Abstract
Dhokla, a popular indigenous savoury dish of India, is prepared by soaking bengalgram dal and rice, grinding separately, mixing the batters, and spontaneously fermenting and steaming of mixed batter. Central composite rotatable response surface designs for soaking, fermentation and steaming at five-level combinations were used for optimising preparation of dhokla to achieve reduced contents of antinutrients. Optimum soaking of bengalgram dal (dal–water ratio of 1:5 w/w, pH 7.0, 23 °C, 20 h) and rice (rice–water ratio of 1:5 w/w, pH 5.6, 16 °C, 18 h) resulted in reduced levels of all the antinutrients, except total biogenic amines in rice. Fermentation of dal–rice (3:1 v/v) mixed batter under optimum condition (added NaCl of 8 g/kg, 32 °C, 18 h) further reduced their levels, but total biogenic amines content was enhanced. However, optimum steaming of dal–rice mixed fermented batter for 20 min was effective in reducing all the tested antinutrients. In dhokla, the content of tannins, phytic acid and total biogenic amines reduced by 100, 94 and 20%, respectively; trypsin inhibitor and haemagglutinating activities reduced by 92 and 100%, respectively, over raw ingredients. The optimally prepared product (dhokla) ranked “excellent” in terms of overall sensory quality.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3119-9) contains supplementary material, which is available to authorized users.
Keywords: Antinutrient, Dhokla, Response surface methodology, Process optimisation, Bengalgram, Rice
Introduction
Legumes are recognised and appreciated for their nutritional quality. However, the presence of antinutrients in legumes presents a challenge prior to consumption. Legume antinutrients include tannins, phytic acid, trypsin inhibitors, haemagglutinin, biogenic amines and so on. Antinutrients interfere with bioavailability of minerals, carbohydrates and proteins, thereby limiting the nutritional quality of foods. Tannins are water-soluble phenolic compounds which readily form complexes with proteins and metal ions, thereby lowering the bioavailability of nutrients (Serrano et al. 2009). Phytic acid, also called myo-inositol hexaphosphoric acid or 1,2,3,4,5,6-hexakis (dihydrogen phosphate) myo-inositol or phytate in salt form, readily forms an insoluble complex with minerals and proteins. Moreover, phytic acid remains undegraded due to the absence of phytase in the gastrointestinal tract and small intestine of humans, thereby hampering enzymic activity, protein solubility and protein digestibility (Kumar et al. 2010). Trypsin inhibitors disturb normal functioning of digestive enzymes, stimulate pancreatic hypertrophy, cause growth retardation and decrease amino acid absorption (Guillamόn et al. 2008; Jezierny et al. 2010). Hemagglutinin or lectin interacts with enterocytes and lymphocytes, causing persistent antigenic responses (van Buul and Brouns 2014). Other physiological manifestations of lectins include bloating, vomiting, diarrhoea, growth suppression and red blood cell agglutination, leading to haemolysis and death (Roy et al. 2010). Migraine, nausea, tachycardia, headaches, rashes, and changes in blood pressure and blood sugar levels often remain associated with the consumption of food rich in biogenic amines (Naila et al. 2010). A level of ≥ 1000 μg amines per gram food is considered hazardous to human health (Kim et al. 2012). Various traditional processing treatments, like soaking, cooking, dehulling and fermentation reduce the levels of antinutrients (Shimelis and Rakshit 2008).
Dhokla is a traditional legume-cereal mixed fermented food, indigenous to the state of Gujarat but is now popular all over India. It is prepared by soaking bengalgram or chickpea (Cicer arietinum L.) dal (dehulled split beans) and rice (Oryza sativa L.) separately. The soaked ingredients are ground, mixed, stirred manually to form a thick batter and added with common salt. The batter is dispensed in a closed container and kept overnight or longer at a warm place for fermentation. Leavening of batter is associated with the development of a pleasant acid flavour, indicating a successful fermentation. Microbiota associated with the fermentation comprises Leuconostoc mesenteroides, Lactobacillus fermentum, Pichia silvicola, Candida sp., Streptococcus faecalis, Torulopsis candida and Torulopsis pullulans. The lactic acid bacteria (LAB) are responsible for the development of the characteristic dhokla flavour, while the yeasts produce folic acid, raise the batter volume and give sponginess to the product (Ray et al. 2016). The fermented batter is poured into a steamer and steamed until soft to obtain a spongy cake. Dhokla is consumed as a snack or breakfast food for all age groups (Das et al. 2012).
Treatments using one variable-at-a-time are less effective, more expensive, fail to determine interaction between the variables and consume more time which might lead to misinterpretation of the results (Nwabueze 2010). Therefore, an effective process optimisation method is necessary to reduce antinutrients. For this purpose, response surface methodology (RSM) can be deployed to study the influence of various factors on response. So, the present work aimed to study a relationship between the various process parameters and their optimisation during dhokla preparation using RSM to reduce antinutrients without unduly affecting sensory attributes.
Materials and methods
Materials
Bengalgram dal and rice, procured from a retailer at Siliguri, were hand-sorted to remove dirt and other unwanted materials, and stored in an aluminium container for a period of maximum 1 month.
Experimental design
The design consisted of a series of experimental runs, fitting mathematical models and finally selecting variable levels by optimising the response. Central composite rotatable designs (CCRDs) for soaking, fermentation and steaming at five-level combinations were used.
The soaking stage consisted of 30 experimental runs, 16 factorial points, eight axial points and six replicates at central points (Tables 1 and 2). The experiments were conducted, and the predicted optimum soaking condition as produced by Design Expert v. 8.0 (Stat-Ease Inc., Minneapolis, MN, USA) was selected.
Table 1.
