Abstract
Background
The aims of this study were to examine the potential association between sleep problems, symptom burden, and survival in advanced cancer patients.
Methods
A prospective study of 294 patients with gastrointestinal cancer were administered questionnaires assessing sleep, depression, anxiety, stress, pain, fatigue, and health-related quality of life. Serum levels of cytokines including Interleukin (IL)-1α, IL-1β, Tumor Necrosis Factor-α, IL-10, IL-2, and IFNγ were measured to assess biological mediation between sleep and survival. Survival was measured as time from diagnosis to death.
Results
Fifty-nine percent of patients reported poor sleep quality, 53% reported poor sleep efficiency, 39% reported sleep latency greater than 30 minutes, and 45% reported sleeping <6 hours or >10 hours. We found a significant association between sleep duration and symptom burden. Shorter sleep duration was significantly associated with higher levels of fatigue (r=−0.169, p=0.01), pain (r=−0.302, p=0.01), anxiety (r=−0.182, p=0.01), depression (r=−0.172, p=0.003) and lower levels of quality of life (r=0.240, p=0.01). After adjustment for demographic, psychological, and disease-specific factors, short sleep duration was associated with reduced survival HR linear = 0.485, 95% CI=0.275–0.857] and there was also evidence for a quadratic pattern [HR quadratic =1.064, 95% CI=1.015–1.115] suggesting a curvilinear relationship between sleep duration and survival. Interleukin-2 was the only cytokine significantly related to survival [HR=1.01, p=0.003] and sleep duration [β=--30.11, p=−0.027]. When serum levels of IL-2 was added to the multivariable model, short and long sleep [β =−0.557, p=0.097; β=0.046, p=0.114] were no longer significantly related to survival, suggesting mediation by IL-2.
Conclusion
Sleep duration was associated with symptom burden and poorer survival and IL-2 was found to mediate the association between sleep and survival. Screening and treatment of sleep problems in patients diagnosed with cancer is warranted.
Keywords: sleep duration, mortality, cytokines, cancer, sleep problems, sleep regulation
Introduction
Sleep problems have been found to be associated with poor quality of life and increased risk of mortality in the general population, and recent research has also began to show similar findings in those diagnosed with cancer.1–6 Quality of life is of utmost importance in patients with advanced cancer and sleep problems are one of the most frequently reported symptoms affecting quality of life in late stage cancer patients.7,8 Studies of sleep in people diagnosed with cancer are underrepresented, yet understanding the effect of sleep problems on quality of life in this population is warranted to develop evidence-based interventions to improve sleep and quality of life.
Palesh and colleagues observed that poor sleep efficiency was related to poorer survival in women diagnosed with breast cancer.9 Despite decades of research concerning sleep and mortality, the underlying biological mechanisms linking sleep and mortality remain unclear. Krueger and colleagues concluded, from decades of animal and human research, that cytokines are key sleep regulatory proteins and may be involved in mediating the association between sleep and mortality.10–12 Evidence from their team suggests that tumor necrosis factor (TNF)-α and Interleukin (IL)-1 stimulate the transcriptional activity of nuclear factor kB (NF-kB) and increases sleep duration.12 In turn, NF-kB promotes the production of IL-2 which has been shown to be somnogenic.12 In contrast, IL-4 and IL-10 inhibit NF-kB, thereby reducing the duration of sleep.12 These proteins work synergistically to maintain sleep homeostasis.12
In the context of cancer, TNF-α and IL-1β are considered key downstream mediators of inflammation regulating the cascade of cytokines, chemokines, adhesion molecules, matrix metalloproteinase (MMP) and pro-angiogenic activities involved in tumor growth and development of metastases. In contrast, IFN-γ and IL-2 have been shown to play a role in cellular mediated immunity; these cytokines have been used to treat some cancer types and extend life.13–20 Interleukin 2 is considered a “master regulator” of the immune system, acting as a growth factor for T cells. Sleep may be considered anti-inflammatory; therefore, sleep deprivation may play a role in disease progression in those diagnosed with cancer.
The majority of human studies that have been conducted regarding sleep and cytokines focused on IL-6 and TNF-α.21 In a recent meta-analysis, Irwin and colleagues observed that sleep disturbances were associated with higher serum levels of C-Reactive Protein (CRP) and the cytokine IL-6, while short sleep duration was associated with higher levels of circulating CRP but not IL-6.21 Long sleep duration was also associated with higher levels of CRP and IL-6.21 Sleep disturbances and sleep duration were not associated with circulating TNF-α.21 This meta-analytic study summarized decades of research examining the link between sleep problems and cytokines. The studies included in the meta-analysis used a mixture of methods to assess sleep problems (e.g., self-report, actigraphy, polysomnography). Importantly, the studies were cross-sectional in design, and only biomarkers of inflammation were included in the meta-analysis.
This prospective study aimed to describe the frequency of sleep problems in advanced cancer patients and their relationship with the most common and debilitating symptoms (e.g., pain, depression, fatigue) associated with quality of life. We expected that shorter sleep duration would be associated with higher symptom burden. We investigated the role of sleep characteristics (e.g., sleep efficiency, sleep duration, sleep quality) and survival while adjusting for demographic, disease-specific, psychological and behavioral factors. Due to curvilinear relationship between sleep duration and survival found in the general population, we expected to find a curvilinear relationship between sleep duration and survival as well as an association between poor sleep efficiency and decreased survival. Finally, we examined the potential biological mediators linking sleep and survival in patients diagnosed with advanced cancer. We expected that TNFα and/or IL-1 would mediate the association between sleep duration and survival.
Methods
Design and Participants
Patients from two prospective studies conducted at a large tertiary medical center were included in this secondary data analysis (R21CA127046; R01CA176809).22 Questionnaires were administered and blood draws were performed at least 7–8 weeks after the patients’ last treatment so that the changes in sleep and biomarkers were not reflective of acute side effects of treatment. We also administered the questionnaires and performed the blood draws at the same time or within a +/− 2 week period.
