Abstract
Objective
The objective of this study was to determine how baseline blood pressure and incident hypertension related to antiretroviral therapy (ART) initiation, HIV-related inflammation, and mortality in HIV-infected adults in a low-income country.
Methods
We conducted long-term follow-up of HIV-infected adults who had participated in a trial of early versus delayed initiation of ART in Port-au-Prince, Haiti. Between 2005-2008, 816 HIV-infected adults were randomized to early (N=408) versus delayed ART (when CD4<200 cells/mm3 or AIDS-defining condition; N=408). Blood pressure was measured every 3 months. Hypertension was diagnosed according to the Joint National Committee (JNC-7) guidelines. Biomarkers of inflammation and coagulation were measured from banked enrollment plasma samples. Survival analyses were performed using Stata 14.
Results
The median age at enrollment was 39 years. The median follow-up time was 7.3 years. The hypertension incidence rate was 3.41 per 100 person years, and was similar in early and delayed ART groups. In multivariable models, independent predictors of incident hypertension were older age, higher BMI, and plasma IL-6 levels (adjusted hazard ratio, aHR=1.23, p<0.001). Systolic pressure >140mmHg at enrollment was associated with increased mortality (aHR=2.47, p=0.03) as was systolic pressure <90mmHg (aHR=2.25, p=0.04). Prevalent and incident hypertension were also significantly associated with mortality.
Conclusions
In a large prospective study of HIV-infected adults we found a high incidence of hypertension associated with HIV-related inflammation. Baseline hypertension conferred a >2-fold increased risk of death. Among HIV-infected adults in low-income countries, hypertension should be considered a serious threat to long-term survival.
Keywords: HIV, mortality, hypertension, interleukin-6, inflammation, Haiti
Condensed Abstract
The objective of this study was to determine how baseline blood pressure and incident hypertension related to inflammation and mortality in HIV-infected adults in a low-income country. We conducted long-term follow-up of 816 HIV-infected adults who had participated in a trial of early versus delayed initiation of ART in Haiti. The hypertension incidence rate was 3.41 per 100 person-years. Independent predictors of incident hypertension were older age, higher BMI, and plasma IL-6 levels. Prevalent and incident hypertension were significantly associated with mortality. In conclusion, hypertension should be considered a serious threat to long-term survival of HIV-infected adults in low-income countries.
INTRODUCTION
Hypertension is the leading risk factor for early mortality worldwide and accounts for nearly 10% of all disability adjusted life years [1]. A disproportionate burden of hypertension and hypertension-related diseases occur in low and middle-income countries (LMIC) [2]. In addition to traditional risk factors such as age and obesity, inflammation is increasingly accepted as an important factor in the pathophysiology of hypertension [3,4]. Infections such as HIV that induce chronic inflammation may increase hypertension risk and may provide insight into the relationship between chronic inflammation and hypertension [5].
Little is known about the predictors and outcomes of hypertension in HIV-infected adults. Recent retrospective and cross-sectional studies have confirmed that the prevalence of hypertension is higher in HIV-infected adults when compared to their uninfected counterparts [6–8]. Cohort studies of HIV-infected adults from several HIC and LMICs have reported a high incidence of hypertension [9–12]. To our knowledge, no prospective data has yet been published describing the relationship between hypertension, ART initiation, HIV-related inflammation and mortality in HIV-infected adults.
We therefore analyzed blood pressure and mortality data from a prospective cohort of HIV-infected Haitian adults. Our primary objective was to determine if high blood pressure is an independent predictor of mortality in HIV-infected adults. Secondary objectives were to determine the incidence rate of hypertension in adults started on either early or delayed antiretroviral therapy (ART) and to determine if inflammatory biomarkers can predict incident hypertension in HIV-infected adults.
METHODS
Study Design
Our study followed subjects enrolled in the CIPRA HT-001 trial [13]. CIPRA HT-001 was a randomized controlled study conducted at the GHESKIO Center in Port-au-Prince, Haiti. Eligibility was limited to HIV-infected, ART-naïve, non-pregnant adults with CD4 T cells between 200-350 cells per mm3 and no history of AIDS-defining conditions. Between August 2005 and July 2008, 816 participants were enrolled and underwent 1:1 randomization to either early or delayed ART initiation. Participants in the early group started treatment within 2 weeks of randomization. Participants in the delayed group started treatment when their CD4 T cells fell below 200 per mm3 or with the development of an AIDS-defining condition. All participants provided informed consent. The study was approved by the Institutional Review Boards at both GHESKIO and Weill Cornell Medicine.
