Abstract
Background/Objective
Guidelines concur that postmastectomy radiation therapy (PMRT) in T1-2 tumors with 1-3 positive (+) lymph nodes (LNs) decreases locoregional recurrence (LRR), but advise limiting PMRT to patients at highest risk to balance against potential harms. Here we identify the risks of LRR after mastectomy in patients with T1-2N1 disease, treated with modern chemotherapy, and to identify predictors of LRR when omitting PMRT.
Methods
Patients with T1-2N1 breast cancer undergoing mastectomy between 1995–2006 were categorized by receipt of PMRT. The Chi-squared test compared the clinicopathologic features between both groups. Kaplan-Meier and Cox regression analysis were used to determine rates of LRR, recurrence-free survival (RFS), and overall survival (OS).
Results
1087 patients (924 no PMRT, 163 PMRT) were included. Median follow-up was 10.8 years (range 0–21). We identified 63 LRRs (56 no PMRT, 7 PMRT). 10-year rates of LRR with and without PMRT were 4.0% and 7.0%, respectively. Patients receiving PMRT were younger (p=0.019), had larger tumors (p=0.013), higher histologic grade (p=0.029), more positive LNs (p<.0001), lymphovascular invasion (LVI) (p<.0001), extranodal invasion (p<.0001), and macroscopic LN metastases (p<.0001). There was no difference in LRR, RFS, or OS between groups. On multivariate analysis, age <40 years (p<.0001) and LVI (p<.0001) were associated with LRR in those not receiving PMRT.
Conclusion
Consistent with the guidelines, 85% of patients with T1-2N1 were spared PMRT at our center, while maintaining low LRR. Age <40 years and presence of LVI are significantly associated with LRR in those not receiving PMRT.
Keywords: postmastectomy radiotherapy, breast cancer, locoregional recurrence, 1-3 positive lymph nodes
INTRODUCTION
Postmastectomy radiation therapy (PMRT) has been shown in various studies to reduce locoregional recurrence (LRR) risk and breast cancer mortality in women with node-positive disease,1 and PMRT use in women with ≥4 positive LNs has been standard of care.2 As the survival benefit from PMRT may be proportional to the reduction of LRR, women with higher LRR risk are expected to benefit most3,4; however, use of PMRT in lower-risk groups, such as patients with 1-3 positive lymph nodes (LNs),5–8 and those with node-negative disease,9–11 remains controversial.
In a recent meta-analysis of randomized trials of women undergoing axillary dissection and mastectomy, there was a 10.6% absolute reduction in 10-year LRR in all patients with any node-positive disease receiving PMRT. This decrease translated to a significant reduction in breast cancer mortality (rate ratio [RR] 0.84, 95% confidence interval [CI] 0.76–0.94, p=0.001). A significant reduction in LRR and breast cancer mortality was seen in women with ≥4 positive nodes and in women with 1-3 positive LNs.1 This meta-analysis included 22 trials conducted from 1965–1986; its results were heavily influenced by studies with 10-year LRR >25%.5,12,13
Recent American Society of Clinical Oncology (ASCO), American Society for Radiation Oncology (ASTRO), and Society of Surgical Oncology (SSO) guidelines concur that PMRT use in patients with early breast cancer (T1-2) and 1-3 positive LNs reduces LRR risk and improves survival. However, it was felt this benefit may be outweighed by side effects in low LRR-risk patients.14
We previously reported LRR risk in patients with T1-2N1 breast cancer who underwent total mastectomy with or without PMRT and received modern adjuvant systemic therapy at a median follow-up of 6.8 years.15 Here we sought to update results beyond 10 years follow-up, as 95% of LRRs occur within 10 years after surgical intervention.3 Here we also evaluated relapse-free survival (RFS), overall survival (OS), and predictors of LRR when PMRT was omitted.
METHODS
Upon institutional review board approval, we queried the Memorial Sloan Kettering Cancer Center (MSKCC) prospectively maintained breast cancer database for patients who underwent mastectomy and had 1-3 positive LNs between 1995–2006. We excluded all patients who had T3 or T4 tumors, received neoadjuvant chemotherapy (NAC), or in whom the axillary node metastases were isolated tumor cells only. In patients with bilateral breast cancer, clinicopathologic data were included for the side that met inclusion criteria. Patients were divided into two groups based on PMRT delivery. 924 (85%) patients received no PMRT; 163 (15%) were selected for PMRT. In the PMRT group, all patients received chest-wall irradiation. Supraclavicular nodal fields were included in 137/163 (84%) patients, axillary field in 7 (4%), and the internal mammary chain was added in 6 (4%).