Antinutrients of bengalgram dal under different soaking conditions as per central composite design
| Run | Processing variable* | Final pH | Response (per gram dry wt) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dal:water (w/w) | t (h) | T (°C) | Initial pH | TC (mg) | PAC (mg) | TIA (kU†) | HA (kU‡) | TBAC (µg) | ||
| 1 | 1:5 (+ 1) | 10 (− 1) | 20 (− 1) | 7.0 (+ 1) | 6.9 ± 0 | 1.06 ± 0.03 | 1.3 ± 0 | 11.1 ± 0.1 | 2.6 ± 0 | 363 ± 2 |
| 2 | 1:5 (+ 1) | 10 (− 1) | 40 (+ 1) | 7.0 (+ 1) | 6.8 ± 0 | 0.96 ± 0.03 | 1.2 ± 0.1 | 10.8 ± 0.1 | 1.7 ± 0.4 | 433 ± 5 |
| 3 | 1:3 (− 1) | 20 (+ 1) | 40 (+ 1) | 5.0 (− 1) | 4.6 ± 0 | 0.49 ± 0.06 | 1.1 ± 0 | 8.1 ± 0.3 | 1.3 ± 0.4 | 404 ± 1 |
| 4 | 1:5 (+ 1) | 20 (+ 1) | 40 (+ 1) | 7.0 (+ 1) | 6.7 ± 0.2 | 0.48 ± 0.03 | 1.1 ± 0 | 8.1 ± 0.4 | 1.3 ± 0.4 | 393 ± 6 |
| 5 | 1:4 (0) | 15 (0) | 30 (0) | 4.0 (− α) | 3.9 ± 0 | 0.59 ± 0.00 | 1.2 ± 0 | 9.1 ± 0.1 | 1.7 ± 0 | 436 ± 2 |
| 6 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.7 ± 0 | 0.58 ± 0.03 | 1.2 ± 0 | 9.7 ± 0.1 | 1.7 ± 0.4 | 387 ± 4 |
| 7 | 1:5 (+ 1) | 20 (+ 1) | 40 (+ 1) | 5.0 (− 1) | 4.6 ± 0 | 0.46 ± 0.03 | 1.1 ± 0 | 8.1 ± 0.3 | 1.3 ± 0.4 | 401 ± 6 |
| 8 | 1:3 (− 1) | 20 (+ 1) | 40 (+ 1) | 7.0 (+ 1) | 6.7 ± 0 | 0.49 ± 0.00 | 1.1 ± 0 | 8.1 ± 0.3 | 1.3 ± 0.4 | 407 ± 2 |
| 9 | 1:3 (− 1) | 20 (+ 1) | 20 (− 1) | 7.0 (+ 1) | 6.7 ± 0 | 0.56 ± 0.03 | 1.1 ± 0 | 8.1 ± 0.1 | 1.7 ± 0.4 | 348 ± 7 |
| 10 | 1:6 (+ α) | 15 (0) | 30 (0) | 6.0 (0) | 5.6 ± 0 | 0.46 ± 0.03 | 1.1 ± 0 | 8.9 ± 0 | 1.7 ± 0.4 | 371 ± 4 |
| 11 | 1:5 (+ 1) | 10 (− 1) | 40 (+ 1) | 5.0 (− 1) | 4.8 ± 0.1 | 0.96 ± 0.03 | 1.2 ± 0 | 10.6 ± 0 | 1.7 ± 0.4 | 432 ± 5 |
| 12 | 1:4 (0) | 5 (− α) | 30 (0) | 6.0 (0) | 6.0 ± 0 | 1.70 ± 0.03 | 1.5 ± 0 | 12.1 ± 0 | 5.1 ± 0 | 425 ± 3 |
| 13 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.7 ± 0 | 0.49 ± 0.00 | 1.2 ± 0 | 9.2 ± 0.3 | 2.1 ± 0.4 | 372 ± 4 |
| 14 | 1:3 (− 1) | 10 (− 1) | 20 (− 1) | 7.0 (+ 1) | 6.9 ± 0 | 1.16 ± 0.03 | 1.3 ± 0 | 11.1 ± 0.1 | 2.6 ± 0 | 366 ± 5 |
| 15 | 1:3 (− 1) | 20 (+ 1) | 20 (− 1) | 5.0 (− 1) | 4.6 ± 0 | 0.56 ± 0.03 | 1.1 ± 0.1 | 8.1 ± 0 | 1.7 ± 0.4 | 353 ± 7 |
| 16 | 1:4 (0) | 15 (0) | 10 (− α) | 6.0 (0) | 5.9 ± 0 | 1.16 ± 0.07 | 1.2 ± 0 | 11.2 ± 0 | 2.1 ± 0 | 376 ± 5 |
| 17 | 1:4 (0) | 15 (0) | 50 (+ α) | 6.0 (0) | 5.4 ± 0 | 1.16 ± 0.03 | 1.1 ± 0 | 9.5 ± 0.1 | 1.1 ± 0.4 | 443 ± 5 |
| 18 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.6 ± 0 | 0.52 ± 0.03 | 1.2 ± 0.1 | 9.8 ± 0.5 | 2.1 ± 0 | 387 ± 4 |
| 19 | 1:4 (0) | 25 (+ α) | 30 (0) | 6.0 (0) | 5.5 ± 0 | 0.46 ± 0.03 | 1.1 ± 0.1 | 8.1 ± 0 | 1.3 ± 0.4 | 345 ± 2 |
| 20 | 1:4 (0) | 15 (0) | 30 (0) | 8.0 (+ α) | 7.9 ± 0.2 | 0.56 ± 0.03 | 1.2 ± 0 | 9.3 ± 0.4 | 1.7 ± 0.4 | 368 ± 6 |
| 21 | 1:5 (+ 1) | 20 (+ 1) | 20 (− 1) | 5.0 (− 1) | 4.7 ± 0 | 0.59 ± 0.10 | 1.1 ± 0 | 8.2 ± 0 | 1.7 ± 0.4 | 353 ± 5 |
| 22 | 1:5 (+ 1) | 10 (− 1) | 20 (− 1) | 5.0 (− 1) | 4.7 ± 0 | 1.06 ± 0.03 | 1.3 ± 0 | 11.1 ± 0.1 | 2.6 ± 0 | 390 ± 6 |
| 23 | 1:3 (− 1) | 10 (− 1) | 40 (+ 1) | 7.0 (+ 1) | 6.8 ± 0 | 1.04 ± 0.03 | 1.3 ± 0 | 10.8 ± 0 | 1.7 ± 0.4 | 436 ± 6 |
| 24 | 1:5 (+ 1) | 20 (+ 1) | 20 (− 1) | 7.0 (+ 1) | 6.7 ± 0 | 0.59 ± 0.03 | 1.1 ± 0 | 8.1 ± 0 | 1.7 ± 0.4 | 349 ± 5 |
| 25 | 1:3 (− 1) | 10 (− 1) | 40 (+ 1) | 5.0 (− 1) | 4.8 ± 0 | 1.06 ± 0.03 | 1.3 ± 0 | 10.9 ± 0 | 1.7 ± 0.4 | 436 ± 2 |
| 26 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.7 ± 0.2 | 0.59 ± 0.10 | 1.2 ± 0 | 9.5 ± 0.3 | 1.7 ± 0.4 | 389 ± 3 |
| 27 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.7 ± 0 | 0.50 ± 0.03 | 1.2 ± 0 | 9.7 ± 0 | 1.7 ± 0 | 398 ± 4 |
| 28 | 1:4 (0) | 15 (0) | 30 (0) | 6.0 (0) | 5.7 ± 0 | 0.50 ± 0.09 | 1.2 ± 0 | 9.6 ± 0.3 | 2.1 ± 0.4 | 382 ± 4 |
| 29 | 1:2 (− α) | 15 (0) | 30 (0) | 6.0 (0) | 5.8 ± 0 | 0.76 ± 0.03 | 1.3 ± 0 | 10.1 ± 0.1 | 2.1 ± 0.4 | 431 ± 5 |
| 30 | 1:3 (− 1) | 10 (− 1) | 20 (− 1) | 5.0 (− 1) | 4.7 ± 0 | 1.10 ± 0.03 | 1.3 ± 0 | 11.2 ± 0 | 2.6 ± 0 | 380 ± 4 |
Values are mean ± SE of three determinations
*Low, center and high levels of each variable were designated as – 1, 0 and + 1, respectively; α (axial distance from the central point) = 1.682
†One unit (U) was defined as a decrease in A280 of 0.01, relative to the blank, in 20 min using a 10 mL assay volume
‡One unit (U) was defined as the least amount of haemagglutinin which produced positive agglutination (1+) under the experimental condition
Table 2.