The CIPRA HT-001 trial officially ended in June 2009 when a scheduled interim analysis showed the hazard ratio for mortality to be 4.0 (p-value <0.01) in the delayed group. This prompted the Data Safety and Monitoring Board to recommend that all participants who had not met study criteria for initiating ART start treatment immediately with continued follow-up [13]. After the end of the CIPRA HT-001 trial, we continued to follow all study participants through June 2016 to determine the incidence of hypertension.
Study methods
A study clinician saw participants in both groups at enrollment, monthly for the first three months after enrollment, and subsequently every three months. At each study visit a research nurse documented vital signs, including blood pressure. Blood pressure measurement was performed after at least 5 minutes of sitting using a mercury sphygmomanometer. If the first blood pressure measurement was elevated, a second blood pressure measurement was taken after at least 15 minutes and the average of these two measurements was recorded.
Prevalent and incident hypertension were defined using the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Report (JNC-7) [14]. Prevalent hypertension was defined as receiving medication for hypertension at the time of enrollment and/or systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg during both of the first 2 study visits. Incident hypertension was defined as having two blood pressure readings ≥140/90mmHg or starting medication for hypertension (among study subjects who did not have prevalent hypertension). In addition to classifying prevalent hypertension and incident hypertension, we also categorized the enrollment SBP as high enrollment SBP (enrollment SBP>140 mmHg), normal enrollment SBP (enrollment SBP 90-140 mmHg) or low enrollment SBP (enrollment SBP<90 mmHg). Treatment for hypertension was provided according to Haitian national guidelines, which are in line with the JNC-7 guidelines; thiazide diuretics were given as first line therapy for most patients with hypertension.
For all participants starting ART, a first-line regimen of lamivudine (150mg every 12 hours) and zidovudine (300mg every 12 hours) in a fixed dose combination, and efavirenz (600mg daily, or 800mg daily if participants were also taking rifampin for treatment of comorbid tuberculosis) was given. Drug substitutions were made according to the WHO guidelines [15].
For study subjects who missed a study visit, a field worker employed by GHESKIO visited the home of the patient. A study subject was only declared “lost to follow-up” if they could not be found after 3 home-visit attempts over a 6-month period. Death was documented by one of the following: obituary, autopsy report, hospital death certificate, or a field worker report documenting verbal communication with subject’s healthcare provider or family member.
Laboratory measurements
CD4 T cell counts, HIV-1 RNA levels, and serum creatinine were measured on-site at GHESKIO. CD4 T cell counts (Becton Dickinson and Company, Franklin Lakes, New Jersey) were performed at enrollment. HIV-1 RNA levels were measured from banked plasma specimens collected at enrollment using the NucliSens EasyQ HIV-1 PCR Test, v1.2 (BioMérieux, Lyon, France). The lower limit of detection for HIV-1 RNA was ≤50 copies/mm3. Serum creatinine was measured before ART initiation and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [16].
The plasma concentrations of CRP, IL-6 and D-dimer were measured from banked plasma specimens collected at enrollment and stored at -80°C. Biomarker analysis was performed in the Core Laboratory of the Clinical and Translational Science Center of Weill Cornell Medicine. All assays were performed in duplicate. CRP and IL-6 were measured using the V-PLEX human CRP and V-PLEX human IL-6 kit respectively from Meso Scale Discovery (Gaithersburg, MD, USA) following the manufacturer’s instructions. The intra-assay variation, inter-assay variation, and measureable ranges for CRP and IL-6 were 4.1% and 4.5%, 10.5% and 7.3%, 14 – 216,500 pg/ml and 0.18 - 749 pg/ml, respectively.
D-dimer was measured using IMUCLONE™ D-dimer ELISA kit (Sekisui Diagnostics LLC, Stamford, CT) following the manufacturer’s protocol. The intra-assay variation, inter-assay variation, and measurable range were 3.6%, 11.7% and 4.88 – 195 ng/ml, respectively.
