Abstract
Endogenous and exogenous enzymatic hydrolysis carried out to obtain vanilla extracts with higher concentrations of vanillin using green vanilla beans. Sequences initiated with freezing of green vanilla beans at − 1 °C for 24 h, followed by endogenous hydrolysis under optimal β-glucosidase activity at 4.2 and 35 °C for 96 h, exogenous hydrolysis with Crystalzyme PML-MX at pH 5.0 and 40 °C for 72 h, and ethanol extraction at 40% (v v−1) for 30 days. In the proposed method, 200 g of fresh green vanilla beans with 84% moisture (32 g dry base) were used to obtain a liter of single fold vanilla extract. This method allowed the release of 82.57% of the theoretically available vanillin from its precursor glucovanillin with 5.78 g 100 g−1 green vanilla beans (dry base). Vanillic acid, p-hydroxybenzaldehyde and vanillyl alcohol were also released and found in commercial and enzymatic extracts. Glucovanillin was detected in commercial and traditional extracts but was absent in enzymatic extracts, indicating incomplete hydrolysis during the curing process. An in vitro assay was conducted to determine if the presence of peroxidase during hydrolysis might affect overall vanillin concentration. Results showed that POD can use vanillin as a substrate under conditions similar to those in which hydrolysis was conducted (pH 5.0 and 50 °C), possibly explaining why vanillin concentration was not complete at the end of the process.
Keywords: Vanillin, Green vanilla beans, Enzymatic hydrolysis, Vanilla extract
Introduction
Vanilla planifolia Andrews is the most valued species of vanilla because of its rich and well-balanced aromatic compounds. However, the main vanilla aromatic compounds are found in the form of glucoside in green vanilla beans. It is hydrolyzed by endogenous β-glucosidase (CE 3.2.1.21) during the curing process (Palama et al. 2011). The process is long and produces cured vanilla beans with 1–2% vanillin even though the amount available is ~ 7% (dry base weight) (Gallage and Møller 2015). This concentration is calculated from its precursor glucovanillin, which is present in a proportion of 14% in green vanilla beans after 9 months of development (Pardío et al. 2009). Cured vanilla beans are processed to obtain vanilla extract designated by Food and drugs Administration (FDA) as that containing 10% of the removable portion of the cured vanilla beans in 35% (v v−1) or more ethylic alcohol. According to the Code of Federal Regulations (Title 21, Part 169.180) vanilla extract quality must be expressed in terms of vanillin content as “fold”; “single fold” strength contains 0.1–0.2% vanillin (FDA 2017). However, the efficiency of vanillin extraction is commonly conditioned by several factors during the traditional curing process: processing temperatures, weather conditions, and loss of aromatic compounds in the conditioning step. Besides, β-glucosidase from green vanilla beans is striking by temperatures used during curing process (Brillouet and Odoux 2010; Baqueiro-Peña and Guerrero-Beltrán 2017). In addition, hydrolysis of glycosylated odor-active compounds is carried out through enzymatic rather than chemical catalysis, which results in low vanillin extraction during the process (Pérez-Silva et al. 2011). Moreover, aromatic compounds, including vanillin, are trapped inside cellulosic structures of the vanilla pods, which block complete extraction, and a lower quality vanilla extract is produced (Waliszewski et al. 2007; Frenkel et al. 2010; Paramita and Yulianto 2013). Other studies have shown gradients of increasing–decreasing concentrations of glucovanillin, β-glucosidase, polyphenol oxidase (EC 1.14.18.1) (PPO) and peroxidase (EC 1.11.1.7) (POD) in the green vanilla beans. PPO and POD play an important role in oxidation during the curing process, but it is not clear whether they oxidize or transform vanillin into dimers in the pod (Brillouet et al. 2010). The demand for natural vanilla in the world is increasing and, as a result, enzymatic methods are being developed to obtain high quality vanilla extracts with high vanillin concentration (Perera and Owen 2010; Naidu et al. 2012; Zhang et al. 2014). Nevertheless, the authors reported that the vanillin released from glucovanillin was less than expected. Moreover, although endogenous β-glucosidase from vanilla beans is responsible for the glucovanillin hydrolysis, its preservation to improve the vanilla extracts has not been taken into account. Most of the studies have used only exogenous enzymes to increase the vanillin concentration in the vanilla extracts. Therefore, considering that high proportions of β-glucosidase and glucovanillin are present in mature green vanilla beans (after 9 months of development), the aim of this study was to obtain a vanilla extract from green vanilla beans with the maximum content of vanillin from glucovanillin hydrolysis, as well as the presence of p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillic acid and vanillyl alcohol using the synergistic activity of endogenous and exogenous enzymes. The method proposal also aims to increase vanilla extract yield and to reduce processing time.
Materials and methods
Plant material
Green vanilla beans with yellow-green apexes were provided by a local producer of Papantla, Veracruz.
Vanilla extract production
This study included the analysis of cellular disruption of the green vanilla beans by freezing to preserve the β-glucosidase activity followed by two phases of hydrolysis. First, endogenous hydrolysis with optimal conditions of reaction to β-glucosidase activity was conducted until higher concentration of vanillin was reached. Afterward, extracts were divided in two groups and a second hydrolysis was carried out adding individually two commercial food-grade preparative enzymes with cellulase activities. The reaction was stopped adding ethanol (40% v v−1) (ethanol extraction) until vanillin reached the maximum concentration and glucovanillin disappeared. Main aromatic compounds was analyzed after 30 days of ethanol extraction and was compared against traditional (Mexican method) using cured vanilla beans and commercial vanilla extract.
Freezing of green vanilla beans
Selected mature green vanilla beans with yellow end-blossom were divided into two lots of 750 g each. All beans were individually wrapped with aluminum foil and placed in a chamber at − 1 and − 15 °C for 24 h. Four pods of each lot were randomly sampled and homogenized following the method reported by Dignum et al. (2001) for β-glucosidase activity and for methanol vanillin extraction by Dignum et al. (2002). Vanillin release was quantified by HPLC method (Waliszewski et al. 2006) and cellular disruption of green vanilla bean cellulosic structures was indirectly measured by the reduced sugars released by 3,5-dinitrosalicylic acid (DNS) method (Ghose 1987). Samples were analyzed at 0, 2, 4, 6, 8, 10, 12, 18, and 24 h of storage.
