Abstract
The effect of calcium impregnation on drip loss, colour, mechanical properties, sensory perception and freezing time on frozen-thawed papaya was studied, evaluating different freezing methods: cryogenic, tunnel and household freezer freezing. Osmotic dehydration as pre-treatment was also evaluated. Freezing in liquid nitrogen was considered an inappropriate method for papaya preservation due to cracking. Calcium impregnation and osmotic dehydration increased tissue firmness and decreased freezing time (freezing time for fresh, calcium impregnated and osmo-dehydrated fruit was 23, 17 and 5 min in a tunnel and 118, 83 and 60 min in a household freezer, respectively). Calcium lactate was the most effective way to protect tissue’s firmness before and after a freeze-thaw cycle (maximum stress values approx. 300–400% of the raw tissue for tunnel freezing and 260% for household freezer). Microstructure analysis showed better tissue integrity retention in papaya samples impregnated with calcium lactate than in those with calcium gluconate, after a freezing–thawing cycle, in agreement with the drip loss results. In spite of these results, consumers preferred frozen papaya without pre-treatment or impregnated with calcium gluconate.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3118-x) contains supplementary material, which is available to authorized users.
Keywords: Tropical fruits, Calcium, Osmotic dehydration, Freezing
Introduction
Ripe papaya (Carica papaya L.) has a delicate aroma and sweetness, besides being a good source of nutrients and bioactive compounds (vitamins A and C, minerals, carotenoids). The fruit, highly perishable, is primarily marketed as a fresh product or processed in syrup, jam, dried fruit or glazed fruit. Different processing alternatives, as vacuum packing combined with refrigeration, have been evaluated to increase its shelf-life (Padmanaban et al. 2014). Freezing is another possible preservation method, prolonging the product’s shelf life. However, especially in fruits, freezing provokes undesirable textural changes and fruit softening (Phothiset and Charoenrein 2014).
Different treatments with calcium solutions (sometimes combined with vacuum or blanching) before freezing have been studied in different fruits with positive results in conserving the texture and reducing drip loss: strawberries (Garcia-Berbari et al. 1998; Suutarinen et al. 2000), melon (Resende and Cal-Vidal 2002), pineapple (Chauhan et al. 2009), and mango (Siramard and Charoenrein 2014).
As for osmotic dehydration before freezing, several researchers have found that drip loss decreases substantially during thawing of fruits such as kiwifruit, apple, pear, melon and mango (Maestrelli et al. 2001; Talens et al. 2002; Marani et al. 2007; Zhao et al. 2014, 2017). Melon cubes’ and kiwi slices’ colour did not change, while lower browning was observed in apples and pears (Talens et al. 2002; Marani et al. 2007; Zhao et al. 2014), compared to frozen fresh fruit. Besides, and specifically in papaya, Udomkun et al. (2014) studied the combined effect of calcium and osmotic dehydration.
Therefore, the aim of this work is to evaluate the influence of two pre-treatments (calcium impregnation and osmotic dehydration) on drip loss, colour, structure, mechanical properties and freezing time in papaya using three different freezing methods.
Materials and methods
Sample preparation
Papaya (Carica papaya L.) fruits were purchased at a local market of Misiones (Argentina). In order to ensure reproducible results, fruits of homogeneous shape (elongated), weight of 1.50 ± 0.35 kg, grade 5 commercial ripeness (76–100% yellow surface, Pereira et al. 2009), were selected. The fruits were carefully washed with distilled water, manually peeled and cut with a stainless steel cork borer into cylinders (10.5 ± 0.5 mm height, 30.0 ± 1.0 mm diameter) from the inner fruit pulp. The average content of soluble solids, aw, pH and moisture of fresh papaya was 8.87 ± 0.66 °Brix, 5.51 ± 0.11, 0.994 ± 0.001 and 89.97 ± 1.47 (%w/w) respectively, analysing 10 fruits.
Pre-treatments
Calcium impregnation
Isotonic aqueous sucrose solutions containing 1.5%w/w calcium lactate or calcium gluconate, at 45 °C and with constant stirring (150 rpm) were used as the impregnation solution. These conditions were selected because they provided appropriate results for calcium incorporation and fruit firmness (Lovera et al. 2014). The solution was isotonic with respect to the soluble solids content of fresh fruit in order to avoid osmotic dehydration. Three series of assays were performed, dipping the papaya cylinders during 4 (both salts) or 8 h (only lactate). The assays are coded according to the details listed in Table 1. After this period, the samples were removed and washed three times with distilled water. Samples randomly selected were used to quantify mechanical properties, colour and moisture content.
Table 1.
Labelling of samples and treatments applied in this study
| Treatments | Denomination |
|---|---|
| Fresh fruit (control) | Fr |
| Dipping in isotonic solution without Ca for 4 h | Isot4 |
| Dipping in isotonic solution with Ca gluconate for 4 h | Glu4 |
| Dipping in isotonic solution with Ca lactate for 4 h | Lac4 |
| Dipping in isotonic solution with Ca lactate for 8 h | Lac8 |
| Osmotic dehydration for 12 h | OD12 |
| Superscripts | |
| Freezing in tunnel | T |
| Freezing in household freezer | HF |
Additionally, an equivalent treatment without calcium (Isot4 in Table 1) was performed in order to evaluate solely the effect of the immersion process at 45 °C.