The Division of Hepatobiliary and Pancreatic Cancer Center evaluates and treats patients with gastrointestinal cancers including hepatocellular, cholangio, gallbladder, and pancreatic carcinoma and other primary tumors that had metastasized to the liver (e.g., colorectal, ovarian, breast). These patients were categorized by diagnosis and prognosis into four groups (i.e., gallbladder, stomach, and pancreatic; hepatocellular and cholangio carcinoma; other primary cancers with liver metastases; and neuroendocrine). Patients were enrolled in the studies between January 2008 to June 2011 (R21CA127046) and November 2013 to June 2015 (R01CA176809). The first study included a study that examined the relationship between symptoms clusters and cytokines.22 The second study was a prospective study examining biobehavioral pathways of stress and disease progression in patients diagnosed with cancer.
The inclusion criteria for both studies were: (1) biopsy or radiographic-proven diagnosis of cancer affecting the hepatobiliary or pancreatic system; (2) age 21 years or older; (3) fluent in English. Exclusion criteria included (1) age under 21 years; (2) evidence of thought disorder, hallucinations, or delusions; (3) chronic steroid use; (4) immunizations in the past months; or (5) infectious illness in the last month. For the purposes of this study, patients who had received a liver transplant or were diagnosed with sleep apnea were excluded from analyses due to differences in survival with these patients. Liver transplant candidacy and a diagnosis of sleep apnea was determined by reviewing the electronic medical record. If the medical record stated the patient was listed for transplant or had a diagnosis of sleep apnea, the patient was excluded from the analyses. The median follow up for patients in these studies was 341 days (range 22–2448 days). Median survival for patients in this study was 1056 days (95% CI: 632 days–1480 days).
Instruments
Demographic, Disease, and Treatment Specific Factors
Sociodemographic variables were reported on a questionnaire designed specifically for this study and included the patients’ age, sex, body mass index (BMI), race, ethnicity, educational level, occupation, income, and health insurance status, and was reported on a questionnaire designed specifically for these studies. Disease-specific and treatment-related information was gathered from the patients’ electronic medical record including: diagnosis, presence or absence of cirrhosis, vascularity of lesions, medical comorbidities, and vascular invasion. Body mass index was gathered from the patients’ medical record. Survival was measured from the time of diagnosis of cancer until death. Death was determined by the electronic medical record or the Social Security Death Index.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is an 18-item self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval.23 The PSQI is composed of 7 component scores describing sleep problems.23 The global PSQI score is a sum of all the component scores. Adequate levels of internal consistency, test-retest reliability, and validity have been reported for the PSQI in cancer patients.23 The component scale scores for the PSQI can be found below in Table 1.
Table 1.
PSQI Component Scores and Interpretation
| Component | Scale | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Subjective Sleep Quality | Very good | Fairly good | Fairly bad | Very bad |
| Sleep Latency | <15 min | 16–30 min | 31–60 min | >60 min |
| Sleep Duration | >7 hours | 6–7 hours | 5–6 hours | < 5 hours |
| Sleep Efficiency | >85% | 75–84% | 65–74% | <65% |
| Sleep Disturbances | 0 | 1–9 | 10–18 | 19–27 |
| Daytime Dysfunction | 0 | 1–2 | 3–4 | 5–6 |
| Sleep Medication | Not during past month | Less than once a week | Once or twice a week | Three or more times a week |
Depressive Symptoms
The Center for Epidemiologic Studies-Depression (CES-D) is a 20-item self-report questionnaire designed to assess depressive symptoms.24 The patient responds on a 4 point scale by reporting weekly frequency of depressive symptoms (“rarely,” ”some days,” ”occasionally,” ”most days”).24 A score of 16 or greater represents depressive symptoms in the clinical range.24 The CES-D has demonstrated adequate construct validity and reliability in cancer patients.25
Anxiety
Anxiety was measured using the short form of the Spielberger State-Trait Anxiety Inventory (STAI-6).26 The 6 items of the questionnaire reflect symptoms of anxiety or the opposite of anxiety (calm, tense, upset, relaxed, content, and worried) and were rated on a four-point scale with a higher scores indicating more anxiety. The STAI-6 is reported to have good reliability and validity.27
Fatigue
The Functional Assessment of Cancer Therapy-Fatigue (FACT-F) subscale is a 13-item questionnaire that is part of the 20-item anemia (FACT-An) module of the FACIT quality of life assessment system.28 The FACT-F has been extensively used in a range of cancer populations. Scores can range between 0 and 52 with higher scores indicating greater fatigue.28 The FACT-Fatigue has been shown to be valid and reliable.28
Pain
The Brief Pain Inventory (BPI) measures both the intensity of pain (sensory dimension) and interference of pain in the patient’s life (reactive dimension).29 It also queries the patient about pain relief, pain quality, and patient perception of the cause of pain.29 The BPI is a widely used instrument that has demonstrated reliability and validity.29
Perceived Stress
The Perceived Stress Scale (PSS) is a 14 item self-report questionnaire of globally perceived stress.30 Each item is rated for the past month on a 5-point Likert-type scale (1 = never to 5 = very often).30 The measure has demonstrated adequate validity and reliability in cancer and non-cancer populations.30
Health Related Quality of Life
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep)24 was used to assess changes in symptoms and side effects of treatment. The FACT-Hepatobiliary includes both the FACT-General25 (a 27-item instrument that measures four dimensions of quality of life) and a module with 18 items specific to hepatobiliary disease.24 The FACT-G has four subscales for a physical (PWB), social and family (SFWB), emotional (EWB), and functional well-being (FWB). The hepatobiliary module (FACT-Hep) includes 18 items that pertain to symptoms of the disease as well as side effects of the treatment.31 The FACT is one of the most widely utilized quality of life questionnaires in clinical trials, and both the FACT-G and the FACT-Hep, have been demonstrated to be a valid and reliable instrument.24,25
Cytokines
Serum levels of cytokines including IL-1α, IL-1β, IL-2, IL-10, TNFα, and IFNγ were measured for the purposes of the present study. The blood draws were performed between 8am and 12am when possible. For serum, blood was drawn into red top vacutainer tubes without anticoagulant and processed in a local laboratory at the University of Pittsburgh upon receipt, allowing >30 minutes for clot formation. Serum was then stored for no more than four hours and aliquoted and then frozen in temperature monitored −80° C freezers without thaw and thawed once before testing by Luminex. The Millipore mulit-plex kit, HCYTOMAG-60K, uses a standard curve range of 10,000 to .064 pg/ml for all cytokines/chemokines. Standard curve concentrations and Minimum Detectable Concentration (MinDC) were calculated using Milliplex Analyst 5.1 software. MinDC is determined by calculating the lowest detection limit assuming an infinite number of standards run under the same assay conditions. For IL-2, the manufacturer lists the MinDC as 1pg/ml, Intra-assay precision coefficient of variation (CV) as 2.1%, and Inter-assay CV as 6.3%. All zero values were below the Minimum Detectable Concentration for their assay run. Two quality control samples of known concentration ranges are assayed with each set of samples. They represent a lower and middle range of the standard curve. All quality control samples were within the acceptable range supplied by the manufacturer for all analytes on all lots of samples analyzed.