Enzyme crude extract
The vanilla beans were cut into pieces of 1 cm approximately and ground in a mill. Ten grams of ground green vanilla beans were mixed with 30 mL extraction buffer containing Bis–Tris-Propane 150 mM, Ethylenediaminetetraacetic acid (EDTA) 2 mM, and Di-thiothreitol (DTT) 3 mM (Dignum et al. 2001). The sample was homogenized (Ultra Turrax® T-25 basic IKA Works, Inc. Wilmington NC, USA) for 5 min at 9000 rpm with 250 mg of Polyvinyl polypyrrolidone (PVPP) g−1 green vanilla beans, dry base. Homogenized samples were centrifuged (Alegra 64R™ Benchtop Beckman Coultier Inc, Brea CA, USA) at 376.32×g for 20 min, filtered through Whatman No. 1 filter paper, and stored in cool conditions until use.
Oxidative enzyme activities
PPO and POD activities were determined during endogenous and exogenous enzymatic extraction. The PPO assay was conducted adding 500 μL of pyrocatechol 20 mM to a mixture of 100 μL enzyme crude extract with 900 μL acetate buffer pH 3.0. Samples were then incubated at 40 °C for 20 min. POD was evaluated by conditioning 100 μL enzyme crude extract in phosphate buffer with a 16 mM solution of hydrogen peroxide at 16 °C for 10 min. Guaiacol 20 mM was then added, and the reaction was conducted at 16 °C for 10 min (Civello et al. 1995). Both reactions were stopped with 10% trichloroacetic acid (TCA) solution at 1:10 (v v−1) and analyzed at 300 and 440 nm for PPO and POD, respectively. Units of enzymatic activity (UEA) were defined as change of absorbance in 0.001 mg protein−1 min−1.
Activity of β-glucosidase endogenous
Glucovanillin was purified according to Odoux et al. (2003) and was used as substrate instead of synthetic p-nitrophenyl-β-d-glucopyranoside (a commonly used substrate). A volume of 100 μL of the enzymatic extract was mixed with 380 μL of the glucovanillin and the reaction was carried out at pH interval of 3.0–7.0 at 35 °C for 4 h, and stopped with 380 μL of Na2CO3. Later, 20 μL of the reaction solution was diluted with 980 μL of mobile phase methanol:water at 60:40 (v v−1) and filtered with Acrodisc® Syringe Filters (0.22 μm) to quantify the vanillin content by HPLC method (Waliszewski et al. 2006). Márquez and Waliszewski (2008) determined 38 °C as the optimum temperature to β-glucosidase activity using p-nitrophenyl-β-d-glucopyranoside as substrate, therefore optimum temperature for β-glucosidase activity using glucovanillin as substrate was analyzed at an interval of 30–55 °C.
Activity of commercial food-grade enzyme preparations
Both food-grade enzyme preparations Cryztalzyme PML-MX and Cellulase 17600L were tested using filter paper assay to identify the Filter Paper Units per milliliter (FPU/mL) (Eveleigh et al. 2009). Analysis of the optimal β-glucosidase activity was similar to that of endogenous β-glucosidase using enzyme preparations instead of crude enzyme extract. Optimal pH of the cellulase activity was determined using 2 g of lyophilized and ground green vanilla beans for each evaluated pH (3.0–7.0); samples were homogenized with 10 mL of the corresponding phosphate or citrate buffer. One milliliter of the homogenized sample was added to a Pyrex test tube (13 × 100) with 1 mL of commercial food-grade enzyme preparations and incubated in a water bath for 2 h at 35, 40, 45 and 50 °C. The enzymatic reaction was stopped by adding ethanol 50% (v v−1). The total protein was estimated by the method of Bradford (1976), using bovine serum albumin (BSA) as standard. Units of enzymatic activity were defined as mg of reduced sugars mg−1 protein min−1.
Enzymatic hydrolysis and alcoholic extraction
Four 50 g lots of green vanilla beans previously frozen were cut into 2 cm pieces and homogenized with water and 5 or 10% ethanol solution at 1:2 (w v−1). Optimal conditions for endogenous β-glucosidase activity were adjusted. Reaction was conducted in a water bath in closed 250 mL Erlenmeyer flasks and vanillin concentration was analyzed every 12 h by HPLC until peak release was observed. At the end of endogenous hydrolysis, the four resulting extracts were divided into two groups, one for each enzyme preparation, Crystalzyme PML-MX and Cellulase 17600L. Also, enzymatic hydrolysis in each group was conducted individually under optimal β-glucosidase and cellulase conditions. Both groups were adjusted to 9.27 IFPU (International Filter Paper Units) g−1 bean dry weight to carry out the exogenous hydrolysis. The reaction was stopped when peak vanillin release was reached. Reduced sugars, vanillin and residual activities of POD, PPO, and β-glucosidase were analyzed during all enzymatic hydrolysis stages. Ethanol extraction was conducted by adding ethanol (95%) until reaching 40% (v v−1) of the total volume. Vanillin and other main compounds, such as p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillic alcohol, and vanillic acid were analyzed. Extraction was carried out with slow magnetic stirring at 30 °C for 30 days. A first order equation Yi = Ye (1 − ekt) was used to determine the constant rate of release (k) and the equilibrium concentration of vanillin (Ye). All trials were conducted in triplicate and statistical significance was determined using Minitab 17.3 statistical package.