Osmotic dehydration
Osmotic dehydration was performed by dipping the papaya cylinders in osmotic sucrose solutions (60 °Brix) for 12 h using a shaken thermostatic bath at 45 °C and 150 rpm (Dubnoff, Vicking, Argentina). The ratio of the fruits to the osmotic solution was 1:5 w/w. Then, the samples were removed from the syrup and washed three times with distilled water.
Freezing and thawing
Three different freezing methods were applied, using both fresh and pretreated papaya samples: cryogenic freezing by immersion in liquid nitrogen at − 196 °C (N2); tunnel freezing, using a prototype air blast freezer (Friotecnología S.R.L., Argentina) with tangential air flow at − 30 °C and 3 m/s (T); and household freezer freezing (Dual Gafa STD L-300 L, Frimetal S.A., Argentina) at − 20 °C (HF).
The temperature was recorded by T-thermocouples, placed at the centre of the samples and connected to a data acquisition system connected to a PC (DAST TC, Keithley, USA). The samples remained in each freezing device until the thermocouples indicated − 30 °C in T, and − 20 °C in N2 and HF.
Thermophysical properties
Thermal conductivity k (W/m °C) was determined by a KD2 instrument (Decagon Devices Inc., USA). The measurement was performed at 25 °C in the fresh samples and at − 20 °C in the frozen samples.
The specific heat of both fresh and frozen samples was calculated using equations from literature (Okos 1986), using experimental moisture content values Xap (%w/w wet basis).
| 1 |
| 2 |
Freezing time
Freezing time tf, defined as the time needed to reach a given temperature (− 10 or − 18 °C) in the coldest point of the sample, was measured from the experimental freezing curves and calculated using software developed by Salvadori and Mascheroni (1997). This software allows selecting the kind of food, and different geometries. For multidimensional foods (such as the flat cylinders used in this work) it calculates a form factor.
Quality attributes
Calcium content
Calcium content was measured by atomic absorption spectrophotometry, using a Perkin Elmer 3110 spectrometer (Perkin ElmerInc., USA). Approximately 2 g of a previously dried sample were calcined in a muffle furnace at 550 °C, the ashes were dissolved in HCL 2N, filtrated, and diluted with deionized water. Calcium content is expressed as mg/100 g fresh fruit, more details in Lovera et al. (2014).
Drip loss
Frozen products were placed on a weighed absorbent paper in a sealed container and thawed at 20 °C for 2 h. Drip loss was evaluated by weighing the absorbent paper before and after thawing (W0) and (Wf) respectively, and was calculated as the percentage loss of the initial weight (Mi) (Eq. 3):
| 3 |
Mechanical properties
A compression test was conducted to determine fruit firmness using a texture analyser (TA.XT2i, Stable Micro Systems, UK). The measurement was performed using a 75 mm circular section stainless steel probe (P/75), with test speed 0.5 mm/s, load cell 5 kg, the sample was compressed until a 70% of deformation was obtained. The reported values correspond to the average of 10 measurements. The data is expressed as corrected stress (σ) and strain (ε) parameters (Eqs. 4, 5) assuming constant volume during compression (Johnson et al. 1980).
| 4 |
| 5 |
where h0 is initial height (m), A0 is initial area (m2), Δh is absolute deformation (m).
The maximum stress (σmax) was determined as the maximum peak of the stress–strain curve. In addition, maximum stress of treated fruit (impregnated, osmotic dehydrated and frozen-thawed) in relation to the value for fresh fruit () was reported.
Colour
Sample colour was measured using a CR-300 colorimeter (Minolta, Japan). The CIELAB parameters L*a*b*(lightness, red-green value, and yellow-blue value respectively) were recorded. From those values, Hue angle, Chroma and ∆E parameters were calculated through Eqs. 6, 7 and 8. ∆E describes total colour change, ΔL*, Δa* and Δb* were calculated as the difference between processed and fresh fruit colour values. The results presented in this work correspond to the average of 8 measurements.
| 6 |
| 7 |
| 8 |
Scanning electronic microscopy
Papaya cubes (5 mm3) were obtained from the central section of each sample, using a scalpel. Frozen samples were thawed at 20 °C for 2 h before sectioning them. The cubes were dehydrated in four dips of 50, 70, 80 and 90% ethanol for 15 min followed by one dip in 100% ethanol for 20 min. The tissues were dried using the critical point drying technique. Microstructural properties of fresh, impregnated and frozen samples were obtained using a scanning electron microscope (ESEM, FEI ESEM Quanta 200, USA) with an accelerated voltage of 12–16 kV.
Sensory analysis
Triangle test: In order to investigate possible organoleptic differences due to the freezing method (T and HF) a triangle test was performed. The sensory panel, constituted by 24 untrained panellists, analysed three randomly organized samples (two of them frozen by one of the tested freezing methods (identical samples) and the other one by the other freezing method (different sample)), and they were asked to identify the different sample.
Acceptability test: This test was performed by 76 judges, members of the Facultad de Ciencias Exactas Químicas y Naturales (UNaM), all of them older than 18 years old and frequent consumers of papaya and ice-cream. With the aim of testing a possible application of the products obtained in this work, the judges tasted a mix of fruit with ice-cream: a sample of papaya was cut in 6 equal portions and mixed with 20 g of cream ice-cream. Three papaya frozen samples (one fresh and the other two with 2 different impregnation treatments) were tested. Each portion of papaya/ice-cream was presented in an individual container randomly coded with three-digit numbers. The judges tasted the product with focus on the fruit attributes. Colour, texture, taste and overall acceptability were evaluated, using a traditional nine point hedonic scale (1 = dislike extremely; 5 = neither like nor dislike; 9 = like extremely). Moreover, they were asked to indicate the sample of “most intense colour” and “firmest tissue” and to add extra comments if they wished. Mineral water was used as a neutralizing agent between tests.