Statistical analysis
Clinical and laboratory information were entered electronically in Haiti and managed by the Frontier Science and Technology Research Foundation (Amherst, New York). Data was exported into Microsoft Excel for cleaning, and analysis was performed using Stata 14 software (StataCorp LP, San Antonio, TX, USA). All analyses were based on intention to treat. Categorical variables were described as proportions (percentages). Continuous variables were described as medians (interquartile ranges, IQR).
Prevalence of hypertension was measured as the proportion of participants in both study arms with hypertension at the time of study enrollment among all participants. The incidence proportion, or cumulative incidence, reflects the number of new diagnoses of hypertension among the population at risk during the study period. Differences in incidence proportion between the early and delayed ART groups were evaluated using two-sample proportions test. The incidence rate was calculated as the number of new diagnoses of hypertension per 100 person-years of follow-up. The two-sample incidence rate comparison is based on the log-rank test. The probability of hypertension-free survival was calculated using standard Kaplan-Meier survival methods and the log-rank test. Participants who were lost during the trial were censored at the time of their last clinic visit, and participants that died were censored on their date of death.
Plots of CRP, IL-6 and D-dimer revealed skewed data and were log transformed to approximate a normal distribution. Cox proportional hazard models using time-independent variables (gender and group assignment) and enrollment values of time-dependent variables (age, HIV Clinical Stage, Body Mass Index (BMI), systolic and diastolic blood pressure creatinine, eGFR, CRP, IL-6, D-dimer, CD4 T cell count, and HIV-1 RNA level) and were used to calculate the hazard ratios for the primary endpoint, incident hypertension. Cox proportional hazard models were used to identify factors associated with incident hypertension. Variables in the Cox models were chosen by backward selection procedure with exit criteria of p-value >0.05. The same final model was selected by forward selection procedure with entry criteria of p-value <0.05. All hazard ratios are reported with a 95% confidence interval (CI). Only p-values <0.05 were considered statistically significant.
RESULTS
Study population
For participation in the original CIPRA HT-001 trial, 1066 individuals were screened and 816 participants were enrolled. In the early group, all 408 participants started ART immediately after enrollment; in the delayed group, 355/408 (87.0%) of participants started ART after a median delay of 1.3 years [13].
Table 1 shows that the enrollment characteristics of participants in the early (N=408) and delayed groups (N=408) were similar. At enrollment, in the whole cohort (N=816): the median age was 39 years (IQR: 32-46 years), 58% (470/816) were female, median BMI was 21.2 kg/m2 (IQR: 19.4 – 23.6 kg/m2), median CD4 T cells were 281 cells/mm3 (IQR: 250-311 cells/mm3), and 32% (260/816) of participants had WHO HIV clinical stage 1 [13,17]. There were no statistically significant differences in the log-normalized values of HIV-1 RNA, CRP, IL-6, or D-dimer at enrollment between the early and delayed groups.
Table 1.