Procedure
Both studies were approved by the University of Pittsburgh’s Institutional Review Board. Patients were referred to the study team by their medical team. If the patient agreed to speak to a member of the study team, the individual was explained the risks and benefits of the study and written informed consent was obtained from the patient prior to completing the questionnaires or blood draw.
Data Analysis
Data were entered, verified, and analyzed using SPSS version 22 (IBM Corp, Armonk, NY), R version 2.15.2 (CRAN, http://cran.r-project.org/), and MPlus 7.0 (Muthén & Muthén, Los Angeles, CA). Descriptive statistics were performed to obtain measures of central tendency, distribution, and proportions for each variable. Undetectable cytokine levels were assigned a “0” for purposes of the analyses. The association between sleep duration and symptoms (e.g., fatigue, depression) were examined using Pearson’s correlations due to the normal distribution of the dependent variable data. Spearman’s rho correlations were utilized to examine the association between cytokines and sleep duration. Predictors included in the multivariable Cox regression analyses included factors found to be significantly (p ≤ .05) associated with survival in univariate Kaplan Meier and stepwise Cox regression survival analyses. Due to the U-shaped, curvilinear relationship between sleep duration and survival identified in previous studies, quadratic and linear terms were included in the analyses. The linear term reflects sleep duration, from short too long. The quadratic term (sleep duration2) assessed for a potential inverted U-shaped relationship between sleep duration and survival which has been observed in the general population.
Results
Sociodemographic and Disease Specific Factors
A total of 294 participants with gastrointestinal cancer (e.g., hepatocellular carcinoma, colorectal cancer with liver metastases), were enrolled in the study. Sociodemographic characteristics can be found in Table 2. Table 3 provides a description of baseline sleep problems and descriptive statistics of other covariates included in the analyses. With regard to sleep medication, 67.6% of patients reported not taking medication for sleep at all in the past month; 7.8% reported taking medication for sleep less than once a week; 4.8% reported taking medication once or twice a week; and 18.8% reported taking medication for sleep three times per week. The median survival for the entire cohort of patients was 2.89 years (95% CI =1.73 – 4.06 years). Patients were enrolled in these studies between 2008–2013 and have been followed between 4–7 years at the time of analyses.
Table 2.
Sample Characteristics
| Age (years) | |
| Mean (S.D.) | 61·9 (10.9) |
| Sex (n, %) | |
| Male | 186 (63.7) |
| Female | 106 (36.3) |
| Race (n, %) | |
| Caucasian | 260 (90.9) |
| Black / African American | 23 (8.0) |
| Other | 3 (1.0) |
| Education Level (n, %) | |
| High School graduate or less | 211 (74.8) |
| Four-year college degree or more | 71 (25.2) |
| Diagnosis (n, %) | |
| Hepatocellular or cholangio carcinoma | 151 (51.4) |
| Neuroendocrine with liver metastases | 32 (11.0) |
| Appendix / Gallbladder / Stomach / Pancreatic | 12 (4.1) |
| Other Primary Cancers (e.g., breast, colorectal, ovarian) with Liver Metastases | 97 (33.6) |
| Vascular Invasion (n, %) | |
| No | 257 (89.5) |
| Yes | 30 (10.5) |
Table 3.
Descriptive statistics of Sleep and Psychological and Behavioral Factors associated with Survival and Immunity
| Mean (S.D.) | ||
|---|---|---|
| Sleep duration (hours) | 6.5 (1.6) | |
| Sleep latency (minutes) | 29.9 (38.3) | |
| Sleep efficiency (time asleep / time in bed) | 79.9 (17.9) | |
| Global PSQI Score | 7.6 (4.4) | |
| PSQI Component Score (0–3) | --- | |
| Sleep Duration | (0=>7 hours, 1=6–7 hours; 2=5–6 hours; 3=<5 hours) | 0.9 (1.1) |
| Sleep Disturbance | (0=not during past month; 1=less than once a week; 2=once or twice a week; 3=three or more times a week) | 1.6 (0.7) |
| Sleep Latency | (0=≤15 min; 1=16–30 min; 2=31–60 min; 3=>60 min) | 1.3 (1.0) |
| Daytime Dysfunction | (1=never; 1=once or twice; 2=once or twice a week; 3=three or more times a week) | 0.9 (0.7) |
| Sleep Efficiency | (0 >85%; 1=75–84%; 2=65–74%; 3=<65%) | 1.1 (1.2) |
| Subjective Sleep Quality | (0=very good; 1=fairly good; 2=fairly bad; 3=very bad) | 1.2 (0.8) |
| Sleep Medication | (0=not during the past month; 1=less than once a week; 2=once or twice a week; 3=three or more times per week) | 0.7 (1.2) |
| Center for Epidemiological Studies-Depression | 19.33 (10.96) | |
| Short State Trait Anxiety Scale | 10.73 (4.25) | |
| Perceived Stress Scale | 30.15 (11.03) | |
| Brief Pain Inventory (average pain score) | 3.27 (2.79) | |
| Functional Assessment of Cancer Therapy-Hep | 75.47 (16.99) | |
| Functional Assessment of Cancer Therapy-Fatigue | 20 (12.22) | |
Sleep Problems and Symptom Burden
We examined the association between sleep duration and symptoms such as fatigue, pain, depression, anxiety and overall quality of life. We found a significant, but relatively small in magnitude, relationships between sleep duration and fatigue (r=−0.169, p=0.01), pain (r=−0.302, p=0.01), anxiety (r=−0.182, p=0.01), and depression (r=−0.172, p=0.003) whereas shorter sleep duration was associated with higher levels of fatigue, pain, anxiety, and depression. We also found a positive relationship between sleep duration and quality of life (r=0.240, p=0.01) with the longer the sleep duration the better quality of life.