All electronic medical records were reviewed to update the follow-up status for each patient. The following variables were collected: age at diagnosis; pathologic T and N stage; histologic grade; nuclear grade; multifocality/multicentricity (defined as presence of tumor in multiple quadrants or same-quadrant discontinuous tumors in the same quadrant, respectively); extensive intraductal component (defined as the presence of intraductal carcinoma in >25% of the tumor); presence of lymphovascular invasion (LVI); number of positive LNs; total number of LNs dissected; size of axillary nodal metastasis whether micrometastases (defined as the presence of metastatic lymph node deposits measuring between 0.2–2mm in dimension) or macrometastasis (deposits measuring >2.0mm,); presence of extracapsular nodal extension (ECE)(defined as growth or spread of tumor cells outside of the lymph node capsule); estrogen receptor (ER) status; progesterone receptor (PR) status; HER2 status; use and protocol of adjuvant chemotherapy; hormonal therapy; or trastuzumab. Pathology slides were not re-reviewed.
Adjuvant treatment, systemic chemotherapy, and/or hormone therapy was given to 98% of patients. The majority (1024; 94.2%) received an axillary lymph node dissection (ALND); the median number of nodes resected was 18 (range 1–51). 805 patients had a sentinel lymph node biopsy (SLNB), of whom 742 (92%) were converted to an ALND.
LRR was defined as recurrence in the ipsilateral chest wall, axillary nodes, internal mammary nodes, infraclavicular nodes, or supraclavicular nodes. Patients were followed up for LRR by routine clinical examination. A recurrence outside those regions was considered distant metastatic disease. Date of last follow-up was defined as the last MSKCC office visit with documented disease status, correspondence documenting disease status, or death notification.
Primary study endpoints were: LRR, defined as time from date of surgery to date of LRR after diagnosis regardless of incidence of other event types, including distant metastasis (DM), that were coincident, before or after the LRR or last follow-up date; RFS, defined as date of surgery to LRR or distant disease, whichever came first; last date of follow-up; and OS, defined as time from surgical intervention to date of death from any cause or last follow-up.
The Chi-square test was used to compare distributions of clinicopathologic features between patients who received PMRT and those who did not. The Kaplan-Meier method and the log-rank test were used to analyze clinicopathologic features associated with LRR, RFS, and OS. Cox regression was used to fit multivariable models for LRR, RFS, and OS. Cumulative incidence was estimated by calculating 1 minus the Kaplan-Meier estimate. P-values <0.05 were considered significant.
RESULTS
We identified 1331 patients who had a mastectomy with 1-3 positive nodes. We excluded 244 who had T3/T4 disease, isolated tumor cells as nodal metastasis, or received NAC. Our final cohort included 1087 patients with T1-2N1 disease (163 [15%] received PMRT, 924 [85%] did not). Median follow-up was 10.8 years (range 0–21) for the entire cohort; 10.8 (range .10–21.4) and 10.7 (range .97–20.9) for the no-PMRT and PMRT groups, respectively. Table 1 summarizes demographic and clinicopathologic cohort parameters. Median age at diagnosis was 51 years (range 20–90). Median tumor size was 1.8cm (range 0.1–5.0cm). Most patients had one or two positive axillary LNs; 58% and 28%, respectively. Only 14% had three. Final surgical margin was clear in 97%, with 3% having close or positive margins. Approximately 74% had macroscopic axillary LN metastasis; 26% had microscopic axillary metastasis.
Table 1.
