult referrals because the process was the same for all consult services in the hospital.
Innovation-Values Fit
Both services exhibited a strong innovation-value fit for palliative care consultation. Across clinician roles, consultation was found to be highly valued and consistent with the providing the best patient care possible. As indicated by one attending clinician, “in medical oncology, it’s a complex hospital. Our people are sick. You have multiple specialists... they’re all key. They’re [palliative care] as key to the team as the thoracic surgeon.” Each service had at least one attending state that every oncology inpatient should have a palliative care consult. Some students and residents spoke about the fit of palliative care consults with their values—the strong desire to learn and gain new skills—while attending clinicians spoke about the fit of palliative care consults with their commitment to educate residents. Clinicians in both services stated that palliative care consults were consistent with “keeping the flow open” and being “vested” in a team-based approach to care for inpatients admitted with complex medical needs. Given that one-third of interview participants in each service reported receiving some palliative care training during their medical education, clinicians’ strong value for consults may have been fostered by this prior exposure.
Although interview participants from the palliative care service generally echoed the findings from medical and gynecologic oncology, several indicated that consults may not always be consistent with oncologists’ priority for chemotherapy treatment or timely discharge from the hospital.
Innovation-Task Fit
Both services reported palliative care consults generally fit well with organizational tasks and workflow. Several themes may explain this finding. First, the main functions of the palliative care service are to address symptom management and facilitate goals of care discussions. Across both services, participants agreed consults added an extra layer of support for symptom management; however, in medical oncology the emphasis was primarily on managing pain while in gynecologic oncology participants identified multiple symptoms that consults aided in managing. As stated by this attending clinician,
I think it’s usually many times symptom management, so if patients are having symptoms from their cancer, especially multiple symptoms from their cancer, there’s pain and nausea and maybe shortness of breath and the things that we know how to do as gynecologic oncologists don’t seem to maybe working the best, I think that’s really probably our number one reason why we call them is for symptom control and help with that.
Likewise, both services considered there to be a strong innovation-task fit if goals of care discussions were needed because clinicians face many competing demands while on-service and lack the time to have lengthier goals of care discussions with patients and their families. Participants mentioned that palliative care consults can help to offset this workload, however our findings across the services suggest there may be a U-shaped relationship between patient volume and innovation-task fit for consults. Specifically, some participants mentioned high patient volume would promote consultation while others commented they would be more likely to use consults when volume was low because there was “more time to think about individual people and some of their broader problems.” Of note, participants often referred to goals of care discussions as “end-of-life care” and indicated they were most compatible only if a patient was transitioning to hospice, however this finding was more pronounced in medical oncology.
Second, both services reported attending clinicians’ preferred roles influenced how well consults fit in the service, particularly as it relates to goals of care discussions. For example, in gynecologic oncology some attending clinicians mentioned wanting to conduct goals of care discussions because they are “my patients.” This comment likely reflects that all clinicians on the service care for the same spectrum of cancer types. In contrast, because attending clinicians in medical oncology specialize in a variety of tumor types, they may be in a better position to discuss prognosis for one cancer type but less comfortable discussing the outlook of patients with other cancer types represented on the service. Participants identified that patient and family preferences may also affect the fit of consults but that this could be addressed by improving the branding of the palliative care service.
Third, participants in gynecologic oncology reported consults were compatible with workflow if they were aware the patient was already receiving palliative care services in the outpatient setting. As one attending clinician stated, “I have a number of my patients that I have palliative care help take care of as an outpatient… so usually they will call the consult and say what is needed.” In contrast, interview participants in medical oncology were more apt to report a poor compatibility if they were unaware whether there was continuity of care with palliative care services in the outpatient setting. As one attending clinician expressed,
Unfortunately what we don’t have yet is a seamless process where the patients are getting these things done in the outpatient setting. And maybe they are, but I get this problem all the time, where is the documentation? It’s the weekend. I can’t reach the primary attending. I have to have these tough conversations now with these folks, so I did them.
Discussion
We studied two initiatives to increase implementation of palliative care consults in inpatient oncology and found empirical support for the role of formal and informal implementation policies and practices as determinants of implementation effectiveness. Specifically, despite the medical oncology service’s use of multiple formal implementation policies and practices, most participants were unaware of the policies and practices, which contributed to a weak implementation climate. In contrast, the gynecologic oncology service employed only one formal implementation policy and instead relied on multiple informal implementation policies and practices, which contributed to broader clinician awareness and a strong implementation climate. Innovation-value fit and innovation-task fit (moderators of implementation climate and implementation effectiveness) were generally strong in both services.
