Abstract
Anxiety disorders impose a staggering burden on public health, underscoring the need to develop a deeper understanding of the distributed neural circuits underlying extreme fear and anxiety. Recent work highlights the importance of the central extended amygdala, including the central nucleus of the amygdala (Ce) and neighboring bed nucleus of the stria terminalis (BST). Anatomical data indicate that the Ce and BST form a tightly interconnected unit, where different kinds of threat-relevant information can be integrated to assemble states of fear and anxiety. Neuroimaging studies show that the Ce and BST are engaged by a broad spectrum of potentially threat-relevant cues. Likewise, mechanistic work demonstrates that the Ce and BST are critically involved in organizing defensive responses to a wide range of threats. Studies in rodents have begun to reveal the specific molecules, cells, and microcircuits within the central extended amygdala that underlie signs of fear and anxiety, but the relevance of these tantalizing discoveries to human experience and disease remains unclear. Using a combination of focal perturbations and whole-brain imaging, a new generation of nonhuman primate studies is beginning to close this gap. This work opens the door to discovering the mechanisms underlying neuroimaging measures linked to pathological fear and anxiety, to understanding how the Ce and BST interact with one another and distal brain regions to govern defensive responses to threat, and to developing improved intervention strategies.
Keywords: affective neuroscience, anxiety disorders, BST/BNST, emotion, individual differences, neuroimaging, nonhuman primate
When extreme, fear and anxiety can become debilitating (Salomon et al., 2015)1. Anxiety disorders impose a staggering burden on public health; they are common, costly, and contribute to the etiology of depression and substance abuse (Craske et al., 2017; DiLuca & Olesen, 2014; Whiteford et al., 2013). Existing treatments are inconsistently effective or associated with significant adverse effects (Bystritsky, 2006; Griebel & Holmes, 2013), underscoring the need to develop a deeper understanding of the distributed neural circuits that control the expression of fear and anxiety. Converging lines of physiological and mechanistic evidence indicate that the central extended amygdala—including the central nucleus of the amygdala (Ce) and bed nucleus of the stria terminalis (BST)—is a key hub in this circuitry and motivates the hypothesis that local alterations in the central extended amygdala can drive changes in remote regions of the brain in ways that promote the development and maintenance of anxiety, mood, and substance use disorders (Fox & Kalin, 2014; Fox, Oler, Tromp, Fudge, & Kalin, 2015; Kalin, 2017; Koob & Mason, 2016; Shackman, Kaplan, et al., 2016; Shackman, Tromp, et al., 2016) (Figure 1).
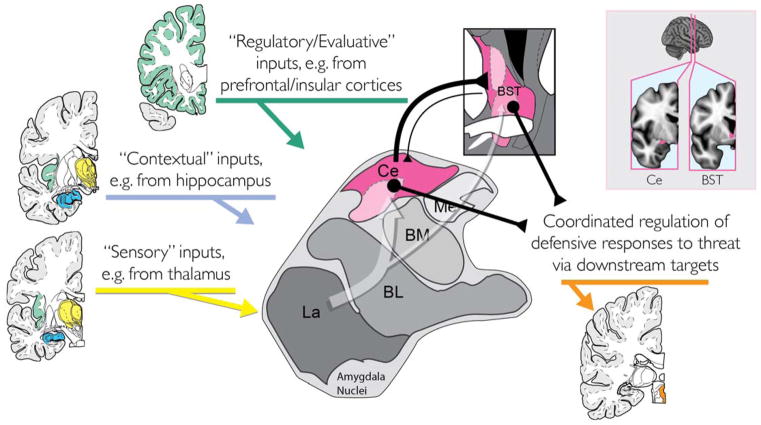
Figure 1. The central extended amygdala helps organize defensive responses to threat.
Simplified schematic of key inputs and outputs to the central extended amygdala (magenta) in humans and other primates. The central extended amygdala encompasses the central nucleus of the amygdala (Ce), which lies in the dorsal amygdala, and the bed nucleus of the stria terminalis (BST), which wraps around the anterior commissure. As shown by the translucent white arrow at the center of the figure, much of the sensory (yellow), contextual (blue), and regulatory (green) inputs to the central extended amygdala are indirect (i.e., poly-synaptic), and often first pass through adjacent amygdala nuclei before arriving at the Ce or BST. In primates, projections linking the Ce with the BST are predominantly from the Ce to the BST. The Ce and BST are both poised to orchestrate or trigger momentary negative affect via projections to downstream target regions (orange), such as the periaqueductal grey (PAG). Inset: Coronal slices depicting the relative locations of the Ce and the BST (magenta). Portions of this figure were adapted with permission from (Mai, Paxinos, & Voss, 2007). The BST region depicted in the inset is described in (Theiss et al., 2017). The Ce region depicted in the inset was kindly provided by Dr. Brendon Nacewicz. Abbreviations: Basolateral (BL), Basomedial (BM), Central (Ce), Lateral (La), and Medial (Me) nuclei of the amygdala; Bed nucleus of the stria terminalis (BST).
Here, we provide an updated mini-review of the contributions of the Ce and the BST to fear and anxiety, focusing on studies in humans and nonhuman primates (for other recent reviews, see Avery, Clauss, & Blackford, 2016; Goode & Maren, 2017; Kalin, 2017; Lebow & Chen, 2016; Shackman & Fox, 2016). This emphasis reflects the fact that anxiety disorders are defined and diagnosed on the basis of subjective symptoms and human studies are essential for understanding the neural mechanisms supporting the experience of fear and anxiety. Human studies are also crucial for identifying the features of animal models that are conserved and, hence, most relevant to understanding human disease and to developing improved interventions for human suffering (‘forward translation;’ Birn et al., 2014; Hyman, 2016). Finally, human studies afford important opportunities for developing objective biomarkers of disease or disease risk (Woo, Chang, Lindquist, & Wager, 2017)—accelerating the development of new diagnostic and treatment strategies (Borsook, Becerra, & Hargreaves, 2006, 2011; Wager & Woo, 2015)—and for generating novel hypotheses that can be mechanistically assessed in animal models (‘reverse translation;’ Ferenczi et al., 2016). Work in monkeys can be conceptualized as a bridge, one that links the precise mechanistic studies that can most readily be performed in rodents to the complexities of human feelings and human disease. The brains of monkeys and humans are genetically, anatomically, and functionally similar, reflecting the two species relatively recent evolutionary divergence (25 million years ago vs. 70 million years ago for rodents; Gibbs et al., 2007; Preuss, 1995; Preuss, 2007; Wise, 2008). Homologous biological substrates, including a well-developed prefrontal cortex (PFC), endow monkeys and humans with a shared repertoire of complex socio-emotional responses to potential threat (Fox & Kalin, 2014; Oler, Fox, Shackman, & Kalin, 2016), increasing the likelihood of successful translation to human disease (C. G. Jennings et al., 2016; Kaiser & Feng, 2015).