Antinutrients of rice under different soaking conditions as per central composite design
| Run | Processing variable* | Final pH | Response (per gram dry wt) | |||||
|---|---|---|---|---|---|---|---|---|
| Rice:water (w/w) | t (h) | T (°C) | Initial pH | TC† (mg) | PAC (mg) | TBAC (µg) | ||
| 1 | 1:1 (− α) | 12 (0) | 26 (0) | 6.0 (0) | 5.8 ± 0 | 0.06 ± 0.03 | 1.3 ± 0 | 81 ± 2.2 |
| 2 | 1:7 (0) | 12 (0) | 4 (− α) | 6.0 (0) | 5.9 ± 0 | 0.03 ± 0.03 | 1.1 ± 0 | 76 ± 1.7 |
| 3 | 1:7 (0) | 24 (+ α) | 26 (0) | 6.0 (0) | 4.9 ± 0 | < dl | 0.9 ± 0 | 78 ± 2.9 |
| 4 | 1:10 (+ 1) | 6 (− 1) | 37 (+ 1) | 4.0 (− 1) | 3.8 ± 0 | < dl | 1.3 ± 0 | 98 ± 0 |
| 5 | 1:10 (+ 1) | 6 (− 1) | 37 (+ 1) | 8.0 (+ 1) | 6.4 ± 0 | 0.03 ± 0.03 | 1.3 ± 0 | 79 ± 0.9 |
| 6 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.8 ± 0 | < dl | 1.4 ± 0 | 77 ± 2.3 |
| 7 | 1:7 (0) | 12 (0) | 26 (0) | 10.0 (+ α) | 6.2 ± 0 | 0.03 ± 0.03 | 1.3 ± 0 | 79 ± 1.0 |
| 8 | 1:10 (+ 1) | 6 (− 1) | 15 (− 1) | 8.0 (+ 1) | 6.6 ± 0 | < dl | 1.3 ± 0 | 75 ± 0 |
| 9 | 1:7 (0) | 0 (− α) | 26 (0) | 6.0 (0) | 6.1 ± 0 | 0.06 ± 0.03 | 1.4 ± 0 | 76 ± 1.0 |
| 10 | 1:4 (− 1) | 18 (+ 1) | 37 (+ 1) | 4.0 (− 1) | 3.3 ± 0 | < dl | 1.4 ± 0 | 96 ± 1.2 |
| 11 | 1:10 (+ 1) | 18 (+ 1) | 37 (+ 1) | 4.0 (− 1) | 3.2 ± 0 | < dl | 1.1 ± 0 | 86 ± 0.9 |
| 12 | 1:10 (+ 1) | 18 (+ 1) | 15 (− 1) | 8.0 (+ 1) | 6.6 ± 0 | < dl | 1.2 ± 0 | 73 ± 1.5 |
| 13 | 1:4 (− 1) | 6 (− 1) | 15 (− 1) | 8.0 (+ 1) | 6.7 ± 0 | 0.06 ± 0.03 | 1.5 ± 0 | 80 ± 0 |
| 14 | 1:4 (− 1) | 6 (− 1) | 15 (− 1) | 4.0 (− 1) | 3.8 ± 0 | 0.03 ± 0 | 1.6 ± 0 | 87 ± 0.8 |
| 15 | 1:10 (+ 1) | 6 (− 1) | 15 (− 1) | 4.0 (− 1) | 3.8 ± 0 | 0.03 ± 0.03 | 1.3 ± 0 | 82 ± 0.7 |
| 16 | 1:10 (+ 1) | 18 (+ 1) | 15 (− 1) | 4.0 (− 1) | 3.7 ± 0.1 | < dl | 0.7 ± 0 | 78 ± 1.0 |
| 17 | 1:7 (0) | 12 (0) | 48 (+ α) | 6.0 (0) | 4.4 ± 0 | 0.03 ± 0.03 | 1.0 ± 0 | 95 ± 1.1 |
| 18 | 1:7 (0) | 12 (0) | 26 (0) | 2.0 (− α) | 3.0 ± 0 | < dl | 1.2 ± 0 | 97 ± 1.7 |
| 19 | 1:10 (+ 1) | 18 (+ 1) | 37 (+ 1) | 8.0 (+ 1) | 5.9 ± 0 | < dl | 1.0 ± 0 | 81 ± 0.9 |
| 20 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | 0.06 ± 0.03 | 1.1 ± 0 | 73 ± 6.0 |
| 21 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | 0.03 ± 0 | 1.3 ± 0 | 74 ± 0 |
| 22 | 1:4 (− 1) | 6 (− 1) | 37 (+ 1) | 8.0 (+ 1) | 6.6 ± 0 | 0.06 ± 0.03 | 1.4 ± 0 | 84 ± 0.2 |
| 23 | 1:13 (+ α) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | < dl | 0.9 ± 0 | 80 ± 0.1 |
| 24 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | < dl | 0.9 ± 0 | 80 ± 0 |
| 25 | 1:4 (− 1) | 6 (− 1) | 37 (+ 1) | 4.0 (− 1) | 3.7 ± 0 | 0.03 ± 0.03 | 1.4 ± 0.1 | 96 ± 0.2 |
| 26 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | 0.06 ± 0.03 | 1.0 ± 0 | 73 ± 0.9 |
| 27 | 1:4 (− 1) | 18 (+ 1) | 15 (− 1) | 8.0 (+ 1) | 6.6 ± 0 | < dl | 1.1 ± 0 | 82 ± 0.3 |
| 28 | 1:7 (0) | 12 (0) | 26 (0) | 6.0 (0) | 5.9 ± 0 | < dl | 1.1 ± 0 | 79 ± 0.1 |
| 29 | 1:4 (− 1) | 18 (+ 1) | 15 (− 1) | 4.0 (− 1) | 3.8 ± 0 | < dl | 1.5 ± 0 | 79 ± 0.1 |
| 30 | 1:4 (− 1) | 18 (+ 1) | 37 (+ 1) | 8.0 (+ 1) | 6.0 ± 0 | < dl | 1.4 ± 0 | 81 ± 0.4 |
Values are mean ± SE of three determinations
*Low, center and high levels of each variable were designated as − 1, 0 and + 1, respectively; α (axial distance from the central point) = 1.682
†dl (detection limit), 0.003 mg/g dry wt
The optimally soaked dal and rice were ground (Bajaj Electricals, Mumbai, India), mixed in different ratios of dal batter-to-rice slurry (4:1–1:4 v/v) with addition of common salt (8 g/kg batter), fermented at 32 °C for 15 h and steamed for 15 min (Moktan et al. 2011). The prepared dhokla was subjected to sensory analysis. This was carried out in two sets, one for the selection of unfermented mixed batter ratio and the other for evaluation of sensory attributes of optimally prepared dhokla. For the selection of ratio, the product (dhokla) was evaluated by a panel of 10 trained judges (regular consumers of dhokla, having knowledge of product defects and scoring patterns) using a 100-point score card.
The selected ratio of ingredients in unfermented mixed batter, producing highest-scored dhokla, was used for optimisation of the subsequent fermentation stage considering three independent variables. The fermentation stage consisted of 20 experimental runs with eight factorial points, six axial points and six replicates at the central points (Table 3). After performing the experiment, the predicted optimum fermentation condition was selected. In addition, pH, acidity, microbial load, such as total aerobic mesophilic bacterial, LAB and yeast count in unfermented mixed batter and fermented batter according to CCRD were also evaluated to study their effect on antinutrients and sensory quality of dhokla.
Table 3.