Results and discussion
Effect of freezing on cellular disruption and enzymatic activities
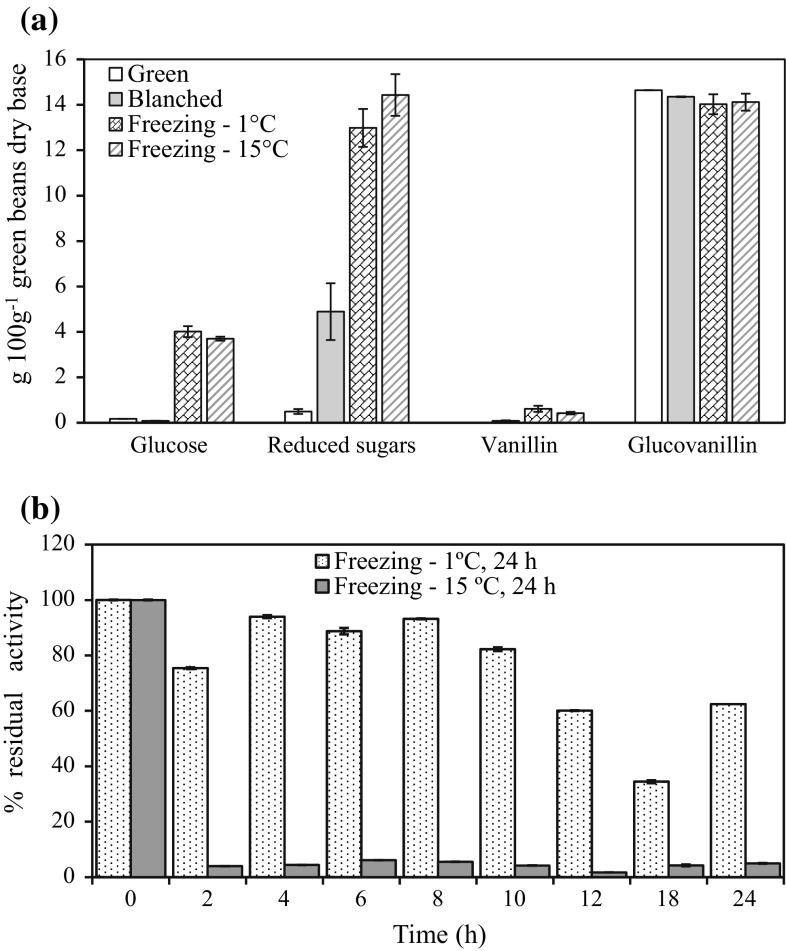
The effect of freezing (− 1 °C and − 15 °C for 24 h), against the traditional blanching method (65 °C for 3 min), on reduced sugars, glucose, vanillin and glucovanillin release are presented in Fig. 1a. Higher quantities of reduced sugar, as an indicator of hydrolysis of cellulosic pod structures, were found when beans were frozen at − 1 and − 15 °C obtaining 12.98 (± 0.83) and 14.43 (± 0.92) g 100 g−1 green vanilla beans, respectively. Moreover, statistical differences between the two freezing treatments were not significant. Pods blanched at 65 °C for 3 min also improved the release of reduced sugars but less efficiently with 4.89 (± 0.008), while green pods released 0.50 (± 0.11) g 100 g−1 dry base. Also, concentration of glucose released from green beans was higher when freezing at − 1 and − 15 °C was used with 4.01 and 3.70 g 100 g−1 dry base, respectively, while was not detected in green beans blanched. Similarly, higher concentration of vanillin was obtained when the pods were frozen at − 1 °C and − 15 °C with 0.61 (± 0.02) and 0.42 (± 0.02) g 100 g−1 dry base after 24 h, respectively. In contrast, vanillin released from blanched pods was 0.08 (± 0.01) 100 g−1 dry base, and it was not detected in fresh green vanilla beans. Besides, residual glucovanillin was quantified and statistical differences were not significant between treatments. Residual β-glucosidase activity (Fig. 1b) was analyzed and 62.44 and 4.94% of residual activity was identified in vanilla beans frozen at − 1 and − 15 °C for 24 h, respectively. However, there was no activity detected in pods blanched at 65 °C for 30 min. Thus, β-glucosidase activity was not drastically affected for temperatures of − 1 °C, and allowed its release from cellular compartments to initiate glucovanillin hydrolysis. These results could explain the low yield of vanillin extracted with the artisanal method where warm temperatures are commonly used. Gradual loss of β-glucosidase activity has been reported in other studies as one of the most significant problems in the vanilla industry because this enzyme is responsible for glucovanillin hydrolysis and vanillin release (Dignum et al. 2002; Waliszewski et al. 2009; Gu et al. 2017). Vanillin concentration is a main factor of the quality of the vanilla extracts; single fold vanilla (containing 0.1–0.2% vanillin) is considered the reference of minimum quality (Gallage and Møller, 2015). Consequently, several studies have been developed to increase vanillin release using exogenous enzymes with cellulase or β-glucosidase activity (Ruiz-Terán et al. 2001; Waliszewski et al. 2007; Perera and Owen 2010; Zhang et al. 2014). As shown in Fig. 1b, the enzymatic activity at − 15 °C decreased after 2 h of freezing, and dark zones along the defrosted pods were detected, probably due to the endogenous oxidative enzymes POD and PPO. These enzymes use phenols as substrate and produce dark pigments, which have been reported as inhibitors of β-glucosidase activity (Waliszewski et al. 2009). Dignum et al. (2001) reported that β-glucosidase was drastically reduced when vanilla pods were stored at − 20 and − 80 °C, while POD activity remains without change for up to 28 days of storage. Our results showed that 62.4% of residual β-glucosidase activity remains when green vanilla beans were frozen at − 1 °C for 24 h. These conditions were selected and were replicated prior to enzymatic hydrolysis of green vanilla beans to improve the release of vanillin from glucovanillin.
Fig. 1.
Effect of the freezing and blanching of green vanilla beans on glucose, reduced sugars and vanillin release (a), and β-glucosidase activity (b)
Endogenous enzymatic hydrolysis
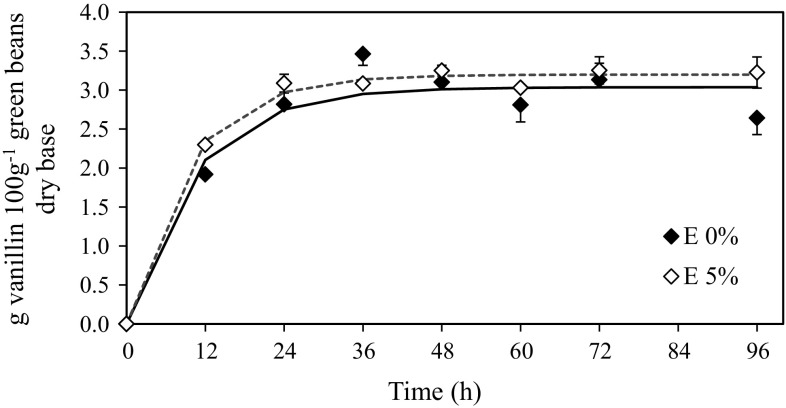
Enzymatic hydrolysis of green vanilla beans was divided in two phases, endogenous and exogenous. Endogenous hydrolysis of green vanilla beans was carried out following freezing step under optimal endogenous β-glucosidase activity at pH 4.2 and 35 °C. Moreover, 5% ethanol (v v−1) was added to enhance vanillin solubility without affecting enzymatic activity. Figure 2a shows the kinetics of vanillin release during endogenous enzymatic hydrolysis, and higher vanillin concentration was detected at 96 h of reaction reaching 3.80 (± 0.20) and 3.23 (± 0.01) g 100 g−1 green pods dry base in hydrolyzed green vanilla beans with and without ethanol, respectively. Vanillin concentration at the end of endogenous hydrolysis was 6.23 times higher than that released after the freezing stage. Moreover, this concentration did not correspond to the ~ 7 g available based on the 14.64 g of glucovanillin present in green pods (Fig. 1a). Although no more vanillin was released after 96 h of endogenous hydrolysis, 7.62 g of glucovanillin was present in the vanilla extract, probably indicating inefficient β-glucosidase activity and the need to reinforce enzymatic activity to assure complete vanillin release. Several studies that used enzymes to extract intracellular metabolites from plants point to the cellulosic structures as a physical barrier to complete extraction (Waliszewski et al. 2007; Frenkel et al. 2010; Paramita and Yulianto 2013). Besides, it was reported that β-glucosidase and glucovanillin are commonly present in different cellular compartments, making glucovanillin and β-glucosidase interaction difficult (Odoux et al. 2003). Therefore, the second stage of enzymatic hydrolysis was carried out using commercial food-grade enzyme preparations under optimal conditions of cellulase and β-glucosidase activities.