Anatomy of the Central Extended Amygdala
The extended amygdala encompasses a heterogeneous collection of subcortical nuclei along the borders of the amygdala and the ventral striatum. Classical studies of structural connectivity first suggested that the central division of the extended amygdala—including the Ce, lateral BST (BSTL), and portions of the sublenticular extended amygdala (SLEA; a neuronal bridge nestled within the substantia innominata)—represents an integrative unit (Alheid & Heimer, 1988). Indeed, it has long been recognized that the amygdala is connected to the BST via two major fiber bundles: the ventral amygdalofugal pathway (VA; sometimes termed the ansa peduncularis) and the stria terminalis (ST) (Nauta, 1961) (Figure 2a). More recent studies in monkeys have confirmed that the Ce and BSTL are structurally interconnected via these two direct pathways (primarily Ce → BSTL) and have identified a novel indirect pathway centered on the SLEA (Ce ↔ SLEA ↔ BSTL) (deCampo & Fudge, 2013; Oler et al., 2017). In parallel, magnetic resonance imaging (MRI) studies have revealed evidence of both structural (Avery et al., 2014; Kamali et al., 2016; Kamali et al., 2015) and functional connectivity between the Ce and BST (Avery et al., 2014; Birn et al., 2014; Gorka, Torrisi, Shackman, Grillon, & Ernst, in press; Oler et al., 2012; Oler et al., 2017; Tillman et al., accepted pending minor revision; Torrisi et al., 2015), reinforcing the hypothesis that they represent a functionally meaningful circuit (Alheid & Heimer, 1988; Fox, Oler, Tromp, et al., 2015).
Figure 2. Imaging the central extended amydala in nonhuman primates and humans.
(A) Diffusion tensor imaging (DTI). Deterministic DTI reveals the evolutionarily conserved white matter pathways (yellow) that link the Ce (magenta) to the BST (magenta) in monkeys (left) and humans (right). Images kindly provided by Do Tromp. (B) Functional neuroimaging of fear and anxiety. In monkeys (left), metabolic activity in the Ce and the BST (magenta arrows) is associated with heightened behavioral and neuroendocrine responses during prolonged (30 min) exposure to a potentially threatening human intruder’s profile (n=592). Likewise, an automated meta-analysis of human imaging studies (right) reveals activation in the BST and the Ce during studies of ‘fear’ and/or ‘anxiety.’ Figure depicts the minimum conjunction (logical ‘AND’) of thresholded forward-inference maps (q<.01) automatically generated using Neurosynth (Yarkoni et al., 2011) for studies tagged with the keywords ‘fear’ (298 studies) and/or ‘anxiety’ (312 studies). Given the strengths and limitations of automated meta-analysis, this finding simply indicates that these regions are routinely recruited by a variety of potentially threat-relevant cues. It does not indicate that these regions are consistently co-activated in particular studies and it does not imply that these regions are similarly responsive to particular kinds of threat (e.g., uncertain or diffuse danger). (C) The Ce and the BST show phasic and sustained responses to potentially threat-related cues in human imaging studies. Left: The BST and neighboring regions (white ring) show sustained (<118-s) activation during the uncertain anticipation of aversive images. Middle: The Ce shows sustained activation (30-s) during exposure to a virtual reality context paired with unpredictable electric shock. Right: The Ce and the BST (black arrows) both show phasic responses to video clips of an approaching tarantula (4-s). Portions of this figure were adapted with permission from (Andreatta et al., 2015; Fox, Oler, Shackman, et al., 2015; Mobbs et al., 2010; Shackman & Fox, 2016; Somerville et al., 2013).
From an anatomical perspective, the central extended amygdala is poised to integrate potentially threat-relevant information and assemble states of fear and anxiety. Invasive tracing studies in rodents and monkeys show that the Ce and the BST receive direct and indirect projections from brain regions that encode sensory, contextual, and regulatory information (Freese & Amaral, 2009) (Figure 1). Both regions are poised to trigger somatomotor and neuroendocrine signs of fear and anxiety via dense mono- and poly-synaptic projections to brainstem and subcortical effector regions (Fox, Oler, Tromp, et al., 2015; Freese & Amaral, 2009) (Figure 1). Leveraging the increased anatomical resolution afforded by ultra-high field strength functional MRI (7 Tesla), human studies indicate that many of these downstream regions show robust functional connectivity with the Ce and the BST (Gorka et al., in press; Torrisi et al., 2015). Other work shows that the Ce and BST contain cells with similar architectonic and neurochemical features and that the two regions show similar patterns of gene expression (for a detailed review, see Fox, Oler, Tromp, et al., 2015). Collectively, these anatomical observations suggest that the Ce and the BST represent an evolutionarily conserved, functionally coherent circuit that is poised to use information about threat, context, and internal states to initiate a range of defensive responses.
Physiology of the Central Extended Amygdala
Studies of nonhuman primates afford an important opportunity to obtain concurrent measures of naturalistic defensive behaviors, neuroendocrine activity, and brain metabolism in response to a range of ethologically relevant threats, including explicit cues (i.e., an unfamiliar human intruder’s profile) and more diffuse contexts (i.e., a novel testing cage) (Fox & Kalin, 2014; Kalin, 2017; Oler et al., 2016). Using a combination of 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) and well-established behavioral assays, we have demonstrated that the Ce and BST are exquisitely sensitive to potential danger. In studies including between 238 and 592 monkeys, elevated levels of metabolic activity in the Ce and BST are associated with heightened signs of fear and anxiety (e.g., freezing, cortisol) during sustained (30-min) exposure to intruder threat (Fox, Oler, Shackman, et al., 2015; Shackman et al., 2013) (Figure 2b). Heightened metabolic activity in the Ce and BST is also associated with elevated defensive responses during sustained exposure to an unfamiliar testing cage (i.e., in the absence of intruder threat; Fox, Shelton, Oakes, Davidson, & Kalin, 2008; Kalin, Shelton, Fox, Oakes, & Davidson, 2005).
Consistent with work in monkeys, imaging research in humans suggests that the Ce and BST are both engaged by a broad range of threat-related cues and contexts. The amygdala responds to a variety of threat-related stimuli (Costafreda, Brammer, David, & Fu, 2008; Fusar-Poli et al., 2009; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Sabatinelli et al., 2011; Sergerie, Chochol, & Armony, 2008)2 and work using high-resolution fMRI indicates that the dorsal region of the amygdala in the vicinity of the Ce is particularly sensitive to aversive visual stimuli (Hrybouski et al., 2016). Increased activation in the dorsal amygdala is, in turn, associated with elevated signs and symptoms of arousal in response to acute threat (e.g., Pavlovian threat cues; Cheng, Knight, Smith, & Helmstetter, 2006; Cheng, Richards, & Helmstetter, 2007; Knight, Nguyen, & Bandettini, 2005; Kragel & LaBar, 2015; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; van Well, Visser, Scholte, & Kindt, 2012; Wood, Ver Hoef, & Knight, 2014). Likewise, multi-voxel classifier analyses suggest that the dorsal amygdala is an important component of a larger circuit that underlies negative affect elicited by aversive images (Chang, Gianaros, Manuck, Krishnan, & Wager, 2015).
Like the Ce, the BST is recruited by a variety of potentially threat-relevant cues in humans, including emotional faces (Sladky et al., in press). In fact, as shown in Figure 2b and described in more detail in the accompanying caption, an automated meta-analysis generated using Neurosynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) reveals that studies tagged with the keywords ‘fear’ and/or ‘anxiety’ consistently reveal activation in the vicinity of the Ce and the BST, although the latter region is rarely labeled as such for a variety of reasons (e.g., omission from automated anatomical labeling tools; Fox, Oler, Tromp, et al., 2015; Shackman & Fox, 2016)3. Like the Ce, BST activation and functional connectivity co-vary with threat-elicited changes in peripheral physiology and self-reported fear and anxiety (Alvarez et al., 2015; Banihashemi, Sheu, Midei, & Gianaros, 2015; McMenamin et al., 2014; Somerville et al., 2013).