Clinicopathologic and Treatment Characteristics
| Characteristic | Variable | No PMRT (N=924) N (%) | PMRT (N=163) N (%) | p-value |
|---|---|---|---|---|
| Age | <40 | 147 (16%) | 33 (20%) | 0.019 |
| 40–50 | 264 (29%) | 62 (38%) | ||
| 50–60 | 232 (25%) | 36 (22%) | ||
| 60–70 | 150 (16%) | 17 (10%) | ||
| >70 | 131 (14%) | 15 (9%) | ||
|
| ||||
| Tumor Size | 0.1–2.0 cm | 561 (61%) | 77 (47%) | 0.0013 |
| 2.1–5.0 cm | 363 (39%) | 86 (53%) | ||
|
| ||||
| Histological Grade* | I | 20 (2%) | 2 (1%) | 0.029 |
| II | 204 (22%) | 23 (14%) | ||
| III | 572 (62%) | 118 (72%) | ||
|
| ||||
| Nuclear Grade* | I | 20 (2%) | 0 (0%) | 0.044 |
| II | 366 (40%) | 57 (35%) | ||
| III | 365 (40%) | 77 (47%) | ||
|
| ||||
| Extensive Intraductal Component* | Yes | 243 (26%) | 41 (25%) | 0.74 |
| No | 678 (73%) | 122 (75%) | ||
|
| ||||
| Multifocal/Multicentric | Yes | 390 (42%) | 74 (45%) | 0.45 |
| No | 534 (58%) | 89 (55%) | ||
|
| ||||
| Lymphovascular Invasion | Yes | 408 (44%) | 104 (64%) | <.0001 |
| No | 516 (56%) | 59 (36%) | ||
|
| ||||
| Extracapsular Extension | Yes | 115 (12%) | 54 (33%) | <.0001 |
| No | 809 (88%) | 108 (66%) | ||
|
| ||||
| Axillary Procedure | SLNB only | 60 (6%) | 3 (2%) | 0.0191 |
| ALND | 864 (94%) | 160 (98%) | ||
|
| ||||
| Number of Positive Nodes | 1 | 577 (62%) | 51 (31%) | <.0001 |
| 2 | 242 (26%) | 61 (37%) | ||
| 3 | 105 (11%) | 51 (31%) | ||
|
| ||||
| Size of Axillary Nodal Metastasis* | Macroscopic | 661 (72%) | 143 (88%) | <.0001 |
| Microscopic | 259 (28%) | 19 (12%) | ||
|
| ||||
| Estrogen Receptor* | Positive | 710 (77%) | 117 (72%) | 0.12 |
| Negative | 197 (21%) | 44 (27%) | ||
|
| ||||
| Progesterone Receptor* | Positive | 552 (60%) | 99 (61%) | 0.84 |
| Negative | 358 (39%) | 62 (38%) | ||
|
| ||||
| HER2 Status* | Amplified | 107 (12%) | 24 (15%) | 0.61 |
| Not Amplified | 611 (66%) | 121 (74%) | ||
|
| ||||
| Systemic Chemotherapy* | Yes | 793 (86%) | 157 (96%) | 0.0002 |
| No | 129 (14%) | 6 (4%) | ||
Percentages are out of total sample size for each group (includes unknowns)
PMRT, postmastectomy radiation therapy; SLNB, sentinel lymph node biopsy; ALND; axillary lymph node dissection
Chemotherapy was delivered to 952 patients (88%); in 76%, the regimen were anthracycline- and/or taxane-based. Of patients with known ER status, 77% (827/1068) were ER positive; all received adjuvant hormone therapy. The HER2/neu oncogene was amplified in 123 (15%) tumors; 51 (41%) of these patients received trastuzumab..
Overall, 15% of the cohort was selected for PMRT. Patients who received PMRT were more likely younger (age ≤50 years [p=0.0011]), to have larger tumor size (p=0.0013), higher histologic grade (p=0.029), and to have LVI (p<.0001). PMRT patients had more positive LNs (p<.0001), and were more likely to have ECE (p<.0001) and macroscopic axillary LN metastases (p<.0001).
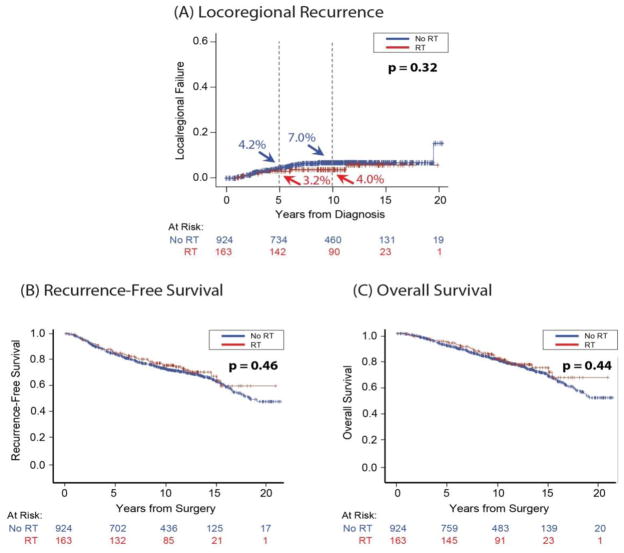
When comparing patients who received PMRT to those who did not, there was no significant difference in LRR, RFS, or OS (Figure 1) (Log-Rank p=0.32, 0.46, 0.44, respectively). Ten-year LRR incidence in the PMRT group was 4.0% versus 7.0% in the no-PMRT group. The 10-year RFS rates for the PMRT and no-PMRT groups were 75% (95% CI 69–82) and 73% (95% CI 69–76), respectively. The OS was 81% (95% CI 73–86) in the PMRT group versus 80% (95% CI 77–82) in the no-PMRT group.