Antinutrients, pH, titratable acidity and microbial load in mixed batter, fermented as per central composite design
| Run | Processing variable* | Response (per gram dry wt) | Final pH | TA (%) | Microbial load (Log cfu/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added salt (g/kg) | t (h) | T (°C) | TC (mg) | PAC (mg) | TIA (kU) | HA (kU) | TBAC (µg) | TAMB | LAB | Yeasts | |||
| 1 | 1.6 (– α) | 20 (0) | 31.5 (0) | 0.10 ± 0.03 | 0.33 ± 0.02 | 2.2 ± 0.3 | 0.19 ± 0.02 | 411 ± 2 | 4.8 ± 0 | 0.06 ± 0.01 | 7.2 ± 0 | 8.2 ± 0 | 5.2 ± 0 |
| 2 | 18.4 (+ α) | 20 (0) | 31.5 (0) | 0.12 ± 0 | 0.34 ± 0.02 | 3.1 ± 0 | 0.16 ± 0.01 | 336 ± 2 | 4.9 ± 0 | 0.05 ± 0 | 7.1 ± 0 | 8.1 ± 0 | 4.9 ± 0 |
| 3 | 10 (0) | 20 (0) | 42.4 (+ α) | 0.06 ± 0.01 | 0.30 ± 0 | 2.0 ± 0 | 0.09 ± 0.01 | 391 ± 2 | 4.3 ± 0 | 0.08 ± 0 | 7.2 ± 0 | 8.1 ± 0 | 4.8 ± 0 |
| 4 | 5 (− 1) | 30 (+ 1) | 38 (+ 1) | 0.06 ± 0.01 | 0.25 ± 0 | 1.9 ± 0 | 0.06 ± 0 | 501 ± 3 | 4.4 ± 0 | 0.08 ± 0 | 7.1 ± 0 | 8.3 ± 0.1 | 5.1 ± 0 |
| 5 | 15 (+ 1) | 30 (+ 1) | 25 (− 1) | 0.09 ± 0 | 0.29 ± 0 | 2.1 ± 0.1 | 0.10 ± 0 | 406 ± 2 | 4.5 ± 0 | 0.05 ± 0.02 | 7.2 ± 0 | 8.2 ± 0 | 5.1 ± 0 |
| 6 | 10 (0) | 20 (0) | 31.5 (0) | 0.12 ± 0.03 | 0.42 ± 0.03 | 3.1 ± 0.1 | 0.26 ± 0.02 | 363 ± 3 | 4.9 ± 0 | 0.06 ± 0.02 | 7.2 ± 0 | 8.1 ± 0 | 5.1 ± 0 |
| 7 | 10 (0) | 3.2 (– α) | 31.5 (0) | 0.29 ± 0 | 1.00 ± 0 | 5.0 ± 0 | 0.90 ± 0.03 | 273 ± 3 | 5.6 ± 0 | 0.04 ± 0.01 | 6.1 ± 0 | 6.1 ± 0 | 3.1 ± 0 |
| 8 | 10 (0) | 36.8 (+ α) | 31.5 (0) | 0.03 ± 0 | 0.23 ± 0 | 1.9 ± 0 | 0.06 ± 0 | 533 ± 6 | 3.1 ± 0 | 0.16 ± 0.01 | 7.4 ± 0 | 8.4 ± 0.1 | 5.3 ± 0.1 |
| 9 | 10 (0) | 20 (0) | 31.5 (0) | 0.12 ± 0.03 | 0.43 ± 0.01 | 2.3 ± 0 | 0.19 ± 0.01 | 354 ± 6 | 4.9 ± 0 | 0.06 ± 0 | 7.2 ± 0 | 8.1 ± 0 | 5.1 ± 0 |
| 10 | 10 (0) | 20 (0) | 31.5 (0) | 0.09 ± 0.03 | 0.34 ± 0.01 | 2.3 ± 0.3 | 0.18 ± 0.01 | 363 ± 5 | 4.9 ± 0 | 0.07 ± 0 | 7.2 ± 0 | 8.1 ± 0 | 5.1 ± 0 |
| 11 | 15 (+ 1) | 10 (− 1) | 25 (− 1) | 0.19 ± 0 | 0.44 ± 0.01 | 3.4 ± 0.2 | 0.34 ± 0.02 | 232 ± 7 | 4.9 ± 0 | 0.05 ± 0 | 6.9 ± 0 | 8.1 ± 0 | 4.8 ± 0 |
| 12 | 10 (0) | 20 (0) | 31.5 (0) | 0.19 ± 0 | 0.30 ± 0 | 3.1 ± 0 | 0.18 ± 0.01 | 356 ± 2 | 4.9 ± 0 | 0.06 ± 0 | 7.2 ± 0 | 8.1 ± 0 | 5.1 ± 0 |
| 13 | 10 (0) | 20 (0) | 20.6 (– α) | 0.19 ± 0.02 | 0.43 ± 0 | 3.4 ± 0 | 0.08 ± 0 | 316 ± 2 | 5.1 ± 0 | 0.05 ± 0 | 7.2 ± 0 | 8.1 ± 0 | 5.2 ± 0 |
| 14 | 10 (0) | 20 (0) | 31.5 (0) | 0.10 ± 0 | 0.44 ± 0 | 2.3 ± 0 | 0.27 ± 0.02 | 356 ± 6 | 4.9 ± 0 | 0.07 ± 0 | 7.2 ± 0 | 8.2 ± 0 | 5.1 ± 0 |
| 15 | 5 (− 1) | 10 (− 1) | 25 (− 1) | 0.19 ± 0 | 0.42 ± 0.04 | 3.6 ± 0.1 | 0.43 ± 0.03 | 319 ± 4 | 4.8 ± 0 | 0.06 ± 0 | 6.6 ± 0 | 8.2 ± 0 | 5.1 ± 0.1 |
| 16 | 15 (+ 1) | 30 (+ 1) | 38 (+ 1) | 0.06 ± 0.01 | 0.26 ± 0.03 | 1.9 ± 0.1 | 0.08 ± 0 | 483 ± 6 | 4.5 ± 0 | 0.07 ± 0 | 7.2 ± 0 | 8.2 ± 0 | 5.1 ± 0 |
| 17 | 15 (+ 1) | 10 (− 1) | 38 (+ 1) | 0.12 ± 0 | 0.40 ± 0.04 | 2.8 ± 0.1 | 0.42 ± 0.03 | 280 ± 4 | 4.9 ± 0 | 0.05 ± 0 | 6.9 ± 0 | 8.2 ± 0 | 5.0 ± 0 |
| 18 | 10 (0) | 20 (0) | 31.5 (0) | 0.10 ± 0 | 0.26 ± 0 | 3.1 ± 0 | 0.23 ± 0.02 | 357 ± 2 | 4.9 ± 0 | 0.07 ± 0.01 | 7.2 ± 0 | 8.1 ± 0 | 5.2 ± 0 |
| 19 | 5 (− 1) | 30 (+ 1) | 25 (− 1) | 0.09 ± 0.01 | 0.28 ± 0 | 2.1 ± 0 | 0.09 ± 0 | 460 ± 2 | 4.5 ± 0 | 0.05 ± 0 | 7.1 ± 0 | 8.4 ± 0 | 5.2 ± 0 |
| 20 | 5 (− 1) | 10 (− 1) | 38 (+ 1) | 0.12 ± 0.03 | 0.40 ± 0.02 | 2.0 ± 0.2 | 0.44 ± 0.03 | 327 ± 3 | 4.8 ± 0 | 0.06 ± 0 | 7.0 ± 0 | 8.2 ± 0 | 5.1 ± 0 |
| Unf† | 5.61 ± 0 | 0.042 ± 0.01 | 6.12 ± 0 | 6.02 ± 0 | 3.12 ± 0 | ||||||||
| Opt‡ | 4.72 ± 0 | 0.101 ± 0.01 | 7.21 ± 0 | 8.31 ± 0 | 5.11 ± 0 | ||||||||
Values are mean ± SE of three determinations. Means, followed by different superscripted numbers in each column for unfermented mixed batter (Unf.) and optimally fermented batter (Opt.), differ significantly (P < 0.05), as determined by Student’s t test
*Low, center and high levels of each variable were designated as − 1, 0 and + 1, respectively; α (axial distance from the central point) = 1.682
†Unfermented mixed batter prepared from optimally soaked dal and rice (3:1 v/v)
‡Batter, fermented under optimum condition (added salt, 8 g/kg; fermentation temp., 32 °C; fermentation time, 18 h)
The optimally fermented batter was used for optimisation of the final steaming stage which consisted of single independent variable (steaming time) having seven experimental runs. The relationship between independent variables and response was visualised by response surface 3D plots, generated for each of the processing stages.