Among researchers focused on humans, it is widely believed that the Ce and BST are functionally dissociable (for critical reviews, see Shackman & Fox, 2016; Shackman, Tromp, et al., 2016). Inspired by an earlier generation of lesion and inactivation studies in rodents (Davis, 2006), this hypothesis suggests that the Ce, or the amygdala more generally, rapidly assembles phasic responses to clear-and-immediate threats (e.g., a cue associated with the imminent delivery of shock), whereas the BST comes on-line more slowly and is responsible for orchestrating sustained responses to dangers that are diffuse, uncertain, or remote. This hypothesis has been adopted with minor modifications by many investigators and commentators and has even been incorporated into the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) as Acute Threat (‘Fear’) and Potential Threat (‘Anxiety’) (https://www.nimh.nih.gov/research-priorities/rdoc/constructs/acute-threat-fear.shtml; https://www.nimh.nih.gov/research-priorities/rdoc/constructs/potential-threat-anxiety.shtml; https://www.nimh.nih.gov/research-priorities/rdoc/negative-valence-systems-workshop-proceedings.shtml).
Consistent with the ‘double-dissociation’ or ‘strict-segregation’ model, several human imaging studies have demonstrated that the BST shows persistent hemodynamic responses during the uncertain anticipation of noxious stimuli, such as shock or aversive images, whereas the dorsal amygdala shows transient responses that are time-locked to the onset of the threat-anticipation period or the actual delivery of the noxious stimulus (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Brinkmann et al., 2017; Grupe, Oathes, & Nitschke, 2013; Herrmann et al., 2016; Klumpers et al., in press; McMenamin et al., 2014; Somerville et al., 2013). In one of the more compelling examples, Somerville and colleagues presented either aversive or neutral images (3-sec) in relatively long blocks (118-sec) where the timing of image presentations was either certain or uncertain (Figure 2c). These unique design features are important because they afford a crucial opportunity to double-dissociate phasic (to 3-sec certain threat) from sustained (i.e., to 118-sec uncertain threat) responses in the same individuals. Analyses revealed transient activation in the amygdala in response to the negative images. In contrast, the BST showed persistent activation for negative-vs.-neutral blocks and for uncertain-vs.-certain blocks. Moreover, the level of sustained activation in the BST closely tracked mean differences in self-reported fear and anxiety across the four blocked conditions (i.e., uncertain-negative > certain-negative > uncertain-neutral > certain-neutral). Despite some important limitations (e.g., perceptual confounds, failing to test the Region × Condition interaction), these results are often taken as strong support for the ‘strict-segregation’ model.
On the other hand, a growing number of imaging studies are difficult to reconcile with the hypothesis of strict functional segregation based on threat uncertainty or duration (Figure 2c). Several studies have reported increased amygdala activation during the anticipation of uncertain threat, both in children (Williams et al., 2015) and in adults (Andreatta et al., 2015; Lieberman, Gorka, Shankman, & Phan, 2017). For example, Andreatta and colleagues observed sustained activation—confirmed using a finite impulse response approach—in the region of the right Ce during exposure to a virtual-reality context (30-sec) paired with unpredictable electric shocks. Leveraging a game-like ‘virtual predator’ paradigm, Mobbs and colleagues observed significantly greater activation in the dorsal amygdala when the predator was first encountered, relative to a subsequent period when shock delivery was imminent and signs and symptoms of fear and anxiety were maximal (Mobbs et al., 2009), which runs counter to the idea that that the amygdala is primarily responsible for organizing transient responses to immediate danger. Other work has revealed phasic responses in the region of the BST to brief threats, such as a 4-sec video clip of an approaching tarantula (Choi et al., 2012; Grupe et al., 2013; Mobbs et al., 2010; Pedersen et al., 2017). Likewise, Brinkmann and colleagues very recently demonstrated that the Ce and the BST show statistically indistinguishable responses to briefly presented (800 ms) aversive images (Brinkmann, Buff, Feldker, Neumeister, et al., in press). The latter result is particularly compelling given the relatively large sample (n=93) and formal test of the Region × Condition interaction. It implies that the magnitude of regional differences (i.e., Ce vs. BST) is much smaller than implied by the strict segregation hypothesis, conditional on unknown moderators, or is simply non-existent, at least for briefly presented aversive images. Another, relatively large imaging study (n=168) reported phasic activation of the BST in response to 4-sec shock-predictive cues (Klumpers et al., 2015), indicating that the BST is sensitive to certain threat. Other recent work suggests that the BST is maximally engaged when threat is psychologically imminent (Meyer et al., 2017). These imaging observations are broadly consistent with evidence from recording and loss-of-function studies in rodents indicating that a substantial proportion of BST neurons exhibit short-latency responses during exposure to both acute threat (e.g., foot- or tail-shock) and diffusely threatening environments (Davis, Walker, Miles, & Grillon, 2010; Goode & Maren, 2017; Gungor & Paré, 2016).
On balance, the brain imaging literature suggests that the Ce and BST, while certainly not interchangeable, are more alike than different. In addition to the anatomical similarities described in the previous section (e.g., connectivity, gene expression), both regions respond to a broad spectrum of threat-related cues and contexts and both are correlated with concurrent changes in physiology and subjective experience. In humans, the Ce and the BST both show transient responses to clear-and-immediate threat and sustained activation in contexts associated with uncertain, longer-lasting threat. Both regions show heightened activation in patients with anxiety disorders and individuals at risk for developing such disorders (Brinkmann, Buff, Feldker, Tupak, et al., in press; Brinkmann et al., 2017; Buff et al., in press; Kaczkurkin et al., 2016; Münsterkötter et al., 2015; Shackman, Kaplan, et al., 2016; Stevens et al., 2017; Straube, Mentzel, & Miltner, 2007; Yassa, Hazlett, Stark, & Hoehn-Saric, 2012), although the studies to date have been small in size, have frequently relied on data acquisition and processing techniques that are less than optimal for resolving subtle differences in regional function (e.g., linear spatial normalization algorithms, large smoothing kernels), and prospective longitudinal data are mostly lacking. In monkeys, individuals expressing more intense signs of fear and anxiety show increased FDG metabolism in the Ce and BST during sustained exposure to novel contexts and potential threat. Although FDG-PET lacks the temporal resolution needed to cleanly dissociate phasic from sustained neural responses, work in monkeys hints at some potential differences between the two regions—activity in the BST is associated with heritable individual differences in fear and anxiety (Fox, Oler, Shackman, et al., 2015) and the BST appears to be involved in organizing persistently elevated signs of fear and anxiety following threat exposure (i.e., mood ‘spillover;’ Shackman et al., 2017). Nevertheless, the critical tests of regional differences have yet to be performed in monkeys (e.g., Region × Condition; Fox et al., 2018; Shackman & Fox, 2016). The upshot of this work is that the available imaging literature provides, at best, mixed evidence for claims of strict functional segregation between the Ce and the BST on the basis of threat uncertainty or duration (i.e., ‘the Ce mediates phasic responses to clear-and-imminent danger; the BST mediates sustained responses to uncertain threat’)—a conclusion that echoes that reached by Gungor and Paré on the basis of mechanistic work in rodents (Gungor & Paré, 2016).