Enrollment characteristics
| All participants (N=816) |
Early group (N=408) |
Delayed group (N=408) |
|
|---|---|---|---|
| Median age (IQR) | 39 (33 - 46) | 39 (33 – 46) | 39 (32 – 46) |
| Female (%) | 470 (58) | 241 (59) | 229 (56) |
| HIV clinical stage (%)* | |||
| Stage 1 | 260 (32) | 137 (34) | 123 (30) |
| Stage 2 | 414 (51) | 193 (47) | 221 (54) |
| Stage 3 | 142 (17) | 78 (19) | 64 (16) |
| Median body mass index (IQR) | 21.2 (19.4 – 23. 6) | 21.3 (19.6 – 23.7) | 21.0 (19.2 – 23.4) |
| Median systolic pressure (IQR) | 110 (100 – 120) | 110 (100 – 120) | 110 (100 – 120) |
| Median diastolic pressure (IQR) | 75 (65 – 80) | 71 (65 – 80) | 75 (65 – 80) |
| eGFR <60 ml/min/1.73m2b | 42 | 15 | 27 |
| Median CD4 T cells per mm3 (IQR) | 281 (250 – 311) | 281 (250 – 307) | 282 (250 – 313) |
| Median HIV-1 RNA level in copies/mm3 (IQR) | 97,000 (30,000–285,000) |
110,000 (34,000–280,000) |
92,000 (26,000–290,000) |
| Median log2CRP in ng/mm3 (IQR) | 10.6 (9.2 – 11.9) | 10.6 (9.3 – 11.9) | 10.6 (9.1 – 12.0) |
| Median log2IL-6 in ng/mm3 (IQR) | −0.7 (−1.5 – 0.1) | −0.8 (−1.6 – 0.1) | −0.6 (−1.4 – 0.1) |
| Median log2D-dimer in μg/mm3 (IQR) | 8.9 (8.2 – 9.6) | 8.9 (8.3 – 9.6) | 8.8 (8.2 – 9.6) |
Participants were followed for a median of 7.3 years (interquartile range (IQR): 5.0-8.3). There were 82 deaths (N=31, early group; N=51, delayed group) and 244 lost (N=123, early group; N=121, late group) over the 11-year study period from the beginning of enrollment in 2005 through the end of follow-up in 2016.
Hypertension outcomes
Table 2 describes the prevalence and incidence of hypertension in whole cohort (all participants) as well as in the 2 study groups (early group vs. delayed group). Only 5.3% (43/816) of participants had prevalent hypertension at the time of study enrollment (N=20, early group vs. N=23, delayed group). The prevalence rate of hypertension in the whole cohort was 5.3% (CI: 3.7 – 6.8%), and was similar in the early and delayed groups (4.9% (CI: 2.8% - 7.0%) vs. 5.6% (CI: 3.4% - 7.9%), p-value 0.64). The median enrollment systolic and diastolic blood pressures at enrollment were 110 (IQR: 100-120) and 75 (IQR: 65-80) mmHg, respectively.
Table 2.
Hypertension prevalence and incidence
| All participants (N=816) |
Early group (N=408) |
Delayed group (N=408) |
p-value | |
|---|---|---|---|---|
| Prevalent Hypertension (CI) | 5.3% (3.7% – 6.8%) |
4.9% (2.8% – 7.0%) |
5.6% (3.4% – 7.9%) |
0.64a |
| Incidence Proportion (CI) | 19.0% (16.3% – 21.8%) |
20.4% (16.4% – 24.4%) |
17.7% (13.9% – 21.5%) |
0.34a |
| Incidence rate per 100 person-years (CI) | 3.41 (2.86 – 3.96) |
3.56 (2.78 – 4.35) |
3.25 (2.48 – 4.03) |
0.58b |
| Median years to incidence (IQR) | 3.24 years (0.59 – 4.86) |
2.76 years (0.48 – 4.79) |
3.57 years (0.69 – 5.10) |
0.42c |
IQR, interquartile range.
p-value by two-sample proportions test.
p-value by log-rank test.
p-value by Wilcoxon Rank Sum test
There were 147 cases of incident hypertension during the study period (N=79, early group vs. N=68, delayed group). The incidence rate of hypertension in the whole cohort was 3.41 (CI: 2.86 – 3.96) per 100 person-years and the incidence proportion was 19.0% (CI: 16.3% – 21.8%). The median time from enrollment to a new diagnosis of hypertension was 3.24 years (IQR: 0.59 – 4.86 years). The incidence rate was similar in the early and delayed groups (3.56 (CI: 2.78 – 4.35) vs. 3.25 (2.48 – 4.03) per 100 person years, p-value 0.58). The incidence proportion and median time to a new diagnosis of hypertension were also similar in the early and delayed groups (see Table 2).
Figure 1A displays the probability of hypertension-free survival for the cohort, which was 70% at 8 years. Figure 1B illustrates that the trends in hypertension-free survival were similar in the early and delayed groups (p-value 0.58 by log-rank test).
Figure 1. Kaplan-Meier estimates for hypertension-free survival.