Numerical optimisation
Numerical optimisation technique was followed to determine the optimum conditions of processing variables after model fitting and generation of polynomial regression equation for response variables. The numerical optimisation finds a point that maximises the desirability function and gives different desirability values. A desirability function determines the level of independent variables that give optimum response. Values closer to 1 are more desirable.
Model verification
Experiments were conducted under the predicted optimum conditions to verify adequacy of response surface models. The experimental and predicted values were compared to check validity of the models by calculating percentage deviation error.
Sample preparation
Raw dal and rice were powdered, while soaked dal and rice were made to paste using a blender. Soak water of both dal and rice were collected for the determination of pH. The dal–rice mixed (unfermented and fermented) batters were used in two sets, one for the estimation of viable cell count, pH and total titratable acidity; the other part of batters, raw powder, paste and macerated dhokla were kept overnight at − 20 °C, lyophilised (Eyela freeze dryer, FDU-506, Tokyo Rikakikai, Tokyo, Japan) and powdered for the estimation of antinutrients.
Estimation of antinutrients
The methods described by Price et al. (1978), Wheeler and Ferrel (1971), Kakade et al. (1969), Liener and Hill (1953) and Yeh et al. (2006), were followed for the estimation of tannins content (TC), phytic acid content (PAC), trypsin inhibitor activity (TIA), haemagglutinating activity (HA) and total biogenic amines content (TBAC), respectively.
Viable cell count
Samples (10 g) of unfermented mixed and fermented dhokla batters were homogenised with 90 mL sterile peptone-physiological saline (1 g/L neutral peptone, 8.5 g/L sodium chloride, pH 7.2) using a stomacher lab-blender 400 (Seward Medical, London, UK) at ‘normal’ speed for 1 min. Suitable dilutions (1 mL each) were pour-plated on plate count agar (M091A; HiMedia Laboratories, Mumbai, India) for the determination of total aerobic mesophilic bacterial count and tryptone glucose yeast extract agar (HiMedia M014) supplemented with 10 IU/mL benzylpenicillin and 12 μg/mL streptomycin sulphate for yeast count. While both types of plates were incubated at 37 °C for 24 h for total aerobic mesophilic bacterial count and yeast count, the MRS agar (HiMedia M641) plates were incubated at 30 °C for 48 h in an anaerobic culture jar (HiMedia LE002A) for the LAB count.
pH and total titratable acidity
The methods described in AOAC (1990) were followed for the determination of pH and total titratable acidity. The analyses were done in triplicate.
The pH of soak water of both dal and rice under different experimental conditions was measured using a CyberScan pH 510 meter (Eutech Instruments, Thermo Fisher Scientific, Mumbai). For pH of mixed batter, a 100 g-sample was mixed with 20 mL of carbon dioxide-free distilled water for 1 min and equilibrated to 25 °C before measuring pH.
Titratable acidity of sample was determined by taking 10 g-sample and blending with 90 mL of carbon dioxide-free distilled water for 1 min. The mixture was filtered. About 25 mL of the filtrate was titrated with 0.1 mol/L sodium hydroxide to an end point of phenolphthalein.
| 1 |
Statistical analysis
Regression analysis and analysis of variance (ANOVA) were conducted for fitting the models and to examine statistical significance of the model terms using a second order polynomial equation. Adequacy of the models was determined using R2 (coefficient of determination) and lack-of-fit test. The significance of the linear, quadratic and interaction effects of independent variables on response was determined at 5% level of confidence.
Results and discussion
Raw ingredients
Compared to raw rice, raw dal contained an appreciable amount of antinutrients (Table 4). Their levels in dried raw dal were in close proximity to the values reported earlier in bengalgram and rice. In bengalgram, PAC, TIA and HA were 1.21 mg, 15–19 kU and 6.22 kU, respectively (Alajaji and El-Adawy 2006; Guillamόn et al. 2008). Rice contained no TIA and HA (Table 4). The values of TC and PAC were similar to the those reported by Hemalatha et al. (2007), where the respective values were 0.05 and 1.6 mg, respectively, per gram dry wt.
Table 4.
Antinutrients in dal and rice at optimum condition of each stage of dhokla preparation
| Parameter | Antinutrient (per gram dry wt)* | ||||
|---|---|---|---|---|---|
| TC (mg) | PAC (mg) | TIA (kU) | HA (kU) | TBAC (μg) | |
| Raw dal | 2.2a ± 0.09 | 1.7a ± 0.1 | 16a ± 0 | 5a ± 0 | 423a ± 2 |
| Soaking (raw dal–water of 1:5 w/w, pH 7.0, 23 °C, 20 h) | |||||
| Predicted | 0.46 | 1.1 | 8 | 2 | 347 |
| Experimental | 0.46b ± 0.03 | 1.1b ± 0 | 8b ± 0 | 2b ± 0 | 348b ± 2 |
| Error% | 0 | 0 | 0 | 0 | 0.29 |
| % change† | − 79.1 | − 35.3 | − 50 | − 60 | − 18 |
| Raw rice | 0.06x ± 0.03 | 1.5x ± 0 | < dl | < dl | 70y ± 4 |
| Soaking (raw rice–water of 1:5 w/w, pH 5.6, 16 °C, 18 h) | |||||
| Predicted | 0.01 | 1.2 | 0 | 0 | 75 |
| Experimental | < dly | 1.1x ± 0 | < dl | < dl | 76x ± 2 |
| Error% | 0 | 9.1 | 0 | 0 | 1.3 |
| % change† | − 83 | − 20 | 0 | 0 | 7 |
| Unfermented mixed batter (dal batter:rice slurry; 3:1 v/v) | |||||
| Experimental | 0.30 ± 0 | 1.11 ± 0 | 61 ± 0 | 1 ± 0 | 2702 ± 1 |
| Fermentation (salt concentration of 8 g/kg, 32 °C 18 h) | |||||
| Predicted | 0.13 | 0.4 | 3 | 0.3 | 355 |
| Experimental | < dl | 0.42 ± 0 | 32 ± 0 | < dl | 3671 ± 1 |
| Error% | 0 | 0 | 0 | 0 | 3.3 |
| % change† | − 57 | − 64 | − 50 | − 70 | 31 |
| Steaming (20 min) | |||||
| Predicted | 0 | 0.1 | 1 | 0 | 267 |
| Experimental | < dl | 0.13 ± 0 | 13 ± 0 | < dl | 2692 ± 2 |
| Error% | 0 | 0 | 0 | 0 | 0.74 |
| % change† | − 100 (− 100) | − 91 (− 75) | − 83 (− 67) | − 100 (− 100) | − 1 (− 25) |
Values are mean ± SE of triplicate determinations in raw/processed samples. Means followed by different superscripts in each column for raw and soaked dal (a and b)/rice (x and y) differ significantly (P < 0.05) as determined by t test, and for unfermented, fermented and steamed dal–rice mixture (1, 2 and 3) differ significantly (P < 0.05) as determined by Duncan multiple range test
*dl (detection limit), 0.003 mg/g dry wt for TC; 0.015 kU/g dry wt for TIA; 0.007 kU/g dry wt for HA
†− and + indicate percent decrease and increase, respectively, of predicted values over raw dal/rice for the soaking stage, and over unfermented mixed batter for fermentation and steaming stages (values within parentheses indicate percentages calculated over the predicted values of fermented batter)
Soaking
The models for soaking stage (Table 1) were analysed using ANOVA. They had significant (P < 0.05) F-value and insignificant (P > 0.05) lack-of-fit, indicating models to be accurate for prediction. The R2-values for TC, PAC, TIA, HA and TBAC were 0.97, 0.90, 0.90, 0.79 and 0.82, respectively. High R2-values indicate that variability of all the responses was well explained by the models which had a good fit. The reduced polynomial equations generated indicate the effect of soaking of dal:
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
where A was dal–water ratio, B was soaking time, C was soaking temperature and D was initial pH of soaking water. The Eqs. (2–6) indicate a linear effect of independent variables of soaking on antinutrients.