According to the Klein and Sorra Organizational Theory of Innovation Implementation, we would expect consult uptake to be suboptimal in medical oncology, however both services exhibited temporal increases. Despite medical oncology clinicians’ lack of awareness, there was a strong upward spike in consult uptake after the initiation of the formal implementation policies and practices in October 2015. Indeed, Figure 3 is illustrative of a significant increase previously reported in a study by the authors which used a difference-in-differences analysis to compare uptake before and after palliative care consult implementation in the oncology services (DiMartino et al., In Press). This disparate finding is surprising and may be attributed to when implementation climate was assessed. Specifically, interviews were conducted several months after initiation of the formal implementation policies and practices in medical oncology and coincided with the declining uptake rates in this service observed at the end of the study (Figure 3). This decline may provide an indication that climate strength in medical oncology weakened over time. Accordingly, our findings from the interviews may not accurately reflect the climate strength that existed soon after the formal implementation policies and practices were initiated. Alternatively, we examined the potential for other initiatives occurring in the oncology services that may have impacted palliative care consult implementation. Participants in both oncology services and the palliative care service were asked if such initiatives had occurred in the past year, but there were no activities reported that would be expected to impact palliative care consult implementation.
Practical Implications
These study findings ultimately point to a broader issue: relying solely on organizationally sanctioned formal implementation policies and practices may not be effective in creating a strong and sustainable climate for implementation in busy, complex healthcare organizations such as the academic oncology services examined in this study (Sommerbakk, Haugen, Tjora, Kaasa, & Hjermstad, 2016). For example, training is a formal implementation policies and practices commonly used by healthcare organizations to promote innovation use, but residents often lack the time outside of their clinical responsibilities to attend skills trainings. In addition, new groups of residents rotate through the oncology services on a frequent (though predictable) schedule. Thus, unless training is mandatory and offered on a continuous and routine basis, exposure will be minimal and ultimately contribute to a weakened implementation climate over time.
From a practical standpoint, our findings support the idea that informal implementation policies and practices may compensate or substitute for formal implementation policies and practices under certain conditions. As we observed in the gynecologic oncology service, this may be more likely to occur in smaller healthcare organizations where there is greater proximity and opportunity for social interaction and information sharing (Klein, Conn, Smith, & Sorra, 2001). For example, one study found small primary care practices achieved effective implementation of the patient-centered medical home using informal care teams rather than more formal care coordination (Berry et al., 2013). Specifically, formal implementation policies and practices may influence implementation climate and subsequent effective implementation insofar as the targeted users of the innovation have the opportunity to develop a shared sense innovation use is expected, supported, and rewarded (Klein & Sorra, 1996). In gynecologic oncology, we found the use of informal implementation policies and practices may have played a critical role in creating that shared sense and a strong and sustainable implementation climate. For example, gynecologic oncology may have exhibited greater awareness of the written guideline because the strong presence of informal implementation policies and practices in the service continually reinforced its enactment. In particular, adopting an emergent bottom-up approach by involving clinicians in all roles in development of the guideline created a greater sense of ownership, which may have contributed to awareness and a more positive view of the guideline. In contrast, informal implementation policies and practices may be less likely to substitute for formal implementation policies and practices in larger organizations, such as medical oncology, where fragmented intra-departmental units have limited opportunity for social interaction (Klein, Conn, Smith, et al., 2001; Weiner, Belden, Bergmire, & Johnston, 2011). As we observed, medical oncology used multiple formal implementation policies and practices developed externally by the palliative care service. The absence of informal implementation policies and practices in combination with a top-down approach may have undermined clinicians’ awareness of the implementation policies and practices, which contributed to a weak shared sense that palliative care consultation was expected, supported, and rewarded. Future research should further investigate the role of formal and informal implementation policies and practices in shaping a strong and sustainable implementation climate, including the interplay between top-down versus bottom-up approaches and subsequent effective implementation of healthcare innovations.