Fig. 1.
Univariate and unadjusted comparison of (A) locoregional recurrence, (B) recurrence-free survival, and (C) overall survival in postmastectomy radiotherapy versus no postmastectomy radiotherapy groups.
There were 63 LRRs in the entire cohort (7 in the PMRT group, 56 for no PMRT). Most were localized to the chest wall (32/63 [51%]) and the supraclavicular nodes (17/63 [27%]). The axillary and internal mammary nodes were the recurrence site in 5 (8%) and 3 (5%) patients, respectively. Recurrence in the axilla and chest wall developed in 5 patients; 1 had recurrence in the supraclavicular and internal mammary nodes (Table 2). None of the 3 patients receiving PMRT after an SLNB had an LRR.
Table 2.
Clinical Outcomes: Locoregional Recurrences by Site, Distant Metastasis, and Death
| Total Cohort (1,087) | No PMRT (924) | PMRT (163) | |||
|---|---|---|---|---|---|
| SLNB (60) | ALND (864) | SLNB (3) | ALND (160) | ||
| Locoregional Recurrence Site | |||||
| Chest wall (CW) | 32 | 3 | 27 | - | 2 |
| Supra-clavicular nodes (SC) | 17 | - | 13 | - | 4 |
| Axillary nodes (AX) | 5 | 2 | 3 | - | - |
| Internal mammary nodes (IM) | 3 | - | 2 | - | 1 |
| AX nodes and CW | 5 | - | 5 | - | - |
| SC and IM nodes | 1 | - | 1 | - | - |
| Distant Metastasis | 184 | 2 | 152 | 1 | 29 |
| Death | 264 | 7 | 222 | 1 | 34 |
PMRT, postmastectomy radiation therapy
In those not receiving PMRT, univariate analysis demonstrated age <40 (p=0.0001), histologic grade (p=0.053), nuclear grade (p=0.010), LVI presence (p<.0001), and macroscopic axillary nodal metastasis (p=0.033) were associated with LRR. T2 tumors approached significance in predicting LRR compared to T1 tumors (p=0.085) (Table 3). On multivariable Cox regression analysis, the only significant factors associated with increased LRR risk were age <40 years (hazard ratio [HR] 4.15; 95% CI 2.42–7.11; p<.0001) and presence of LVI (HR 3.47; 95% CI 1.92–6.29; p<.0001).
Table 3.
Univariate Analysis of 10-Year Kaplan-Meier Locoregional Recurrence-Free Outcomes in the No-PMRT Group
| Characteristic | Variable | n | LRR-free survival (95% CI) | p-value |
|---|---|---|---|---|
| All patients | 924 | |||
|
| ||||
| Gender | Female | 898 | 0.93 (0.91–0.95) | 0.61 |
| Male | 26 | 0.94 (0.67–0.99) | ||
|
| ||||
| Age | <40 | 147 | 0.81 (0.73–0.87) | <.0001 |
| 40–50 | 264 | 0.93 (0.90–0.96) | ||
| 50–60 | 232 | 0.95 (0.91–0.97) | ||
| 60–70 | 150 | 0.98 (0.93–0.99) | ||
| >70 | 131 | 0.95 (0.87–0.98) | ||
|
| ||||
| Tumor Size | 0.1–2.0 cm | 638 | 0.94 (0.92–0.96) | 0.085 |
| 2.1–5.0 cm | 449 | 0.91 (0.87–0.94) | ||
|
| ||||
| Histological Grade | I | 20 | 1 | 0.053 |
| II | 204 | 0.95 (0.91–0.98) | ||
| III | 572 | 0.91 (0.88–0.93) | ||
|
| ||||
| Nuclear Grade | I | 20 | 1 | 0.010 |
| II | 366 | 0.95 (0.92–0.97) | ||
| III | 365 | 0.90 (0.85–0.93) | ||
|
| ||||
| Extensive Intraductal Component | Yes | 243 | 0.92 (0.88–0.95) | 0.39 |
| No | 678 | 0.93 (0.91–0.95) | ||
|
| ||||
| Multifocal/Multicentric | Yes | 390 | 0.93 (0.90–0.95) | 0.89 |
| No | 534 | 0.93 (0.90–0.95) | ||
|
| ||||
| Lymphovascular Invasion | Yes | 408 | 0.88 (0.85–0.91) | <.0001 |