Understanding the neurobiology of human fear and anxiety is important, both conceptually and clinically. As reviewed elsewhere (Fox et al., 2018; Fox, Oler, Tromp, et al., 2015; Shackman & Fox, 2016), drawing strong inference about the neural circuits supporting phasic and sustained responses to different dimensions of threat requires the use of well-matched tasks. Parametric manipulations of threat probability (if threat will occur), imminence (when or where it will occur), and duration (as in Bradford, Shapiro, & Curtin, 2013; Meyer et al., 2017; Mobbs et al., 2009; Mobbs et al., 2007; Mobbs et al., 2010) would be particularly useful. The use of dynamic parametric tasks (e.g., where threat imminence or probability is smoothly and continuously varied) would also afford powerful new opportunities for understanding the kinds of uncertainty most relevant to fear and anxiety and for identifying circuits involved in triggering behavioral and physiological ‘phase transitions’ (e.g., from vigilance to behavioral inhibition to active defense; Mobbs, Hagan, Dalgleish, Silston, & Prevost, 2015; Mobbs & Kim, 2015). Putative double dissociations need to be rigorously assessed using the appropriate Region × Condition interaction (as in Brinkmann et al., 2017; Somerville, Whalen, & Kelley, 2010), preferably in adequately powered samples (Munafò et al., 2017; Poldrack et al., 2017; Szucs & Ioannidis, 2017). Absent that, claims of anatomical dissociation are unwarranted. Likewise, concluding that a particular brain region is ‘not involved’ in a complex, multidimensional psychological function, like ‘fear’ or ‘anxiety,’ based on a null statistical test or a single assay is unwarranted. Given mounting evidence that fear and anxiety, like other emotions, reflect widely distributed neural circuits (e.g., Kragel, Knodt, Hariri, & LaBar, 2016; Nummenmaa & Saarimaki, in press; Pessoa, 2017; Shackman & Fox, 2018; Shackman, Fox, & Seminowicz, 2015; Wager et al., 2015), one of the most important challenges for future research will be to extend models of fear and anxiety to encompass interactions between the central extended amygdala and distal regions of the brain, a point that we discuss in more detail in the final section.
Mechanistic Studies of the Central Extended Amygdala
There is ample evidence that that the Ce and the BST are critical for assembling states of fear and anxiety. Summarizing the data available nearly a decade ago, just prior to the widespread adoption of high-precision optogenetic and chemogenetic techniques, Davis and colleagues outlined a ‘partial-dissociation’ model, hypothesizing that the Ce plays a critical role in organizing both immediate and longer-lasting responses to threat (Davis et al., 2010). This model suggests that phasic responses are mediated by circuits coursing from the basolateral amygdala (BL) to the medial division of the Ce (CeM) and from there to downstream effector regions. In contrast, responses to more persistent kinds of danger—those that are uncertain, psychologically diffuse, or remote in time in time or space—were thought to be mediated by circuits passing from the lateral division of the Ce (CeL) to the lateral division of the BST (BSTL) and, ultimately, to effector regions.
More recent work in rodent models has refined our understanding of the brain bases of fear and anxiety (e.g., Paré & Quirk, 2017; Yu et al., 2017), while highlighting the anatomical and functional complexity of the central extended amygdala (e.g., Fadok et al., 2017; Gungor & Paré, 2016; S. Y. Kim et al., 2013; Viviani et al., 2011). It has become abundantly clear that the Ce and the BST, like many other brain regions, harbor a variety of distinct cell ‘types’—groups of neurons that can be distinguished based on protein expression, firing characteristics, connectivity, and other features—and that different cell types within the same region of the central extended amygdala (e.g., Ce) are functionally dissociable (e.g., S. Y. Kim et al., 2013; Viviani et al., 2011). Some of these neurons are response-specific, while others are threat-specific. For example, PAG-projecting cells in the CeM trigger freezing, whereas an independent, but anatomically intermingled, set of medulla-projecting neurons trigger changes in cardiovascular activity (Viviani et al., 2011). These response-specific neurons can be activated by different threat-specific neurons. For example, serotonin receptor 2a-expressing neurons in the CeL play a key role in regulating the competition between innate and learned defensive responses: amplifying freezing elicited by sustained exposure (10-min) to innate threat (i.e. an odor derived from fox feces) and attenuating freezing to learned threat (i.e., a neutral odor associated with foot-shock) (Isosaka et al., 2015). These kinds of observations underscore the conceptual importance of understanding how different cell types in the central extended amygdala contribute to fear and anxiety.
Despite this complexity, mechanistic work in rodents reinforces the general conclusion that the microcircuits responsible for assembling phasic and sustained responses to threat overlap in the central extended amygdala (Calhoon & Tye, 2015; Gungor & Paré, 2016; Tovote et al., 2015). For example, the Ce and the BST have both been shown to be important for assembling sustained responses to diffuse threat (e.g., Botta et al., 2015; Crowley et al., 2016; Duvarci, Bauer, & Paré, 2009; Glangetas et al., 2017; J. H. Jennings et al., 2013; S. Y. Kim et al., 2013; Mazzone et al., in press; Moller, Wiklund, Sommer, Thorsell, & Heilig, 1997; Zimmerman & Maren, 2011; Zimmerman, Rabinak, McLachlan, & Maren, 2007). Projections from the BL to the Ce exert bi-directional control over defensive responses to the elevated-plus maze (EPM) and open-field test, which can be considered diffusely threatening contexts (Tye et al., 2011); chemical inactivation of the Ce reduces defensive responses to the elevated-plus maze (Moreira, Masson, Carvalho, & Brandao, 2007); and CRF-expressing neurons in the Ce modulate conditioned freezing to threatening contexts and longer-lasting (30-s) threat cues (Asok et al., in press; Pitts & Takahashi, 2011). With regard to the BST, serotonergic projections from the dorsal raphe to the BST modulate the recall of conditioned defensive responses to both contextual and cued threats (Marcinkiewcz et al., 2016). Moreover, work using temporally unpredictable shock paradigms demonstrates that cannabinoid projections from the BL and the Ce to the BST are necessary for sustained defensive responses in response to uncertain danger (Lange et al., 2017). This observation, which harnesses a task adapted from that of Davis, Walker, and colleagues (Daldrup et al., 2015; Miles, Davis, & Walker, 2011; Walker & Davis, 2008), provides important evidence that the BL, Ce, and BST all play a role in responding to uncertain or diffuse threat, consistent with other recent work (e.g., Felix-Ortiz et al., 2013; Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, & Tye, 2016; Lee, Amir, Haufler, & Pare, 2017). While our understanding remains far from complete, taken together, these observations show that specific microcircuits within and between the Ce and the BST are important for orchestrating defensive responses to a variety of threats.
