Abstract
Peony seed oil has recently been introduced as a high-quality food oil. Because the high price of peony seed oil may tempt unscrupulous merchants to dilute it with cheaper substitutes, a rapid detection method for likely adulterants is required. In this study, the fatty acid composition of peony seed oil and four less expensive edible oils (soybean oil, corn oil, sunflower oil, and rapeseed oil) were measured by gas chromatography mass spectrometry. Peony oil adulterated by other edible oils was assessed using iodine values to estimate the extent of adulteration. Adulteration was also measured using an electronic nose (E-nose) combined with principal component analysis (PCA) or linear discriminant analysis (LDA). Results indicated that peony seed oil was highly enriched in α-linolenic acid. Although the iodine value can be used to detect some adulterants by measuring unsaturation, it was not able to detect all four potential adulterants. In contrast, the E-nose can rapidly identify adulterated peony seed oil by sampling vapor. Data analyses using PCA and LDA show that LDA more effectively clusters the data, discriminates between pure and adulterated oil, and can detect adulteration at the 10% level. E-nose combined with LDA suitable for detection of peony seed oil adulteration.
Keywords: Peony seed oil, Adulteration, GC–MS, Electronic nose
Introduction
Tree peony (Paeonia section Moutan DC.), a well-known woody ornamental indigenous to China, has recently been exploited as an oilseed plant (Li 2011; Xue et al. 2015). The oil content of peony’s black elliptical seeds exceeds 20%, and more than 90% of the oil is unsaturated fatty acids (UFA). Surprisingly, α-linolenic acid (ALA), a type of omega-3 fatty acid, constitutes up to 40% of the oil. ALA cannot be synthesized in the human body but it is indispensable. ALA provides significant health benefits due to its hypolipidemic effects and antioxidant properties; it is also protective against rheumatoid arthritis, stroke, fatal ischemic heart disease, and some forms of cancer (Connor 1999; Han et al. 2016; Su et al. 2016; Wang et al. 2015). Although ALA is present in other edible oils, it is relatively less abundant. The ALA content of rapeseed oil is 9.46%, sunflower oil is 1%, olive oil is 0.72%, sesame oil is 0.29%, and camellia oil less than 0.27%. Some researchers suggest that the nutritional quality of peony seed oil is superior to olive oil and camellia oil (Xie et al. 2013; Rincóncervera et al. 2016; Samman et al. 2008; Wang and Yuan 2015). In 2011, the National Health and Family Planning Commission approved peony seed oil as a new food resource for the marketplace in China.
Peony seed oil adulteration has rarely been reported, but other high-quality oils such as olive oil, camellia seed oil, and flaxseed oil are often illicitly blended with inexpensive oils to increase profits (Dourtoglou et al. 2003; Xie et al. 2013; Sun et al. 2015). Adulteration of edible oils not only harms the interests of consumers, but may also harm their health. Because peony seed oil commands a high price, a strong incentive exists to dilute it with cheaper edible oils. Oil adulteration is most commonly detected using spectroscopic and chromatographic methods, including near-infrared (NIR) (Kuriakose and Joe 2013; Özdemir and Öztürk 2007), mid-infrared spectroscopy (MIR) (Souza et al. 2015; Gurdeniz and Ozen 2009), nuclear magnetic resonance (NMR) (Xu et al. 2014), fluorescence spectroscopy (Ge et al. 2014; Kunz et al. 2011; Mabood et al. 2016), gas chromatography mass spectrometry (GC–MS) (Sun et al. 2015), and high-performance liquid chromatography (HPLC) (Salghi et al. 2014). These instruments are expensive, and the data analysis requires specialized software and algorithms, making it time-consuming and difficult for ordinary food inspectors to master. A simple and efficient method is therefore needed to detect peony seed oil adulteration. Ideally, the test should be inexpensive, rapid, and effective.
The “electronic nose” (E-nose), a detection device containing metal oxide semiconductor sensors, has been used to analyze food products (Berna 2010; Servili et al. 2008). The E-nose has several advantages over other techniques. It is portable, not too expensive, and is simple, fast, and sensitive (Cosio et al. 2006). An E-nose is composed of a set of sensor arrays that detect different kinds of volatile compounds (Lerma et al. 2013; Tian et al. 2013). Briefly, the device works as follows. Odor molecules are drawn into the E-nose by an odor sampler, and converted into electrical signals by the gas sensors. The signals are transformed into digital values and subjected to feature extraction. Additional processing is accomplished using a pre-loaded signal pattern recognition system for qualitative and quantitative identification of the gas signatures (Zheng and Lin 2012). The analyses typically applied to the data are Cluster analysis (CA), principal component analysis (PCA), and linear discriminant analysis (LDA) (Wilson and Baietto 2009). Electronic noses have been used successfully to identify the geographical origin of extra virgin olive oils and to detect corn oil adulteration in sesame oil (Hai and Wang 2006b; Melucci et al. 2016). However, the E-nose has not yet been used to detect peony seed oil adulteration.
In this study, the fatty acid compositions of five edible oils (peony seed oil, soybean oil, corn oil, sunflower oil, and rapeseed oil) were first characterized by GC–MS. Peony seed oil was then mixed with different proportions and kinds of edible oil, and the adulteration was detected using a traditional method (iodine value) and an E-nose.
