Abstract
The effect of freeze- and spray-drying on physico-chemical characteristics, phenolics compounds and antioxidant activity of papaya pulp was investigated. The frozen pulp was freeze dried at − 62 °C during 48 h. Papaya pulp with 14% DE maltodextrin was also dried in a spray dryer. The organic acids, sugars, color, total soluble solids, pH, carotenoids, phenolic and flavonoid compounds, and antioxidant capacity values were determined. The changes in color, pH and lycopene were negligible. However, lower retention (86.5%) of vitamin C and sugars (glucose—79.7% and fructose—66.1%) was observed in spray dried products. Phenolic and flavonoid compounds were identified and quantified in dried papaya products by UHPLC-QqQ-MS/MS. Spray dried products presented a higher retention of phenolic and flavonoid compounds compared to the freeze dried products. Despite some variations in the parameters studied, the use of freeze- and spray-drying has proven viable options for the drying of papaya pulp.
Keywords: Papaya pulp, Freeze-drying, Spray-drying, Phenolics, Flavonoids
Introduction
Fruit consumption has become an imminent necessity due to its vital nutrients content, and currently, it is an important part of the human diet (Silva et al. 2014). Papaya (Carica papaya L.) has attained a certain prominence among the tropical fruits and its culture is widely spread in the world market. The fruit has high contents of carotenoids, sugars and vitamins and exhibit a pleasant aromatic flavor. Moreover, this fruit is an important source of functional nutrients including minerals (Udomkun et al. 2015). In addition, papaya is rich in bioactive compounds such as amines (Santiago-Silva et al. 2011), phenolic and flavonoid compounds (Sancho et al. 2011), which are responsible for various medicinal properties such as antioxidant, anti-inflammatory, antimicrobial, antifungal, diuretic and anti-ulcer (Vij and Pashar 2015).
Most tropical fruits are highly perishable, and its post-harvest losses are very high to about 30% of its production. These losses can be reduced by processing the fruits in a variety of products (Sousa et al. 2010). In this context, food researchers have focused their interest on the development of new processing methods which preserve the bioactivity and availability of certain constituents, besides the fruit´s nutritional and sensorial characteristics (Zhang et al. 2016).
Drying has several benefits, but it can also result in vitamin degradation, antioxidant activity reduction, and undesirable changes in color, texture and flavor of the fresh product, besides in some cases involving with high costs of operation (Fijalkowska et al. 2016).
Freeze-drying is a technique based on water removal by sublimation and is used to obtain several industrial products (Santo et al. 2013). On the other hand, spray drying consists of maximizing heat transfer (Nedovic et al. 2011). In spray drying, the selection of a proper encapsulation agent is of high importance for an efficient drying process. Among the available materials, maltodextrins are widely used as encapsulation agents, since these are extremely cost effective (Apintanapong and Noomhorm 2003).
The effect of freeze-drying on physico-chemical characteristics of papaya peel was reported (Morais et al. 2015) and a substantial decrease in phenolic contents, organic acids concentration and antioxidant activity (DPPH and FRAP) was observed.
Despite several publications existing on drying of papaya, few relate to the physico-chemical characteristics of the minor constituents in papaya pulp. Furthermore, no work has yet been published to evaluate the effect of freeze- and spray-drying on the quality and stability of papaya pulp (cv. “Sunrise Solo”). Thus, the objective of this study was to evaluate the effect of freeze- and spray-drying techniques on physico-chemical characteristics, organic acids, sugars, carotenoids, phenolic and flavonoid compounds and on antioxidant activity of papaya pulp and dried products.
Materials and methods
Papaya samples and drying process
Papaya fruits (cv. “Sunrise Solo”) were obtained from the municipal market from Aracaju, SE, Brazil. The pulp was obtained by manual peeling and removing the seeds. Pulp samples were stored at − 20 °C.
Papaya pulp was dehydrated in a freeze-dryer (Christ, Osterode, Germany, Alpha 1-4 LD Plus) at − 62 °C and 6.11 mbar for 48 h, according to the methodology proposed by Chranioti et al. (2016). For spray-drying, maltodextrin (14% DE with respect to the weight of the fresh papaya pulp) was added directly to the papaya pulp. Drying was carried out in a spray dryer (LABMAQ, Riberão Preto, Brazil, model MSDI 1.0) operating under inlet temperature of 150 °C, air flow of 4.00 m3/min and air pressure of 3 kgf/cm2. The feeding in the spray dryer was done by using a peristaltic pump at a rotation speed adjusted to its maximum speed (100 rpm), and flow of 0.4 L/h, according to the methodology proposed by Tan et al. (2011).
For physico-chemical and chromatographic analysis in the final dried products, the powders were rehydrated to achieve the same °Brix of papaya fresh pulp. However, for spray dried product, pulp was considered without maltodextrin addition.