Study Limitations and Conclusion
This study was conducted at a single academic medical center, which limits generalizability. However, case study research, which emphasizes depth over breadth, is appropriate for the purposes of theory refinement (Eisenhardt, 1989). Second, interview data were gathered after the initiation of the formal implementation policies and practices in both services. Therefore, we are unable to provide a longitudinal assessment of how the organizational context for palliative care consults may have changed over time or determine whether the sharp decline in palliative care consult uptake rates in medical oncology observed at the end of the study would persist or eventually rebound. Third, the medical oncology residents we interviewed described implementation climate at the time of the interview and may not be representative of residents who were rotating when the palliative care skills training was initiated. Had we interviewed residents soon after initiation of the training we may have found different climate perceptions. Fourth, although development of quantitative measures of implementation climate are underway (Weiner et al., 2011), they have not been fully tested. Thus, we were unable to specify with precision how the services compared on this construct. Finally, there were contextual differences identified between the two services that may not be modifiable by administrators (e.g. service size). Understanding these differences could be helpful in guiding adaptation of the implementation policies and practices to accommodate varying contexts.
Despite these limitations, this study makes a novel contribution to the implementation science literature by offering preliminary evidence for the role of both formal and informal implementation policies and practices as determinants of implementation, suggesting refinements to the Klein and Sorra Organizational Theory of Innovation Implementation. There is precedent for elaborating on this theory (Birken, Lee, & Weiner, 2012; Helfrich et al., 2007). This study also adds to the small body of implementation research adapting the theory to include innovation-task fit to provide an indication of congruence of the innovation with the organization and is a critical determinant of implementation (Helfrich et al., 2007; Weiner et al., 2012). Future research examining whether our study findings are a function of other aspects of the theory, including readiness to change, management support, and/or resource availability within the service may lead to more robust results.
To date, the influence of formal policies and practices on healthcare innovation implementation has garnered more attention in the implementation science literature than informal policies and practices. However, by providing an in-depth exploration of the organizational determinants of palliative care consult implementation in inpatient oncology, our findings suggest informal policies and practices for promoting effective implementation should be encouraged in certain contexts such as smaller healthcare organizations. The results from our study may help organizations to identify optimal strategies to improve effectiveness of healthcare innovation implementation and minimize gaps between evidence and practice.
Acknowledgments
Dr. DiMartino’s work on this research was supported by funding from the National Cancer Institute Cancer Control Education Program (R25 CA057726). Ms. Clary’s work on this research was supported by the National Cancer Institute at the National Institute of Health (R25CA116339). The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number TL1TR001110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would also like to thank Kathryn Wessell and Kemi Doll, MD for their assistance with this research.
The research was approved by the University of North Carolina at Chapel Hill Institutional Review Board (IRB# 16-0087). All participants provided verbal informed consent prior to participation in the study.
Contributor Information
Lisa D. DiMartino, Social and Health Organizational Research and Evaluation Program, Research Triangle Institute, International, 3040 East Cornwallis Road, PO Box 12194, Research Triangle Park, NC 27709-2194, 919-316-3331, ldimartino@rti.org.
Sarah A. Birken, Department of Health Policy and Management, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, 1103E McGavran-Greenberg, 135 Dauer Drive, Campus Box 7411, Chapel Hill, NC 27599-7411, birken@unc.edu.
Laura C. Hanson, Division of Geriatric Medicine, Center for Aging and Health, UNC Palliative Care Program, 5003 Old Clinic Building, Campus Box 7550, Chapel Hill, NC 27599-7550, lhanson@med.unc.edu.
Justin G. Trogdon, Department of Health Policy and Management, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, 1101B McGavran-Greenberg, 135 Dauer Drive, Campus Box 7411, Chapel Hill, NC 27599-7411, justintrogdon@unc.edu.
Alecia S. Clary, Department of Health Policy and Management, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, 135 Dauer Drive, Campus Box 7411, Chapel Hill, NC 27599-7411, alecias@live.unc.edu.
Morris Weinberger, Department of Health Policy and Management, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, 1105B McGavran-Greenberg, 135 Dauer Drive, Campus Box 7411, Chapel Hill, NC 27599-7411, mweinber@email.unc.edu.
Katherine Reeder-Hayes, 170 Manning Drive, Campus Box 7305, Chapel Hill, NC 27599, kreederh@email.unc.edu.
Bryan J. Weiner, Department of Global Health, Department of Health Services, Box 357965, University of Washington, Seattle, WA 98195-7965, bjweiner@uw.edu.