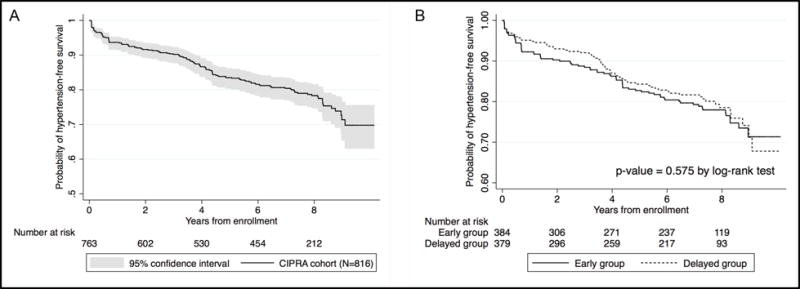
Panel A presents hypertension-free survival in the whole CIPRA cohort (N=816). Panel B presents hypertension-free survival in the early group (N=408) and delayed groups (N=408).
Predictors of incident hypertension
Table 3 shows both the unadjusted and adjusted predictors of incident hypertension at enrollment. In multivariate analyses, the 4 independent predictors of incident hypertension were older age (adjusted HR (aHR) = 1.32 per decade, p-value 0.002), higher BMI (aHR = 1.07 per 1 unit increase in BMI, p<0.001), higher SBP (aHR = 2.02 per 10 mmHg increase in SBP, p-value <0.001), and higher IL-6 level (aHR 1.24 per log2 increase, p-value <0.001). The same final multivariate model was reached with both forward and backward selection procedures.
Table 3.
Enrollment predictors of incident hypertension among all participants
| Unadjusted hazard ratio (CI, p-value) |
Adjusted hazard ratiof (CI, p-value) |
|
|---|---|---|
| Delayed group | 0.91 (0.66 – 1.26, 0.575) | – |
| Agea | 1.72 (1.45 – 2.03, <0.001) | 1.32 (1.10 – 1.59, 0.002) |
| Female | 0.94 (0.68 – 1.30, 0.707) | – |
| HIV clinical stage | ||
| Stage 1 | 1 (reference) | – |
| Stage 2 | 1.18 (0.85 – 1.64, 0.314) | – |
| Stage 3 | 0.75 (0.46 – 1.22, 0.243) | – |
| Body Mass Index (BMI)b | 1.12 (1.07 – 1.16, <0.001) | 1.07 (1.03 – 1.12, < 0.001) |
| Systolic pressure (SBP)c | 2.09 (1.88 – 2.33, <0.001) | 2.02 (1.79 – 2.27, < 0.001) |
| Diastolic pressure (SBP)c | 2.74 (2.28 – 3.30, <0.001) | – |
| eGFR <60ml/min/1.73m2 | 1.68 (0.88 – 3.20, 0.114) | – |
| CD4 T cell countd | 1.04 (0.84 – 1.30, 0.721) | – |
| Log2HIV-1 RNA levele | 1.02 (0.96 – 1.09, 0.556) | – |
| Log2C-reactive proteine | 1.05 (0.97– 1.12, 0.225) | – |
| Log2Interleukin-6e | 1.09 (0.98 – 1.21, 0.123) | 1.24 (1.12 – 1.38, < 0.001) |
| Log2D-dimere | 1.15 (0.99 – 1.34, 0.069) | – |
Hazard ratio, HR reported per 10-year increment.
HR reported per 1 kg/m2 increase in BMI.
HR reported per 10 mm of mercury increment.
HR reported per 50 cell/mm3 increment.
HR reported per log2 increase.
Adjusted for all the variables in the adjusted model (age, BMI, SBP and Log2Interleukin-6).
Blood pressure and mortality
Blood pressure was an independent predictor of mortality by multiple measures including: prevalent hypertension, prevalent hypertension or early incident hypertension (within the first year of study enrollment), and baseline SBP. These associations remained statistically significant after adjusting for age and sex. Additional controlling for other factors such as randomization group, HIV clinical stage and CD4 T-cell count lead to similar results. These data are displayed in Table 4.
Table 4.