Fig. 2.
Effect of endogenous hydrolysis at pH 3.4 and 35 °C with and without ethanol on vanillin release. Points refer to experimental values and lines refer to predicted values by means linear regression. E0%: sample without ethanol; E5%: sample with ethanol solution at 5% (v v−1)
Exogenous enzymatic hydrolysis
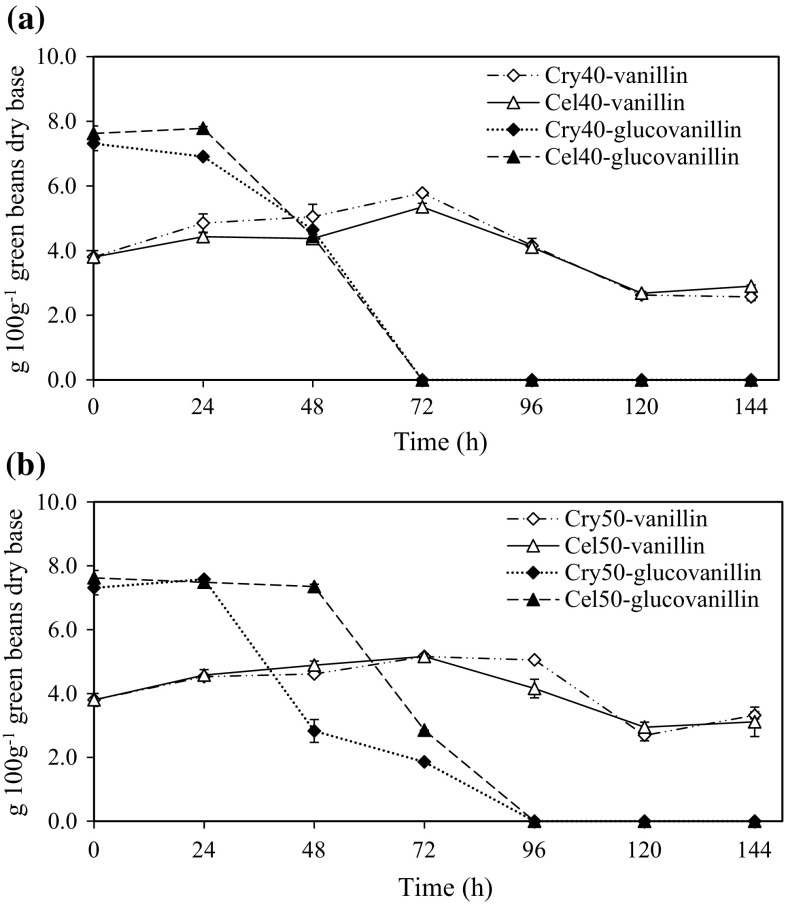
Cellulase conditions were indirectly evaluated on reduced sugars released during enzymatic reaction. Higher concentrations of reduced sugars were obtained when Cryztalzyme PML-MX (Cry50) and Cellulase 17600L (Cel50) were used at pH 3.4 and 50 °C, and at pH 4.7 and 50 °C, respectively. Optimal conditions of β-glucosidase activity were identified at pH 5.0 and 40 °C for both Cryztalzyme PML-MX (Cry40) and Cellulase 17600L (Cel40) (Data not shown). Reaction conditions were tested using 5% (v v−1) ethanol (95%), considering a non-inhibitory effect on hydrolytic activity and a preservative effect against microorganism growth and vanillin solubility (Waliszewski et al. 2007). The effect of adding enzyme preparations under β-glucosidic conditions on vanillin release and glucovanillin hydrolysis is presented in Fig. 3. Greater release of vanillin and disappearance of glucovanillin was observed in both Cry40 and Cel40 treatments at 72 and 96 h, respectively, indicating the effectiveness of the enzymatic treatments. However, final quantity of vanillin obtained after 72 h was not in accord with the theoretically available glucovanillin concentration in fresh green vanilla beans, as mentioned. The highest vanillin concentration was obtained when β-glucosidic conditions were used in both Cry40 and Cel40 treatments, reaching 5.78 and 5.35 g vanillin 100 g−1 green vanilla beans dry base, respectively.
Fig. 3.
