Abstract
Fluorescence spectrometry, combined with principle component analysis, partial least-squares regression (PLSR) and artificial neural network (ANN), was applied for the analysis of Maltese extra virgin olive oil (EVOO) adulterated by blending with vegetable oil (corn oil, soybean oil, linseed oil, or sunflower oil). The novel results showed that adjusted PLSR models based on synchronised spectra for detecting the % amount of EVOO in vegetable oil blends had a lower root mean square error (0.02–6.27%) and higher R2 (0.983–1.000) value than those observed when using PLSR on the whole spectrum. This study also highlights the use of ANN as an alternative chemometric tool for the detection of olive oil adulteration. The performance of the model generated by the ANN is highly dependent both on the type of data input and the mode of cross validation; for spectral data which had a variable importance plot value > 0.8 the excluded row cross validation was more appropriate while for complete spectral analysis k-fold or CV-10 was more appropriate.
Keywords: Adulteration, Chemometrics, EVOO, Spectrofluorimetry, Synchronised spectra, ANN, PLSR, PCA
Introduction
Virgin olive oils are obtained from the olive tree Olea europaea L drupes, widely diffused across the Mediterranean region (Solari and Vernet 1992; Terral and Arnold-Simard 1996). The classification of olive oils is governed by strict controls issued by the European Union Commission (EEC 2013) for the verification of its quality and purity. In order for an olive oil to be classified as virgin, it must be obtained solely by mechanical means without any extra treatment or refining processes. Only minimal treatment such as washing, pressing, decantation, centrifugation and filtration is allowed. Unlike the common seed oils, it is one of the few vegetable oils consumed in its natural state without the need of further refining process, such as bleaching and deodorisation (Rahmani and Saari-Csallany 1998).
The high investment required to produce high quality olive oil results in a higher market price than other refined seed oils. Due to their lower price, refined oils are sometimes used to adulterate olive oils of better quality, such as extra-virgin olive oil, in order to increase the volume of oil and obtain a larger output at the expense of quality. The most common edible oils such as soybean, corn, canola, cotton, sunflower, and peanut are likely to be used as illicit adulterants of olive oil. Therefore, a rapid method to detect adulteration is important for purposes of quality control and to check the veridicity of labelling of extra virgin olive oil (Guimet et al. 2005).
Numerous analytical practices have been established in recent years to safeguard the authenticity of olive oil. These include chromatographic techniques (Bosque-Sendra et al. 2012; Baccouri et al. 2008) and spectroscopic techniques, such as mass spectrometry (Calvano et al. 2012), nuclear magnetic resonance (Fragaki et al. 2005), near-infrared spectroscopy (Mignani et al. 2011), Raman spectroscopy (Dong et al. 2012), chemiluminescence (Papadopoulos et al. 2002), UV spectrometry (Jiang et al. 2013), fluorescence spectroscopy (Sikorska et al. 2012), and synchronous fluorescence (Poulli et al. 2007). The application of different DNA based methods have been shown to be very effective in ensuring both the traceability of EVOO from the tree to the oil but also when it comes to varietal adulteration (Pasqualone et al. 2016). Compared to the other analytical techniques both UV and fluorescence spectroscopic techniques are ideal for the determination of olive oil adulteration, owing to their simplicity, cost-effectiveness, rapidity and non-destructive nature of the analysis. Fluorescence spectroscopy is more sensitive and selective in terms of organic and inorganic compounds than the other spectroscopic methods (Sikorska et al. 2004).
The fluorescence emission spectra of olive oils reveals five major bands. The 300–390 nm band provides information about their polyphenolic and tocopherol content (Zandomeneghi 2006; Giungato et al. 2004). While the low intensity doublet at 440 and 455 nm corresponds to oxidised fatty acids and phenolic antioxidants in virgin olive oils, which provide greater protection against oxidation of monounsaturated fatty acids (Kyriakidis and Skarkalis 2000). The strong band at 525 nm corresponds to the vitamin E content. The medium intensity band at 681 nm corresponds to the chlorophyll band which most of the time is absent in the rest of the seed oils.
In the present study we focused on the use of a single wavelength synchronised fluorescence spectroscopy rather than the entire EEM (Maggio et al. 2010) or the total synchronous fluorescence (Poulli et al. 2006) for the determination of different olive oil adulterants. The aim of this study was to determine the potential of fluorescence spectroscopy and chemometric methods as a tool for the assessment of olive oil adulterants on local EVOOs. However in this study, a variable selection process was carried out and its potential effect on the overall performance of the partial least squares regression models was assessed. Furthermore, the performance of the PLSR models was compared to those obtained using higher modelling power methods namely artificial neural networks.