Hypertension and enrollment blood pressure as a predictor of mortality
| Unadjusted hazard ratio (CI, p-value) |
Adjusted hazard ratioa (CI, p-value) |
|
|---|---|---|
| Prevalent hypertension | 2.41 (1.20–4.81, 0.01) | 2.12 (1.01–4.44, 0.046) |
| Prevalent hypertension or incident hypertension within 1 year of enrollment | 2.12 (1.19–3.77, 0.01) | 1.93 (1.04–3.59, 0.04) |
| Enrollment SBP | ||
| Normal SBP (90 to 140 mmHg) | 1 (reference) | 1 (reference) |
| High SBP (>140 mmHg) | 3.03 (1.39–6.61, 0.005) | 2.47 (1.10–5.57, 0.03) |
| Low SBP (<90 mmHg) | 2.17 (1.00–4.73, 0.050) | 2.25 (1.03–4.95, 0.04) |
Adjusted for age and sex.
Of all of the measures of blood pressure, the participant’s SBP at enrollment was the strongest predictor of mortality. Kaplan Meier curves for the relationship between enrollment SBP and mortality are displayed in Figure 2. We also performed multivariate Cox regression analysis to confirm these results.
Figure 2. Kaplan Meier estimates for survival by systolic blood pressure (SBP) measurement at enrollment.
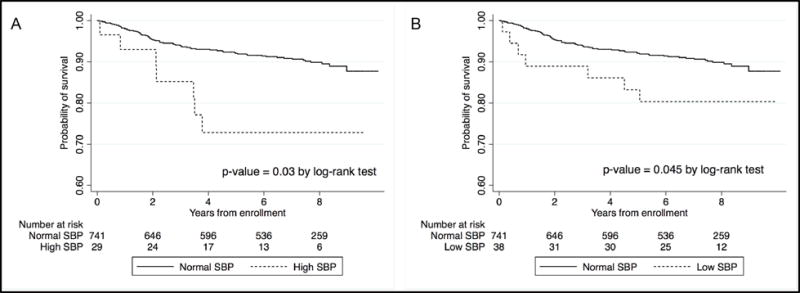
Panel A presents survival in participants with normal SBP (90 – 140 mmHg) vs. high SBP (>140 mmHg) at enrollment. Panel B presents survival in participants with normal SBP (90 – 140 mmHg) vs. low SBP (<90 mmHg) at enrollment.
Participants with high SBP (>140 mmHg) at enrollment had higher mortality when compared to individuals with normal SBP (90–140 mmHg) (HR 3.03; CI: 1.39 – 6.61, p-value 0.005). This association remained statistically significant after adjusting for age and sex (aHR=2.47; CI: 1.10-5.57, p-value 0.03); and was still significant after controlling for other factors such as randomization group, HIV clinical stage and CD4 T cell count.
Participants with low SBP (<90 mmHg) at enrollment also had increased mortality risk when compared to individuals with normal SBP (90–140 mmHg). This relationship remained statistically significant after adjusting for age and gender in a Cox regression model (aHR 2.25; CI: 1.03-4.95, p-value 0.042).
DISCUSSION
In our study, HIV-infected adults with hypertension at enrollment had a >2-fold higher risk of mortality. Independent predictors of incident hypertension included traditional risk factors (age and adiposity) and HIV-related inflammation (plasma IL-6).
Our study provides the first prospective evidence that high blood pressure is an important determinant of long-term survival in HIV-infected adults. In our study both prevalent hypertension and early incident hypertension were associated with increased mortality during the 10-year study period. These results confirm the hypothesis generated from a retrospective analysis of electronic medical records for HIV-infected adults in Kenya between 2005-2010 [18]. In that study, a high SBP measurement recorded in an electronic medical record was associated with increased mortality in HIV-infected men with WHO clinical stage 1 or 2 disease. Based on these findings, HIV clinics in LMIC should ensure that blood pressure is measured regularly and accurately in order to accomplish early diagnosis and early treatment for hypertension [19].
Both high and low SBP at enrollment was also associated with higher mortality in our study population. HIV-infected adults with a SBP of <90 mmHg at study enrollment had a 2-fold higher mortality than participants with normal SBP. Low SBP has previously been reported as a strong predictor of mortality in HIV-infected adults in Kenya, particularly those with advanced HIV disease (WHO clinical stage 3 or 4) [18]. The relationship between low blood pressure and mortality is possibly due to adrenal insufficiency, autonomic dysfunction, concomitant infections, or bacterial translocation [18]. Both high and low SBP, therefore, may be helpful in identifying HIV-infected patients who would benefit from differentiated models of care with tailored services to reduce their risk of mortality [20]. The WHO has recommended that, “differentiated care for HIV requires delivery of different care packages for people based on their needs” but current models of differentiated care have started with widely-available markers of HIV disease severity such as CD4 T cell count or WHO clinical stage. SBP may be an additional, simple, widely-available biomarker indicating the need for new differentiated care pathways.