Hydrolysis of glucovanillin and release of vanillin using exogenous enzymes preparations under β-glucosidic (a) and cellulosic (b) conditions. Cry40 = Sample extracted with 5% ethanol (v v−1) using Crystalzyme PML-MX at pH 5.0 and 40 °C; Cel40 = Sample extracted with 5% ethanol (v v−1) using Cellulase 17600L at pH 5.0 and 40 °C. Cry50 = Sample extracted with 5% ethanol (v v−1) using Crystalzyme PML-MX at pH 3.4 and 50 °C; Cel50 = Sample extracted with 5% ethanol (v v−1) using Cellulase 17600L at pH 4.7 and 50 °C
The increased release of vanillin observed up to 72 h of reaction indicated that commercial enzymes and β-glucosidic activities improved the quality of vanilla extracts. However, after 72 h, vanillin gradually decreased in both extracts to 2.73 and 3.05 g vanillin 100 g−1 of green vanilla beans dry base. A similar effect was detected in the extracts under cellulase conditions (Cry50 and Cel50). Gatfield et al. (2006) evaluated the curing process of harvested green vanilla beans, indicated that more than half the vanillin was lost as a result of enzymatic oxidation by peroxidase at pH 5.0 and 40 °C, remaining 2–3 g 100 g−1 vanilla beans dry base. Other studies have reported that dark zones on vanilla beans developed during the curing process is possibly attributed to POD o because the entire POD system (phenolic substrate-peroxide-enzyme) was found in the green pods, impeding its total quantification (Márquez et al. 2008; Nishimura et al. 2016). Because of these results, POD, β-glucosidase, and PPO activities were analyzed during enzymatic hydrolysis to corroborate the lack of vanillin.
POD, PPO and β-glucosidase activities
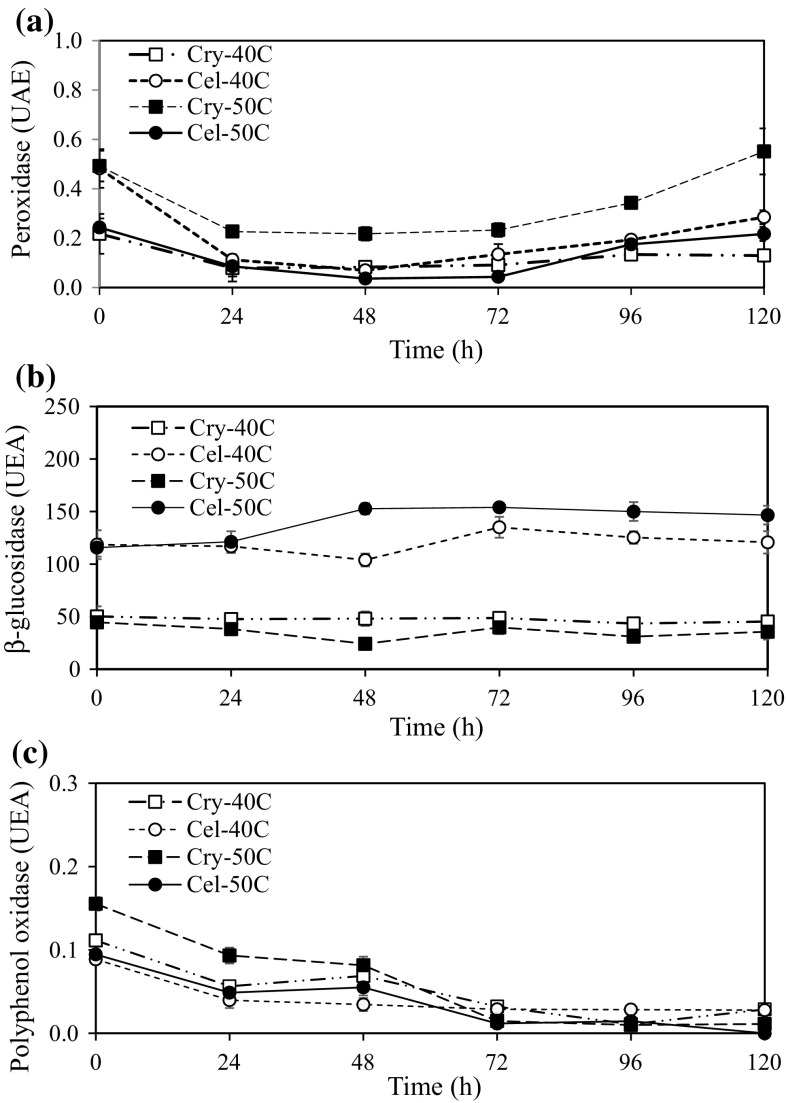
A gradual decrease of POD activity during first 72 h of hydrolysis, followed by an increase until 120 h was identified (Fig. 4). Thus, POD was not inhibited by hydrolysis and was more active in Cry50 extracts, maintaining 0.55 (± 0.093) UEA at the end of the hydrolysis phase. Even samples with lower POD activity (Cry40) showed activities of 0.29 (± 0.028) UAE at the same time. Cel50 and Cel40 extracts also presented POD activities of 0.22 (± 0.029) and 0.13 (± 0.024) UAE. Thermostability and reactivation under low and high temperatures are two of the main characteristics reported for POD in several studies and is considered responsible for the deterioration of flavor, color and nutritional quality in foods. POD has been studied in vanilla beans during traditional curing by Dignum et al. (2002), who determined that POD activity gradually decreases through the process but does not disappear entirely, remaining until the last steps of curing. Márquez et al. (2008) studied POD from green vanilla beans and reported 26% activity loss at 40 °C and severe loss of POD activity at 50 °C, indicating a probable alteration in its catalytic mechanism. Brillouet et al. (2010) also defined a hypothetical sequence of events leading to the blackish-brown vanilla during curing process, consisting of tissular decompartmentation, hydrolysis of glucovanillin conducted by β-glucosidase, rapid oxidation of the vanillin released by POD, and diffusion of brown oxidation products outward until the whole pod turns deep brown. Hence, it is suggested that enzymatic hydrolysis stimulated the release of the remaining POD, its reactivation coinciding with decreasing vanillin concentration in all treatments. Reduced POD activity at the beginning of the reaction could due to the effect of the low temperatures used during freezing stage. This is a characteristic described as inherent to this enzyme in many vegetables (Okcu et al. 2013).
Fig. 4.
Peroxidase (a), β-glucosidase (b), and polyphenol oxidase (c) activities during exogenous hydrolysis with Crystalzyme PML-MX and Cellulase 17600L. Cry40 = Sample extracted with 5% ethanol (v v−1) using Crystalzyme PML-MX at pH 5.0 and 40 °C; Cel40 = Sample extracted with 5% ethanol (v v−1) using Cellulase 17600L at pH 5.0 and 40 °C. Cry50 = Sample extracted with 5% ethanol (v v−1) using Crystalzyme PML-MX at pH 3.4 and 50 °C; Cel50 = Sample extracted with 5% ethanol (v v−1) using Cellulase 17600L at pH 4.7 and 50 °C
On the other hand, both treatments Cel40 and Cel50 exhibited higher residual activity of β-glucosidase until the end of the reaction with 120.78 (± 10.67) and 146.66 (± 21.97) UEA, respectively. In contrast, the Cry40 and Cry50 samples had lower β-glucosidase activity, with 45.35 (± 4.29) and 35.62 (± 7.40) UEA, but a higher quantity of vanillin after 72 h of hydrolysis (Fig. 4b). On the basis of these results, and taking into account that β-glucosidase activity remains practically without change during enzymatic hydrolysis, the hypothesis that a β-glucosidase deficiency is the main reason that the maximum vanillin theoretically available from glucovanillin is not obtained can be rejected.