Sleep Duration and Survival
To test the relationship between sleep duration and survival, univariate Cox regression and Kaplan Meier survival analyses were performed examining the association between demographic (age, sex, race, education), disease, specific factors (diagnostic group, vascular invasion), medical comorbidities (body mass index, history of cardiovascular disease, hypertension, and diabetes), psychological and behavioral factors (depression, stress, pain, anxiety) and survival. We found that age (HR=1.002, p=0.002), diagnostic group (cancer type) (Chi-Square=9.352, p=0.025), vascular invasion (Chi-Square=28.974, p<0.001), depression (HR=1.038, p=0.037), sleep duration linear term (HR=0.611, p=0.049) and sleep duration quadratic term (HR=1.042, p=0.041), and snoring (Chi-Square=8.808, p=0.032) to be associated with survival. See Table 4 and 5.
Table 4.
Univariate Cox regression of potential factors associated with survival
| β | p-value | HR | 95% CI | |
|---|---|---|---|---|
| Age | 0.002 | 0.817 | 1.002 | 0.984–1.021 |
| Body Mass Index | −0.026 | 0.436 | 0.974 | 0.912–1.040 |
| Depression | 0.037 | 0.001 | 1.038 | 1.014–1.061 |
| Sleep Quality | 0.129 | 0.274 | 1.138 | 0.903–1.435 |
| Sleep Latency | −0.002 | 0.609 | 0.998 | 0.992–1.005 |
| Sleep Efficiency | −0.001 | 0.911 | 0.999 | 0.988–1.011 |
| Global Sleep (PSQI Total) | 1.366 | 0.243 | 1.028 | 0.981–1.078 |
| Pain | −0.012 | 0.544 | 0.988 | 0.907–1.077 |
| Anxiety | 0.031 | 0.148 | 1.032 | 0.989–1.076 |
| Stress | 0.005 | 0.742 | 1.005 | 0.974–1.038 |
| Sleep Duration: Linear | −0.493 | 0.049 | 0·611 | 0·374–0·997 |
| Sleep Duration: Quadratic | 0.041 | 0.050 | 1·042 | 1·000–1·085 |
Table 5.
Univariate Kaplan Meier Survival Analysis of Potential Factors Associated with Survival
| Chi-Square | p-value | |
|---|---|---|
| Sex | 1.609 | 0.205 |
| Race | 1.428 | 0.839 |
| Education | 2.427 | 0.119 |
| History of Cardiovascular Disease | 0.423 | 0.516 |
| Hypertension | 0.167 | 0.683 |
| Diabetes | 2.201 | 0.138 |
| Diagnosis | 9.352 | 0.025 |
| Vascular Invasion | 28.974 | <0.001 |
| Snoring | 8.808 | 0.032 |
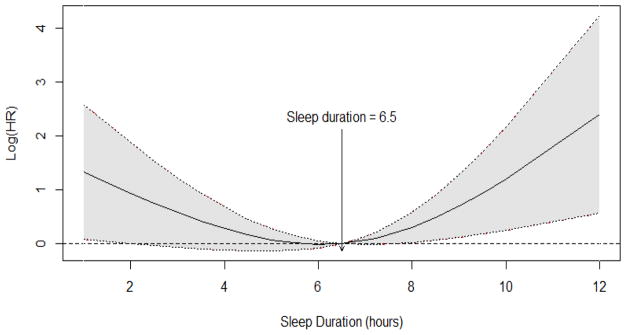
The variables that were significantly related to survival in univariate analyses were entered into the multivariable Cox regression model. After adjusting for demographic, psychological, and disease-specific factors, short sleep duration was associated with reduced survival HR linear = 0.485, 95% CI=0.275–0.857] and there was also evidence for a quadratic pattern [HR quadratic =1.064, 95% CI=1.015–1.115] suggesting a curvilinear relationship between sleep duration and survival. Figure 1.
Figure 1.
Smooth log hazard ratio across sleep duration.
Using Cox regression, we tested each of the six cytokines that were measured in the serum to determine if they were associated with survival. Interleukin-10 [HR=0.998, 95% CI=0.993–1.003, p=0.481], IL-1β [HR=1.00, 95% CI=1.00–1.00, p=0.999], and TNF-α [HR=0.999, 95% CI=0.995–1.002, p=0.485] were not found to be significantly related to survival. Serum levels of Interleukin-2 [HR=1.01, 95% CI=1.00–1.02, p=0.003], IL-1α [HR=1.002, 95% CI=1.001–1.004, p=0.008], and IFN γ [HR=1.003, 95% CI=1.001–1.004, p<0.001] were significantly related to survival. Due to the non-normal distribution of cytokines, Spearman rho correlations were used to test the link between sleep duration and cytokines. Serum levels of Interleukin-10 [rho=−0.115, p=0.087], TNFα [rho=−0.021, p=0.759], and IFN γ [rho=−0.083, p=0.216] were not significantly associated with sleep duration; however, circulating levels of IL-2 [rho=−0.148, p=0.027] was significantly related to longer sleep duration whereas IL-1 β [rho=−0.149, p=0.026], and IL-1-α [rho=−0.138, p=0.039] were significantly linked to shorter sleep duration.
Interleukin-2 Mediation of Sleep Duration and Survival
Interleukin-2 was the only cytokine significantly related to survival [HR=1.01, p=0.003] and sleep duration [Standardized β= −30.11, p=−0.027] and therefore was tested as a potential mediating factor linking sleep duration and survival using Baron & Kenny’s mediational analyses.33 Using Cox survival analyses we tested the mediation of IL-2 while adjusting for all factors significantly linked survival (i.e., age, diagnostic group, vascular invasion, depression, and snoring). When circulating IL-2 was entered into the model, sleep duration, including both the linear [β= −0.557, p=0.097] and quadratic terms [β=0.046, p=0.114], was no longer significantly related to survival, suggesting mediation (See Table 6).