Although the causal contribution of the BST to fear and anxiety has yet to be explored in humans or other primates, monkeys with fiber-sparing excitotoxic lesions of the Ce show a marked reduction in defensive behaviors and endocrine activity during sustained exposure to human intruder threat and during acute exposure to a live snake (Kalin, 2017; Kalin, Shelton, & Davidson, 2004; Oler et al., 2016). Likewise, humans with amygdala damage exhibit a profound lack of fear and anxiety in response to both diffusely threatening contexts (e.g., walking through a haunted house, foraging in the presence of uncertain threat) and acute threat (e.g., spiders, snakes, conditioned threat cues) (Bechara et al., 1995; Feinstein, Adolphs, Damasio, & Tranel, 2011; Feinstein, Adolphs, & Tranel, 2016; Korn et al., 2017). A major caveat is that such deficits may reflect damage to axonal fibers passing through the Ce en route to other regions, such as the BST, or more subtle kinds of long-range functional disconnection (Davis & Whalen, 2001), a point that we take up more fully in the next section.
Closing the Gap between Mechanistic and Imaging Research
The Ce and the BST are anatomically complex and can be partitioned into multiple sub-regions (Figure 1), each containing a variety of intermingled cell types (Fox, Oler, Tromp, et al., 2015; Gungor & Paré, 2016). Although unfamiliar to many brain imagers, recently developed opto- and chemogenetic tools provide numerous opportunities for deciphering this complexity and identifying the specific circuit components that control defensive responses to threat (Gomez et al., 2017; C. K. Kim, Adhikari, & Deisseroth, 2017; Roth, 2016; Smith, Bucci, Luikart, & Mahler, 2016; Wiegert, Mahn, Prigge, Printz, & Yizhar, 2017). Developing a basic understanding of these methods is a key step to dissolving artificial academic ‘silos’ and developing more thoughtful hypotheses about the role of the central extended amygdala in human emotion and emotional disorders. Typically, a DNA vector encoding a target ligand or receptor is engineered into a virus. The virus is injected into the brain, inducing expression of the target protein in the infected region (e.g., BST). Regional and cell-type specificity is achieved using recombinase-dependent viruses or cell type-specific promoter viruses. For example, a virus containing a Cre-dependent vector can be injected into the Ce of transgenic mice engineered to express Cre recombinase in somatostatin-expressing neurons, resulting in selective expression of the targeted receptor protein in somatostatin-expressing neurons in the Ce. More sophisticated approaches enable the inclusion (Boolean AND) or exclusion (Boolean NOT) of cells with specific efferent or afferent projections, specific behavioral profiles (e.g., activated by reward vs. punishment), or combinations of these criteria. By overexpressing receptors that respond to light (‘optogenetics’) or designer drugs with minimal off-target effects (‘chemogenetics’), it is possible to reversibly activate or silence specific cell populations on demand. The application of these approaches to rodent models of fear and anxiety has revealed a level of architectural intricacy in the central extended amygdala far beyond what can be resolved using existing neuroimaging techniques, including mutually inhibitory circuits within the Ce that control freezing and fleeing (Fadok et al., 2017; Isosaka et al., 2015) and neuronal populations within the BST that can promote or dampen signs of fear and anxiety (Garcia-Garcia et al., in press; S. Y. Kim et al., 2013).
These exciting observations raise two very important questions. First, are these mechanisms conserved in humans and other primates? If so, then they are likely to be relevant to our understanding of anxiety disorders and could guide the development of improved treatments (Hyman, 2016). Second, what role do these extended amygdala mechanisms play in the kinds of large-scale brain circuits that have been linked to maladaptive fear and anxiety in humans and monkeys? Which molecules and micro-circuits underlie heightened activation in the central extended amygdala and how do they influence the function (i.e., activity, functional connectivity) of distal regions of the brain implicated in pathological fear and anxiety? Reconciling these two levels of analysis—one global, the other local—is mandatory, if we are to develop a complete and clinically useful understanding of fear and anxiety.
Nonhuman primate research provides a powerful opportunity to combine focal perturbation techniques with whole-brain surveys of brain function and has begun to address some of these fundamental questions. For example, imaging studies in monkeys demonstrate that metabolic activity in the posterior orbitofrontal cortex (OFC)/anterior insula is associated with heightened passive avoidance of threat (i.e., freezing; Fox, Oler, Shackman, et al., 2015). Although surgical lesions of the OFC markedly reduce threat-elicited freezing, suggesting a causal role (Kalin, Shelton, & Davidson, 2007; Rudebeck, Saunders, Prescott, Chau, & Murray, 2013), neuroimaging measures collected before and after surgery suggest that this anxiolytic effect is proximally mediated by ‘downstream’ alterations in BST metabolism (Fox et al., 2010) (Figure 3a). Reduced BST activity has also been observed in humans with OFC damage (Motzkin et al., 2015), suggesting that this circuit is conserved across primate species (Figure 3b). In more recent work, Kalin, Fox, and colleagues have extended this strategy to gain-of-function studies (Kalin et al., 2016). Harnessing a viral vector approach, they demonstrated that overexpression of corticotropin-releasing hormone (CRH) in the dorsal amygdala increases defensive behaviors during sustained exposure to potential threat, consistent with work in rodents highlighting the importance of the Ce CRH system for responding to diffusely threatening contexts, such as the elevated plus-maze (Regev, Tsoory, Gil, & Chen, 2012). These behavioral changes were associated with increased metabolism in the dorsal amygdala and posterior OFC as well as enhanced functional connectivity between the two regions, highlighting the importance of a distributed brain network underlying fear and anxiety (Figure 4a).
Figure 3. Focal damage to the ventral PFC is associated with distal changes in BST function.
(A) Monkeys. Experimental lesions of the OFC reduce threat-elicited freezing (not depicted) and BST metabolism (magenta arrow). The orbitofrontal regions targeted by the surgery (maroon) can be seen from the lateral (far left) and basal views (middle). Bar-plot depicts the significant Group × Time interaction for BST metabolism. (B) Humans. Damage to the ventromedial PFC (vmPFC) is associated with reduced perfusion in the BST (magenta arrow). The ventromedial regions showing damage can be seen from the mid-sagittal (far left) and basal views (middle). Bar-plot depicts the significant reduction in right BST perfusion in the patient group. Portions of this figure were adapted with permission from (Fox et al., 2010; Motzkin et al., 2015).
Figure 4. Nonhuman primate research provides a powerful opportunity to combine focal manipulations of the amygdala with whole-brain surveys of brain function.
(A). Molecular activation. Using MRI-guided injections of a viral vector (upper left), Kalin, Fox and colleagues overexpressed corticotropin-releasing hormone (CRH) in the dorsal amygdala. MRI image depicts the gadolinium flume (white) in the target region. Photomicrograph shows CRH-expressing cells in the same region (upper right). CRH overexpression in the amygdala enhanced threat-elicited defensive responses (not shown) and increased metabolism (yellow clusters) in the dorsal amygdala (magenta arrow) and the posterior OFC (green arrows). CRH-induced increases in defensive responses and metabolism were correlated in both regions (red clusters). (B) Chemogenetic inhibition. Leveraging a chemogenetic approach, Grayson and colleagues reversibly inhibited the amygdala while using fMRI to assess intrinsic functional connectivity across the brain. A viral vector encoding an inhibitory designer receptor exclusively activated by a designer drug with minimal off-target effects (DREADD) was injected into the amygdala (upper left). Systemic administration of the designer drug reversibly inactivated the amygdala (upper right). DREADDs-mediated inhibition of the amygdala was associated with decreased amygdala-BST connectivity (magenta arrow), decreased amygdala-OFC connectivity, and increased corticocortical coupling (lower panels). In the coronal section, clusters (yellow) depict the minimum conjunction (logical ‘AND’) of regions significant for four designer-drug vs. vehicle contrasts that were made available by Grayson and colleagues on the publisher’s website. Portions of this figure were adapted with permission from (Grayson et al., 2016; Kalin et al., 2016).