Similarly, the models for rice soaking (Table 2) were subjected to ANOVA which showed an insignificant lack-of-fit. The models explained 50% of the variation of TC and PAC. In case of TBAC, the model explained 92% of the variation. The polynomial equation generated after removing insignificant terms of the models were:
| 7 |
| 8 |
| 9 |
where A was rice–water ratio, B was soaking time, C was soaking temperature and D was initial pH of soaking water.
The Eqs. 7 and 8 indicate a linear effect, while the Eq. 9 indicates an interaction effect of independent variables of soaking on antinutrients.
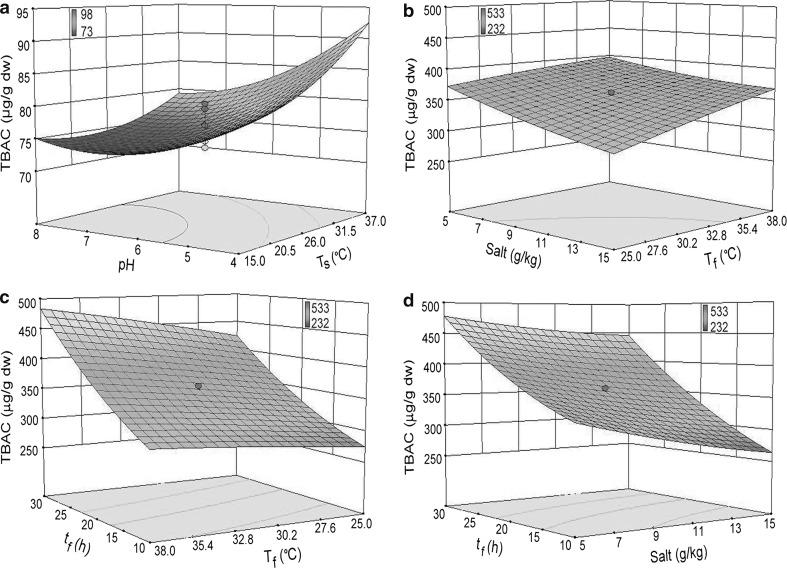
The predicted values revealed minimum TC of 0.37 mg/g dry wt when the dal–water ratio was increased from 1:3 w/w to 1:5 w/w and soaking time was increased from 10 to 20 h, keeping temperature and initial pH of soaking water fixed at 30 °C and 6.0, respectively. When rice–water ratio was increased from 1:4 w/w to 1:10 w/w and soaking time was increased from 6 to 10 h, the TC reduced below the detection limit. An increase in dal/rice-to-water ratio and soaking time might have caused the softening of dal/grains and simultaneous leaching of tannins into the soaking medium. An increase in ratio and time might have enhanced leaching of tannins from dal (beans having no external barrier, i.e. seed coat) into the soak water (Sharma et al. 2017). Further, the activation of polyphenol oxidase might be the cause of its reduction (Khandelwal et al. 2010). The reduction of TC in the present study was almost four times (83%) higher compared to the value (22%) reported by Khandelwal et al. (2010) in bengalgram. Besides, in soaked redgram, greengram and lentil, the reduction was 23, 39 and 24%, respectively (Khandelwal et al. 2010). The minimum PAC of dal was 1.07 mg/g dry wt, when dal was soaked at a ratio of 1:4 w/w for 20 h at 40 °C and initial pH 6.0. Hydrolysis of phytate, during soaking, is greatly influenced by temperature and pH (Greiner and Konietzny 1999). The PAC of rice was minimised to 0.98 mg/g dry wt at 1:10 w/w of rice–water when soaked for 18 h. Hydration of seeds, activation of phytase and diffusion of the products might be responsible for the reduction of PAC (Kumar et al. 2010). The minimum TIA was 8.2 kU/g dry wt, when dal was soaked in 1:5 w/w ratio for 20 h, at 30 °C and initial pH 6.0. In the present study 49% reduction of TIA was observed, the value which is much higher than already reported TIA levels (9%) in chickpea (El-Hady and Habiba 2003). High reduction of TIA in this study might be governed by the difference in the seed coat permeability of dehulled seeds used. Since trypsin inhibitors are low-molecular weight, they get easily diffused into the soaking medium (Grewal and Jood 2006). HA was minimised to 1.4 kU/g dry wt, by soaking dal at 40 °C for 20 h. The reduction in HA was in line with an earlier report in different legumes (Kalpanadevi and Mohan 2013). Incomplete destruction of HA might be due to the presence of high levels of other antinutrients which may interfere with the lectin destruction (Kalpanadevi and Mohan 2013). Minimum value of TBAC (370 μg/g dry wt) was obtained when dal was soaked at a ratio of 1:5 w/w for 20 h at 30 °C and pH 6.0. An interaction of soaking temperature and initial pH showed minimum level of TBAC in soaked rice (74 µg/g dry wt) at 20.5 °C and pH 7.0, when rice–water ratio and soaking time were kept constant at 1:7 w/w and 12 h, respectively (Fig. 1a). During soaking, pH is an important factor influencing microbial decarboxylase activity; pH 3.0–6.0 is considered optimum for bacteria to produce decarboxylase that induces formation of biogenic amines in soaked products (Silla Santos 1996). Low temperature adversely affects proteolytic and decarboxylating reactions, resulting in a decreased amine concentration (Joosten and van Boekel 1988; Sharma et al. 2015). Besides, the reduction might be due to leaching of amines in the soaking medium.
Fig. 1.
Response surface 3D plots showing significant effect of independent variables on total biogenic amines content: a influence of rice soaking temperature (Ts) and initial pH when rice–water ratio and rice soaking time (ts) were constant at 1:7 w/w and 12 h, respectively; b influence of added salt concentration and fermentation temperature (Tf) when fermentation time (tf) was constant at 20 h; c influence of tf and Tf when salt concentration was constant at 10 g/kg mixed dal–rice batter; d influence of salt concentration and tf when Tf was constant at 31.5 °C
After numerical optimisation, the optimum condition for soaking of dal was 1:5 w/w of dal-to-water ratio for 20 h at 23 °C and initial pH 7.0 of soak water. For soaking of rice, the optimum condition was 1:5 w/w for 18 h at 16 °C and initial pH 5.6 of soak water. The predicted reduction of antinutrients from raw ingredients is shown in Table 4.
The reduction of antinutrients might also be related to the change in pH of soaked water. The initial pH for dal ranged from 4 to 8 and that for rice ranged from 2 to 10. The final pH of soak water of dal was 3.9–7.9 (run numbers: 20–5; Table 1) and that of rice was 3.0–6.7 (run numbers: 18–13; Table 2). The decrease in pH might be due to accumulation of organic acids produced due to the growth of microbiota during soaking (Ashenafi and Busse 1991).
Unfermented mixed batter
The mixed batter, prepared from optimally soaked dal and rice, had TC, PAC, TIA, HA and TBAC of 0.3 mg, 1.1 mg, 6 kU, 1 kU and 270 µg, respectively, per gram dry wt (Table 4). Compared to dal, rice contained a negligible amount of antinutrients. Since in mixed batter dal constituted three-fourth by volume, the level of antinutrients in mixed batter was correspondingly lower than that in soaked dal only.