Statistical analysis
Statistical analysis was performed with Statgraphics Plus 5.1 (StatPoint Technologies Inc., USA) software. Analyses of variance (ANOVA) were applied. Average values were compared applying Fisher multiple range test (LSD) when ANOVA showed statistical difference (p < 0.05).
Results and discussion
Freezing curves
In the first instance, the feasibility of liquid nitrogen (N2), air-blast tunnel (T) and household freezer (HF) freezing on fresh and impregnated papaya was evaluated. T and HF were the most appropriate freezing methods to preserve the sample’s geometry and size; on the contrary N2 freezing produced notorious cracking in most samples, perhaps due to the surface crust formed at the beginning of the freezing process, which prevents volume expansion when the phase change of the inner portions occurs. Agnelli and Mascheroni (2001) observed cracking on mushrooms surface during liquid nitrogen freezing. Chassagne-Berces et al. (2009) reported cracking in apples frozen by immersion in N2. Based on these facts, liquid N2 freezing was considered an inappropriate method for the preservation of papaya fruit.
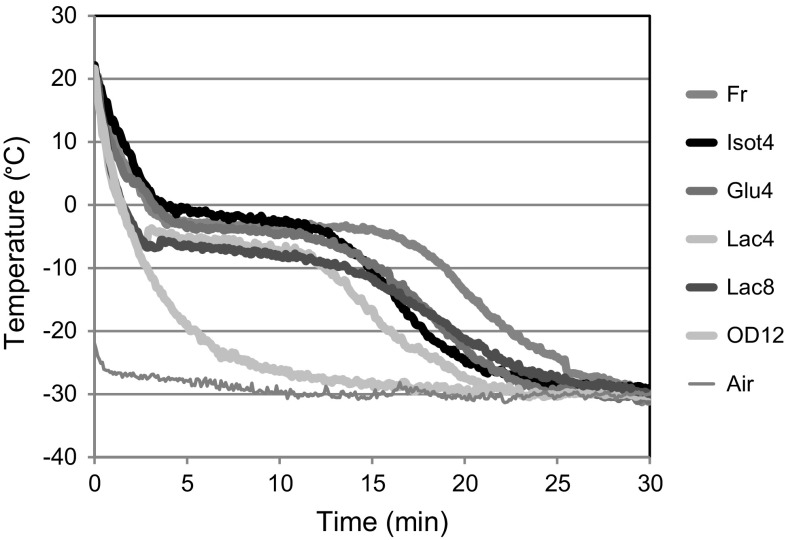
Figure 1 shows the experimental temperature profiles of the different samples frozen in air-blast tunnel. The samples pre-treated with lactate (Lac4, Lac8) present lower initial cryoscopic temperature (Tcr) than those pre-treated with calcium gluconate (Glu4) or untreated (Fr). Besides, the freezing of samples treated with calcium was significantly faster than the freezing of fresh fruit, independently of the impregnation time and the dipping solution. A lower freezing time was also observed in the samples dipped in isotonic solution without calcium (Isot4T), suggesting that it is the dipping treatment per se which induces structural changes in the fruit, reducing its freezing time. Osmotic dehydration caused a noticeable decrease in freezing time and the freezing curve does not show the typical plateau (OD12), this result is attributed to the low moisture content of the samples (34 g water/100 g fruit) after OD. In this sense, Ramallo and Mascheroni (2010) found that an increase in the osmotic dehydration time reduces the total process time required to freeze pineapple slices.
Fig. 1.
Freezing curves of papaya samples frozen in tunnel, labelling detailed in Table 1
The temperature profiles recorded during household freezer freezing of both fresh and treated fruit samples presented similar behaviours, although the freezing times were significantly higher. Again, the osmo-dehydrated samples froze faster than the impregnated ones.
Thermophysical properties and freezing time
There were no significant differences between experimental values of papaya moisture content before and after the calcium treatment; and similar behaviours were observed in thermal conductivity and density measurements. The average value for experimental moisture content Xap (%w/w wet basis) was 88.65 ± 1.44 for the fresh samples (Fr, Glu4, Lac4 and Lac8) and 87.30 ± 0.70 for the frozen ones (FrHF, Glu4HF, Lac4HF and Lac8HF). The average experimental value for thermal conductivity of Fr, Glu4, Lac4 and Lac8 samples was 0.50 ± 0.01 W/m °C; for the frozen ones the average value was 1.30 ± 0.02 W/m °C. From these results, it can be inferred that calcium intake has no influence on the thermal conductivity. For osmo-dehydrated samples, the thermal conductivity was calculated using the equation proposed by Ilicali and Icier (2010), lower values were obtained for OD12 (0.38 W/m °C) and OD12HF (1.12 W/m °C) as a consequence of the lower moisture content of these samples (33.76 ± 0.45 and 31.36 ± 0.92%w/w w.b. respectively). Ilicali and Icier (2010) reported thermophysical properties of papaya pulp, fresh and microwave dehydrated, similar to those obtained in this work. Finally, the calculated values of specific heat (J/kg °C) were 3807 (Fr, Lac4, Lac8); 2010 (OD12), 1934 (FrHF, Lac4HF, Lac8HF), and 1277 (OD12HF).