Other recent work demonstrates the feasibility of using opto- and chemogenetic approaches in nonhuman primates (e.g., Afraz, Boyden, & DiCarlo, 2015; Eldridge et al., 2016; Gerits et al., 2012; Jazayeri, Lindbloom-Brown, & Horwitz, 2012; Nagai et al., 2016; Yazdan-Shahmorad et al., 2016)—including cell-type specific perturbations in wild-type (i.e., genetically unmodified) monkeys (Stauffer et al., 2016)—and highlights the value of combining mechanistic interventions and cellular recordings with whole-brain imaging techniques (Mazzone et al., in press; Michaelides & Hurd, 2015; Park et al., 2017; Shiba et al., 2017). In a landmark study, Grayson and colleagues showed that chemogenetic inactivation of the amygdala produces widespread alterations in intrinsic functional connectivity, including decreased amygdala-BST connectivity, decreased amygdala-OFC connectivity, and increases in corticocortical coupling (Grayson et al., 2016) (Figure 4b). This finding is consistent with work in rodents (Ferenczi et al., 2016; Otchy et al., 2015) and neurological patients (Carrera & Tononi, 2014; Corbetta, Kincade, Lewis, Snyder, & Sapir, 2005; Fornito, Zalesky, & Breakspear, 2015) demonstrating that the behavioral consequences of focal brain damage can emerge from physiological alterations in distal brain regions (for a related perspective, see Pessoa, 2017). These findings highlight the importance of a distributed circuit centered on, but not limited to, the central extended amygdala and they underscore the added value of combining the focal perturbation strategies that are widely used in rodent studies with the whole-brain imaging techniques that are routinely used in basic and clinical research in humans.
Conclusions
The central extended amygdala plays a crucial role in evaluating and responding to a broad spectrum of threat-related cues and contexts. While they are certainly not interchangeable, the Ce and the BST show similar patterns of connectivity, cellular composition, neurochemistry, and gene expression. Both are sensitive to uncertain or temporally remote threat; both co-vary with threat-elicited changes in behavior, physiology, and experience; both show phasic responses to acute threat; and both show heightened activity during sustained exposure to diffusely threatening contexts. Work in rodents indicates that both regions play a critical role in organizing sustained defensive responses to a range of potentially threatening cues and contexts. More generally, studies leveraging opto- and chemogenetic techniques have begun to reveal the specific molecules, cells, and microcircuits within the central extended amygdala that support signs of fear and anxiety in rats and mice. A major challenge is to understand the relevance of these discoveries to human experience and human disease. Recent work in nonhuman primates provides a bridge to addressing this fundamental issue. Using a combination of focal perturbations and whole-brain imaging, this new generation of nonhuman primate research sets the stage for discovering the mechanisms within the central extended amygdala that underlie neuroimaging metrics linked to extreme fear and anxiety in humans; for understanding how the Ce and BST functionally interact with one another and with remote regions of the brain, such as the OFC; and ultimately for accelerating the development of improved strategies for diagnosing, treating, and preventing pathological fear and anxiety.
Acknowledgments
We gratefully acknowledge assistance from L. Brinkmann, L. Friedman, B. Nacewicz, and D. Tromp; critical feedback from J. Blackford, L. Pessoa, S. Padmala, and 3 anonymous reviewers; and financial support from the California National Primate Center; National Institute of Mental Health (DA040717, MH107444); University of California, Davis; and University of Maryland, College Park. Authors declare no conflicts of interest.
Footnotes
It has become increasingly common to draw a distinction between ‘fear’ and ‘anxiety’ (e.g., LeDoux, 2015). Yet lay people, scholars in other areas, the American Psychiatric Association’s Diagnostic and Statistical Manual (American Psychiatric Association, 2013), and even domain experts often use these terms in interchangeable, inconsistent, or overly inclusive ways (Kagan, in press; Shackman, Tromp, et al., 2016; Watson, Stanton, & Clark, 2017). To avoid misunderstanding, we have adopted the undifferentiated term ‘fear and anxiety’ (for a more detailed discussion of nomenclature, see Fox, Lapate, Davidson, & Shackman, 2018; Shackman & Fox, 2016). Understanding the neurobiology of fear and anxiety is both theoretically and clinically important and requires that we determine how the Ce, the BST, and other brain regions work together to evaluate and respond to different kinds of threat. We urge other researchers to eschew potentially problematic redefinitions of everyday language and, instead, focus on the specific parameters of the threat, the context in which it occurs (e.g., prospects for escape), and the neurobehavioral response (e.g., time course), including subjective experience.
Interestingly, the amygdala is not consistently recruited by conditioned threat cues in human fMRI studies (Fullana et al., 2016; Mechias, Etkin, & Kalisch, 2010), contrary to electrophysiological and mechanistic work in rodents, monkeys, and humans (Antoniadis, Winslow, Davis, & Amaral, 2007; Bechara et al., 1995; Tovote, Fadok, & Luthi, 2015). In addition, several groups have reported ‘de-activation’ of the amygdala in a variety of aversive paradigms (Choi, Padmala, & Pessoa, 2012; Derbyshire et al., 1997; Grupe, Wielgosz, Davidson, & Nitschke, 2016; McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Meyer, Padmala, & Pessoa, 2017; Mobbs et al., 2009; Petrovic, Carlsson, Petersson, Hansson, & Ingvar, 2004; Pruessner et al., 2008; Wager et al., 2009). The mechanisms underlying these effects remain enigmatic.
Although automated anatomical labeling tools do not yet include the BST, probabilistic masks are now available for the supracapsular portion (Theiss, Ridgewell, McHugo, Heckers, & Blackford, 2017; Torrisi et al., 2015), as shown in Figure 1. It can also be helpful to assess whether provisional BST clusters lie outside of neighboring regions incorporated in probabilistic atlases (a Boolean NOT with nucleus accumbens, pallidum, caudate, putamen, thalamus, and ventricles) (Klumpers, Kroes, Baas, & Fernandez, in press; Shackman & Fox, 2016).