Materials and methods
Samples and sample preparation
Fresh extra virgin olive oil (EVOO) (12 samples), which had been previously analysed for its peroxide value, free acidity, K232 and K270 parameters in order to establish its quality, was obtained from a small-scale local press. Samples of six commonly used adulterants, namely sunflower (SFO) (4 samples), peanut (PO) (2 samples), soya bean (SBO) (3 samples), linseed (LSO) (3 samples), corn (CO) (3 samples) and rapeseed (RSO) (2 samples) oil, were purchased from local supermarkets. Solutions of olive oil, pure, and adulterated (0.5, 5, 20, 25, 50, 60, 75, 80% w/w) with increasing levels of seed oil up to a composition of pure seed oil, were prepared and diluted 50% in spectrophotometrically pure 2,2,4-trimethylpentane (Sigma- Aldrich).
Fluorescence spectroscopy and synchronous fluorescence spectra measurement
A three-dimensional (3D) matrix excitation-emission matrix (EEM) was obtained for each sample using a Jasco FP-8300 fluorescence spectrophotometer, with both the excitation and the emission bandwidths set at 5 nm for a measurement range between 210 and 750 nm. The acquisition interval and the integration time were maintained at 0.5 nm and 10 ms, respectively, with a scan speed of 5000 nm min−1. The oil samples were examined by means of right-angle geometry. Synchronous fluorescence spectra were acquired by simultaneous scanning of the excitation and the emission monochromators, with a constant distance, ∆λ, of 24 nm. All analyses were carried out in duplicates, and the results reported as mean values. Fluorescence intensities were plotted as a function of the excitation wavelength.
Statistical analysis and model building
Principal component analysis (PCA)
PCA is a multivariate projection method, generally used in chemometrics to compress large dimensional data into a smaller-dimensional space with the smallest loss of information (Aguado et al. 2008). PCA used to visualize differences in the various seed oils and olive oil on the basis of the whole spectral difference observed in the excitation, emission and synchronised spectra data by using Past 3.06 (Øyvind Hammer, University of Oslo).
Partial least square regression analysis (PLSR)
PLSR is a type of multiple linear regression analysis which is used to extract latent factors that account for much of the apparent factor (Zheng and Lu 2011). In this study, the synchronised spectral data obtained at 24 nm from 210 to 750 nm were used. The PLSR prediction models were built using JMP® 10 statistical software package (SAS) using leave-one-out as the validation method. The PLSR was optimised using an adjusted PLSR, by using wavelengths which had a variable importance plot value (VIP) larger than 0.8.
Artificial neural networks (ANN)
ANN is a nonlinear statistical model whereby the response variable, in this case the % amount of olive oil present and the absorbance at the different wavelength, were considered as factors, used to build up a prediction model. The purposes of using an artificial neural network was to process information supplied by the spectral data and predict the concentration of the adulterant. This method was cross validated using three methods the k-fold, holdback and excluded rows. For the k-fold cross validation the original data was randomly divided into k equivalent subsamples, and a single subsample retained as the validation data for testing the model, while the remaining k − 1 subsamples were used as training data. Similarly, holdback validation was carried out by randomly selecting a portion of the data for training whilst 0.33% of the data was used as a holdback portion for testing. Unlike the other cross validation techniques whereby the testing and training portions were randomly selected, in the case of the excluded row holdback the testing portion of data is chosen by the user; in this experiment the 20, 40 and 80% adulterant concentration data were used to testing the model, since these concentrations would cover a substantial range in order to assess the performance of the model. Similar to PLSR, ANN model building was carried out on both the whole spectral region and the wavelengths which had a VIP > 0.8.
Assessment of model performance
The residual difference between experimental values and prediction values was plotted and validation of the models was performed by comparing differences in root mean square error (RMSE), root mean standard deviation (RMSD), coefficient of linearity (R2) and mean biased error (MBE).
Results and discussion
Excitation and emission fluorescence spectra of adulterants and olive oil
Three-dimensional excitation and emission fluorescence spectra showed variations in both the excitation and emission wavelengths and their corresponding intensity. In general, with the exception of linseed oil, as the concentration of the adulterating seed oil increased there was a shifting of the EEM fluorescence towards 350 nm excitation and 450 nm emission coupled with an increase in the intensity. Figure 1 illustrates the changes observed from 100% seed oil (left side) to 25% seed oil. In the majority of the spectra the decrease in the intensity observed within the 350 nm (Ex) and 450 nm (Em) was coupled with an increase in the intensity of bands appearing at an excitation of 330–440 nm and an emission of 660–700 nm. This peak is attributed to the presence of chlorophyll pigment and their degraded analogue pheophytins, present predominantly in olive oil and to some extent also in cold pressed linseed oil (Gliszczyńska-Świgło et al. 2007 and Herchi et al. 2012). Since the samples were freshly prepared, this peak would be expected to be more pronounced in aged samples.
Fig. 1.