Freezing time was defined as the time needed for the sample, initially at ambient temperature, to reach a final temperature, set as − 10 or − 18 °C, at its thermal centre. Initial freezing or cryoscopic temperature () and experimental freezing time () were measured from the experimental freezing curves and are detailed in Table 2. Values of papaya fruit (FrHF and FrT) were lower than those published by Telis et al. (2007) for papaya (− 1.24 °C) and mango (− 1.69 °C).
Table 2.
Experimental freezing time (tc), cryoscopic temperature (Tcr) and drip loss (%), labelling detailed in Table 1
| Sample | tf (− 10 °C) (min) | tf (− 18 °C) (min) | Tcr (°C) | Drip loss (%) |
|---|---|---|---|---|
| FrT | 18.73 ± 1.50e | 23.06 ± 2.03d | − 2.31 ± 0.52e | 4.26 ± 0.83b |
| Isot4T | 16.08 ± 1.41d | 18.38 ± 1.36c | − 0.50 ± 0.07d | 21.00 ± 3.44d |
| Glu4T | 14.58 ± 0.59cd | 17.63 ± 0.53c | − 3.09 ± 0.08c | 21.00 ± 0.33d |
| Lac4T | 12.50 ± 1.12b | 14.96 ± 0.53b | − 4.00 ± 0.58b | 14.12 ± 1.91c |
| Lac8T | 13.42 ± 1.30bc | 18.04 ± 0.76c | − 5.71 ± 0.34a | 3.29 ± 1.11b |
| OD12T | 3.21 ± 0.53a | 5.00 ± 0.71a | 1.74 ± 0.07a | |
| FrHF | 84.81 ± 1.87e | 118.14 ± 0.42e | − 2.60 ± 0.06b | 9.83 ± 3.72b |
| Isot4HF | 68.30 ± 9.19cd | 109.20 ± 8.48e | − 4.33 ± 0.06a | 21.11 ± 3.13d |
| Glu4HF | 60.30 ± 2.12c | 80.10 ± 2.97c | − 3.93 ± 0.18a | 18.68 ± 0.68d |
| Lac4HF | 50.10 ± 1.42b | 73.50 ± 2.12b | − 2.36 ± 0.64b | 14.31 ± 0.53c |
| Lac8HF | 71.94 ± 0.47d | 96.69 ± 1.00d | − 2.65 ± 0.13b | 5.83 ± 0.36b |
| OD12HF | 18.15 ± 1.87a | 59.57 ± 1.80a | 2.16 ± 0.77a |
Different letters within the same column and same freezing method (T or HF) indicate significant difference (p < 0.05)
The predicted freezing times until reaching − 18 °C at the samples’ centre were 20.18 and 147.8 min for fresh papaya in tunnel and household freezer respectively, using the thermophysical properties detailed above. Predicted osmo-dehydrated papaya freezing times were 6.34 and 47.4 min in T and HF, respectively. The average relative error between experimental and predicted freezing time was 16% in T tests and 29% in HF ones, in this case the large error can be attributed to the small difference between product temperature and freezer temperature at the end of freezing.
Papaya freezing time was significantly reduced (p < 0.05) by calcium uptake as it is shown in Fig. 1 and Table 2. This effect was more noticeable when calcium lactate instead of calcium gluconate was used in the impregnation treatment.
Even though different values were measured from the experimental temperature profiles (Table 2), the effect of calcium impregnation conditions on those values was not clear: in tunnel freezing they decreased as papaya calcium content increased, and this effect was more pronounced when calcium lactate was used. On the contrary, in fruit samples frozen in a household freezer, the cryoscopic temperature significantly decreased for the fruit samples treated with the isotonic solution or impregnated with calcium gluconate.
Quality characteristics
Drip loss
Drip loss was measured in thawed-frozen fruits in order to compare the different treatments; the results are detailed in Table 2. As it was expected, drip loss was significantly lower in samples frozen by T than HF, as a higher freezing rate produces lower dripping loss.
When comparing the effect of dipping in isotonic solutions without calcium (Isot4) and with calcium (Glu4, Lac4, Lac8), a significant decrease of drip loss was measured only in the samples impregnated with calcium lactate. In general, the immersion treatment at 45 °C favors drip loss, with values 2–5 times higher (frozen in T and HF respectively) when compared with fruit frozen without any pretreatment. Only Lac8 samples showed values of drip loss that were lesser than or equal to those of fresh fruit. The structure preservation obtained in the samples treated with calcium lactate is shown in the photograph given as Online Resource 1. On the contrary, the softening effect of dipping in isotonic aqueous solution is notorious.
Abd-Elhady (2014) studied the influence of an immersion pretreatment with citric acid and calcium lactate for 5 min at room temperature in the quality of frozen strawberries, the author found that calcium lactate reduced drip loss and increased firmness after thawing.
Calcium content
Experimental values of calcium content, measured in papaya samples prior to both freezing assays, are detailed in Table 3. From these values, it can be inferred that a higher calcium uptake was obtained when calcium lactate was used as mineral source; also the impregnation time influenced this value. Osmo-dehydrated papaya presented similar calcium content than fresh fruit.
Table 3.