PPO, an enzyme important to improving the flavor of vanilla using phenolic components (Waliszewski et al. 2009), was analyzed to evaluate its effect on vanillin release. The kinetics displayed in Fig. 4c shows a decreasing trend in activity during hydrolysis, considered to be insufficient to support vanillin loss. On basis of these results, the low quantity of vanillin obtained at the end of the extraction was not due to the lack of β-glucosidase activity. Therefore, in this study POD is probably responsible for this phenomenon. In order to determine if POD uses vanillin as substrate, POD activity was analyzed using synthetic vanillin (99%) and partially purified POD from green vanilla beans. POD activity was defined as a change of 0.001 in the absorbance of vanillin (231 nm) mg protein−1 min−1. Optimal activity was obtained at pH 8.0 as shown the Fig. 5a, meaning that the alkaline medium stimulated loss of vanillin. Nevertheless, all enzymatic extracts were elaborated at pH 5.0, which only correspond to 20% of residual activity, but enough to interact with vanillin. Because of this, the following assays were adjusted to pH 5.0 to determine the ideal temperature of POD.
Fig. 5.
Optimal conditions of pH (a) and temperature (b) of peroxidase activity using vanillin and hydrogen peroxide as substrate. Residual activity was defined as the change of absorbance in 0.001 mg protein−1 min−1
Results showed in the Fig. 5b indicated higher POD activity at 50 °C while samples adjusted to 40 °C had 52.33% residual activity. Both temperatures, 40 and 50 °C, were used during enzymatic hydrolysis and could explain why vanillin concentration was lesser than expected to the theoretically available from glucovanillin, even when glucovanillin was completely hydrolyzed. These results agree with those reported by Gatfield et al. (2006), who found that 50 °C and pH 5.0 were the best conditions for the formation of vanillin dimers in presence of hydrogen peroxide and POD. In our study, divanillin was not measured but should be considered in subsequent studies.
Ethanol extraction
At the end of enzymatic hydrolysis both Cry40 and Cel40 extracts were adjusted with ethanol (95%) to a final 40% (v v−1) and vanillin release was analyzed for 30 days. Although around 250 aromatic components that affect the complete bouquet have been identified (Frenkel et al. 2010), vanillin, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillyl alcohol, and vanillic acid are considered the major volatile compounds found in vanilla beans (Pérez-Silva et al. 2011; Santos et al. 2017).
Table 1 shows the main aromatic compounds identified in vanilla extracts after hydrolysis and ethanol extraction compared against Virginia Dare commercial vanilla extract and a traditional extract obtained from cured vanilla beans. Glucovanillin was quantified to determine whether it was completely hydrolyzed using the different process studied. Among the aromatic compounds evaluated, p-hydroxybenzoic acid was not identified in any of the extracts, and glucovanillin was identified only in traditional and commercial extracts. However, as previously mentioned, glucovanillin was absent in both extracts of green vanilla beans Cry40 and Cel40, with maximum concentrations of vanillin of 0.169 (± 0.005) and 0.153 (± 0.008) g 100 mL−1, respectively. Moreover, vanillin concentration was not statistically different between enzymatic treatments, but it was higher than that in commercial and traditional extracts with 0.135 (± 0.015) and 0.136 (± 0.008) g 100 mL−1, respectively. On the other hand, vanillyl alcohol was identified in the commercial extract with 0.382 (± 0.135) mg 100 mL−1 and in both enzymatic extracts Cry40 and Cel40 with 1.521 (± 0.111) and 1.450 (± 0.192) mg 100 mL−1, respectively. Furthermore, vanillic acid was detected only in the commercial and Cry40 extracts. Odoux (2011) indicated that vanillic acid and p-hydroxy benzoic acid present in vanilla beans could increase by oxidation of vanillin and p-hydroxy benzaldehyde, respectively. However, Baqueiro-Peña and Guerrero-Beltrán (2017) identified β-d-glucosides of vanillin, vanillic acid, vanillyl alcohol, and p-hydroxy benzaldehyde in mature green vanilla beans concluding that these compounds are released during the curing process by β-glucosidase enzymes which improve the quality of the extracts. Therefore, enzymatic reactions such as glucoside hydrolysis and oxidation of volatile compounds are important to achieve the overall vanilla flavor. Thus, control of the conditions during the curing process is crucial to obtaining the characteristic aroma and flavor of the vanilla extract. In this context, our study presents an alternative method for obtaining vanilla extracts from mature green vanilla beans using exogenous enzymes, which guarantees the complete hydrolysis of the volatile glucoside compounds.
Table 1.