Determination of pH, total soluble solids (°Brix) and color
The pH of the pulp was determined by direct measurement in microprocessor pH/ORP Meter (Hanna instruments, Barueri, Brazil).
Total soluble solids content was determined by a direct reading in a manual refractometer (Instrutherm, São Paulo, Brazil, RT-30 ATC). The soluble solids were quantified using two drops of pulp spread in a digital refractometer reading and expressed in degrees Brix. After performing each analysis the refractometer prism was cleaned thoroughly with distilled water.
The color of the samples was measured using a colorimeter (Konica Minolta®, Chroma Meter, Japan, model CR-400). The instrument operates on CIELAB (L*, a* and b*). The L, a, b values were used to calculate the chromaticity and the hue angle.
Antioxidant activity
Papaya pulp (1 g of fresh, freeze- or spray-dried) was homogenized in 10 mL of methanol, sonicated for 30 min in an ultrasonic bath and then centrifuged for 15 min at 4000g at 20 °C. The clear supernatant so obtained was used for the estimation of antioxidant activity.
DPPH assay
DPPH assay was conducted according to the methodology of Moo-Huchin et al. (2015) with slight modifications. The stock solution was prepared by mixing 2.5 mg of DPPH radical with 100 mL of methanol. The solution absorbance was adjusted to 0.7 ± 0.02 at 515 nm using an UV–Vis spectrophotometer (Molecular Devices, Sunnyvale, CA, USA; SpectraMax M2). Briefly, 3.9 mL of DPPH radical were placed in a test tube and 100 µL of the antioxidants extract or standard were added while methanol was used as a blank. The decrease in absorbance at 515 nm was measured at 1 min intervals for the first 10 min, and then at 5 min intervals until stabilization.
ABTS assay
The ABTS assay was performed according to the methodology proposed by Moo-Huchin et al. (2015). The ABTS radical cation (ABTS°+) was prepared by taking 19.2 mg of ABTS dissolved in 5 mL of deionized water and 88 µL of 0.0378 g/mL of potassium persulfate. This stock solution was allowed to react for 16 h in dark. The ABTS activated radical was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. After the addition of 30 µL of antioxidants extract or standard to 2970 µL of diluted ABTS solution, absorbance values were recorded after 6 min.
Ferric reducing antioxidation power (FRAP)
The FRAP assay was carried out according to the methodology reported by Thaipong et al. (2006) with slight modifications. The fresh FRAP reagent was prepared by mixing 10 mM 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl, 20 mM FeCl3 aqueous solution and acetate buffer (3.1 g C2H3NaO2 3H2O and 16 mL C2H4O2; pH: 3.6). Fifty microliter of sample was mixed with 950 µL of the FRAP reagent. The increase in absorbance was measured at 593 nm (29/30 °C) until stabilization and compared against the stable FRAP reagent.
Antioxidant activities (DPPH, ABTS and FRAP) were expressed in both units as Trolox equivalent (TE) and ascorbic acid equivalent (AAE) per g mass of extract. Samples analysis was performed in triplicate.
Identification and quantification of organic acids
The organic acids quantification was done according to the methodology proposed by Gomes et al. (2017). Papaya pulp (1 g) was diluted in 2 mL of distilled water and centrifuged at 4000g and 20 °C. Supernatant was passed through in a C-18 SPE cartridge and filtered using a 0.22 µm cellulose membrane (Merck Millipores Ltd, USA). High Performance Liquid Chromatographic (HPLC) analysis was used to separate the organic acids. A HPLC with UV detector system (Shimadzu Corporation, Japan) equipped with a degasser DGU-20A5, a pump system (LC-20AT), a column oven (CTO-20A), autosampler (SIL-20A HT), and UV-DAD detector (SPD-20A) was used and wavelength was read at 210 nm. Organic acids were separated onto an Agilent Hi-Plex column (300 × 7.8 mm) (Agilent Technologies) and kept at 50 °C. The isocratic elution was performed with 0.01 M sulfuric acid in deionized water as mobile phase for 30 min at a flow rate of 0.6 mL/min. The injection volume was 20 µL.
For quantification of malic and ascorbic acids in papaya pulp and in dried products, calibration curves were prepared by analyzing a mixture of these acids at different concentrations (0.2–2.0 g/L for malic acid and 0.1–1.0 g/L for ascorbic acid). Samples analysis was performed in triplicate.
Identification and quantification of sugars
The sugars quantification was performed according to the methodology proposed by Tingirikari et al. (2017). Papaya pulp (1 g) was diluted in 2 mL of distilled water and centrifuged at 4000g and 20 °C. Supernatant was passed through in a C-18 SPE cartridge and filtered using a 0.22 µm cellulose membrane (Merck Millipore, Barueri, Brazil).