References
- Berry CA, Mijanovich T, Albert S, Winther CH, Paul MM, Ryan MS, Shih SC. Patient-centered medical home among small urban practices serving low-income and disadvantaged patients. Ann Fam Med. 2013;11(Suppl 1):S82–89. doi: 10.1370/afm.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken SA, Lee SY, Weiner BJ. Uncovering middle managers' role in healthcare innovation implementation. Implement Sci. 2012;7:28. doi: 10.1186/1748-5908-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Curry LA, Cherlin EJ, Talbert-Slagle K, Horwitz LI, Bradley EH. Integrating new practices: a qualitative study of how hospital innovations become routine. Implement Sci. 2015;10:168. doi: 10.1186/s13012-015-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center to Advance Palliative Care. The National Palliative Care Registry: Trends in Hospital Palliative Care Since 2008. 2014 Retrieved from https://palliativeinpractice.org/palliative-pulse/palliative-pulse-september-2016/national-palliative-care-registry-trends-hospital-palliative-care-since-2008/
- DiMartino LD, Weiner BJ, Hanson LC, Weinberger M, Birken SA, Reeder-Hayes KR, Trogdon JG. The Impact of Two Triggered Palliative Care Consultation Approaches on Consult Implementation in Oncology. Healthcare: The Journal of Delivery Science and Innovation. doi: 10.1016/j.hjdsi.2017.12.001. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt KM. Building theories from case study research. Academy of management review. 1989;14(4):532–550. [Google Scholar]
- Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs-principles and practices. Health Serv Res. 2013;48(6 Pt 2):2134–2156. doi: 10.1111/1475-6773.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich CD, Weiner BJ, McKinney MM, Minasian L. Determinants of implementation effectiveness: adapting a framework for complex innovations. Med Care Res Rev. 2007;64(3):279–303. doi: 10.1177/1077558707299887. [DOI] [PubMed] [Google Scholar]
- Klein KJ, Conn AB, Smith DB, Sorra JS. Is everyone in agreement? An exploration of within-group agreement in employee perceptions of the work environment. J Appl Psychol. 2001;86(1):3–16. doi: 10.1037/0021-9010.86.1.3. [DOI] [PubMed] [Google Scholar]
- Klein KJ, Conn AB, Sorra JS. Implementing Computerized Technology: An Organizational Analysis. Journal of Applied Psychology. 2001;86(5):811–824. doi: 10.1037/0021-9010.86.5.811. [DOI] [PubMed] [Google Scholar]
- Klein KJ, Sorra JS. The Challenge of Innovation Implementation. The Academy of Management Review. 1996;21(4):1055–1080. [Google Scholar]
- Okhuysen GA, Bechky BA. Coordination in Organizations: An Integrative Perspective. The Academy of Management Annals. 2009;3(1):463–502. [Google Scholar]
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, … Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerbakk R, Haugen DF, Tjora A, Kaasa S, Hjermstad MJ. Barriers to and facilitators for implementing quality improvements in palliative care - results from a qualitative interview study in Norway. BMC Palliat Care. 2016;15:61. doi: 10.1186/s12904-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal R, Bergmire DM, Johnston M, Weiner BJ. Implementing community-based provider participation in research: an empirical study. Implement Sci. 2012;7:41. doi: 10.1186/1748-5908-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke W, Volgering M, Hessels M. Exploring the conceptual expansion within the field of organizational behavior: Organizational climate and organizational culture. Journal of Management Studies. 1998;35:303–329. [Google Scholar]
- Weiner BJ, Belden CM, Bergmire DM, Johnston M. The meaning and measurement of implementation climate. Implement Sci. 2011;6:78. doi: 10.1186/1748-5908-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner BJ, Haynes-Maslow L, Kahwati LC, Kinsinger LS, Campbell MK. Implementing the MOVE! weight-management program in the Veterans Health Administration, 2007–2010: a qualitative study. Prev Chronic Dis. 2012;9:E16. [PMC free article] [PubMed] [Google Scholar]
- Wolfenden L, Jones J, Williams CM, Finch M, Wyse RJ, Kingsland M, … Yoong SL. Strategies to improve the implementation of healthy eating, physical activity and obesity prevention policies, practices or programmes within childcare services. Cochrane Database Syst Rev. 2016;10:CD011779. doi: 10.1002/14651858.CD011779.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin RK. Case Study Research Design and Methods. 5. Thousand Oaks, CA: Sage Publications; 2014. [Google Scholar]