Aromatic compounds identified in commercial, traditional and enzymatic vanilla extracts
| Extract | Vanillin (g 100 mL−1) | Glucovanillin (mg 100 mL−1) | p-hydroxybenzaldehyde (mg 100 mL−1) | Vanillyl alcohol (mg 100 mL−1) | Vanillic acid (mg 100 mL−1) |
|---|---|---|---|---|---|
| Traditional | 0.135ª (± 0.015) | 1.430ª (± 0.123) | 4.772ª (± 0.371) | ND | ND |
| V. Dare | 0.136ª (± 0.008) | 6.408b (± 0.191) | 5.327ab (± 0.328) | 0.382a (± 0.135) | 1.342a (± 0.082) |
| Cry40 | 0.169b (± 0.005) | ND | 5.934b (± 0.285) | 1.521b (± 0.111) | 0.278b (± 0.047) |
| Cel40 | 0.153b (± 0.008) | ND | 5.540b (± 0.198) | 1.450b (± 0.192) | ND |
Traditional = Vanilla extract from cured beans; V. Dare = Commercial extract Virginia Dare; Cry40 = Vanilla extract from green beans hydrolyzed with Crystalzyme PML-MX at pH 5.0 and 40 °C; Cel40 = Vanilla extract from green beans hydrolyzed with Cellulase 17600L at pH 5.0 and 40 °C
Different letters indicated statistically differences (p < 0.05) for each treatment (row)
Quality and strength of the vanilla extracts are defined by the FDA (2017) on the basis of the total sapid and odorous principles extractable from vanilla beans, and define a single fold vanilla extract as “a solution with not less than 35% of ethyl alcohol by volume, and containing the total sapid and odorous principles extractable from 13.35 oz of vanilla pods with not more than 25% moisture per gallon”. Vanillin concentration in single fold extracts is to 0.1–0.2% and is dependent on vanilla pod quality. In this study, although enzymatic extracts Cry40 and Cel40 were single fold, the content of vanillin was higher than commercial and traditional extracts. Moreover, only 50 g of green vanilla beans with 84% moisture per 250 mL (200 g l−1) were used to obtain the enzymatic vanilla extracts. In contrast, the vanilla extract industry employs 13.35 oz of cured vanilla beans per gallon (100 g l−1) with 25% of moisture. In comparison, 75 g dry base of cured vanilla are used to make traditional vanilla extract, while only 32 g dry base of green vanilla beans were used for enzymatic vanilla extracts, indicating higher performance with this alternative method. In addition, only 9 days were necessary to carry out the process that included the sequential steps of freezing at − 1 °C for 24 h, endogenous hydrolysis at pH 3.4 and 35 °C for 96 h with 5% of ethanol 95% (v v−1), and hydrolysis assisted with endogenous enzymes (Cellulase 17600L and Crystalzyme PML-MX) at pH 5.0 and 40 °C, using green vanilla beans. In contrast, 2–3 months are required for the traditional curing process. The results indicate that the enzymatic method improves vanilla extract quality based on vanillin concentration. However, other components reported to be responsible for the overall aroma profile were also identified in the enzymatic vanilla extracts and in the commercial extract Virginia Dare as well. It was also demonstrated in this study that POD reacts with vanillin during enzymatic hydrolysis, limiting its extraction, and could be producing divanillin, a flavoring that gives a taste impression of creaminess, milk fattiness, butteriness and sweetness (Krings et al. 2015).
Conclusion
Food grade enzyme preparations allowed use of a smaller quantity of green vanilla beans to obtain single strength vanilla extract with higher vanillin concentration than that found in commercial extracts. The freezing process of the vanilla beans at − 1 °C for 24 h supported residual β-glucosidase activity at 62.44%, which gradually decreased to 9.28% during endogenous hydrolysis, improving vanillin release up to 3.8 g (± 0.20) 100 g−1 dry base of green vanilla beans of the total vanillin (~ 7 g 100 g−1) available from 14.67 g 100−1 of glucovanillin in green vanilla beans, suggesting that reaction rate is slow and directly depends on β-glucosidase activity. However, complete hydrolysis of glucovanillin were achieved by adding the food grade enzyme preparation Crystalzyme PML-MX at pH 5.0 and 40 °C for 72 h, reaching 5.78 (± 0.12) g vanillin 100 g−1. Moreover, the effect of POD on vanillin oxidation impeding its total extraction, with probable divanillin formation, was demonstrated. Aromatic compounds identified in the vanilla extracts obtained by means of enzymatic treatment with Crystalzyme PML-MX were similar to those in the commercial vanilla extracts, implying similar impact on flavor. However, further studies must be conducted to evaluate its quality in food.
Acknowledgements
This study was funded by the Dirección General de Educación Superior Tecnológica (DGEST/SEP) (Project 2180.09P). We gratefully acknowledge Diane Fumiko Miyoshi Udo, M.A. (English editor) for reviewing this manuscript.
Footnotes
Krzysztof N. Waliszewski: Deceased.
Contributor Information
Violeta T. Pardío, Email: vpardio@uv.mx
Argel Flores, Phone: (229) 9 34 20 75, Email: mopri02@yahoo.com.mx, Email: argflores@uv.mx.
Karla M. López, Email: karlop_80@yahoo.com.mx
David I. Martínez, Email: dmartinez@uv.mx
Ofelia Márquez, Email: ofeliammolina@yahoo.com.
Krzysztof N. Waliszewski, Email: kw@itver.edu.mx
References
- Baqueiro-Peña I, Guerrero-Beltrán JA. Vanilla (Vanilla planifolia Andr.), its residues and other industrial by-products for recovering high value flavor molecules: a review. J Appl Res Med Aromat Plants. 2017 [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brillouet JM, Odoux E. In vivo kinetics of β-glucosidase towards glucovanillin and related phenolic glucosides in heat-treated vanilla pod (Vanilla planifolia, Orchidaceae) Fruits. 2010;65:85–95. doi: 10.1051/fruits/20010004. [DOI] [Google Scholar]
- Brillouet JM, Odoux E, Conejero G. A set of data on green, ripening and senescent vanilla pod (Vanilla planifolia; Orchidaceae): anatomy, enzymes, phenolics and lipids. Fruits. 2010;65:221–235. doi: 10.1051/fruits/2010018. [DOI] [Google Scholar]
- Civello PM, Martínez GA, Chaves AR, Añón MC. Peroxidase from strawberry fruit (Fragaria ananasa Duch.): partial purification and determination of some properties. J Agric Food Chem. 1995;43:2596–2601. doi: 10.1021/jf00058a008. [DOI] [Google Scholar]