Mechanical properties and colour parameters of fresh, calcium impregnated and osmotic dehydrated fruit before and after freezing in tunnel and household freezer, before and after freezing in tunnel and household freezer, labelling detailed in Table 1
| Sample | Calcium content** mg/100 g Fr | ɛ max | Hue | ΔE | |
|---|---|---|---|---|---|
| Fr | 20.6 ± 3.9a | 0.17 ± 0.04a | 1.00 ± 0.06c | 57.15 ± 2.20de | |
| Glu4 | 39.5 ± 6.3b | 0.22 ± 0.03a | 1.12 ± 0.17 cd | 55.62 ± 1.91cde | 6.86 ± 1.56a |
| Lac4 | 150.1 ± 7.4d | 0.49 ± 0.07c | 5.10 ± 0.77f | 57.57 ± 1.10de | 4.92 ± 1.41a |
| Lac8 | 238.0 ± 26.3e | 0.53 ± 0.12cde | 6.01 ± 0.54 g | 58.31 ± 3.71ef | 5.09 ± 1.49a |
| OD12 | 19.1 ± 5.4a | 0.56 ± 0.10de | 1.50 ± 0.38d | 38.08 ± 3.27a | 14.67 ± 2.17de |
| FrT | 19.8 ± 3.9a | 0.37 ± 0.07b | 0.47 ± 0.12b | 53.50 ± 6.54bc | 9.98 ± 2.82b |
| Glu4T | 31.2 ± 5.1b | 0.70 ± 0.01f | 0.51 ± 0.03b | 54.45 ± 3.62bcd | 10.80 ± 1.83bc |
| Lac4T | 129.1 ± 9.2c | 0.70 ± 0.02f | 2.94 ± 0.47e | 56.52 ± 4.73cde | 12.51 ± 2.24cd |
| Lac8T | 230.5 ± 28.1e | 0.70 ± 0.01f | 4.20 ± 0.37f | 61.70 ± 4.05f | 14.25 ± 2.38de |
| OD12T | 18.7 ± 5.3a | 0.66 ± 0.04f | 1.32 ± 0.24d | 37.91 ± 3.89a | 17.24 ± 3.06f |
| FrHF | 18.7 ± 4.3a | 0.33 ± 0.07b | 0.19 ± 0.06a | 52.01 ± 5.82b | 10.21 ± 3.69b |
| Glu4HF | 32.2 ± 5.4b | 0.67 ± 0.06f | 0.41 ± 0.05b | 54.82 ± 1.74bcd | 14.71 ± 1.99de |
| Lac4HF | 128.7 ± 7.1c | 0.66 ± 0.02f | 2.48 ± 0.51e | 58.13 ± 3.78def | 14.96 ± 2.01ef |
| Lac8HF | 224.2 ± 25.6e | 0.61 ± 0.09ef | 2.52 ± 0.43e | 57.75 ± 1.9def | 14.29 ± 1.28de |
| OD12HF | 18.6 ± 5.4a | 0.50 ± 0.03 cd | 1.49 ± 0.29d | 35.57 ± 1.41a | 15.92 ± 1.38ef |
** Papaya calcium content was calculated in frozen fruit, taking into account the drip loss
Different letters within the same column and same freezing method (T or HF) indicate significant difference (p < 0.05)
The calcium content of frozen samples informed in Table 3 was estimated assuming that calcium loss is directly related to drip loss.
Mechanical properties
Fresh fruit presented the typical stress–strain curve with a defined maximum peak (data not shown). The maximum stress and the elasticity modulus (initial slope of the curve) became lower in samples dipped in aqueous solution, without calcium (Isot4). Tunnel freezing significantly reduced both the maximum peak and the elasticity modulus of papaya (FrT), denoting a more elastic behaviour; and maintaining the shape of the stress–strain curve. Also, the combined treatment of dipping in isotonic solution followed by freezing in tunnel, Isot4T, resulted in drastic changes in the shape stress–strain curve, showing an increase of elasticity without a defined maximum peak. Similar results were observed in household freezing samples.
The values of the maximum deformation (ɛmax) and maximum stress related to fresh fruit maximum stress () recorded are detailed in Table 3. The maximum stress value recorded in fresh fruits varied widely between 12 and 72 Pa, because of which is used to evaluate the treatment effect. Before freezing, calcium impregnated samples registered higher maximum stress values than fresh fruit, depending on the calcium salt and the impregnation time, reaching values of up to 6 times greater than the fresh fruit. The freezing process reduced the firmness of fresh and treated fruit, however a firmness protective effect of impregnation treatments with calcium lactate was observed, reaching values of 4.20 and 2.52 for frozen fruits in T and in HF respectively. As it was just noted in drip loss, calcium lactate seems to be more favourable than calcium gluconate to protect the plant tissue integrity and thus to preserve the mechanical properties of the fruit. As it was expected, in untreated samples (FrT and FrHF) a higher freezing rate has a less negative impact than a slower one.
Concerning osmo-dehydrated fruit, this pre-treatment produced samples that were more resistant and elastic than fresh fruit, as it can be seen from the results in Table 3. Besides, both freezing modes had no significant effect on the mechanical properties, only a slight change of the stress–strain curve was observed (OD12T and OD12HF samples), confirming that moisture content reduction of osmo-dehydrated fruits diminishes the negative impact of freezing–thawing on cell structure integrity.