| No | 516 | 0.97 (0.94–0.98) | ||
|
| ||||
| Extracapsular Extension | Yes | 115 | 0.95 (0.88–0.98) | 0.43 |
| No | 809 | 0.93 (0.91–0.94) | ||
|
| ||||
| Axillary Procedure | SLNB Only | 60 | 0.89 (0.77–0.96) | 0.47 |
| ALND | 864 | 0.93 (0.91–0.95) | ||
|
| ||||
| Number of Positive Nodes | 1 | 577 | 0.94 (0.91–0.96) | 0.099 |
| 2 | 242 | 0.93 (0.89–0.96) | ||
| 3 | 105 | 0.87 (0.78–0.93) | ||
|
| ||||
| Size of Axillary Nodal Metastasis | Macroscopic | 661 | 0.91 (0.89–0.94) | 0.033 |
| Microscopic | 259 | 0.96 (0.92–0.98) | ||
|
| ||||
| Estrogen Receptor | Positive | 710 | 0.93 (0.91–0.95) | 0.60 |
| Negative | 197 | 0.93 (0.88–0.96) | ||
|
| ||||
| Progesterone Receptor | Positive | 552 | 0.94 (0.91–0.96) | 0.25 |
| Negative | 358 | 0.92 (0.88–0.94) | ||
PMRT, postmastectomy radiation therapy; LRR, locoregional recurrence; CI, confidence interval; SLNB, sentinel lymph node biopsy; ALND; axillary lymph node dissection
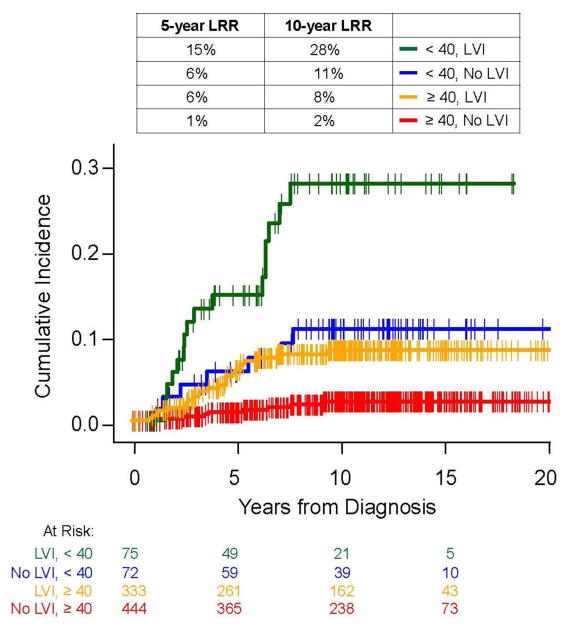
We further examined the relationship between age and LVI presence with LRR in patients who did not receive PMRT. When patients were stratified on the basis of age <40 years and LVI presence, we found that at 10 years, LRR rates for patients with no LVI and age >40 years was 2% (95% CI 0.7–3.8) compared to 28% (95% CI 11.0–22.1) in patients with both LVI and age <40 years (p<.0001) (Figure 2)..
Fig. 2.
Comparison of locoregional recurrence rates in the no-PMRT group by age and lymphovascular invasion.
PMRT, postmastectomy radiotherapy; LVI, lymphovascular invasion
DISCUSSION
With data supporting its benefit, PMRT use in patients with T1-2 tumors and 1-3 positive nodes has increased over time.16 We have noticed a similar trend at MSKCC: the percentage of patients receiving PMRT with T1-2N1 disease increased from 9.6% before 2000 to 17% afterward.
In an EBCTCG meta-analysis, PMRT significantly improved LRR, OS, disease-free survival (DFS), and breast-cancer–specific survival in all patients with positive nodes. When limiting the analysis to the 1314 patients with 1-3 positive nodes, PMRT use decreased the LRR rate from 20.3% to 3.8% at 10 years. This reduction translated into an absolute breast cancer mortality reduction of 7.9% at 20 years. When further limiting the analysis to the 1133 patients with 1-3 positive LNs who received systemic therapy, the benefit of PMRT persisted.1 The 20.3% LRR without PMRT in the meta-analysis is significantly higher than the currently reported LRR rates of 4–10% in more modern series.11,15,17–19 The data presented in this study are from a single institution, and hence reflect a unique patient population and may not be generalized; our LRR rate without PMRT (7.0%), however, is comparable to those reported in more modern series. Furthermore, with 10.8 years median follow-up, we believe we have captured the overwhelming majority of LRR, as these events are infrequent beyond the first 10 years follow-up.3 The difference in LRR rates between the trials in the meta-analysis and our study may be partially attributed to two factors: more modern chemotherapy and endocrine treatment, and axillary clearance completeness.