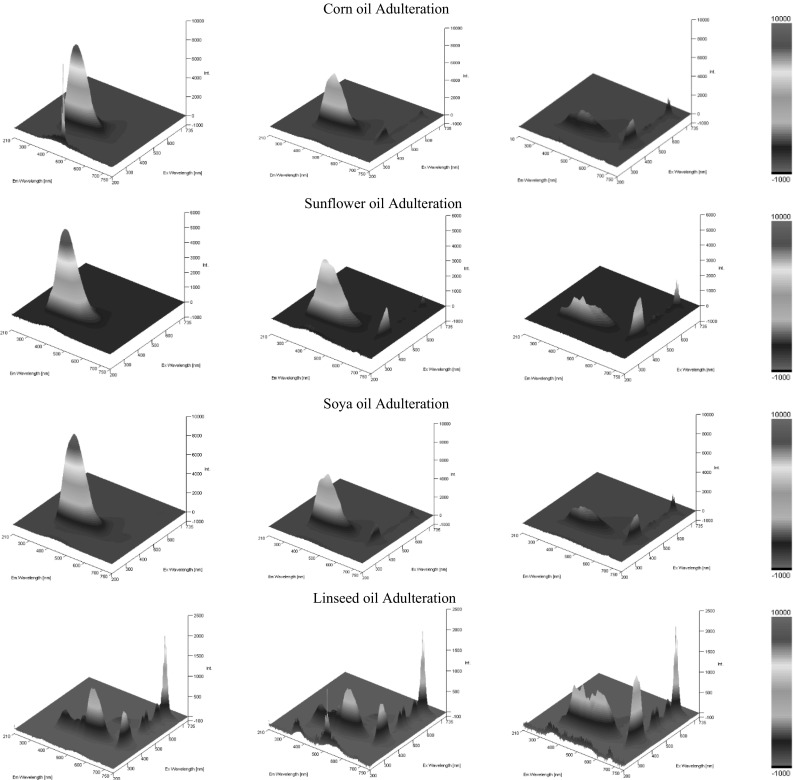
3D EEM’s between 210 to 750 nm excitation (axis z) and 210 to 750 nm emission (x axis) against intensity (y axis) for different levels of olive oil adulteration concentrations, 100, 75, and 25% (left to right), for corn (1st row), sunflower (2nd row), soya bean oil (3rd row) and linseed (4th row)
Both the seed oil adulterants and olive oil samples studied displayed a strong characteristic band with excitation at 300–360 nm and emission at about 350–400 nm. This band has been attributed to tocopherols and tocotrienols (Sikorska et al. 2004). The maxima of tocopherol emission vary slightly from one oil to another due to differences in the tocopherol composition. The intensity in the absorbance reflects the amount of tocopherols and tocotrienols present in the oil, with the seed adulterating oils showing a much higher concentration of tocopherols, as displayed by the increase in the intensity within this region when compared to seed: olive oil mixtures. Since tocopherols and tocotrienols are a vast class of compounds their spectral characteristics vary. Figure 1 shows that there are only small changes in excitation and emission maxima, which was attributed to the different extraction procedures to which the seeds were subjected. However, the EEM spectrum of linseed oil differs greatly from the rest of the seed oils; in fact, apart from the emission peak corresponding to the tocopherols and tocotrienols a stronger band was observed at a longer wavelength of 520 nm, which decreased in intensity as the % content of olive oil increased, whilst the peak corresponding to the tocopherols and tocotrienols increased. This is probably due to the presence of fluorophores present in linseed but not in olive oil. These compounds are most probably omega-3,6,9 fatty acids, present in very large amounts in cold pressed linseed oil but that are found at much lower concentrations in olive oil (Sauci et al. 1994).
Principal component analysis (PCA)
Using PCA, we found that the use of only two principal components explained 91.1% of the variance of the data for the difference between the emission spectra, 99.5% for the excitation spectra and 96.3% for the synchronised spectra at 24 nm. The score plots obtained for each analysis classified the oils in four distinct areas; olive oil and linseed oil separated from the other seed oils (Fig. 2). This separation is due to the negative scores obtained in PC1, attributed to its high content of compounds emitting at 420 to 485 nm and 600 to 700 nm, together with compounds excited at 680 to 700 nm. Apart from chlorophyll pigments, the presence of a different fatty acid profile will also contribute to variation in the emission and excitation spectra (Maggio et al. 2009; Matthäus and Özcan 2011). Virgin olive oil is rich in oleic acid (55–83%), which is monounsaturated, while corn, soybean and sunflower oils predominantly contain polyunsaturated fatty acids. The clustering of olive oil and linseed oil is attributed to the higher levels of other fluorophores such as tocopherols, β-carotene and phenolic compounds that are refined out of other oils. Under both the excitation and emission PCA, peanut and sunflower oil clustered together whilst soya and rapeseed oil formed a separate cluster, due to similar classes of tocopherols and fatty acids (Kamal-Eldin and Andersson 1997). The pair combination of these two types of oils is due to an emission wavelength maxima centred at 350 nm and excitation wavelength maxima at 420 nm for soya and rapeseed oil, while peanut and sunflower oil showed an emission maxima at a lower wavelength of 330 nm and an excitation centred at 410 nm. This hypsochromic shift is attributed to the different extraction procedures and refining process (Sikorska et al. 2004).