HPLC-Refractive index detector analysis was used to detect and quantify the sugars. A Shimadzu LC System (Shimadzu Corporation; Japan) equipped with a pump system (LC-20AT) and Refractive Index detector (RID-10A) was used. Sugars were separated onto a Supelcogel Ca column (300 × 7.8 mm) (Supelco Inc., USA) maintained at 80 °C. The injection volume was 20 µL. The isocratic elution was performed with deionized water as mobile phase for 40 min at a flow rate of 0.5 mL/min.
In order to quantify glucose and fructose, calibration curves were constructed by analyzing a mixture of these sugars at different concentrations (1.0–10 g/L). Samples analysis was performed in triplicate.
Identification and quantification of carotenoids
Papaya pulp (1 g) was diluted in 2 mL of acetone, centrifuged at 4000 g and at 20 °C, and supernatant was filtered using a 0.22 µm cellulose membrane (Merck Millipores, Barueri, SP, Brazil), according to the methodology reported by Ajila et al. (2007).
Ultra Performance Liquid Chromatography (UPLC) analysis was used to detect and quantify the carotenoids. UPLC analysis was carried out on a Waters system (model Waters ACQUITY UPLC H-Class) equipped with a quaternary pump, a four-channel in-line vacuum degasser and an autosampler injector. The injection volume was of 10 µL and the flow rate was adjusted to 0.5 mL/min. Carotenoids were separated and analyzed using an Acquity UPLC BEH C18 column 1.7 µm (2.1 × 50 mm) (Waters, Milford, MA, USA) maintained at 30 °C. The mobile phase consisted of acetonitrile (A), methanol (B) and ethyl acetate (C). The gradient profile was as follows: 95% A, 5% B, 0% C to 60% A, 20% B, 20% C in 20 min, maintaining the last proportion until the end of the run.
The quantification of lycopene was performed using a calibration curve prepared with the standard (1.0–100 mg/L). Samples analysis was performed in triplicate.
Identification and quantification of phenolic and flavonoid compounds
Papaya pulp (2 g) was extracted with 75 mL of ethanol solution (70%). The solution was heated at 55 °C and maintained on a water bath for 5 min. Later it was kept on a rotary shaker (150 rpm, 27 °C) for 24 h. The samples were centrifuged at 4000g and 20 °C and the supernatant was evaporated in a rotary evaporator at 40 °C. Methanol was used to resuspend the extracts, which was later filtered using 0.22 µm cellulose membrane (Merck, Barueri, Brazil).
The phenolic compounds analysis was performed using an Ultra-high Performance Liquid Chromatography coupled with tandem mass spectrometry (UHPLC-QqQ-MS/MS) system model 1290 Infinity coupled to a 6490 Triple Quadruple mass spectrometer equipped with an electrospray ionization (Agilent Technologies, Palo Alto, USA). The chromatographic separation was performed on an Ascentis Express F5, 2.7 µm particle size and 150 × 2.1 mm i.d. column (Sigma Aldrich, Saint Louis, MO, USA). The mobile phase consisted of 0.1% (v/v) aqueous formic acid solution (A) and acetonitrile (B), with a flow rate of 0.2 mL/min, under gradient elution at 40 °C. The gradient profile was as follows: 0–1 min, 15% B; 1–7 min, 25% B; 7–9 min, 25% B; 9–13 min, 30% B; 13–16, 30% B; 16–21 min, 40% B; 21–23, 40% B; 23–25, 45% B; 25–28, 50% B; 28–33, 60% B; 33–37, 75% B; 37–38, 15% B. The injection volume was 2 µL. The UHPLC-QqQ-MS/MS data were acquired using Mass Hunter software (Agilent; version B.07.00).
Mass spectrometric conditions were source temperature: 200 °C; gas flow: 12 L/h; nebulizer: 20 psi; sheet gas temperature: 400 °C; gas flow: 11 L/min; capillary: 3500 V; nozzle: 500 V; acceleration cell voltage: 5 V; dwell time: 9.8. In order to obtain maximum sensitivity for identification and detection of all target compounds, two SRM (Selected Reaction Monitoring) transitions were optimized for each compound. Electrospray ionization source was operated in positive mode, except for caffeic acid phenethyl ester (CAPE), which was monitored in negative mode.
Statistical analysis
Statistical analysis was performed using the statistical software Statistica (Statsoft) version 10.0. The results were compared by applying Tukey test at a 95.0% confidence level.
Results and discussion
Effect of drying on pH and color of papaya pulp
The soluble solids content of fresh papaya pulp was 10.9 °Brix. The atomized and lyophilized powders were rehydrated to achieve the same brix value as that of the fresh pulp. Table 1 shows the pH and color parameter values of the papaya pulp samples and dried powders by freeze- and spray-drying. Drying did not induce any change in the pH of papaya pulp compared with the control sample. The pH values were in the range of 3.78–3.80.
Table 1.