Table 6.
Cox regression analysis of sleep duration and survival
| β | p-value | HR | 95% CI | |
|---|---|---|---|---|
| Sex: female versus male | −0.413 | 0.106 | 0.661 | (0.400–1.092) |
| Age at Diagnosis | −0.005 | 0.680 | 0.995 | (0.970–1.020) |
| Diagnosis | 0.206 | |||
| Stomach, pancreatic, gallbladder | 0.984 | 0.207 | 0.207 | (0.579–12.355) |
| Hepatocellular and cholangio carcinoma | 0.096 | 0.726 | 0.726 | (0.644–1.880) |
| Other primaries (e.g., colorectal, breast, ovarian) with liver metastases | −1.961 | 0.058 | 0.058 | (0.019–1.069) |
| Neuroendocrine with liver metastases | 0.517 | 0.623 | 0.623 | (0.213–13.179) |
| Vascular Invasion at Diagnosis: | 0.759 | 0.007 | 2.135 | (1.229–3.170) |
| Depression at Diagnosis | 0.036 | 0.009 | 1.036 | (1.009–1.063) |
| Snoring at Diagnosis | 0.014 | |||
| Not during the past month | ||||
| Less than 1x/week | 0.227 | 0.424 | 1.255 | (0.718–2.193) |
| 1–2x/week | −1.105 | 0.036 | 0.331 | (0.118-.931) |
| 3+/week | −0.885 | 0.029 | 0.413 | (0.187-.912) |
| Interleukin-2 at Diagnosis | 0.000 | 0.059 | 1.00 | (1.00–1.001) |
| Sleep Duration at Diagnosis | ||||
| Sleep Duration (linear) | −0.557 | 0.097 | 0.573 | (0.297–1.106) |
| Sleep Duration (quadratic) | 0.046 | 0.114 | 1.047 | (0.989–1.107) |
Discussion
While sleep problems have been studied in the context of cancer, few studies have assessed the frequency of sleep problems in cancer patients, their association with symptom burden, or the underlying biological mechanisms that are associated with sleep with survival.34,35 A high rate of sleep problems including poor sleep efficiency and quality, long sleep latency, frequent sleep disturbances, and shorter and longer than average (7–8 hours) sleep duration were observed in this cohort of advanced cancer patients. These findings were consistent with prior research in that a high rate of sleep problems were reported by patients in the palliative care setting.2,9,36–40
We also found that sleep duration, particularly short sleep duration, was associated with higher levels of symptom burden and lower levels of quality of life. Similar to studies in the general population, we observed a U-shaped relationship suggesting both short and long sleep duration were associated with poorer survival.41–43 We did not find sleep efficiency to be associated with poorer survival as have others found in cancer patients using actigraphy.9 Similar to studies by our team and others, vascular invasion and higher levels of depressive symptoms, were associated with poorer survival in both univariable and multivariable analyses.44–45
We observed that longer sleep duration was associated with IL-2, an anti-inflammatory cytokine, and shorter sleep duration was related to the pro-inflammatory cytokines. This was the first study to our knowledge to observe a mediational role of IL-2 with regard to sleep and survival in any population. Despite decades of animal research involving IL-2, few human studies have been conducted concerning sleep and IL-2.12 Irwin and colleagues found that sleep deprivation was associated with decreased natural killer (NK) cell number and activity and suppression of IL-2.46 However, after a night of recovery from sleep deprivation, NK cell activity returned to baseline but IL-2 remained suppressed possibly suggesting the importance of this cytokine with regard to sleep regulation.46 Born and colleagues showed that stimulated ex vivo production of IL-2 is enhanced during sleep compared to when an individual was awake.47 This effect is not dependent on the circadian rhythm but rather sleep duration.45 Conversely, sleep loss that occurred in association with partial night sleep deprivation induces decrements in production of IL-2 and natural killer cells.48–49
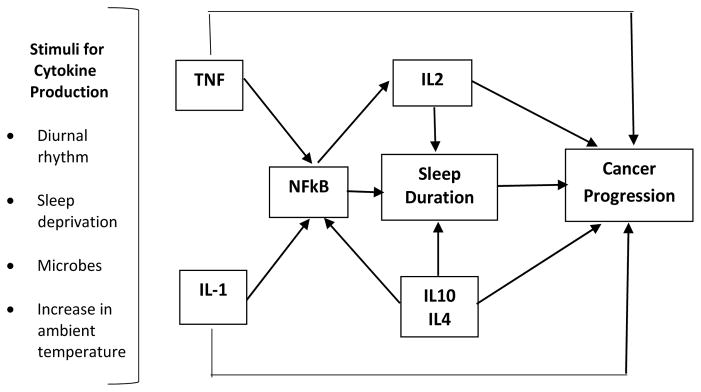
Our interpretation of the mechanisms linking short sleep duration and survival comes from two disparate literatures. First, Krueger and others have shown that sleep deprivation results in an upregulation of TNF and IL1, which in turn stimulates NFkB, which increases the production of IL-2.11,12 See Figure 2. Moreover, IL-2 has been demonstrated in animals and humans to have a homeostatic effect on sleep regulation.12 In the cancer literature, exogenous administration of IL-2 is associated with improved survival across multiple cancer types.16,50–53 Therefore, higher levels IL-2 appears to be involved in both the regulation of sleep and increased survival after a diagnosis from cancer.
Figure 2.
Role of cytokines in sleep regulation and cancer progression (adapted from Krueger and colleagues, 2001).
An alternative explanation may be that IL-2 mediates the association between sleep duration and survival by increasing the production of T lymphocytes and natural killer cells, which are also associated with increased survival in cancer. Interleukin-2 also improves the function of lymphokine-activated killer cells and tumor-infiltrating lymphocytes, which are all critical in slowing tumor growth and development of metastatic disease.17,18,52–55 However, some investigators have found that IL-2 decreases T lymphocytes so further research is warranted.56–58
Due to the curvilinear relationship between sleep and survival that was observed, one explanation with regard to why IL-2 may mediate long sleep duration which was associated with decreased survival may come from the studies focused on sleep fragmentation. Studies that have included both self-report and actigraphy to measure sleep have found a correlation between long self-reported sleep duration and sleep fragmentation as measured by actigraphy.59 Sleep fragmentation has been shown to be associated with higher levels of serum TNFα, tumor progression, upregulation of oncogenes, and tumor growth in animal studies.60 However, it should be noted that sleep fragmentation is defined and measured differently across studies.