Our study is the first to report that HIV-related inflammation (elevated plasma IL-6) precedes and predicts incident hypertension in HIV-infected adults. IL-6 levels are commonly elevated in HIV-infected adults due to dysregulation of T cells [5]. In fact, because the enrollment IL-6 levels were high in our cohort, our finding may underestimate the predictive role of elevated IL-6 for incident hypertension in HIV-infected adults. In HIV-uninfected adults, two studies have reported an association between IL-6 levels and incident hypertension [21,22]. In addition, lowering blood pressure in HIV-uninfected adults has been shown to reduce IL-6 levels [23]. One smaller case-control study of HIV-infected adults in Uganda demonstrated a trend toward higher IL-6 predicting incident hypertension, but the results were not statistically significant [24]. The relationship between IL-6 and hypertension in HIV-infected adults may be important for two reasons. First, the IL-6 pathway may provide targets for interventions to prevent hypertension [4]. Second, hypertension could be a modifiable mediator in the relationship between higher IL-6 levels and higher rates of cardiovascular events [25]. Additional prospective cohort studies are needed to determine the relationship between IL-6, hypertension and cardiovascular disease and to compare between HIV-infected and HIV-uninfected populations.
In our cohort of HIV-infected Haitian adults, the incidence of hypertension was 3.41 per 100 person-years and over 20% of participants were diagnosed with hypertension by 8 years. This hypertension incidence rate is nearly twice the incidence rate that has been reported in HIV-uninfected adults of similar age in the United States [26–28]. This finding is particularly striking because, at enrollment, our study participants had relatively low blood pressure and BMI. As expected from the observation of premature aging in HIV-infected adults, the hypertension incidence rates in our cohort are similar to the incidence rates reported for adults 10 to 20 years older than the individuals in our cohort [26–29]. Two other longitudinal studies from Africa have also reported similarly high incidence rates of hypertension in cohorts of HIV-infected adults [9,10]. These findings are particularly concerning because HIV-infected adults with hypertension are at even higher risk of hypertension-related complications than HIV-uninfected adults with similar blood pressures [30]. Therefore, HIV clinicians in LMIC must be better equipped to diagnose and treat hypertension [31].
One limitation of our study is that 244 study participants were lost to follow-up during the course of our 10-year study (2005 – 2016). We do not think that this had a major impact on our findings, though, for several reasons. First, the average lost to follow-up rate was only ~3% per year. Second, the average follow-up time contributed by participants that were lost was still 4.6 years (IQR: 1.8–6.2 years). If participants developed incident hypertension prior to being lost, the time from enrollment to incident hypertension was considered their person time at risk. If participants did not develop hypertension prior to being lost, the time from enrollment to last known date alive was considered as their person time at risk. Third, baseline characteristics among study participants who were lost and participants who completed the study were similar. Another limitation of the current study is that some additional risk factors for mortality, such as dyslipidemia, type 2 diabetes mellitus, serum uric acid and lipodystophy, were not considered. Future studies should include these risk factors.
In conclusion, hypertension incidence is high in HIV-infected adults in Haiti. Both high and low SBP at enrollment are associated with higher mortality. SBP may be useful biomarkers in identifying patients who need differentiated care to reduce mortality. Higher enrollment IL-6 is associated with increased risk of incident hypertension. Further research is needed to determine the mechanism for how IL-6 leads to hypertension in HIV-infected adults and to explore prevention strategies that target the IL-6 pathway.
Acknowledgments
All authors were involved in developing the study concept and design, data acquisition, data management and interpretation of results. Data analysis was performed by ML, ASB and RP. This submission was drafted by ASB, RP and ML; all other authors were involved in editing and review. All authors have approved of the final version of this submission.