Fig. 2.
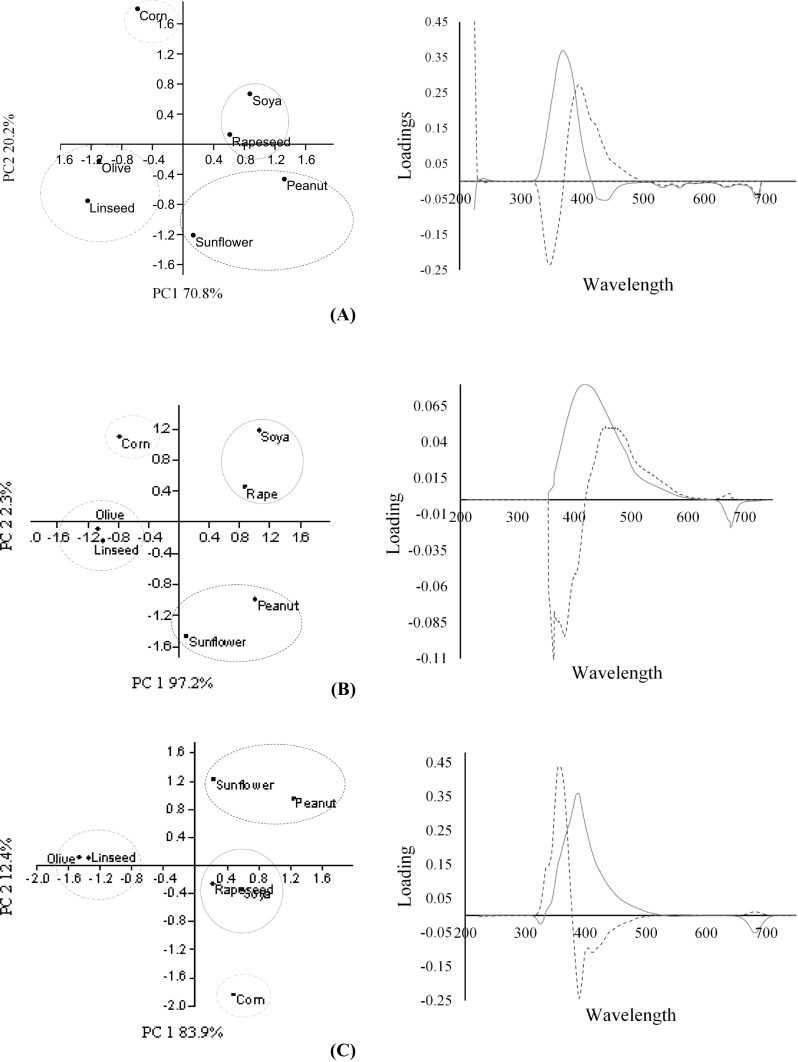
PCA scores plots (left column) for the discrimination of EVOO and vegetable oil adulterants based on emission spectral data (a), excitation spectral data (b) and synchronised spectral data at 24 nm (c), and their corresponding loading plots (right column). The blue solid lines represent loading scores for PC1 while the red dotted lines represent loading scores for PC2 for the different wavelengths (x-axis)
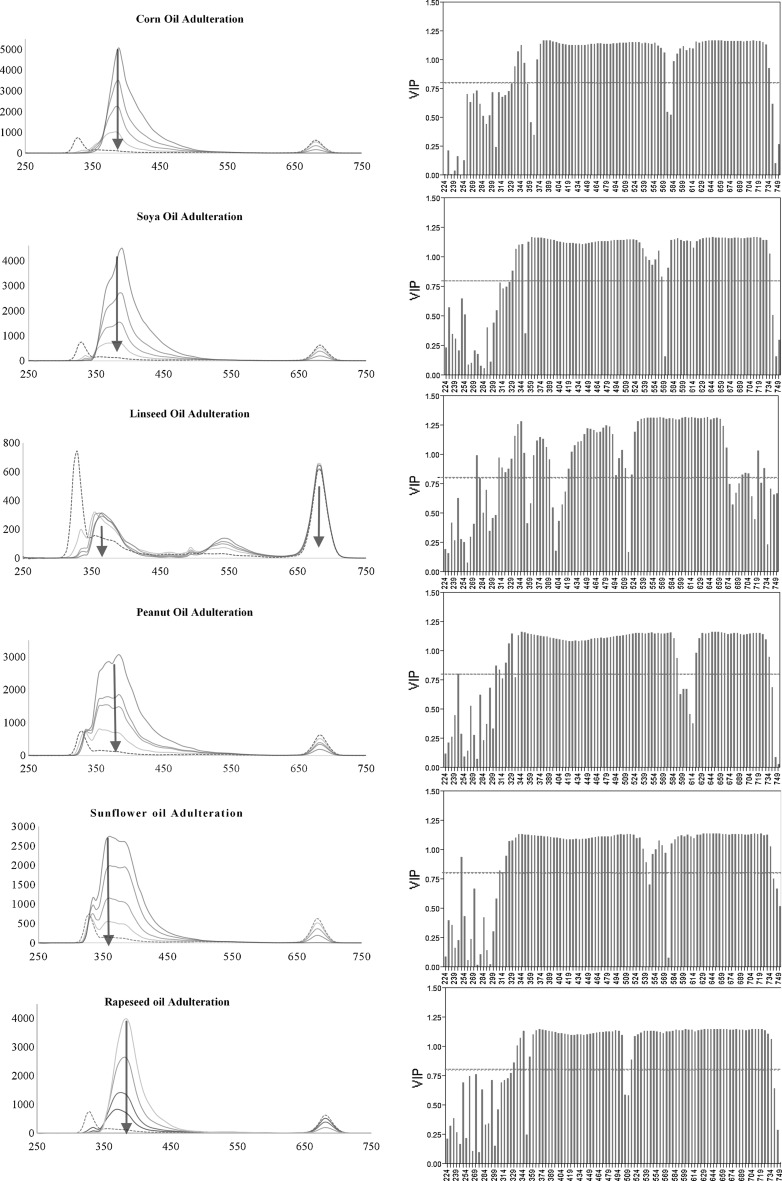
Synchronous spectra and partial least squares analysis (PLSR)
PLSR was performed on the oil-adulterant mixture samples’ synchronised spectra. This method models both the dependent (absorbance value at each wavelength) and independent (concentration of olive oil present within the mixture) variables simultaneously to find the latent variables (wavelengths) that will best predict the concentration of olive oil in the mixture. The optimum number of factors calculated using the leave-one-out cross-validation varied depending on the seed oil adulterant. VIP score values for each wavelength are a measure of a variable’s importance in modelling both wavelength absorbance and % concentration present in olive oil (Fig. 3). A value of 0.8 is generally considered to be a small VIP (Eriksson et al. 2006) and a red line is drawn on the plot at 0.8. From the variable importance plots one can see that not every wavelength in the spectrum has a VIP > 0.8, however there was a small region in all spectra which did not vary on changing to a different adulterant. This region was found between 360 to 500 nm and another one at 579–664 nm; these regions correspond to the tocopherols/tocotrienols band and the chlorophylls/pheopytins respectively. The performance of the prediction model varied depending on the choice of the adulterant, the root mean square error ranging from 7.2 for peanut oil adulteration to 0.7 for soya bean oil (Table 1). Although the RMSE was acceptable we repeated the PLSR using only the wavelengths which had a VIP score > 0.8. The results showed a great improvement on the predicted model and with the exception of peanut oil adulteration, the RMSE was decreased by more than 28% for the remaining oil adulterants.
Fig. 3.
Synchronised spectra (left column) obtained at 24 nm with increasing concentration of seed oil adulteration (solid blue lines) and olive oil (black dotted line). Arrows indicate the maxima obtained for the different seed oils. The variable importance plots (right column) obtained on using PLSR, where the wavelengths (x-axis) and their corresponding VIP (y-axis) are plotted. The red dotted line indicates the VIP at 0.8 (color figure online)
Table 1.