The EBCTCG meta-analysis trials were predominantly conducted in the 1970s/1980s with chemotherapy regimens considered era-appropriate, primarily, cyclophosphamide, methotrexate, and 5-fluorouracil (CMF). In our current cohort, women received more modern chemotherapy,20 mostly (77%) including a dose-dense regimen of Adriamycin, cyclophosphamide, and Taxol (ACT). Moreover, after 2004, anti-HER2 therapy became standard at our institution due to its survival benefit.21–23 Anti-HER2 therapy has also been shown to significantly improve local control.24 Furthermore, endocrine therapy used in the 1970s/1980s was limited to tamoxifen compared to the modern era, in which aromatase inhibitors are favored in postmenopausal women.25 In our patient population, 60% of patients receiving endocrine therapy had an aromatase inhibitor included. Additionally, the use of tamoxifen, in some of the meta-analysis trials, was limited to one year, which is currently considered sub-optimal.5,12,13
94% of our patients received an ALND; the median number of nodes removed was 18 versus <10 in some of the EBCTCG meta-analysis trials. Proper axillary clearance leads to better staging of the axilla, hence identifying patients with ≥4 nodes in whom PMRT is standard and beneficial. Furthermore, complete ALND lowers the chance of residual nodal disease that may contribute to a higher axillary nodal recurrence. In one of the meta-analysis’s largest trials, the median number of axillary nodes resected was 7, and axillary nodal recurrence rates reached 33.7%.13 In our cohort, the most common nodal recurrence site was the supraclavicular area; only 10/63 (15.9%) of the recurrences involved the axilla (5 isolated, 5 combined with chest-wall recurrence).
In our cohort, PMRT was delivered at the treating physician’s discretion, limiting its use to high-risk patients (Table 1). At 10.8 years median follow-up, age <40 years and LVI presence were the strongest LRR predictors. The extent and morphologic features of LVI did not seem to predict outcome, however.26
Age9,27–29 and LVI 8,9,27,30 have been similarly reported in multiple studies as LRR predictors in patients with T1-T2 breast cancer and 1-3 positive LNs. The 10-year LRR rate in the age <40 years with LVI group was 28%; such a high rate warrants PMRT. Arguably, in patients with clinically negative axillas and <40 years who have T1-2 tumors with LVI, a positive sentinel node may not dictate a completion ALND, as the axilla may be equally controlled with radiation therapy with lower rate of lymphedema. The AMAROS study reported, in cT1-2N0 patients with positive sentinel nodes, an axillary recurrence of 0.4% in the ALND group and 1.2% in the radiotherapy arm, with respective lymphedema rates of 23% and 11% at 5 years.31 The vast majority of our patients (92.4%) had an axillary node dissection, hence omission of PMRT is not applicable to patients who had only 1-3 positive sentinel nodes without completion axillary node dissection.
In the current analysis, tumor size lost its significance in predicting LRR on multivariate analysis, but remained significant for OS (p<.0001). Prior studies, however, have reported increased tumor size is an LRR predictor.9,32,33 The lack of significance of tumor size as an LRR predictor in patients not receiving RT in our study, may be related to selection bias, as patients with larger tumors were selected to receive PMRT.
Our study is limited by its retrospective nature and the potential ensuing bias. Additionally, Figure 1, which compares outcomes in the RT vs no-RT groups, is not based on a randomized trial and should not be interpreted as such. Despite our study’s retrospective nature, our reported LRR rates are consistent with those in modern series.11,15,17–19
The EORTC 22922 prospective randomized trial34 has shown an improvement in DFS and distant DFS, and a reduction in breast cancer mortality with internal mammary and medial supraclavicular irradiation. The study included 4004 patients; 955 underwent a mastectomy. It is difficult to compare the results of EORTC 22922 to ours for various reasons. EORTC 22922 included patients with stage I–III disease, of whom 43.1% had 1-3 positive nodes35; our study focused on T1-2N1 disease. Additionally, chemotherapy was given to 88% of our study’s patients compared to 55% in EORTC 22922. Finally, 77% of our study’s patients had an ER positive tumor, all of whom received hormonal therapy; in EORTC 22922, 78% of patients with known ER status had an ER positive tumor; only 59% of these patients received hormonal treatment.35 As previously stated, we believe modern chemotherapy and adequate hormonal treatment were important in lowering the LRR rate and improving survival in those without PMRT. Lastly, the EORTC34 reported the HR of death for the different subgroups, while comparing the irradiated arm versus the control (0.91 for the mastectomy group, 0.73 for the lumpectomy group). Hence, if the EORTC’s analysis had been limited to the 955 patients who underwent a mastectomy, it is unclear if the same conclusion could be reached.