In the study by Zavodny and colleagues, sleep fragmentation was induced for two consecutive nights. The interruption occurred every 2 minutes from the onset of undisturbed stage 2, stage 3/4, or REM sleep. The disturbances were caused by a tone series of 1000 Hz generated by an audiometer until an arousal response was observed. If no arousal was noted within 15 seconds, a second tone was sent at 60–90 dB lasting 10 seconds.59 Each tone series was terminated by the technicians upon signs of electroencephalographic arousal or after a total of 11 tones were presented.59 In the contrast in the study by Hakim, sleep fragmentation in the mice was induced by using a near-silent motorized mechanical sweeper.60 This method eliminates the need for human or foreign objects touching the animals during sleep. Two-minute interval between each sweep were implemented during the light period (7:00a.m. to 7:00 p.m.). Mice were housed in group cages to prevent the isolation stress.60
Smagula and colleagues reported that sleep problems in men were linked to greater inflammatory burden and the inflammatory markers also mediated the association between sleep duration and survival.61 While IL-1β and TNFα are often referred to as “pro-inflammatory” cytokines and we observed an association between short sleep duration and IL-1β; these cytokines are in fact pleiotropic cytokines that have receptor families, which help to regulate the balance of the inflammatory response. Redundancy is built into the inflammatory cytokine network. Inflammatory cytokines work together to promote and subdue the inflammatory, phagocytic, and coagulatory stages involved in protecting the body and maintaining immune system homeostasis.
Bjurström and colleagues observed in a cohort of patients diagnosed with rheumatoid arthritis that sleep maintenance and depth have a countervailing relationship with evening and morning levels of monocytic production of TNFα and IL-6, respectively.62 The findings support the hypothesis of a feedback loop between sleep maintenance, slow-wave sleep, and cellular inflammation that is cytokine specific.60 However, further research is warranted, particularly in humans, to understand the complex and dynamic associations between sleep, cytokines, and health.
Our study had several strengths including the large sample size; as well as the ability to covary factors associated with cytokines and survival, such as demographic, disease-specific, psychological and behavioral factors. However, limitations of this study included a sample that was predominantly male and Caucasian, the majority of whom had a diagnosis of hepatocellular or cholangiocarcinoma. Yet, we found no differences in the type or rates of sleep problems by sex or race. We did find a significant link between diagnostic group (cancer type) and survival but adjusted for this in the survival analyses. Inconsistent with prior research, we found that snoring was associated with better survival. One explanation may be that other studies did not exclude patients with sleep apnea as we did in the present study. Sleep apnea is associated with cardiovascular disease, diabetes, obesity, and poorer survival. We believe that the snoring in the patients included in this study may be secondary to higher body mass index. Higher body mass index, which is in contrast to cachexia, is associated with better survival in the context of many cancers.63
Despite the high correlation between self-report estimates of sleep with actigraphy and polysomnography, the lack of objective measures of sleep was a limitation.64–65 A combination of the use of structured clinical interviews and actigraphy is recommended for future research concerning the role of sleep, cytokines, and disease progression in the context of cancer. Sleep diaries may also be utilized to better quantify daily changes in sleep. Sleep diaries, particularly the Consensus Sleep Diary, are reliable methods to assess insomnia and have adequate agreement with polysomnography data.66–67 Sleep diaries have also been recommended to be a useful screening tool for assessment of primary insomnia.68 Furthermore, the one-time assessment of sleep duration is a significant limitation of these analyses and future research by our team will include the analysis of our longitudinal sleep data. Finally, it should be noted that we chose the covariates in the multivariable model based on the significant association with survival. Although this is a common practice, Babyak has recommended that covariates be based on theory rather than univariate statistically significant associations to avoid model overfitting.69
The clinical implications of this study are noteworthy. Sleep problems, particularly short sleep duration, are associated with greater symptom burden and poorer survival. The development of effective interventions to improve sleep quality is warranted to improve sleep and quality of life and to decrease psychological and physical morbidity in advanced cancer patients. Although low doses of IL-2 have been shown to improve sleep in healthy individuals, IL-2 administration was demonstrated to reduce lymphocyte subsets and NK cells, therefore it is unclear if IL-2 to improve sleep would be a good option for all cancer patients.70 Cognitive-behavioral interventions have been demonstrated to produce sustained improvements in sleep quality and duration over time without the potential adverse effects of medication.29 If our findings are replicated, screening and treatment of sleep disorders is recommended to be integrated in the oncology setting to identify, and potentially mitigate, a modifiable risk factor for mortality.
Acknowledgments
Funding: This study was supported by grants from the National Cancer Institute (K07CA118576; R21CA127046; R01CA176809) and utilized the UPCI Immunologic Monitoring and Cellular Products Laboratory, partially funded by P30CA047904-24.
Footnotes
Statement of Protection of Human Subjects: University of Pittsburgh Institutional Review Board
No authors disclose any conflicts of interest
Author Contributions:
Jennifer. L. Steel: Conceptualization, design, writing, analyses, interpretation
Lauren Terhorst: Analyses, editing
Kevin P. Collins: Analyses, editing
David A. Geller: Conceptualization, referral of patients, editing
Yoram Vodovotz: Processing and testing of biological samples, editing
Juliana Kim: Data entry and management, literature review
Andrew Krane: Data entry and management, literature review
Michael Antoni: Writing and editing
James W. Marsh: Referral of patients, editing
Lora Burke: Editing
Lisa H. Butterfield: Processing and testing biological samples, editing
Frank Penedo: Conceptualization and Editing
Daniel J. Buysse: Conceptualization and editing
Allan Tsung: Referral of patients and editing
Contributor Information
Jennifer. L. Steel, University of Pittsburgh, Department of Surgery, Psychiatry, and Psychology.
Lauren Terhorst, University of Pittsburgh, Department of Occupational Therapy.