Root mean square error, root mean standard deviation, R2 and bias error obtained using PLSR and adjusted PLSR whereby the model was re-evaluated using only the wavelengths which had a VIP > 0.8
| Oil Adulterant | RMSE | RMSD | R2 | MBE | ||||
|---|---|---|---|---|---|---|---|---|
| PLSR | Adjusted PLS | PLSR | Adjusted PLS | PLSR | Adjusted PLS | PLSR | Adjusted PLS | |
| Sunflower | 3.490 | 0.646 | 1.028 | 0.587 | 0.995 | 1.000 | − 0.400 | − 0.034 |
| Linseed | 3.411 | 2.453 | 0.625 | 2.340 | 0.995 | 0.997 | 0.364 | 0.417 |
| Corn | 2.928 | 1.257 | 0.623 | 1.661 | 0.996 | 0.999 | − 0.404 | − 0.127 |
| Soya | 0.773 | 0.002 | 1.509 | 0.002 | 1.000 | 1.000 | − 0.021 | 0.000 |
| Peanut | 7.665 | 6.274 | 2.207 | 4.732 | 0.975 | 0.983 | − 0.200 | − 0.327 |
| Rapeseed | 3.349 | 1.850 | 1.292 | 0.108 | 0.995 | 0.999 | − 0.329 | − 0.105 |
Artificial neural networks and prediction model analysis (ANN)
Similar to the PLSR, ANN process large amounts of data in order to obtain a model with lowest error. ANN starts by input signals; in this experiment the wavelengths were designed to receive various absorbance values from the whole or part of the spectrum. The network processed the data in order to give an output signal which corresponded to the % concentration of olive oil in the adulterated mixture. In this experiment we tested three different kinds of cross validation in the neural network. It was found that on using wavelengths which had a VIP > 0.8 as previously determined by the PLSR, the model reached its optimal performance on using the excluded row validation (Table 2). This confirms that the concentrations chosen by the experimenter for cross validation covered a good concentration range for modelling and testing. For the majority of the seed adulterants the excluded row cross validation method had a lower RMSE, RMSD, MBE and higher R2 when compared to the other cross validation methods. Table 3 shows that on using the whole synchronised spectrum, the model reached optimum prediction on using cross validated using k-fold or CV-10 rather than on using the excluded row holdback. This indicates that whilst PLSR improved on using variables which could explain the maximum variation, in the case of ANN the validation method was more dependent on the type of data.
Table 2.
The Root mean square error, root mean standard deviation and R2 value obtained using the artificial neural network, constructed on the synchronised spectral wavelengths which had a VIP > 0.8. The model was cross validated using different methods k-fold or CV-10 (k), Holdback at 0.33% (HB) and excluded row (ER)
| Oil adulterant | RMSE | RMSD | R2 | MBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | HB | ER | k | HB | ER | k | HB | ER | k | HB | ER | |
| Sunflower | 6.803 | 2.940 | 1.694 | 1.004 | 3.727 | 0.356 | 0.984 | 0.998 | 0.999 | − 0.111 | 0.061 | − 0.075 |
| Linseed | 1.609 | 3.231 | 1.031 | 1.231 | 6.043 | 0.037 | 0.999 | 0.996 | 1.000 | − 0.015 | − 0.027 | − 0.009 |
| Corn | 1.254 | 1.780 | 0.420 | 0.810 | 2.804 | 0.003 | 1.000 | 0.999 | 1.000 | − 0.027 | 0.217 | 0.001 |
| Soya | 3.018 | 3.546 | 1.330 | 0.704 | 7.177 | 0.007 | 1.000 | 0.996 | 0.995 | − 0.004 | 0.379 | 0.146 |
| Peanut | 6.314 | 6.808 | 2.618 | 3.014 | 7.411 | 0.000 | 0.987 | 0.980 | 0.997 | − 0.088 | 0.132 | − 0.171 |
| Rapeseed | 0.001 | 6.928 | 1.458 | 2.967 | 5.949 | 0.000 | 1.000 | 0.980 | 0.999 | 0.000 | − 0.265 | − 0.006 |
Table 3.
The root mean square error, root mean standard deviation and R2 value obtained using the artificial neural network, constructed on the whole synchronised spectrum at 24 nm. The model was cross validated using different methods k-fold or CV-10 (k), Holdback at 0.33% (HB) and excluded row (ER)
| Oil Adulterant | RMSE | RMSD | R2 | MBE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | HB | ER | k | HB | ER | k | HB | ER | k | HB | ER | |
| Sunflower | 3.202 | 7.414 | 3.110 | 0.066 | 0.000 | 4.230 | 0.997 | 0.977 | 0.996 | − 0.083 | − 0.098 | − 0.080 |
| Linseed | 1.655 | 6.199 | 7.597 | 0.017 | 1.948 | 2.797 | 0.999 | 0.988 | 0.989 | − 0.106 | 0.241 | 1.468 |
| Corn | 0.090 | 6.351 | 1.023 | 0.007 | 0.005 | 1.126 | 1.000 | 0.989 | 1.000 | − 0.026 | − 0.096 | − 0.059 |
| Soya | 0.001 | 4.478 | 3.321 | 0.000 | 3.332 | 1.200 | 1.000 | 0.995 | 0.996 | 0.000 | − 0.061 | − 0.372 |
| Peanut | 0.097 | 11.449 | 21.201 | 0.018 | 5.149 | 1.195 | 1.000 | 0.944 | 0.789 | − 0.007 | 0.087 | 0.001 |
| Rapeseed | 0.862 | 5.409 | 1.658 | 0.000 | 1.123 | 1.624 | 1.000 | 0.992 | 0.999 | 0.036 | 1.275 | 0.062 |
Conclusion
We present a first report that coupling a single synchronised fluorescence spectra rather than the entire EEM to chemometrics can provide a quick and easy, determination of seed oil adulterants in Maltese EVOOs without the need for any sample pre-treatment of the oil sample. The results also show that application of a variable selection process greatly improved the PLSR models based on synchronised spectra for detecting the % amount of EVOO in vegetable oil blends, with a lower RMSE than those observed on using PLSR on the whole spectrum. This study also showed that ANN has use as an alternative chemometric tool for the detection of olive oil adulteration, with the performance of the model generated by the ANN being however highly dependent both on the type of data input and the mode of cross validation.