Conclusions
Recent ASCO/ASTRO/SSO guidelines on PMRT in women with T1-T2 disease and 1-3 positive LNs strongly recommend a multidisciplinary decision to use PMRT and that the PMRT-use decision be made in a multidisciplinary manner. PMRT use lowers LRR and improves survival, but is associated with higher lymphedema rates,36,37 radiation-induced cardiac disease,38–40 and more complications after breast reconstruction, especially in implant cases.41,42 There is also a risk, albeit small, of secondary cancers with PMRT.43 This study supports these guidelines, as limiting PMRT to high-risk groups resulted in low LRR among those not receiving PMRT, and similar RFS and OS in those treated with and without PMRT.
Synopsis.
Here we evaluate the risk of locoregional recurrence in patients with T1–T2 tumors and 1-3 positive lymph nodes treated in the modern era, while identifying predictors of locoregional recurrence in patients not receiving postmastectomy radiation therapy. We find that age <40 years and presence of lymphovascular invasion are significant predictors of locoregional recurrence in those not receiving postmastectomy radiation.
Acknowledgments
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. The authors have no conflict of interest disclosures to report.
Footnotes
Conflict of Interest Disclosures: The preparation of this study was supported by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. This study was presented in part of an oral presentation at the Society of Surgical Oncology 2017 Annual Cancer Symposium, March 15–18, 2017, Seattle WA. The authors have no conflict of interest disclosures to report.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frasier LL, Holden S, Holden T, Schumacher JR, Leverson G, Anderson B, Greenberg CC, et al. Temporal Trends in Postmastectomy Radiation Therapy and Breast Reconstruction Associated With Changes in National Comprehensive Cancer Network Guidelines. JAMA Oncol. 2016;2(1):95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.The National Institutes of Health Consensus Development Conference: Adjuvant Therapy for Breast Cancer. Bethesda, Maryland, USA. November 1–3, 2000. Proceedings. J Natl Cancer Inst Monogr. 2001;(30):1–152. [PubMed] [Google Scholar]
- 5.Killander F, Anderson H, Ryden S, Moller T, Aspegren K, Ceberg J, Danewid C, et al. Radiotherapy and tamoxifen after mastectomy in postmenopausal women -- 20 year follow-up of the South Sweden Breast Cancer Group randomised trial SSBCG II:I. Eur J Cancer. 2007;43(14):2100–8. doi: 10.1016/j.ejca.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82(3):247–53. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald SM, Abi-Raad RF, Alm El-Din MA, Niemierko A, Kobayashi W, McGrath JJ, Goldberg SI, et al. Chest wall radiotherapy: middle ground for treatment of patients with one to three positive lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2009;75(5):1297–303. doi: 10.1016/j.ijrobp.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Yang PS, Chen CM, Liu MC, Jian JM, Horng CF, Liu MJ, Yu BL, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77(2):516–22. doi: 10.1016/j.ijrobp.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Raad R, Boutrus R, Wang R, Niemierko A, Macdonald S, Smith B, Taghian AG. Patterns and risk factors of locoregional recurrence in T1-T2 node negative breast cancer patients treated with mastectomy: implications for postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(3):e151–7. doi: 10.1016/j.ijrobp.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghian AG, Jeong JH, Mamounas EP, Parda DS, Deutsch M, Costantino JP, Wolmark N. Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2006;24(24):3927–32. doi: 10.1200/JCO.2006.06.9054. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Bedrosian I, Lucci A, Hwang RF, Rourke LL, Qiao W, Buchholz TA, et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010;17(11):2899–908. doi: 10.1245/s10434-010-1089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson M, Kamby C, Jensen MB, Mouridsen H, Ejlertsen B, Dombernowsky P, Rose C, et al. Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer co-operative Group DBCG 82B Trial. Eur J Cancer. 1999;35(12):1659–66. doi: 10.1016/s0959-8049(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–8. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 14.Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, Hudis CA, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol. 2016;34(36):4431–42. doi: 10.1200/JCO.2016.69.1188. [DOI] [PubMed] [Google Scholar]
- 15.Moo TA, McMillan R, Lee M, Stempel M, Patil S, Ho A, El-Tamer M. Selection criteria for postmastectomy radiotherapy in t1-t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20(10):3169–74. doi: 10.1245/s10434-013-3117-0. [DOI] [PubMed] [Google Scholar]