Physico-chemical and chemical characteristics in papaya pulp and dried powders
| Characteristics | Fresh pulp | Dried powders | |
|---|---|---|---|
| Freeze drying | Spray drying | ||
| Color parameters | |||
| L* | 9.48 ± 0.24c | 12.41 ± 0.05b | 14.07 ± 0.00a |
| a | 11.84 ± 0.20a | 6.24 ± 0.03b | 3.99 ± 0.05c |
| b | 11.28 ± 0.17c | 15.76 ± 0.0a | 14.49 ± 0.19b |
| C | 16.35 ± 0.18a | 16.95 ± 0.05a | 15.03 ± 0.17b |
| h° | 52.41 ± 0.06b | 68.82 ± 0.08a | 73.91 ± 0.11a |
| pH | 3.79 ± 0.01a | 3.78 ± 0.01a | 3.80 ± 0.00a |
| Brix | 10.91 ± 0.01a | 10.90 ± 0.01a | 10.91 ± 0.02a |
| Sugars | |||
| Glucose (mg/g of pulp) | 20.79 ± 0.01a | 19.43 ± 0.01a | 16.57 ± 0.15b |
| Fructose (mg/g of pulp) | 25.16 ± 0.06a | 27.65 ± 0.04a | 16.62 ± 0.01b |
| Carotenoids | |||
| Lycopene (µg/g of pulp) | 24.80 ± 0.02a | 22.70 ± 0.01b | 21.62 ± 0.01b |
Mean ± standard deviation (n = 3)
Values with different lower-case superscript letters (a–c) in the same line are significantly different (p < 0.05)
The L parameter, which indicates the luminosity of the product, had significant difference (p < 0.05) between spray dried products (14.07 ± 0.0) and the fresh pulp (9.48 ± 0.24). An increase in hue parameter in freeze (68.82 ± 0.08) and spray dried (73.91 ± 0.11) products in comparison to the fresh pulp (52.41 ± 0.06) was also observed.
One of the parameters, which affected color, was the addition of maltodextrin in the papaya pulp for spray drying. It has been earlier reported that the color of products was significantly affected by maltodextrin concentration. Since maltodextrin is of white color, lightness in color of powders, represented by a higher L* value was reported at higher concentrations of maltodextrin in samples of gac fruit (Kha et al. 2010). In other studies, it was concluded that increased drying temperatures resulted in lower retention of the redness in carrot (Chen et al. 1995).
Despite the increase in the color parameters in dried products compared to the fresh pulp, there was no significant difference (p < 0.05) in the values of color in the two types of dried products.
Antioxidant activity
The antioxidant capacity of papaya pulp and dried products was measured by DPPH, FRAP and ABTS free radical assays. The results are presented in Table 2. No significant difference (p < 0.05) was observed in FRAP values among the samples subjected to drying techniques in comparison to control sample. DPPH and ABTS analyses revealed an increase in the antioxidant activity in lyophilized products. However, a decrease in antioxidant activity in spray dried products analyzed by DPPH and ABTS assays was observed.
Table 2.
Antioxidant activity in papaya pulp and dried powders
| Samples | DPPH (mg TE/g) | DPPH (mg AAE/g) | ABTS (mg TE/g) | ABTS (mg AAE/g) | FRAP (mg TE/g) | FRAP (mg AAE/g) |
|---|---|---|---|---|---|---|
| Fresh pulp | 0.56 ± 0.05a | 0.45 ± 0.01a | 0.48 ± 0.02b | 0.31 ± 0.01b | 0.37 ± 0.03a | 0.21 ± 0.01a |
| Freeze drying | 0.61 ± 0.03a | 0.49 ± 0.02a | 0.58 ± 0.01a | 0.37 ± 0.00a | 0.42 ± 0.00a | 0.23 ± 0.00a |
| Spray drying | 0.51 ± 0.01b | 0.42 ± 0.03b | 0.45 ± 0.00b | 0.28 ± 0.01b | 0.35 ± 0.01a | 0.20 ± 0.02a |
Mean ± standard deviation (n = 3)
AAE ascorbic acid equivalent, TE trolox equivalent
Values with different lower-case superscript letters (a–b) in the same column are significantly different (p < 0.05)
The effect of microwaves, hot air and freeze drying on antioxidant activity (DPPH and ABTS) of cacao husks was reported (Valedez-Carmona et al. 2017). An increase in antioxidant activity values was observed in all dried products. Among these, the freeze dried products presented higher value of antioxidant activity.