Colour
CIELAB parameters, Hue angle and Chroma were obtained for the papaya samples after the different treatments detailed in Table 1, only Hue and ∆E are shown in Table 3.
L*, Chroma and Hue were not significantly affected by calcium impregnation. Freezing induced the decrease of lightness (L*), in fruits with and without calcium impregnation, independently of the freezing method. Concerning the Hue angle, the effect of freezing was more notorious in untreated fruits (p > 0.05). Chroma values increased in HF samples, but no clear trend was observed in T samples (data not shown). Both freezing and impregnation treatments have a significant influence on the total color difference respect to fresh fruit, ΔE.
Hue angle and lightness of osmo-dehydrated fruits presented noticeable changes respect to the fresh ones. OD produced more reddish fruits, possibly due to the previous dehydration that increased the pigment concentration in the tissue. Consequently, the highest colour change was observed in osmo-dehydrated samples before (ΔE = 14.67 ± 2.12) and after freezing (ΔE = 17.24 ± 3.06 in T and ΔE = 15.92 ± 1.38 in HF).
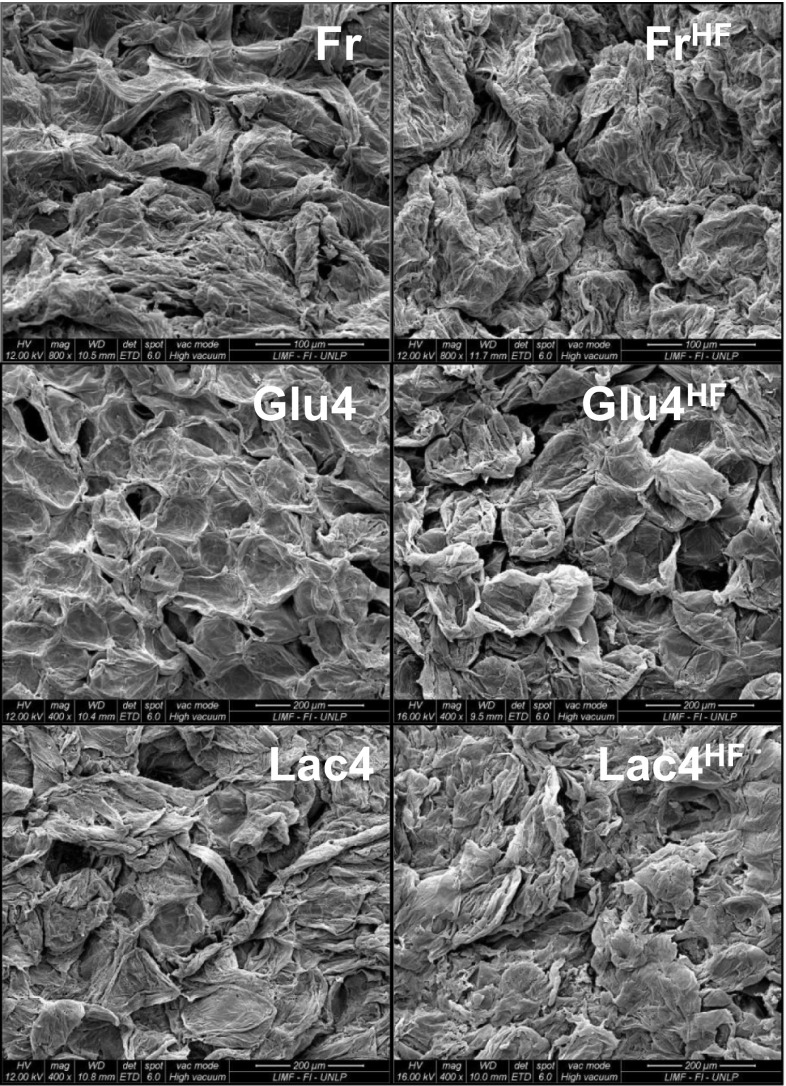
Scanning electronic microscopy
The microstructures of fresh, calcium impregnated and frozen fruits (Fr, Glu4, Lac4, FrHF, Lac4HF and Glu4HF), were observed using SEM. The results are shown in Fig. 2. Defined cells and cellular membranes of fresh fruit (Fr) were visible. After the freezing–thawing cycle, the FrHF papaya microstructure showed a contraction of its tissue, but without remarkable disruptions. This could be due to cellular collapse during the freezing–thawing cycle (Phothiset and Charoenrein 2014). Conversely, the samples impregnated with calcium gluconate showed significant cellular disruption due to the freezing–thawing process. These observations are in agreement with the results of drip loss (Table 2), where drip loss values of Glu4 and Isot4 samples were the highest recorded. Likewise, Glu4HF showed more intercellular spaces and more evidence of cellular disruption than Lac4HF, this fact could explain the higher compression resistance of Lac4HF samples.
Fig. 2.
SEM micrographs showing the effect of different treatments on the structure of papaya samples, labelling detailed in Table 1
It should be noted that the quantitative analysis by SEM recorded calcium peaks in impregnated and frozen samples (Glu4, Lac4, Lac4HF and Glu4HF) confirming the incorporation of calcium in tissue; no peaks were recorded in the frozen fruit without impregnation (FrHF).
Sensory analysis
Triangle test
In order to evaluate if the freezing method has some influence on the sensory characteristics of frozen papaya, the triangle test was applied. Samples with the same calcium treatment, frozen in tunnel (Glu4T) and household freezer (Glu4HF) were used. 11 of 24 judges correctly identified the different sample; this result indicates that there were no significant differences between samples at a significance level of 95% (Roessler et al. 1978). Therefore, both freezing methods can be considered equivalent from a sensory approach.