- 16.Shirvani SM, Pan IW, Buchholz TA, Shih YC, Hoffman KE, Giordano SH, Smith BD. Impact of evidence-based clinical guidelines on the adoption of postmastectomy radiation in older women. Cancer. 2011;117(20):4595–605. doi: 10.1002/cncr.26081. [DOI] [PubMed] [Google Scholar]
- 17.Hamamoto Y, Ohsumi S, Aogi K, Shinohara S, Nakajima N, Kataoka M, Takashima S. Are there high-risk subgroups for isolated locoregional failure in patients who had T1/2 breast cancer with one to three positive lymph nodes and received mastectomy without radiotherapy? Breast Cancer. 2014;21(2):177–82. doi: 10.1007/s12282-012-0369-7. [DOI] [PubMed] [Google Scholar]
- 18.Tendulkar RD, Rehman S, Shukla ME, Reddy CA, Moore H, Budd GT, Dietz J, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1-3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83(5):e577–81. doi: 10.1016/j.ijrobp.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 19.McBride A, Allen P, Woodward W, Kim M, Kuerer HM, Drinka EK, Sahin A, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89(2):392–8. doi: 10.1016/j.ijrobp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative G. Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Jr, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 23.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 24.Lanning RM, Morrow M, Riaz N, McArthur HL, Dang C, Moo TA, El-Tamer M, et al. The Effect of Adjuvant Trastuzumab on Locoregional Recurrence of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Treated with Mastectomy. Ann Surg Oncol. 2015;22(8):2517–25. doi: 10.1245/s10434-014-4321-2. [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 26.Hilliges C, Hsu M, Gallagher M, Stempel M, El-Tamer M, Brogi E. Morphologic features and prognostic value of lymphovascular invasion in lymph node positive breast carcinoma. Lab Invest. 2014;94:54A. [Google Scholar]
- 27.Yildirim E, Berberoglu U. Local recurrence in breast carcinoma patients with T(1-2) and 1-3 positive nodes: indications for radiotherapy. Eur J Surg Oncol. 2007;33(1):28–32. doi: 10.1016/j.ejso.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1337–47. doi: 10.1016/j.ijrobp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lu C, Xu H, Chen X, Tong Z, Liu X, Jia Y. Irradiation after surgery for breast cancer patients with primary tumours and one to three positive axillary lymph nodes: yes or no? Curr Oncol. 2013;20(6):e585–92. doi: 10.3747/co.20.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Botteri E, Gentilini O, Rotmensz N, Veronesi P, Ratini S, Fraga-Guedes C, Toesca A, et al. Mastectomy without radiotherapy: outcome analysis after 10 years of follow-up in a single institution. Breast Cancer Res Treat. 2012;134(3):1221–8. doi: 10.1007/s10549-012-2044-2. [DOI] [PubMed] [Google Scholar]
- 31.Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, Hortobagyi G, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18(15):2817–27. doi: 10.1200/JCO.2000.18.15.2817. [DOI] [PubMed] [Google Scholar]
- 33.Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, Falkson G, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17(6):1689–700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 34.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, Collette L, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med. 2015;373(4):317–27. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 35.Musat E, Poortmans P, Van den Bogaert W, Struikmans H, Fourquet A, Bartelink H, Kirkove C, et al. Quality assurance in breast cancer: EORTC experiences in the phase III trial on irradiation of the internal mammary nodes. Eur J Cancer. 2007;43(4):718–24. doi: 10.1016/j.ejca.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, Vallis KA, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015;373(4):307–16. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, Sadek BT, Shenouda MN, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88(3):565–71. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 39.Gyenes G, Rutqvist LE, Liedberg A, Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol. 1998;48(2):185–90. doi: 10.1016/s0167-8140(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 40.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowen D, Gross E, Rouannet P, Teissier E, Ellis S, Resbeut M, Tallet A, et al. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat. 2010;121(3):627–34. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- 42.Kelley BP, Ahmed R, Kidwell KM, Kozlow JH, Chung KC, Momoh AO. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practics ideal? Ann Surg Oncol. 2014;21(5):1732–8. doi: 10.1245/s10434-014-3494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiother Oncol. 2013;106(1):42–9. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]