Identification and quantification of organic acids
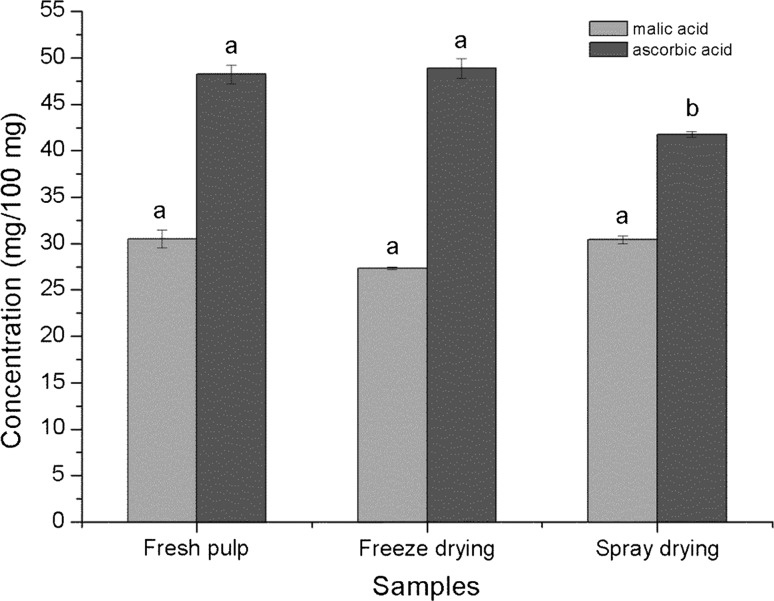
These results did show some differences in the concentration of the ascorbic acid after spray-drying in comparison with the fresh pulp and freeze dried products (Fig. 1). It should be noted that there was a total retention of ascorbic acid in freeze dried products as compared to spray dried product which had only 86.5% retention. No significant change (p < 0.05) was observed in the malic acid concentration after drying.
Fig. 1.
Concentration (mg/100 mg) of organic acids in papaya pulp and dried powders
Marques et al. (2006) reported a reduction in vitamin C concentration of papaya during freeze-drying from 87.24 ± 0.02 to 66.13 ± 0.03 g/100 g, which was significant (p < 0.05), thus corroborating the results obtained in this work. Other studies have reported the effect of microencapsulation of passion fruit juice using spray (Borrmann et al. 2013) and freeze drying (Borrmann et al. 2011). Microencapsulated samples showed a significant loss in vitamin C concentration after spray drying.
Identification and quantification of sugars
The results of monosaccharides concentration in papaya powder obtained after freeze-drying did not present any significant (p < 0.05) changes when compared to fresh pulp (Table 1). The concentrations of glucose and fructose after freeze-drying (19.43 ± 0.01 and 27.65 ± 0.04 mg/g of pulp, respectively) were similar to the concentrations obtained in the pulp samples (20.79 ± 0.01 and 25.16 ± 0.06 mg/g of pulp, respectively). The monosaccharides concentration after spray drying presented a decrease in comparison to the papaya fresh pulp. The concentrations of glucose and fructose after spray drying (16.57 ± 0.15 and 16.62 ± 0.01 mg/g of pulp, respectively) were lower to the concentrations in the unprocessed papaya pulp. This decrease can be attributed to the addition of 14% maltodextrin, in the pulp mixture used for spray drying.
Moßhammer et al. (2006) studied the evaluation of different methods for the production of juice concentrates and fruit powders from cactus pear. For both forms of concentrations such as evaporation and freeze-drying, glucose and fructose contents slightly increased during processing while it did not change the glucose/fructose ratio. However, they did not find any significant (p < 0.05) changes in these monosaccharides between pear fruit pulp and its spray dried powder.
Identification and quantification of carotenoids
A decrease in the lycopene concentration was observed after freeze-drying (22.70 ± 0.01 µg/g of pulp) and spray-drying (21.62 ± 0.01 µg/g of pulp), compared to the fresh papaya pulp (24.80 ± 0.01 µg/g of pulp) (Table 1).
These results on lycopene corroborate with those found in the color parameters, wherein there was loss in color intensity. Changes in temperature of drying processes may be associated with oxidation of pigments. The decrease in lycopene concentration is also associated with carotenoid degradation. Despite the decrease in lycopene concentration, freeze- and spray-drying presented a good retention percentage (91.53 and 87.17%, respectively) in the dried products.
Kha et al. (2010) evaluated the effect of spray drying conditions on total carotenoid content of gac fruit and reported the effects of inlet drying air temperature and maltodextrin addition. The total carotenoid content in dried powder reduced from 1.95 to 0.61 mg/g of powder which was correlated to the maltodextrin concentration which increased from 10 to 30%. Moreover, the total carotenoid content decreased with the increase in temperature.
Some studies have reported the degradation of carotenoids in papaya after conventional drying (50–80 °C). Udomkun et al. (2015) reported that drying parameters, like temperature, can decompose carotenoids in papaya compared to fresh pulp. In other study, Calvache et al. (2016) compared the difference of total carotenoids content in papaya pulp after microwave and freeze-drying. It was observed that the lyophilized product contained 14.0 mg/100 g and papaya pulp after microwave contained 5.10 mg/100 g of total carotenoids content.