Acceptability test
Frozen papaya can be consumed directly or as an ingredient in fruit salads or as a blend with ice-cream. In this sense, to analyse the sensory acceptability of frozen papaya, an acceptability test was performed. Three frozen papaya samples were tested: fresh (FrHF) and pretreated fruit (Glu4HF and Lac4HF), always mixed with ice-cream, the results are presented in Table 4.
Table 4.
Sensory analysis on acceptability of frozen papaya, also the percentage of samples rated higher than 5 (% > 5) is informed
| Sample | Colour | Taste | Texture | Overall acceptability | ||||
|---|---|---|---|---|---|---|---|---|
| Mean score | % > 5 | Mean score | % > 5 | Mean score | % > 5 | Mean score | % > 5 | |
| FrHF | 6.80a | 73.68 | 6.32a | 65.79 | 6.39a | 63.16 | 6.78a | 75.00 |
| Glu4HF | 6.30ab | 77.63 | 5.72ab | 52.63 | 6.43a | 69.74 | 6.62a | 76.32 |
| Lac4HF | 5.91b | 53.95 | 5.07b | 42.11 | 5.74b | 53.95 | 5.82b | 61.84 |
Different letters within the same column and same freezing method (T or HF) indicate significant difference (p < 0.05)
Consumers assigned a score higher than 5 to all samples, for every attribute, which indicates that there was no product rejection. Particularly, the mean score for the attribute “Taste” of both calcium impregnated fruits did not differ significantly. However, 40 judges, of a total of 76, considered acceptable (score > 5) the taste of fruit impregnated with calcium gluconate, while only 30 of them valued as acceptable the taste of fruit treated with calcium lactate. The highest mean score for “Overall Acceptability” was obtained for the untreated fruit, FrHF, followed by Glu4HF, no significant differences (p < 0.05) between this two samples were found. Finally, Lac4HF samples had the lowest score in all attributes, and significant differences with untreated frozen samples were found in all of them.
On the basis of additional information provided by the judges, 57% of them considered that the fresh FrHF sample had “the most intense colour”, and 83% of them considered that the Lac4HF sample had “the firmest tissue”.
Conclusion
Calcium lactate as mineral source is more effective than calcium gluconate. In this sense, calcium content increased 200 and 750% with respect to fresh fruit. Regarding freezing, cracking was observed in all the samples frozen in liquid nitrogen; consequently only tunnel and slow freezing were compared.
Impregnated papaya required lower freezing times and presented higher resistance to fracture than fresh fruit, these characteristics being more notorious in fruits treated with calcium lactate. On the other hand, osmotic dehydration also had beneficial effect on papaya quality after a freezing–thawing cycle (drip loss decrease, firmness preservation, more intense colour). Moreover, a significant reduction of the freezing time was observed.
According to the sensory analysis, the effect of the freezing rate is not perceived by the consumers, when tasting frozen papaya impregnated with calcium gluconate. Besides, the acceptability test indicates that frozen papaya without pre-treatment or impregnated with calcium gluconate has greater overall acceptability, in spite of the fact that the fruit treated with calcium lactate had the firmest tissue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Visual aspect of papaya samples, after dipping (without and with calcium) and freezing, labelling detailed in Table 1 (PDF 167 kb)
Acknowledgements
Authors acknowledge CONICET, UNaM and UNLP from Argentina for their financial support.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3118-x) contains supplementary material, which is available to authorized users.
References
- Abd-Elhady M. Effect of citric acid, calcium lactate and low temperature prefreezing treatment on the quality of frozen strawberry. Ann Agric Sci. 2014;59:69–75. [Google Scholar]
- Agnelli ME, Mascheroni RH. Cryomechanical freezing. A model for the heat transfer process. J Food Eng. 2001;47:263–270. doi: 10.1016/S0260-8774(00)00126-6. [DOI] [Google Scholar]
- Chassagne-Berces S, Poirier C, Devaux MF, Fonseca F, Lahaye M, Pigorini G, Girault C, Marin M, Guillon F. Changes in texture, cellular structure and cell wall composition in apple tissue as a result of freezing. Food Res Int. 2009;42:788–797. doi: 10.1016/j.foodres.2009.03.001. [DOI] [Google Scholar]
- Chauhan OP, Shah A, Singh A, Raju PS, Bawa AS. Modeling of pre-treatment protocols for frozen pineapple slices. LWT-Food Sci Technol. 2009;42:1283–1288. doi: 10.1016/j.lwt.2009.02.019. [DOI] [Google Scholar]
- Garcia-Berbari SA, Nunes-Nogueira J, Da Silva-Campos SD. Effect of different pre-freezing treatments on the quality of frozen strawberries variety Chandler. Ciencia Tecnol Alime. 1998;18:82–86. [Google Scholar]