Kevin P. Collins, University of Pittsburgh, Department of Surgery, Mathematica Policy Research.
David A. Geller, University of Pittsburgh, Department of Surgery.
Yoram Vodovotz, University of Pittsburgh, Department of Surgery
Juliana Kim, University of Pittsburgh, Department of Surgery.
Andrew Krane, University of Pittsburgh, Department of Surgery.
Michael Antoni, University of Miami, Department of Psychology.
James W. Marsh, University of Pittsburgh, Department of Surgery.
Lora E. Burke, University of Pittsburgh, School of Nursing.
Lisa H. Butterfield, University of Pittsburgh, Department of Medicine, Surgery and Immunology
Frank J. Penedo, Northwestern University, Department of Medical Social Sciences, Psychology, and Psychiatry and Behavioral Sciences.
Daniel J. Buysse, University of Pittsburgh, Department of Psychiatry.
Allan Tsung, University of Pittsburgh, Department of Surgery.
References
- 1.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues in Clinical Neuroscience. 2008;10(4):473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior, and Immunity. 2013 Mar;30(Suppl):S58–67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Medicine Clincs. 2002 Jul;3(4):305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002 Feb;59(2):131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979 Jan;36(1):103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 6.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011 Mar;12(3):215–21. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Perlis ML, Morrow GR. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 8.Mercadante S, Girelli D, Casuccio A. Sleep disorders in advanced cancer patients: Prevalence and factors associated. Supportive Care Cancer. 2004 May;12(5):355–9. doi: 10.1007/s00520-004-0623-4. [DOI] [PubMed] [Google Scholar]
- 9.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-Measured Sleep Disruption as a Predictor of Survival among Women with Advanced Breast Cancer. Sleep. 2014 May 1;37(5):837–42. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger JM, Huang YH, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. European Journal of Neuroscience. 2013 Jul;38(2):2199–209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger JM, Majde JA, Obál F. Sleep in host defense. Brain, behavior, and immunity. 2003 Feb;17(Suppl 1):S41–7. doi: 10.1016/s0889-1591(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 12.Krueger JM, Obál FJ, Fang J, Kubota T, Taishi P. The role of cytokines in sleep regulation. Ann NY Acad Sci. 2001 Mar;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 13.Berghella AM, Pellegrini P, Piancatelli D, Maccarone D, Del Beato T, Giubilei D, Pomidori A, Adorno D, Casciani CU. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunology, immunotherapy. 1994 Mar;38(3):160–6. doi: 10.1007/BF01525636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzio C, De Palma G, Passalacqua R, Potenzoni D, Ferrozzi F, Cattabiani MA, Manenti L, Borghetti A. Effectiveness of very low doses of immunotherapy in advanced renal cell cancer. Br J Cancer. 1997;76(4):541–4. doi: 10.1038/bjc.1997.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan B, Lee W, Hu CX, Ng P, Li KW, Lo G, Ho G, Yeung DW, Woo D. Adoptive cellular immunotherapy for non-small cell lung cancer: a pilot study. Cytotherapy. 2003;5(1):46–54. doi: 10.1080/14653240310000074. [DOI] [PubMed] [Google Scholar]
- 16.Clary BM, Coveney EC, Philip R, Blazer DG, 3rd, Morse M, Gilboa E, Lyerly HK. Inhibition of established pancreatic cancers following specific active immunotherapy with interleukin-2 gene-transduced tumor cells. Cancer gene therapy. 1997 Mar-Apr;4(2):97–104. [PubMed] [Google Scholar]
- 17.Dillman RO, Church C, Oldham RK, West WH, Schwartzberg L, Birch R. Inpatient continuous-infusion interleukin-2 in 788 patients with cancer. The National Biotherapy Study Group experience. Cancer. 1993 Apr 1;71(7):2358–70. doi: 10.1002/1097-0142(19930401)71:7<2358::aid-cncr2820710730>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Eklund JW, Kuzel TM. A review of recent findings involving interleukin-2-based cancer therapy. Current opinion in oncology. 2004 Nov;16(6):542–6. doi: 10.1097/01.cco.0000142070.45097.68. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Yamaguchi Y. Adjuvant immunotherapy with interleukin 2 and lymphokine-activated killer cells after noncurative resection of primary lung cancer. Lung Cancer. 1995 Aug;13(1):31–44. doi: 10.1016/0169-5002(95)00478-j. [DOI] [PubMed] [Google Scholar]
- 20.Yano T, Sugio K, Yamazaki K, Kase S, Yamaguchi M, Ondo K, Yoshino I, Sugimachi K. Postoperative adjuvant adoptive immunotherapy with lymph node-LAK cells and IL-2 for pathologic stage I non-small cell lung cancer. Lung Cancer. 1999 Dec;26(3):143–8. doi: 10.1016/s0169-5002(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 21.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry. 2016 Jul 1;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel JL, Geller DA, Kim KH, Butterfield LH, Spring M, Grady J, Sun W, Marsh W, Antoni M, Dew MA, Helgeson V, Schulz R, Tsung A. A web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016 Apr 15;122(8):1270–82. doi: 10.1002/cncr.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Radoff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 25.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) Journal of Psychosomatic Research. 1999 May;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992 Sep;31(Pt 3):301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 27.Van der Bij AK, De Weerd S, Cikot RJ, Steegers EA, Braspenning JC. Validation of the Dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genet. 2003;6(2):84–7. doi: 10.1159/000073003. [DOI] [PubMed] [Google Scholar]
- 28.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. Journal of Pain and Symptom Management. 1997 Feb;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994 Mar;23(2):129–38. [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983 Dec;24(4):385–96. [PubMed] [Google Scholar]
- 31.Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. Journal of Clinical Oncology. 2002 May 1;20(9):2229–39. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 32.Kenny DA. Cross-lagged panel correlation: A test for spuriousnes sychological. Bulletin. 1975;82:887–903. [Google Scholar]
- 33.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986 Dec;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 34.Hearson B, McClement S, Hearson B, McClement S. Sleep disturbance in family caregivers of patients with advanced cancer. International journal of palliative nursing. 2007 Oct;13(10):495–501. doi: 10.12968/ijpn.2007.13.10.27493. [DOI] [PubMed] [Google Scholar]