Likewise, some studies reported the importance of stability of carotenoids in freeze-dried papaya. It was reported that 32.78% of papaya pigment was lost at room temperature and 42.0% at 37 °C after 45 days period (Arya et al. 1983).
Identification and quantification of phenolic and flavonoid compounds
Five phenolic compounds (protocatechuic acid, vanillic acid, ferulic acid, caffeic acid and artepillin C) were identified in papaya fresh pulp and these were also found in papaya spray dried powders (Table 3). These results are similar to those obtained by Sancho et al. (2011), who identified ferulic acid (277.49–186.63 mg/100 g DW) and caffeic acid (175.51–112.89 mg/100 g DW) as principal phenolic compounds in papaya (Carica papaya L., cv. “Maradol”) pulp. To the best of our knowledge, no work has been reported which quantified and compared the phenolic acids and flavonoids profile in freeze- and spray-drying products of papaya. Moreover, the presence of artepillin C is being reported in papaya pulp and dried products for the first time in the present study.
Table 3.
Concentration (ng/g) of phenolic and flavonoid compounds in papaya pulp and dried powders
| Compounds | Fresh pulp | Dried powders | |
|---|---|---|---|
| Freeze drying | Spray drying | ||
| p-coumaric acid | < MDL | < MDL | 57.09 ± 0.40 |
| Protocatechuic acid | 19.14 ± 1.99a | 21.06 ± 0.11a | 18.32 ± 1.46a |
| Vanillic acid | 46.04 ± 0.50a | 15.82 ± 0.12c | 30.73 ± 0.35b |
| Ferulic acid | 7.24 ± 0.04b | < MDL | 11.26 ± 0.32a |
| Naringenin | < MDL | < MDL | 0.46 ± 0.02 |
| Caffeic acid | 9.93 ± 0.29a | < MDL | 9.45 ± 1.12a |
| Artepillin C | 12.77 ± 0.06b | 13.52 ± 0.58b | 45.02 ± 1.69a |
Mean ± standard deviation (n = 3)
MDL method detection limit
Values with different lower-case superscript letters (a–c) in the same line are significantly different (p < 0.05)
The drying method affected the phenolic concentration of dried papaya products. Freeze-drying resulted in adverse effects, with concentrations lower than the method detectable limit (MDL) for ferulic, caffeic, p-coumaric acids, and also naringenin, when compared with the fresh pulp and spray dried product. Vanillic acid had a drastic decrease (76%) in its concentration after freeze-drying. A slight increase was observed for protocatechuic acid in freeze dried papaya but this increase was not significant (p < 0.05) when compared to the fresh pulp and spray dried products. Oxidative reactions are known to affect the phenolic losses in drying process. In freeze-drying, enzymatic reactions with action of oxidative enzymes, like polyphenol oxidase and peroxidase are likely to occur. In general, the structure of papaya cells is disrupted in freeze-drying and due to the lower exposure to oxygen; such conditions may lead to the liberation of these kinds of enzymes, in addition to the cells injury caused by ice crystals formation (Gümüsay et al. 2015). Moreover, modifications in chemical structure of phenolic compounds or protein binding with matrix components might result in difficulty in the extraction of these compounds (Miranda et al. 2010).
The spray dried papaya product contained slightly higher concentrations of polyphenolic compounds than the freeze dried one. Naringenin and p-coumaric acid were identified and quantified in spray dried products, but these were not found in fresh pulp. In addition, artepillin C concentration was increased by more than three times when compared with fresh pulp, inferring the positive result of this drying technique. A similar effect was observed by Horszwald et al. (2013), who found a higher content of total polyphenols in chokeberry after drying at high temperatures, than after freeze-drying. High temperature applications deactivate oxidative and hydrolytic enzymes, consequently resulting in avoiding losses of polyphenolic components. In addition, it is well known that thermal processing with high temperatures may release more bound phenolic acids from the breakdown of cellular constituents, leading to the presence of phenolic compounds which were not detected in fresh pulp samples (Dewanto et al. 2002). The choice of drying method and optimization of drying parameters influence significantly the product quality as related to retention of bioactive compounds and antioxidant activity.
Finally, it is concluded from the present work that spray dried papaya products presented a higher retention of flavonoids and phenolic compounds compared with the freeze dried products.
Conclusion
Freeze- and spray-drying showed promising results in the preservation of bioactive compounds and physico-chemical proprieties of papaya pulp. Mostly the phenolic and flavonoid compounds were retained in both dried products. The color parameters, pH and lycopene did not show any major changes after drying. Ascorbic acid presented less retention (86.5%) in spray dried powder compared to freeze dried products. Monosaccharides (glucose and fructose) decreased in spray dried products. Freeze-drying resulted in lower retention of some prominent phenolic compounds (vanillic acid, ferulic acid and caffeic acid). Artepillin C was identified in papaya pulp and dried products for the first time in the present study. Finally, our results suggest both spray and freeze-drying are suitable for the production of papaya powders, since these techniques preserved main nutrients of fresh papaya, making them viable options for pulp processing.