- Ilicali C, Icier F. Freezing time prediction for partially dried papaya puree with infinite cylinder geometry. J Food Eng. 2010;100:696–704. doi: 10.1016/j.jfoodeng.2010.05.022. [DOI] [Google Scholar]
- Johnson EA, Segars RA, Kapsalis JG, Normand MD, Peleg M. Evaluation of the compressive deformability modulus of fresh and cooked fish flesh. J Food Sci. 1980;45:1318–1320. doi: 10.1111/j.1365-2621.1980.tb06545.x. [DOI] [Google Scholar]
- Lovera NN, Ramallo L, Salvadori VO. Effect of processing conditions on calcium content, firmness, and color of papaya in syrup. J Food Process. 2014 [Google Scholar]
- Maestrelli A, Lo Scalzo R, Lupi D, Bertolo G, Torreggiani D. Partial removal of water before freezing: cultivars and pre-treatments as quality factors of frozen muskmelon (Cucumis melo, cv reticulates Naud) J Food Eng. 2001;49:255–260. doi: 10.1016/S0260-8774(00)00211-9. [DOI] [Google Scholar]
- Marani C, Agnelli ME, Mascheroni RH. Osmo-frozen fruits: mass transfer and quality evaluation. J Food Eng. 2007;79:1122–1130. doi: 10.1016/j.jfoodeng.2006.03.022. [DOI] [Google Scholar]
- Okos MR. Physical and chemical properties of foods. St. Joseph: ASAE American Society of Agricultural Engineers; 1986. [Google Scholar]
- Padmanaban G, Singaravelu K, Annavi ST. Increasing the shelf-life of papaya through vacuum packing. J Food Sci Technol. 2014;51:163–167. doi: 10.1007/s13197-011-0468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira T, de Almeida PSG, de Azevedo IG, Da Cunha M, de Oliveira JG, Da Silva MG, Vargas H. Gas diffusion in ‘Golden’ papaya fruit at different maturity stages. Postharvest Biol Technol. 2009;54:123–130. doi: 10.1016/j.postharvbio.2009.07.010. [DOI] [Google Scholar]
- Phothiset S, Charoenrein S. Effects of freezing and thawing on texture, microstructure and cell wall composition changes in papaya tissues. J Sci Food Agric. 2014;94:189–196. doi: 10.1002/jsfa.6226. [DOI] [PubMed] [Google Scholar]
- Ramallo LA, Mascheroni RH. Dehydrofreezing of pineapple. J Food Eng. 2010;99:269–275. doi: 10.1016/j.jfoodeng.2010.02.026. [DOI] [Google Scholar]
- Resende JV, Cal-Vidal J. Frutos de melão submetidos a pré-tratamentos com hidrocolóides: efeitos do processo de congelamento sobre a microestrutura celular. Ciencia Tecnol de Alime. 2002;22:295–304. doi: 10.1590/S0101-20612002000300017. [DOI] [Google Scholar]
- Roessler EB, Pangborn RM, Sidel JL, Stone H. Expanded statistical tables for estimating significance in paired-preference, paired-difference, duo–trio and triangle tests. J Food Sci. 1978;43:940–943. doi: 10.1111/j.1365-2621.1978.tb02458.x. [DOI] [Google Scholar]
- Salvadori VO, Mascheroni RH (1997) Tiempos: Un software de cálculo de tiempos de refrigeración y congelación de alimentos. Herramientas de Cálculo para la Ingeniería de Alimentos, vol III. CYTED, UPV, Spain, pp 25–31
- Siramard S, Charoenrein S. Effect of ripening stage and infusion with calcium lactate and sucrose on the quality and microstructure of frozen mango. Int J Food Sci Technol. 2014;49:2136–2141. doi: 10.1111/ijfs.12553. [DOI] [Google Scholar]
- Suutarinen J, Heiska K, Moss P, Autio K. The effects of calcium chloride and sucrose prefreezing treatments on the structure of strawberry tissues. LWT-Food Sci Technol. 2000;33:89–102. doi: 10.1006/fstl.1999.0616. [DOI] [Google Scholar]
- Talens P, Martínez-Navarrete N, Chiralt A. Changes in optical and mechanical properties during osmodehydrofreezing of kiwi fruit. Innov Food Sci Emerg Technol. 2002;3:191–199. doi: 10.1016/S1466-8564(02)00027-9. [DOI] [Google Scholar]
- Telis VRN, Telis-Romero J, Sobral PJA, Gabas AL. Freezing point and thermal conductivity of tropical fruit pulp: mango and papaya. Int J Food Prop. 2007;10:73–84. doi: 10.1080/10942910600744007. [DOI] [Google Scholar]
- Udomkun P, Mahayothee B, Nagle M, Müller J. Effects of calcium chloride and calcium lactate applications with osmotic pretreatment on physicochemical aspects and consumer acceptances of dried papaya. Int J Food Sci Technol. 2014;49:1122–1131. doi: 10.1111/ijfs.12408. [DOI] [Google Scholar]
- Zhao J, Hu R, Xiao HW, Yang Y, Liu F, Gan ZL, Ni YY. Osmotic dehydration pretreatment for improving the quality attributes of frozen mango: effects of different osmotic solutes and concentrations on the samples. Int J Food Sci Technol. 2014;49:960–968. doi: 10.1111/ijfs.12388. [DOI] [Google Scholar]
- Zhao J, Xiao HW, Ding Y, Nie Y, Zhang Y, Zhu Z, Tang XM. Effect of osmotic dehydration pretreatment and glassy state storage on the quality attributes of frozen mangoes under long-term storage. J Food Sci Technol. 2017;54:1527–1537. doi: 10.1007/s13197-017-2584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual aspect of papaya samples, after dipping (without and with calcium) and freezing, labelling detailed in Table 1 (PDF 167 kb)