References
- Afraz A, Boyden ES, DiCarlo JJ. Optogenetic and pharmacological suppression of spatial clusters of face neurons reveal their causal role in face gender discrimination. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6730–6735. doi: 10.1073/pnas.1423328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, Drevets WC. Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry. 2015;5:e591. doi: 10.1038/tp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5 2013. [Google Scholar]
- Andreatta M, Glotzbach-Schoon E, Muhlberger A, Schulz SM, Wiemer J, Pauli P. Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex. 2015;63:352–363. doi: 10.1016/j.cortex.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27(28):7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Draper A, Hoffman AF, Schulkin J, Lupica CR, Rosen JB. Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Molecular Psychiatry. doi: 10.1038/mp.2017.79. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014;91:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, Gianaros PJ. Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Soc Cogn Affect Neurosci. 2015;10:474–485. doi: 10.1093/scan/nsu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, … Kalin NH. Evolutionarily conserved dysfunction of prefrontal-amygdalar connectivity in early-life anxiety. Molecular Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nature Reviews Drug Discovery. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discovery Medicine. 2011;11:197–207. [PubMed] [Google Scholar]
- Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, … Luthi A. Regulating anxiety with extrasynaptic inhibition. Nature Neuroscience. 2015;18:1493–1500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychol Sci. 2013;24:2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Neumeister P, Heitmann CY, Hofmann D, … Straube T. Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage. doi: 10.1016/j.neuroimage.2017.10.054. in press. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak SV, Becker MPI, Herrmann MJ, Straube T. Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychological Medicine. :1–14. doi: 10.1017/S0033291717001192. in press. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Neumeister P, Tupak SV, Becker MP, Herrmann MJ, Straube T. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Human Brain Mapping. 2017;38:2190–2205. doi: 10.1002/hbm.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Bruchmann M, Becker MPI, Tupaka S, Herrmann MJ, Straube T. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in Generalized Anxiety Disorder. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsx103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Molecular Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nature Neuroscience. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59:1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nature Neuroscience. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU. Anxiety disorders. Nat Rev Dis Primers. 2017;3:17024. doi: 10.1038/nrdp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, … Kash TL. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup T, Remmes J, Lesting J, Gaburro S, Fendt M, Meuth P, … Seidenbecher T. Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes Brain Behav. 2015;14:281–291. doi: 10.1111/gbb.12211. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- deCampo DM, Fudge JL. Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. Journal of Comparative Neurology. 2013;521:3191–3216. doi: 10.1002/cne.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- DiLuca M, Olesen J. The cost of brain diseases: a burden or a challenge? Neuron. 2014;82:1205–1208. doi: 10.1016/j.neuron.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, … Richmond BJ. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nature Neuroscience. 2016;19(1):37–39. doi: 10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, … Luthi A. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Current Biology. 2011;21:1–5. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Tranel D. A tale of survival from the world of Patient S.M. In: Amaral DG, Adolphs R, editors. Living without an amygdala. New York: Guilford; 2016. [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, … Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nature Reviews Neuroscience. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fox AS, Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Lapate RC, Davidson RJ, Shackman AJ. The nature of emotion: A research agenda for the 21st century. In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. New York: Oxford University Press; 2018. [ http://shackmanlab.org/wp-content/uploads/2017/2007/fox_shackman_NoE_Epilogue_070917Final.pdf]) [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, … Kalin NH. Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences USA. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DP, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends in Neurosciences. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. Journal of Neuroscience. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. NY: Guilford; 2009. pp. 3–42. [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, Radua J. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Molecular Psychiatry. 2016;21:500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, … Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Canetta S, Stujenske JM, Burghardt NS, Ansorge MS, Dranovsky A, Leonardo ED. Serotonin inputs to the dorsal BNST modulate anxiety in a 5-HT1A receptor-dependent manner. Molecular Psychiatry. doi: 10.1038/mp.2017.165. in press. [DOI] [PMC free article] [PubMed]
- Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Current Biology. 2012;22:1722–1726. doi: 10.1016/j.cub.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, … Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, … Georges F. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun. 2017;8:14456. doi: 10.1038/ncomms14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, … Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning and Memory. 2017;24:480–491. doi: 10.1101/lm.044206.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, Ernst M. Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage. doi: 10.1016/j.neuroimage.2017.03.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, … Amaral DG. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91:453–466. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nature Reviews Drug Discovery. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB. Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychological Medicine. 2016;46:1885–1895. doi: 10.1017/S0033291716000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D. Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience. 2016;36:8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MP, Tupak SV, Guhn A, Schmidt B, … Straube T. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping. 2016;37:1091–1102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, Shafer AT, Baron CA, Seres P, … Malykhin NV. Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 2016;133:98–110. doi: 10.1016/j.neuroimage.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Back to basics: luring industry back into neuroscience. Nature Neuroscience. 2016;19:1383–1384. doi: 10.1038/nn.4429. [DOI] [PubMed] [Google Scholar]
- Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, Kobayakawa K. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell. 2015;163:1153–1164. doi: 10.1016/j.cell.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nature Neuroscience. 2012;15:1368–1370. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings CG, Landman R, Zhou Y, Sharma J, Hyman J, Movshon JA, … Feng G. Opportunities and challenges in modeling human brain disorders in transgenic primates. Nature Neuroscience. 2016;19:1123–1130. doi: 10.1038/nn.4362. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, … Satterthwaite TD. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry. 2016;80:775–785. doi: 10.1016/j.biopsych.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Brain and emotion. Emotion Review in press. [Google Scholar]
- Kaiser T, Feng G. Modeling psychiatric disorders for developing effective treatments. Nature Medicine. 2015;21:979–988. doi: 10.1038/nm.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH. Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. European Neuropsychopharmacology. 2017;27:543–553. doi: 10.1016/j.euroneuro.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, … Oler JA. Overexpressing corticotropin-releasing hormone in the primate amygdala increases anxious temperament and alters its neural circuit. Biological Psychiatry. 2016;80:345–355. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Sair HI, Blitz AM, Riascos RF, Mirbagheri S, Keser Z, Hasan KM. Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct. 2016;221:3561–3569. doi: 10.1007/s00429-015-1119-3. [DOI] [PubMed] [Google Scholar]
- Kamali A, Yousem DM, Lin DD, Sair HI, Jasti SP, Keser Z, … Hasan KM. Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neuroscience Letters. 2015;608:45–50. doi: 10.1016/j.neulet.2015.09.035. [DOI] [PubMed] [Google Scholar]
- Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nature Reviews Neuroscience. 2017;18:222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, … Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SE, Oosting RS, … Baas JM. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry. 2015;78:582–589. doi: 10.1016/j.biopsych.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, Baas J, Fernandez G. How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3830-16.2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annual Review of Pharmacology and Toxicology. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Korn CW, Vunder J, Miró J, Fuentemilla L, Hurlemann R, Bach DR. Amygdala lesions reduce anxiety-like behavior in a human benzodiazepine-sensitive approach-avoidance conflict test. Biological Psychiatry. 2017;82:522–531. doi: 10.1016/j.biopsych.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Knodt AR, Hariri AR, LaBar KS. Decoding Spontaneous Emotional States in the Human Brain. PLoS Biol. 2016;14:e2000106. doi: 10.1371/journal.pbio.2000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, LaBar KS. Multivariate neural biomarkers of emotional states are categorically distinct. Soc Cogn Affect Neurosci. 2015;10:1437–1448. doi: 10.1093/scan/nsv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lange MD, Daldrup T, Remmers F, Szkudlarek HJ, Lesting J, Guggenhuber S, … Pape HC. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Molecular Psychiatry. 2017;22:1422–1430. doi: 10.1038/mp.2016.156. [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Anxious. Using the brain to understand and treat fear and anxiety. NY: Viking; 2015. [Google Scholar]