Acknowledgements
All authors gratefully acknowledge financial support received from CNPq, Brazil vide research project ‘National Institute of Science & Technology for Tropical Fruits’ (Process 57573781/2008-7) in developing this work. Authors acknowledge and thank CAPES (Ministry of Education, Brazil) and CNPq for their fellowships.
Contributor Information
Wesley Faria Gomes, Email: profwgomes@hotmail.com.
Fernanda Rocha Morais França, Email: moraisfr@yahoo.com.br.
Marina Denadai, Email: marrydenadai@gmail.com.
Julianna Karla Santana Andrade, Email: andrade.julianna@hotmail.com.
Ester Maria da Silva Oliveira, Email: hadassa-ester@hotmail.com.
Edy Sousa de Brito, Email: edy.brito@embrapa.br.
Sueli Rodrigues, Email: suelir@gmail.com.
Narendra Narain, Phone: +5579 31946514, Email: narendra.narain@gmail.com.
References
- Ajila CM, Naidu KA, Bhat SG, Rao UJSR. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007;105:982–988. doi: 10.1016/j.foodchem.2007.04.052. [DOI] [Google Scholar]
- Apintanapong M, Noomhorm A. The use of spray drying to microencapsulate 2-acetyl-1-pyrroline, a major flavor component of aromatic rice. Int J Food Sci Technol. 2003;38:95–102. doi: 10.1046/j.1365-2621.2003.00649.x. [DOI] [Google Scholar]
- Arya SS, Natesan V, Parihar DB, Vijayaraghavan PK. Stability of carotenoids in freeze-dried papaya (Carica papaya) J Food Technol. 1983;18:177–181. doi: 10.1111/j.1365-2621.1983.tb00258.x. [DOI] [Google Scholar]
- Borrmann D, Leite SGF, Leão MHMR. Microencapsulation of passion fruit (Passiflora) juice in capsule. Int J Fruit Sci. 2011;11:376–385. doi: 10.1080/15538362.2011.630299. [DOI] [Google Scholar]
- Borrmann D, Pierucci APTR, Leite SGF, Leão MHMR. Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatized starch using spray-drying. Food Bioprod Proc. 2013;91:23–27. doi: 10.1016/j.fbp.2012.08.001. [DOI] [Google Scholar]
- Calvache JN, Cueto M, Farroni A, Pla ME, Gerschenson LN. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.) J Funct Foods. 2016;27:319–328. doi: 10.1016/j.jff.2016.09.012. [DOI] [Google Scholar]
- Chen BH, Peng HY, Chen HE. Changes of carotenoids, color, and vitamin A contents during processing of carrot juice. J Agric Food Chem. 1995;43:1912–1918. doi: 10.1021/jf00055a029. [DOI] [Google Scholar]
- Chranioti C, Chranioti S, Tzia C. Comparison of spray, freeze and oven drying as a means of reducing bitter aftertaste of steviol glycosides (derived from Stevia rebaudiana Bertoni plant)—evaluation of the final products. Food Chem. 2016;190:1151–1158. doi: 10.1016/j.foodchem.2015.06.083. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Fijalkowska A, Nowacka M, Wiktor A, Sledz M, Witrowa-Rajchert D. Ultrasound as a pretreatment method to improve drying kinetics and sensory properties of dried apple. J Food Process Eng. 2016;39:256–265. doi: 10.1111/jfpe.12217. [DOI] [Google Scholar]
- Gomes WF, Tiwari BK, Rodriguez O, Brito ES, Fernandes FAN, Rodrigues S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017;218:261–268. doi: 10.1016/j.foodchem.2016.08.132. [DOI] [PubMed] [Google Scholar]
- Gümüsay ÖA, Borazan AA, Ercal N, Demirkol O. Drying effects on the antioxidant properties of tomatoes and ginger. Food Chem. 2015;173:156–162. doi: 10.1016/j.foodchem.2014.09.162. [DOI] [PubMed] [Google Scholar]
- Horszwald A, Julien H, Andlauer W. Characterization of Aronia powders obtained by different drying processes. Food Chem. 2013;141:2858–2863. doi: 10.1016/j.foodchem.2013.05.103. [DOI] [PubMed] [Google Scholar]
- Kha TC, Nguyen MH, Roach PD. Effects of spray drying conditions on the physicochemical and antioxidant properties of the gac (Momordica cochinchinensis) fruit aril powder. J Food Eng. 2010;98:385–392. doi: 10.1016/j.jfoodeng.2010.01.016. [DOI] [Google Scholar]
- Marques LG, Silveira AM, Freire JT. Freeze-drying characteristics of tropical fruits. Drying Technol. 2006;24:457–463. doi: 10.1080/07373930600611919. [DOI] [Google Scholar]