Sources of Funding
This work was supported by the NIH: T32AI07613, K24AI098627, K01TW010281, and UL1-TR000457.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. A global brief on hypertension. Geneva, Switzerland: 2013. [Google Scholar]
- 3.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130-38–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–40. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–47. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 6.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–97. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 7.Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 8.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okello S, Kanyesigye M, Muyindike WR, Annex BH, Hunt PW, Haneuse S, et al. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens. 2015;33:2039–2045. doi: 10.1097/HJH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania – A prospective cohort study. PLoS One. 2017;12:e0172089. doi: 10.1371/journal.pone.0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manner IW, Baekken M, Oektedalen O, Os I. Hypertension and antihypertensive treatment in HIV-infected individuals. A longitudinal cohort study. Blood Press. 2012;21:311–319. doi: 10.3109/08037051.2012.680742. [DOI] [PubMed] [Google Scholar]
- 12.Thiébaut R, El-Sadr WM, Friis-Møller N, Rickenbach M, Reiss P, Monforte AD, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10:811–23. doi: 10.1177/135965350501000706. [DOI] [PubMed] [Google Scholar]
- 13.Severe P, Juste MAJ, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chobanian AAV, Bakris GL, Black HR, Cushman WC, Green L a, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1–104. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach: 2006 revision. WHO; 2006. [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO case definitions for AIDS surveillance in adults and adolescents. Relev Epidemiol Hebd. 1994;69:273–5. [PubMed] [Google Scholar]
- 18.Bloomfield GS, Hogan JW, Keter A, Holland TL, Sang E, Kimaiyo S, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. doi: 10.1186/1471-2334-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Iguacel R, Negredo E, Peck R, Friis-Moller N. Hypertension Is a Key Feature of the Metabolic Syndrome in Subjects Aging with HIV. Curr Hypertens Rep. 2016;18:46. doi: 10.1007/s11906-016-0656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNairy ML, Joseph P, Unterbrink M, Galbaud S, Mathon J-E, Rivera V, et al. Outcomes after antiretroviral therapy during the expansion of HIV services in Haiti. PLoS One. 2017;12:e0175521. doi: 10.1371/journal.pone.0175521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 23.Vázquez-Oliva G, Fernández-Real JM, Zamora A, Vilaseca M, Badimón L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens. 2005;19:457–62. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 24.Okello S, Asiimwe SB, Kanyesigye M, Muyindike WR, Boum Y, Mwebesa BB, et al. D-Dimer Levels and Traditional Risk Factors Are Associated With Incident Hypertension Among HIV-Infected Individuals Initiating Antiretroviral Therapy in Uganda. J Acquir Immune Defic Syndr. 2016;73:396–402. doi: 10.1097/QAI.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordell AD, McKenna M, Borges ÁH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dannenberg AL, Garrison RJ, Kannel WB. Incidence of hypertension in the Framingham Study. Am J Public Health. 1988;78:676–679. doi: 10.2105/ajph.78.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med (Baltim) 1987;16:235–51. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 28.Vargas C, Ingram DD, Gillum RF. Incidence of Hypertension and Educational Attainment. Am J Epidemiol. 2000;152:272–278. doi: 10.1093/aje/152.3.272. [DOI] [PubMed] [Google Scholar]
- 29.Rywik SL, Williams OD, Pajak A, Broda G, Davis CE, Kawalec E, et al. Incidence and correlates of hypertension in the Atherosclerosis Risk in Communities (ARIC) study and the Monitoring Trends and Determinants of Cardiovascular Disease (POL-MONICA) project. J Hypertens. 2000;18:999–1006. doi: 10.1097/00004872-200018080-00002. [DOI] [PubMed] [Google Scholar]
- 30.Armah KA, Chang CCH, Baker JV, Ramachandran VS, Budoff MJ, Crane HM, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and-uninfected veterans. Clin Infect Dis. 2014;58:121–129. doi: 10.1093/cid/cit652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peck R, Mghamba J, Vanobberghen F, Kavishe B, Rugarabamu V, Smeeth L, et al. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Heal. 2014;2:e285–e292. doi: 10.1016/S2214-109X(14)70033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