Fermentation
The levels of antinutrients during fermentation are shown in Table 3. The model showed an insignificant lack-of-fit. The R2-values for TC, PAC, TIA, HA and TBAC were 0.77, 0.53, 0.61, 0.96 and 0.99, respectively. The low R2-values of PAC and TIA indicate that while only 53 and 61% of the irrespective variations are influenced by fermentation, the remaining percentage of variations can be attributed to other factors. The reduced polynomial equations for each response indicate the effect of fermentation:
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
where A was amount of added common salt, B was fermentation time and C was fermentation temperature.
Equations 10–13 indicate a linear effect, while the Eq. 14 indicates an interaction effect of independent variables of fermentation on antinutrients.
The predicted values revealed that with the decrease in salt concentration from 15 to 5 g/kg and with the increase in fermentation time from 10 to 30 h, the minimum TC, PAC and TIA per gram dry wt of fermenting mixed dal–rice batter were 0.06 mg, 0.24 mg and 1.9 kU, respectively, keeping fermentation temperature fixed at 31.5 °C. The elimination of TC is attributed to soaking and concurrent activity of polyphenol oxidase during fermentation (Luo et al. 2012). The reduction of PAC might be due to the activity of phytase, produced by the growth of microorganisms during fermentation (Kumar et al. 2010). Bacteria produce lactic acid and lower the pH, thus favouring phytase activity, resulting in the reduction of PAC (Shimelis and Rakshit 2008). Lactic acid fermentation is also effective in reducing TIA in legumes (Shimelis and Rakshit 2008). The minimum HA (0.05 kU/g dry wt) was obtained when the fermentation time and temperature were increased from 10 to 30 h and 25 to 38 °C, keeping added salt concentration fixed at 10 g/kg. The elimination of HA signifies its hydrolysis into simpler and more soluble products, brought about by microorganisms during fermentation (Martín-Carbrejas et al. 2004). While studying the interaction effect of fermentation temperature (25 °C) and added salt concentration (15 g/kg), the lowest level of TBAC at fixed fermentation time (20 h) was high (306 μg/g dry wt; Fig. 1b). When fermentation time was decreased to 10 h at 27.6 °C, keeping added salt concentration constant at 10 g/kg, TBAC decreased to 282 μg/g dry wt (Fig. 1c), indicating fermentation time playing a major role in this case. However, no published report could be found in support of this surmise. The minimum TBAC (265 μg/g dry wt) was observed when the added salt concentration was increased to 15 g/kg at 31.5 °C and the duration of fermentation was 10 h (Fig. 1d). The formation of biogenic amines might be due to the growth of several microbial groups, including LAB, possessing decarboxylase activity (Naila et al. 2010). However, the formation of amines was minimised at increased salt concentration. A high salt concentration reduces cell yield and disturbs the membrane-located microbial decarboxylase (Gardini et al. 2016). LAB readily grow in most food substrates and can lower the pH rapidly to a point where competing microorganisms are no longer able to grow (Shimelis and Rakshit 2008). This lowering of pH favours yeasts (Nout 2009), which is evident from their growth (P < 0.05) during fermentation. Reduction in biogenic amines can be also related to the decrease in pH and growth of microbiota during fermentation (Table 3). Rapid acidification could reduce the levels of biogenic amines production and decrease in the growth of decarboxylating microorganisms (Gardini et al. 2001).
Optimum fermentation conditions comprised 9.4 g/kg as added salt concentration, and fermentation temperature and time of 38 °C and 24 h, respectively. Although fermentation in this condition gave maximum reduction of antinutrients, a shorter fermentation time, a lower temperature and a lower concentration of salt were selected without affecting (P < 0.05) desirability. This is because a faster production is preferred, ambient temperature at night usually is lower and addition of salt in diet is usually not recommended. Thus, the predicted optimum fermentation conditions selected were 18 h, 32 °C and 8 g/kg added salt. The predicted reduction of antinutrients under this condition is shown in Table 4.
The values of microbial load, initial pH and total titratable acidity of unfermented mixed batter changed rapidly during the course of fermentation (Table 3). It was further observed that LAB outnumbered non-LAB, causing a reduction (P < 0.05) in pH of fermented batter.
Steaming
After steaming of optimally fermented batter, the lower and higher levels of PAC, TIA and TBAC were 0.09 and 0.17 mg, 1.1 and 1.2 kU, and 267 and 354 μg, respectively, per gram dry wt. The steamed product was devoid of TC and HA. The model showed insignificant lack-of-fit. The R2-values for all the antinutrients were high, indicating that the models could explain 100% of variation for the reduction of antinutrients during steaming. The reduced polynomial equations generated were:
| 15 |
| 16 |
| 17 |
where A was steaming time.
All the Eqs. (15–17) indicate a linear effect of the independent variables on antinutrients.
Increase in steaming time (20 min) caused a reduction (P < 0.05) of PAC, TIA and TBAC (Table 4). Reduction of PAC might be due to the degradation of myo-inositol hexa-phosphate (phytic acid) into penta- and tetra-phosphates (Vadivel and Biesalski 2012). Due to their heat-sensitive nature, trypsin inhibitors and haemagglutinin might have lost their activities during steaming (Kalpanadevi and Mohan 2013). Reactions involving deamidation and splitting of covalent bonds, such as hydrolysis of peptide bonds, and interchange or destruction of disulphide bonds, might be involved in the thermal inactivation of trypsin inhibitors (Alonso et al. 1998). Thermal deactivation of HA in bengalgram has been reported earlier (Alajaji and El-Adawy 2006). The TBAC in dhokla was 269 µg/g dry wt, which is about four times lower than the threshold value (1000 µg/g dry wt) for human consumption (Kim et al. 2012). Therefore, the optimised steaming time of 20 min is effective in reducing the accumulated amines during fermentation and the optimised dhokla can safely be consumed by humans.
Sensory analysis
Dhokla of good quality should have a mild acidic taste and flavour, and yellowish colour with spongy texture. Dhokla, prepared from batter having 8 g/kg added salt and fermented under optimised condition (32 °C for 18 h), scored maximum (95), ranking “excellent”, the same grade as of traditional one which scored 94 in terms of overall quality.
Conclusion
Traditional dhokla prepared by soaking dal and rice at 25 °C for 10 h, grinding, mixing and fermenting at 32 °C for 15 h and steaming for 15 min had TC, PAC, TIA, HA and TBAC of 0.03 mg, 0.39 mg, 3 kU, 0 kU and 508 μg, respectively, per gram dry wt, while the respective values in optimally prepared dhokla were 0 mg, 0.1 mg, 1 kU, 0 kU and 269 μg. The mathematical model-based optimisation technique in the present study will be useful in ensuring quality and safety of the food products. The outcome of the work can be used in scaling up to medium-scale industrial production of dhokla under better controlled conditions than used traditionally resulting in products of constant quality and enhanced consumer safety.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Grant, F.3–4/2013 (SAP-II), from the University Grants Commission, New Delhi, India.
Abbreviations
- ANOVA
Analysis of variance
- CCRD
Central composite rotatable design
- HA
Haemagglutinating activity
- LAB
Lactic acid bacteria
- PAC
Phytic acid content
- RSM
Response surface methodology
- TA
Titratable acidity
- TAMB
Total aerobic mesophilic bacteria
- TBAC
Total biogenic amines content
- TC
Tannins content
- TIA
Trypsin inhibitor activity
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3119-9) contains supplementary material, which is available to authorized users.