- 35.Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care. 2005 Mar;3(1):23–31. doi: 10.1017/s1478951505050042. [DOI] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dimsdale J, Cohen-Zion M, Fiorentino L. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Supportive Care in Cancer. 2006 Mar;14(3):201–9. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton-Burke M. Cancer-related fatigue and sleep disturbances. Cancer Nursing. 2006 Mar-Apr;29(2 Suppl):72–7. doi: 10.1097/00002820-200603002-00025. [DOI] [PubMed] [Google Scholar]
- 38.Carter PA, Chang BL. Sleep and Depression in Cancer Caregivers. International Journal of Palliative Nursing. 2000 Dec;23(6):410–5. doi: 10.1097/00002820-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008 Dec;62(3):391–400. doi: 10.1016/j.lungcan.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. Journal of Clinical Oncology. 2001 Feb 1;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 41.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European Heart Journal. 2011 Jun;32(12):1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 42.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of Sleep Research. 2009 Jun;18(2):148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 43.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010 May;33(5):585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol. 2007 Jun 10;25(17):2397–405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 45.Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer. 2017 Nov;6(4):360–374. doi: 10.1159/000481315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996 Apr;10(5):643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 47.Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. Journal of Immunology. 1997 May 1;158(9):4454–64. [PubMed] [Google Scholar]
- 48.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999 Jun;84(6):1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 49.Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995 Mar-Apr;57(2):97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Wahab M, El-Shennawy F, Agha S, Ragab E, Fathi O, Sultan A, Elghawalby N, Ezzat F. Evaluation of cell mediated immunity in advanced pancreatic carcinoma before and after treatment with interleukin-2 (IL-2) Hepatogastroenterology. 1999 May;46(Suppl 1):1293–6. [PubMed] [Google Scholar]
- 51.Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M, Dorbic T, Neubauer A, Wittig B, Huhn D. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999 Nov;81(6):1009–16. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–84. doi: 10.1097/00000658-198910000-00008. discussion 484–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. The cancer journal from Scientific American. 2000 Feb;6(Suppl 1):S55–7. [PubMed] [Google Scholar]
- 54.Scudeletti M, Filaci G, Imro MA, Motta G, Di Gaetano M, Pierri I, Tongiani S, Indiveri F, Puppo F. Immunotherapy with intralesional and systemic interleukin-2 of patients with non-small-cell lung cancer. Cancer Immunol Immunother. 1993 Jul;37(2):119–24. doi: 10.1007/BF01517044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratto GB, Costa R, Maineri P, Alloisio A, Piras MT, D’Agostino A, Tripodi G, Rivabella L, Dozin B, Bruzzi P, Melioli G. Neo-adjuvant chemo/immunotherapy in the treatment of stage III (N2) non-small cell lung cancer: a phase I/II pilot study. International journal of immunopathology and pharmacology. 2011 Oct-Dec;24(4):1005–16. doi: 10.1177/039463201102400418. [DOI] [PubMed] [Google Scholar]
- 56.Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol. 2015 Dec;36(12):763–77. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, Huse M, Sun JC, Rudensky AY. IL-2–dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. JEM. 2013 Jun 3;210(6):1167–78. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin Z, Chen J, Zeng J, Niu L, Xie S, Wang X, Liang Y, Wu Z, Zhang M. Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: A preliminary clinical study. Cancer biology & therapy. 2017 May 4;18(5):323–330. doi: 10.1080/15384047.2017.1310346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zavodny J, Roth C, Bassetti CL, Mathis J, Douglas NJ, Gugger M. Effects of sleep fragmentation on the arousability to resistive loading in NREM and REM sleep in normal men. Sleep. 2006 Apr;29(4):525–32. doi: 10.1093/sleep/29.4.525. [DOI] [PubMed] [Google Scholar]
- 60.Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, Khalyfa A, Shirwan H, Almendros I, Gozal D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Research. 2014 Mar 1;74(5):1329–37. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smagula SF, Stone KL, Redline S, Ancoli-Israel S, Barrett-Connor E, Lane NE, Orwoll ES, Cauley JA; Osteoporotic Fractures in Men (MrOS) Research Group. Actigraphy- and Polysomnography-Measured Sleep Disturbances, Inflammation, and Mortality Among Older Men. Psychosomatic Med. 2016 Jul-Aug;78(6):686–96. doi: 10.1097/PSY.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjurström MF, Olmstead R, Irwin MR. Reciprocal Relationship Between Sleep Macrostructure and Evening and Morning Cellular Inflammation in Rheumatoid Arthritis. Psychosom Med. 2017 Jan;79(1):24–33. doi: 10.1097/PSY.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003;21(3):198–213. doi: 10.1159/000073337. [DOI] [PubMed] [Google Scholar]
- 64.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: Comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005 Nov;76(11):1058–63. [PubMed] [Google Scholar]
- 65.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004 May 1;27(3):440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 66.Rogers AE, Caruso CC, Aldrich MS. Reliability of sleep diaries for assessment of sleep/wake patterns. Nurs Res. 1993 Nov-Dec;42(6):368–72. [PubMed] [Google Scholar]
- 67.Maich KHG, Lachowski AM, Carney CE. Psychometric Properties of the Consensus Sleep Diary in Those With Insomnia Disorder. Behavioral Sleep Medicine. 2018 Mar-Apr;16(2):117–134. doi: 10.1080/15402002.2016.1173556. [DOI] [PubMed] [Google Scholar]
- 68.Natale V, Léger D, Bayon V, Erbacci A, Tonetti L, Fabbri M, Martoni M. The consensus sleep diary: quantitative criteria for primary insomnia diagnosis. Psychosom Med. 2015 May;77(4):413–8. doi: 10.1097/PSY.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 69.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004 May-Jun;66(3):411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 70.Lange T, Marshall L, Späth-Schwalbe E, Fehm HL, Born J. Systemic immune parameters and sleep after ultra-low dose administration of IL-2 in healthy men. Brain, behavior, and immunity. 2002 Dec;16(6):663–74. doi: 10.1016/s0889-1591(02)00018-1. [DOI] [PubMed] [Google Scholar]