Acknowledgements
The research carried out was funded by the Malta Government Scholarships Post-Graduate Scheme for 2014 (MGSS-PG 2014).
References
- Aguado D, Montoya T, Borras L, Seco A, Ferrer J. Using SOM and PCA for analysing and interpreting data from a P-removal SBR. Eng Appl Artif Intell. 2008;21:919–930. doi: 10.1016/j.engappai.2007.08.001. [DOI] [Google Scholar]
- Baccouri O, Guerfel M, Baccouri B, Cerretani L, Bendini A, Lercker G, Zarrouk M, Miled DDB. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008;109(4):743–754. doi: 10.1016/j.foodchem.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Bosque-Sendra JM, Cuadros-Rodríguez L, Ruiz-Samblás C, De La Mata AP. Combining chromatography and chemometrics for the characterization and authentication of fats and oils from triacylglycerol compositional data: a review. Anal Chim Acta. 2012;724:1–11. doi: 10.1016/j.aca.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Calvano CD, De Ceglie C, D’Accolti L, Zambonin CG. MALDI-TOF mass spectrometry detection of extra-virgin olive oil adulteration with hazelnut oil by analysis of phospholipids using an ionic liquid as matrix and extraction solvent. Food Chem. 2012;134(2):1192–1198. doi: 10.1016/j.foodchem.2012.02.154. [DOI] [PubMed] [Google Scholar]
- Dong W, Zhang Y, Zhang B, Wang X. Quantitative analysis of adulteration of extra virgin olive oil using Raman spectroscopy improved by Bayesian framework least squares support vector machines. Anal Methods. 2012;4:2772–2777. doi: 10.1039/c2ay25431j. [DOI] [Google Scholar]
- EEC (2013) Commission Implementing Regulation (EU) No 1348/2013 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis
- Eriksson L, Kettaneh-Wold N, Trygg J, Wilkstrom C, Wold S. Multi- and megavariate data analysis, basic principles and application. 2. Umea, Sweden: Umetrics AB; 2006. [Google Scholar]
- Fragaki G, Spyros A, Siragakis G, Salivaras E, Dais P. Detection of extra virgin olive oil adulteration with lampante olive oil and refined olive oil using nuclear magnetic resonance spectroscopy and multivariate statistical analysis. J Agric Food Chem. 2005;53(8):2810–2816. doi: 10.1021/jf040279t. [DOI] [PubMed] [Google Scholar]
- Giungato P, Aveni M, Rana R, Notarnicola L. Modifications induced by extra virgin olive oil frying processes. Ind Aliment. 2004;43:369–375. [Google Scholar]
- Gliszczyńska-Świgło A, Sikorska E, Khmelinskii I, Sikorski M. Tocopherol content in edible plant oils. Pol J Food Nutr Sci. 2007;57(4):157–161. [Google Scholar]
- Guimet F, Ferré J, Boqué R. Rapid detection of olive pomace oil adulteration in extra virgin olive oils from the protected denomination of origin Siurana using excitation–emission fluorescence spectroscopy and three-way methods for analysis. Anal Chim Acta. 2005;544:143–152. doi: 10.1016/j.aca.2005.02.013. [DOI] [Google Scholar]
- Herchi W, Arráez-Román D, Boukhchina S, Kalle H, Segura-Carretero A, Fernández-Gutierrez A. A review of the methods used in the determination of flaxseed components. Afr J Biotechnol. 2012;11(4):724–731. [Google Scholar]
- Jiang L, Zheng H, Lu H. Application of UV spectrometry and chemometric models for detecting olive oil-vegetable oil blends adulteration. J Food Sci Technol. 2013;52:479–485. doi: 10.1007/s13197-013-1003-1. [DOI] [Google Scholar]
- Kamal-Eldin A, Andersson R. Multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J Am Oil Chem Soc. 1997;74:375–380. doi: 10.1007/s11746-997-0093-1. [DOI] [Google Scholar]
- Kyriakidis NB, Skarkalis P. Fluorescence spectra measurement of olive oil and other vegetable oils. J Am Oil Chem Soc. 2000;83:1435–1439. [PubMed] [Google Scholar]
- Maggio RM, Kaufman TS, Carlo MD, Cerretani L, Bendini A, Cichelli A, Compagnone D. Monitoring of fatty acid composition in virgin olive oil by Fourier transformed infra-red spectroscopy coupled with partial least square. Food Chem. 2009;114:1549–1554. doi: 10.1016/j.foodchem.2008.11.029. [DOI] [Google Scholar]