References
- Alajaji SA, El-Adawy TA. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Comp Anal. 2006;19:806–812. doi: 10.1016/j.jfca.2006.03.015. [DOI] [Google Scholar]
- Alonso R, Orúe E, Marzo F. Effects of extrusion and conventional processing methods on protein and antinutritional factor contents in pea seeds. Food Chem. 1998;63:505–512. doi: 10.1016/S0308-8146(98)00037-5. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of the AOAC. 15. Arlington, VA: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Ashenafi M, Busse M. Microflora of soak water during tempeh production from various beans. J Appl Bacteriol. 1991;70:334–338. doi: 10.1111/j.1365-2672.1991.tb02945.x. [DOI] [PubMed] [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R. Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol. 2012;49:665–672. doi: 10.1007/s13197-011-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hady A, Habiba RA. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci Technol. 2003;36:285–293. doi: 10.1016/S0023-6438(02)00217-7. [DOI] [Google Scholar]
- Gardini F, Martuscelli M, Caruso MC, Galgano F, Crudele MA, Favati F, Guerzoni ME, Suzzi G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int J Food Microbiol. 2001;64:105–117. doi: 10.1016/S0168-1605(00)00445-1. [DOI] [PubMed] [Google Scholar]
- Gardini F, Özogul Y, Suzzi G, Tabanelli G, Özogul F. Technological factors affecting biogenic amine content in foods: a review. Front Microbiol. 2016;7:1218–1235. doi: 10.3389/fmicb.2016.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner R, Konietzny U. Improving enzymatic reduction of myo-inositol phosphates with inhibitory effects on mineral absorption in black beans (Phaseolus vulgaris var Preto) J Food Process Preserv. 1999;23:249–261. doi: 10.1111/j.1745-4549.1999.tb00383.x. [DOI] [Google Scholar]
- Grewal A, Jood S. Effect of processing treatments on nutritional and antinutritional contents of green gram. J Food Biochem. 2006;30:535–546. doi: 10.1111/j.1745-4514.2006.00080.x. [DOI] [Google Scholar]
- Guillamόn E, Pedrosa MM, Burbano C, Cuadrado C, de Cortes Sánchez M, Muzquiz M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chem. 2008;107:68–74. doi: 10.1016/j.foodchem.2007.07.029. [DOI] [Google Scholar]
- Hemalatha S, Platel K, Srinivasan K. Zinc and iron contents and their bioaccessibility in cereals and pulses consumed in India. Food Chem. 2007;102:1328–1336. doi: 10.1016/j.foodchem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Jezierny D, Mosenthin R, Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2010;157:111–128. doi: 10.1016/j.anifeedsci.2010.03.001. [DOI] [Google Scholar]
- Joosten HMLJ, van Boekel MAJS. Conditions allowing the formation of biogenic amines in cheese. A study of the kinetics of histamine formation in an infected Gouda cheese. Neth Milk Dairy J. 1988;42:3–24. [Google Scholar]
- Kakade ML, Simons N, Liener IE. An evaluation of natural vs. synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1969;46:518–526. [Google Scholar]
- Kalpanadevi V, Mohan VR. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. unguiculata. LWT Food Sci Technol. 2013;51:455–461. doi: 10.1016/j.lwt.2012.09.030. [DOI] [Google Scholar]
- Khandelwal S, Udipi SA, Ghugre P. Polyphenols and tannins in Indian pulses: effect of soaking, germination and pressure cooking. Food Res Int. 2010;43:526–530. doi: 10.1016/j.foodres.2009.09.036. [DOI] [Google Scholar]
- Kim B, Byun BY, Mah JH. Biogenic amine formation and bacterial contribution in natto products. Food Chem. 2012;135:2005–2011. doi: 10.1016/j.foodchem.2012.06.091. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: a review. Food Chem. 2010;120:945–959. doi: 10.1016/j.foodchem.2009.11.052. [DOI] [Google Scholar]
- Liener IE, Hill EG. The effect of heat treatment on the nutritive value and hemagglutinating activity of soybean oil meal. J Nutr. 1953;49:609–620. doi: 10.1093/jn/49.4.609. [DOI] [PubMed] [Google Scholar]
- Luo Y, Xie W, Xu M, Luo F. Effects of phytase and polyphenol oxidase treatments on in vitro iron bioavailability in faba bean (Vigna faba L.) CyTA J Food. 2012;10:165–171. doi: 10.1080/19476337.2011.631222. [DOI] [Google Scholar]
- Martín-Carbrejas MA, Sanfiz B, Vidal A, Molla E, Esteban R, López-Andreu J. Effect of fermentation and autoclaving on dietary fibre fractions and antinutritional factors of beans (Phaseolus vulgaris L.) J Agric Food Chem. 2004;52:261–266. doi: 10.1021/jf034980t. [DOI] [PubMed] [Google Scholar]
- Moktan B, Roy A, Sarkar PK. Antioxidant activities of cereal-legume mixed batters as influenced by process parameters during preparation of dhokla and idli, traditional steamed pancakes. Int J Food Sci Nutr. 2011;62:360–369. doi: 10.3109/09637486.2010.532116. [DOI] [PubMed] [Google Scholar]
- Naila A, Flint S, Fletcher G, Bremer P, Meerdink G. Control of biogenic amines in food—existing and emerging approaches. J Food Sci. 2010;75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nout MJR. Rich nutrition from the poorest—cereal fermentation in Africa and Asia. Food Microbiol. 2009;26:685–692. doi: 10.1016/j.fm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Nwabueze TU. Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int J Food Sci Technol. 2010;45:1768–1776. doi: 10.1111/j.1365-2621.2010.02256.x. [DOI] [Google Scholar]
- Price ML, van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem. 1978;26:1214–1218. doi: 10.1021/jf60219a031. [DOI] [Google Scholar]
- Ray M, Ghosh K, Singh S, Mondal KC. Folk to functional: an explorative overview of rice-based fermented foods and beverages in India: a review. J Ethnic Foods. 2016;3:5–18. doi: 10.1016/j.jef.2016.02.002. [DOI] [Google Scholar]
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int. 2010;43:432–442. doi: 10.1016/j.foodres.2009.09.002. [DOI] [Google Scholar]
- Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:310–329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumari S, Nout MJR, Wongputtisin P, Sarkar PK. Optimization of soybean processing into kinema, a Bacillus-fermented alkaline food, with respect to a minimum level of antinutrients. J Appl Microbiol. 2015;119:162–176. doi: 10.1111/jam.12826. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumari S, Nout MJR, Sarkar PK. Minimization of antinutrients in idli by using response surface process optimization. J Food Process Preserv. 2017;41:13099–13111. doi: 10.1111/jfpp.13099. [DOI] [Google Scholar]
- Shimelis EA, Rakshit SK. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L.) Int J Food Sci Technol. 2008;43:658–665. doi: 10.1111/j.1365-2621.2006.01506.x. [DOI] [Google Scholar]
- Silla Santos MH. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Vadivel V, Biesalski HK. Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. J Food Sci Technol. 2012;49:673–684. doi: 10.1007/s13197-010-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul VJ, Brouns FJPH. Health effects of wheat lectins: a review. J Cereal Sci. 2014;59:112–117. doi: 10.1016/j.jcs.2014.01.010. [DOI] [Google Scholar]
- Wheeler EL, Ferrel RE. A method of phytic acid determination in wheat and wheat fractions. Cereal Chem. 1971;48:312–320. [Google Scholar]
- Yeh CY, Lin SJ, Hwang DF. Biogenic amines, histamine and label of dressed fried fish meat products in Taiwan. Food Control. 2006;17:423–428. doi: 10.1016/j.foodcont.2005.02.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.