- Miranda M, Vega-Gálvez A, López J, Parada G, Sanders M, Aranda M, Uribe E, Di Scala K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.) Ind Crops Prod. 2010;32:258–263. doi: 10.1016/j.indcrop.2010.04.019. [DOI] [Google Scholar]
- Moo-Huchin VM, Moo-Huchin MI, Estrada-León RJ, Cuevas-Glory L, Estrada-Mota IA, Ortiz-Vásquez E, Betancur-Ancona D, Sauri-Duch E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015;166:17–22. doi: 10.1016/j.foodchem.2014.05.127. [DOI] [PubMed] [Google Scholar]
- Morais DR, Rotta EM, Sargi SC, Schmidt EM, Bonafe EG, Eberlin MN, Sawaya ACHF, Visentainer JV. Antioxidant activity, phenolics and UPLC–ESI(–)MS of extracts from different tropical fruits parts and processed peels. Food Res Int. 2015;77:392–399. doi: 10.1016/j.foodres.2015.08.036. [DOI] [Google Scholar]
- Moßhammer MR, Stintzing FC, Carle R. Evaluation of different methods for the production of juice concentrates and fruit powders from cactus pear. Innov Food Sci Emerg Technol. 2006;7:275–287. doi: 10.1016/j.ifset.2006.04.003. [DOI] [Google Scholar]
- Nedovic V, Kalusevic A, Manojlovic V, Levic S, Bugarski B. An overview of encapsulation technologies for food applications. Proc Food Sci. 2011;1:1806–1815. doi: 10.1016/j.profoo.2011.09.265. [DOI] [Google Scholar]
- Sancho LEG, Yahia EM, González-Aguilar GA. Identification and quantification of phenols carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res Int. 2011;44:1284–1291. doi: 10.1016/j.foodres.2010.12.001. [DOI] [Google Scholar]
- Santiago-Silva P, Labanca RA, Gloria MBA. Functional potential of tropical fruits with respect to free bioactive amines. Food Res Int. 2011;44:1264–1268. doi: 10.1016/j.foodres.2010.11.026. [DOI] [Google Scholar]
- Santo EFE, Lima LKF, Torres APC, Oliveira G, Ponsano EHG. Comparison between freeze and spray drying to obtain powder Rubrivivax gelatinosus biomass. Food Sci. Technol. 2013;33:47–51. doi: 10.1590/S0101-20612013005000008. [DOI] [Google Scholar]
- Silva LMR, Figueiredo EAT, Ricardo NMPS, Vieira IGP, Figueiredo RW, Brasil IM, Gomes CL. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Sousa PHM, Ramos AM, Maia GA, Brito ES, Garruti DS, Fonseca AVV. Addition of Ginkgo biloba and Panax ginseng extracts to mixed tropical fruit nectars. Food Sci Technol. 2010;30:463–470. doi: 10.1590/S0101-20612010000200025. [DOI] [Google Scholar]
- Tan LW, Ibrahim MN, Kamil R, Taip FS. Empirical modeling for spray drying process of sticky and non-sticky products. Proc Food Sci. 2011;1:690–697. doi: 10.1016/j.profoo.2011.09.104. [DOI] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallosc L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Tingarikari JMR, Gomes WF, Rodrigues S. Efficient production of prebiotic gluco-oligosaccharides in orange juice using immobilized and co-immobilized dextransucrase. Appl Biochem Biotechnol. 2017;183:1–17. doi: 10.1007/s12010-017-2427-2. [DOI] [PubMed] [Google Scholar]
- Udomkun P, Nagle M, Mahayothee B, Nohr D, Koza A, Müller J. Influence of air drying properties on non-enzymatic browning, major bio-active compounds and antioxidant capacity of osmotically pretreated papaya. Food Sci technol. 2015;60:914–922. [Google Scholar]
- Valedez-Carmona L, Plazola-Jacinto CP, Hernández-Ortega M, Hernández-Navarro MD, Villarreal F, Necoechea-Mondragón H, Ortiz-Moreno A, Ceballos-Reyes G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.) Innov Food Sci Emerg Technol. 2017;41:378–386. doi: 10.1016/j.ifset.2017.04.012. [DOI] [Google Scholar]
- Vij T, Pasha Y. A review on medicinal properties of Carica papaya Linn. Asian Pac J Trop Dis. 2015;5:1–6. doi: 10.1016/S2222-1808(14)60617-4. [DOI] [Google Scholar]
- Zhang L, Liu T, Xue Y, Liu C, Ru H, Dong M, Yu Z. Effects of drying methods on the aroma components and quality of Capsella Bursa-pastoris L. J Food Process Eng. 2016;39:107–120. doi: 10.1111/jfpe.12204. [DOI] [Google Scholar]