- Dignum MJW, Kerler J, Verpoorte R. β-glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochem Anal. 2001;12:174–179. doi: 10.1002/pca.578. [DOI] [PubMed] [Google Scholar]
- Dignum MJW, Kerler J, Verpoorte R. Vanilla curing under laboratory conditions. Food Chem. 2002;79:165–171. doi: 10.1016/S0308-8146(02)00125-5. [DOI] [Google Scholar]
- Eveleigh DE, Mandels M, Andreotti R, Roche C. Measurement of saccharifying cellulose. Biotechnol Biofuels. 2009;2:1–8. doi: 10.1186/1754-6834-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2017) CFR-Code of Federal Regulations, Title 21 Part 169 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=169. Accessed 5 Mar 2018
- Frenkel C, Ranadive AS, Vázquez JT, Havkin-Frenkel D. Curing of vanilla. In: Havkin-Frenkel D, Belanger FC, editors. Handbook of vanilla science and technology. Oxford: Wiley-Blackwell; 2010. pp. 79–106. [Google Scholar]
- Gallage NJ, Møller BL. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol Plant. 2015;8:40–57. doi: 10.1016/j.molp.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Gatfield I, Reib I, Krammer G, Schmidt CO, Kindel G, Bertram HJ. Novel taste-active component of fermented vanilla beans. Perfum Flavor. 2006;31:18–20. [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- Gu F, Chen Y, Hong Y, Fang Y, Tan L. Comparative metabolomics in vanilla pod and vanilla bean revealing the biosynthesis of vanillin during the curing process of vanilla. AMB Exp. 2017;7:1–9. doi: 10.1186/s13568-016-0313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings U, Esparan V, Berger RG. The taste enhancer divanillin: a review on sources and enzymatic generation. Flavour Fragr J. 2015;30:362–365. doi: 10.1002/ffj.3251. [DOI] [Google Scholar]
- Márquez O, Waliszewski KN. The effect of thermal treatment on β-glucosidase inactivation in vanilla bean (Vanilla planifolia Andrews) Int J Food Sci Technol. 2008;43:1993–1999. doi: 10.1111/j.1365-2621.2008.01804.x. [DOI] [Google Scholar]
- Márquez O, Waliszewski KN, Oliart RM, Pardío VT. Purification and characterization of cell wall-bound peroxidase from vanilla bean. LWT Food Sci Technol. 2008;41:1372–1379. doi: 10.1016/j.lwt.2007.08.017. [DOI] [Google Scholar]
- Naidu MM, Kumar PVS, Shyamala BN, Sulochanamma G, Prakash M, Thakur MS. Enzyme-assisted process for production of superior quality vanilla extracts from green vanilla pods using tea leaf enzymes. Food Bioprocess Technol. 2012;5:527–532. doi: 10.1007/s11947-009-0291-y. [DOI] [Google Scholar]
- Nishimura RT, Giammanco CH, Vosburg DA. Green, enzymatic syntheses of divanillin and diapocynin for the organic, biochemistry, or advanced general chemistry laboratory. J Chem Educ. 2016;87:526–527. doi: 10.1021/ed8001607. [DOI] [Google Scholar]
- Odoux E. Developing the aromatic quality of cured vanilla beans (Vanilla planifolia G. Jackson) In: Odoux E, Grisoni M, editors. Vanilla. Boca Raton: CRC Press; 2011. pp. 189–204. [Google Scholar]
- Odoux E, Escoute J, Verdeil J, Brillouet JM. Localization of β-d-glucosidase activity and glucovanillin in vanilla bean (Vanilla planifolia Andrews) Ann Bot. 2003;92:437–444. doi: 10.1093/aob/mcg150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okcu Z, Keleş F, Şat IG. Changes in chemical composition and peroxidase activity of turnip (Brassica rapa), during processing and frozen storage. Int J Food Agric Environ. 2013;11:91–94. [Google Scholar]
- Palama TL, Khatib A, Choi YH, Come B, Fock I, Verpoorte R, Kodja H. Metabolic characterization of green pods from Vanilla planifolia accessions grown in La Réunion. Environ Exp Bot. 2011;72:258–265. doi: 10.1016/j.envexpbot.2011.03.015. [DOI] [Google Scholar]
- Paramita V, Yulianto ME. Effect of β-glucosidase activity on vanillin enzymatic formation by using rumen liquid for cell walls degradation. J Food Res. 2013;2:65–69. doi: 10.5539/jfr.v2n2p65. [DOI] [Google Scholar]
- Pardío VT, Mariezcurrena MD, Waliszewski K, Sánchez V, Janczur MK. Effects of killing conditions of vanilla (Vanilla planifolia, Andrews) pods during the curing process on aroma composition of pod ethanol extract. Int J Food Sci Technol. 2009;44:2417–2423. doi: 10.1111/j.1365-2621.2009.01944.x. [DOI] [Google Scholar]
- Perera CO, Owen E. Effect of tissue disruption by different methods followed by incubation with hydrolyzing enzymes on the production of vanilla from Tongan vanilla beans. Food Bioprocess Technol. 2010;3:49–54. doi: 10.1007/s11947-007-0048-4. [DOI] [Google Scholar]
- Pérez-Silva A, Gunata Z, Lepoutre JP, Odoux E. New insight on the genesis and fate of odor-active compounds in vanilla beans (Vanilla planifolia G. Jackson) during traditional curing. Food Res Int. 2011;44:2930–2937. doi: 10.1016/j.foodres.2011.06.048. [DOI] [Google Scholar]
- Ruiz-Terán F, Pérez-Amador I, López-Munguía A. Enzymatic extraction and transformation of glucovanillin to vanillin from vanilla green pods. J Agric Food Chem. 2001;49:5207–5209. doi: 10.1021/jf010723h. [DOI] [PubMed] [Google Scholar]
- Santos IC, Smuts J, Schug KA. Rapid profiling and authentication of vanilla extracts using gas chromatography-vacuum ultraviolet spectroscopy. Food Anal Methods. 2017;10:4068–4078. doi: 10.1007/s12161-017-0976-1. [DOI] [Google Scholar]
- Waliszewski KN, Pardío VT, Ovando SL. A simple and rapid HPLC technique for vanillin determination in alcohol extract. Food Chem. 2006;101:1059–1062. doi: 10.1016/j.foodchem.2006.03.004. [DOI] [Google Scholar]
- Waliszewski KN, Ovando SL, Pardío VT. Effect of hydration and enzymatic pretreatment of vanilla beans on the kinetics of vanillin extraction. J Food Eng. 2007;78:1267–1273. doi: 10.1016/j.jfoodeng.2006.01.029. [DOI] [Google Scholar]
- Waliszewski KN, Márquez O, Pardío VT. Quantification and characterisation of polyphenol oxidase from vanilla beans. Food Chem. 2009;117:196–203. doi: 10.1016/j.foodchem.2009.03.118. [DOI] [Google Scholar]
- Zhang Y, Mo L, Chen F, Lu M, Dong W, Wang Q, Xu F, Gu F. Optimized production of vanillin from green vanilla pods by enzyme-assisted extraction combined with pre-freezing and thawing. Molecules. 2014;19:2181–2198. doi: 10.3390/molecules19022181. [DOI] [PMC free article] [PubMed] [Google Scholar]