- Maggio RM, Cerretani L, Chiavaro E, Kaufman TS, Bendini A. A novel chemometric strategy for the estimation of extra virgin olive oil adulteration with edible oils. Food Control. 2010;21(6):890–895. doi: 10.1016/j.foodcont.2009.12.006. [DOI] [Google Scholar]
- Matthäus B, Özcan MM. Determination of fatty acid, tocopherol, sterol contents and 1,2- and 1,3-diacylglycerols in four different virgin olive oil. J Food Process Technol. 2011;2:117. doi: 10.4172/2157-7110.1000117. [DOI] [Google Scholar]
- Mignani AG, Ciaccheri L, Ottevaere H, Thienpont H, Conte L, Marega M. Visible and near-infrared absorption spectroscopy by an integrating sphere and optical fibers for quantifying and discriminating the adulteration of extra virgin olive oil from Tuscany. Anal Bioanal Chem. 2011;399(3):1315–1324. doi: 10.1007/s00216-010-4408-y. [DOI] [PubMed] [Google Scholar]
- Papadopoulos K, Triantis T, Tzikis CH, Nikokavoura A, Dimotikali D. Investigations of extra virgin olive oils with seed oils using weak chemiluminescence. Anal Chim Acta. 2002;464(1):135–140. doi: 10.1016/S0003-2670(02)00436-1. [DOI] [Google Scholar]
- Pasqualone A, Montemurro C, di Rienzo V, Summo C, Paradiso VM, Caponio F. Evolution and perspectives of cultivar identification and traceability from tree to oil and table olives by means of DNA markers. J Sci Food Agric. 2016;96(11):3642–3657. doi: 10.1002/jsfa.7711. [DOI] [PubMed] [Google Scholar]
- Poulli KI, Mousdis GA, Georgiou CA. Synchronous fluorescence spectroscopy for quantitative determination of virgin olive oil adulteration with sunflower oil. Anal Bioanal Chem. 2006;386(5):1571–1575. doi: 10.1007/s00216-006-0729-2. [DOI] [PubMed] [Google Scholar]
- Poulli KI, Mousdis GA, Georgiou CA. Rapid synchronous fluorescence method for virgin olive oil adulteration assessment. Food Chem. 2007;105:369–375. doi: 10.1016/j.foodchem.2006.12.021. [DOI] [Google Scholar]
- Rahmani M, Saari-Csallany A. Role of minor constituents in the photoxidation of virgin olive oil. J Am Oil Chem Soc. 1998;75:837–843. doi: 10.1007/s11746-998-0234-1. [DOI] [Google Scholar]
- Souci SW, Fahman W, Kraut H. Food composition and nutrition tables. 5. Florida, United States: CRC Press; 1994. [Google Scholar]
- Sikorska E, Romaniuk A, Khmelinskii IV, Herance R, Bourdelande JL, Sikorski M, Koziol J. Characterization of edible oils using total luminescence spectroscopy. J Fluoresc. 2004;14:25–35. doi: 10.1023/B:JOFL.0000014656.75245.62. [DOI] [PubMed] [Google Scholar]
- Sikorska E, Khmelinskii I, Sikorski M. Analysis of olive oils by fluorescence spectroscopy: Methods and applications. In: Dimitrios Boskou., editor. Agricultural and Biological Sciences – Olive Oil – Constituents, Quality, Health Properties and Bioconversions. Urbana, Illinois, United States: AOCS Press; 2012. pp. 63–88. [Google Scholar]
- Solari ME, Vernet JL. Late glacial and Holocene vegetation of the Corbières based on charcoal analysis at the Cova de L’Espérit (Salses, Pyrénées Orientales, France) Rev Palaeobot Palynol. 1992;71:111–120. doi: 10.1016/0034-6667(92)90159-E. [DOI] [Google Scholar]
- Terral JF, Arnold-Simard G. Beginnings of olive cultivation in eastern Spain in relation to Holocene bioclimatic changes. Quat Sci Rev. 1996;46:176–185. [Google Scholar]
- Zandomeneghi M. Fluorescence spectroscopy excitation-emission methods combined with three-way analysis as a complementary technique for the characterization of olive oil. J Agric Food Chem. 2006;54(14):5214–5215. doi: 10.1021/jf0605648. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lu HF. Use of kinetic, Weibull and PLSR models to predict the retention of ascorbic acid, total phenols and antioxidant activity during storage of pasteurized pineapple juice. LWT Food Sci Technol. 2011;44:1273–1281. doi: 10.1016/j.lwt.2010.12.023. [DOI] [Google Scholar]