- Lee SC, Amir A, Haufler D, Pare D. Differential recruitment of competing valence-related amygdala networks during anxiety. Neuron. 2017;96:81–88. e85. doi: 10.1016/j.neuron.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, Shankman SA, Phan KL. Impact of panic on psychophysiological and neural reactivity to unpredictable threat in depression and anxiety. Clin Psychol Sci. 2017;5(1):52–63. doi: 10.1177/2167702616666507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the human brain. 3. San Diego, CA: Academic Press; 2007. [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, … Kash TL. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, … Kash TL. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Molecular Psychiatry. doi: 10.1038/mp.2016.218. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Padmala S, Pessoa L. Tracking dynamic threat imminence. bioRxiv 2017 [Google Scholar]
- Michaelides M, Hurd YL. DREAMM: a biobehavioral imaging methodology for dynamic in vivo whole-brain mapping of cell type-specific functional networks. Neuropsychopharmacology. 2015;40:239–240. doi: 10.1038/npp.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. 2011;36:1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Dalgleish T, Silston B, Prevost C. The ecology of human fear: survival optimization and the nervous system. Front Neurosci. 2015;9:55. doi: 10.3389/fnins.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Kim JJ. Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Current Opinion in Behavioral Sciences. 2015;5:8–15. doi: 10.1016/j.cobeha.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, … Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. Journal of Neuroscience. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, … Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Acadademy of Sciences USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Research. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Moreira CM, Masson S, Carvalho MC, Brandao ML. Exploratory behavior of rats in the elevated plus maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Research Bulletin. 2007;71:466–474. doi: 10.1016/j.brainresbull.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex. 2015;64:281–288. doi: 10.1016/j.cortex.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Nosek BA, Bishop DVM, Button KS, Chambers CD, du Sert NP, … Ioannidis JPA. A manifesto for reproducible science. Nature Human Behaviour. 2017;1:21. doi: 10.1038/s41562-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterkötter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, … Dannlowski U. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety. 2015;32:656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Kikuchi E, Lerchner W, Inoue KI, Ji B, Eldridge MA, … Minamimoto T. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun. 2016;7:13605. doi: 10.1038/ncomms13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ. Fibre degeneration following lesions of the amygdaloid complex in the monkey. Journal of Anatomy. 1961;95:515–531. [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Saarimaki H. Emotions as discrete patterns of systemic activity. Neuroscience Letters. doi: 10.1016/j.neulet.2017.07.012. in press. [DOI] [PubMed] [Google Scholar]
- Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA, … Kalin NH. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage. 2012;61:1059–1066. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shackman AJ, Kalin NH. The central nucleus of the amygdala is a critical substrate for individual differences in anxiety. In: Amaral DG, Adolphs R, editors. Living without an amygdala. NY: Guilford; 2016. [Google Scholar]
- Oler JA, Tromp DP, Fox AS, Kovner R, Davidson RJ, Alexander AL, … Fudge JL. Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Struct Funct. 2017;222:21–39. doi: 10.1007/s00429-016-1198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, … Olveczky BP. Acute off-target effects of neural circuit manipulations. Nature. 2015;528:358–363. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ. When scientific paradigms lead to tunnel vision: lessons from the study of fear. Science of Learning. 2017;2:6. doi: 10.1038/s41539-017-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Russ BE, McMahon DBT, Koyano KW, Berman RA, Leopold DA. Functional subpopulations of neurons in a macaque face patch revealed by single-unit fMRI mapping. Neuron. 2017;95:971–981. doi: 10.1016/j.neuron.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WS, Balderston NL, Miskovich TA, Belleau EL, Helmstetter FJ, Larson CL. The effects of stimulus novelty and negativity on BOLD activity in the amygdala, hippocampus, and bed nucleus of the stria terminalis. Soc Cogn Affect Neurosci. 2017;12:748–757. doi: 10.1093/scan/nsw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. A network model of the emotional brain. Trends in Cognitive Sciences. 2017;21:357–371. doi: 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. Journal of Cognitive Neuroscience. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiology of Learning and Memory. 2011;95:86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, … Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience. 2017;18:115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cog Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Sytems. Vol. 4. NY: Elsevier; 2007. pp. 3–34. [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, … Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biological Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, … Jeffries J. Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, … Vos T. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience. 2016;36:8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. How are emotions organized in the brain? In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. New York, NY: Oxford University Press; 2018. [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Molecular Psychiatry. 2017;22:724–732. doi: 10.1038/mp.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Seminowicz DA. The cognitive-emotional brain: Opportunities and challenges for understanding neuropsychiatric disorders. Behavioral and Brain Sciences. 2015;38:e86. doi: 10.1017/S0140525X14001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Kaplan CM, Stockbridge MD, Tillman RM, Tromp DPM, Fox AS, Gamer M. The neurobiology of anxiety and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology. 2016;7:311–342. doi: 10.5127/jep.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, Fox AS. Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin. 2016;142:1275–1314. doi: 10.1037/bul0000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Oikonomidis L, Sawiak S, Fryer T, Hong YT, Cockcroft G, … Roberts AC. Converging prefronto-insula-amygdala pathways in negative emotion regulation in marmoset monkeys. Biological Psychiatry. 2017;82:895–903. doi: 10.1016/j.biopsych.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Geissberger N, Pfabigan DM, Kraus C, Tik M, Woletz M, … Windischberger C. Unsmoothed functional MRI of the human amygdala and bed nucleus of the stria terminalis during processing of emotional faces. Neuroimage. doi: 10.1016/j.neuroimage.2016.12.024. in press. [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDs: Use and application in behavioral neuroscience. Behavioral Neuroscience. 2016;130:137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W. Dopamine neuron-specific optogenetic stimulation in rhesus macaques. Cell. 2016;166:1564–1571. e1566. doi: 10.1016/j.cell.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, … Ressler KJ. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological Psychiatry. 2017;81:1023–1029. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Szucs D, Ioannidis JP. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol. 2017;15:e2000797. doi: 10.1371/journal.pbio.2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss JD, Ridgewell C, McHugo M, Heckers S, Blackford JU. Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. Neuroimage. 2017;146:288–292. doi: 10.1016/j.neuroimage.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, Shackman AJ. Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping. doi: 10.1002/hbm.23917. accepted pending minor revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, … Ernst M. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Human Brain Mapping. 2015;36:4076–4088. doi: 10.1002/hbm.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, … Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci. 2012;12:499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, … Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF. A Bayesian model of category-specific emotional brain responses. PLoS Comput Biol. 2015;11:e1004066. doi: 10.1371/journal.pcbi.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Woo CW. fMRI in analgesic drug discovery. Sci Transl Med. 2015;7:274fs276. doi: 10.1126/scitranslmed.3010342. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Watson D, Stanton K, Clark LA. Self-report indicators of negative valence constructs within the research domain criteria (RDoC): A critical review. Journal of Affective Disorders. 2017;216:58–69. doi: 10.1016/j.jad.2016.09.065. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. Silencing neurons: Tools, applications, and experimental constraints. Neuron. 2017;95:504–529. doi: 10.1016/j.neuron.2017.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MA, … Kalin NH. Fear of the unknown: Uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2015;40:1428–1435. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends in Neurosciences. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nature Neuroscience. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion. 2014;14:693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdan-Shahmorad A, Diaz-Botia C, Hanson TL, Kharazia V, Ledochowitsch P, Maharbiz MM, Sabes PN. A large-scale interface for optogenetic stimulation and recording in nonhuman primates. Neuron. 2016;89:927–939. doi: 10.1016/j.neuron.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, … Li B. The central amygdala controls learning in the lateral amygdala. Nature Neuroscience. 2017;20:1680–1685. doi: 10.1038/s41593-017-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiology of Learning and Memory. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learning and Memory. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]