Materials and methods
Materials
Peony seeds (P. ostii ‘Feng Dan’) were collected from the Tree Peony and Herbaceous Peony Demonstration Station, at the Institute of Vegetables and Flowers of the Chinese Academy of Agricultural Sciences (Beijing, China). Freshly dried seeds were shelled, frozen in liquid nitrogen, and then ground into powder using a freeze grinder (IKA A11 basic, Germany). The powder was filtered using a 40 mesh screen and then stored at − 20 °C. Peony seed oil was obtained by supercritical carbon dioxide (SC-CO2) extraction using a laboratory-scale system (Spe-ed SFE-2, USA) from Automatic Systems, Inc. Jinlongyu brand Soybean oil (Sb), corn oil (C), sunflower oil (Sf), and rapeseed oil (R) were produced by Yihai Kerry Oils and Grains Co., Ltd.
Fatty acid composition of edible oils by GC–MS
Analysis of fatty acid composition was conducted using a GC–MS (GCMS-QP5050A, SHIMADZU) equipped with an AOC-5000 auto injector (SHIMADZU). A SP-2560 polydimethylcyanopropylsiloxane packed capillary column was used (100 m × 0.25 mm × 0.2 μm, Supelco, USA).
Oven temperature was initially 100 °C for 5 min, then ramped to 240 °C at a rate of 4 °C min−1 and maintained for 15 min at 240 °C. The transfer line and ion source were held at 280 and 150 °C, respectively. GC was performed in constant flow mode. The mass spectrometer was operated in electron impact (EI) mode at 70 eV. Retention times for fatty acid methyl ester (FAME) standards were obtained using a 37-component FAME Mix. An internal standard (methyl heptadecanoate) was used to quantitate fatty acid content (Li et al. 2015).
Pretreatment and derivatization was described previously (Li et al. 2013, 2015). Briefly, weigh 25 mg oil was placed into a 10 mL centrifuge tube, 3.0 mL chloroform–methanol (1:2 v/v) was added, and the mixture shaken for 1 h at 4 °C. Following the addition of 1.0 mL chloroform and 1.8 mL KCl (1 M), the contents were centrifuged at 2500 rpm for 10 min. The chloroform layer (lower layer) was recovered from the centrifuge tube and dried using a stream of nitrogen. The residue was dissolved in 1.0 mL methanol solution containing 5% H2SO4, vortexed for 1 min, and incubated in an 80 °C water bath for 1 h. After the incubation, 1.0 mL of distilled water was added to stop the reaction. 5 mL of n-hexane was added to extract FAMEs, and the n-hexane component was then centrifuged at 2500 rpm for 10 min. 0.1 mL of each supernatant was placed into a 1.5 mL vial, along with 20 μL of methyl heptadecanoate (1.0 mg mL−1 in hexane) as an internal standard.
Preparation of adulterated samples of peony seed oil
The four edible oils used as adulterants were soybean oil (Sb), corn oil (C), sunflower oil (Sf), and rapeseed oil (R). Peony seed oil was adulterated with one other edible oil to generate a series of mixtures, designated according to the % concentration (v/v) of contaminating oil in the mixture. For example, 0% indicates pure peony oil, 30% indicates a mixture containing 30% adulterant oil and 70% peony oil, and 100% indicates pure adulterant oil.
Determination of iodine values for adulterated oils
Iodine value was determined using a standard method (Standardization Administration of China 2008). Briefly, 0.150 ± 0.001 g adulterated oil was placed into an iodometric flask. 20 mL of cyclohexane and glacial acetic acid (1:1 v/v) were added, followed by the addition of 25 mL Wijs reagent (25 g of iodine monochloride in 1500 mL glacial acetic acid). The mixture was shaken thoroughly and then placed in the dark at 25 °C for 1 h. Sodium thiosulfate standard solution (0.1 M) was used to titrate the sample. Controls contained no oil but were treated identically otherwise. Tests were repeated three times. Iodine values are expressed as averages ± standard deviation (SD).
E-nose system and sampling procedure
A PEN3 E-nose (portable electronic nose 3, Airsense Analytics, Germany) was used in our test. This novel analysis instrument can detect and identify most complex odors and volatile components. The E-nose consists of a sensor array and an automatic pattern recognition system. The sensor array system is composed of 10 different metal oxide semiconductor (MOS)-type chemical sensors: MOS1 (S1, aromatic), MOS2 (S2, broad range), MOS3 (S3, aromatic), MOS4 (S4, hydrogen), MOS5 (S5, aromatic aliphatics), MOS6 (S6, broad methane), MOS7 (S7, sulfur organic), MOS8 (S8, broad alcohol), MOS9 (S9, sulfur chlorinate), and MOS10 (S10, methane aliphatics) (Rizzolo et al. 2013).
Briefly, samples were prepared as follows for measurement by the E-nose system. 2 g of adulterated oil was transferred to a 250 mL beaker. The beaker was sealed using plastic wrap and incubated in an 80 ± 1 °C water bath for 15 min. E-nose sampling for volatiles was performed at a flow rate of 200 mL min−1 and a measurement recorded once per second. The measurement phase lasted 60 s to allow the sensors to reach stable signal values. Before every sampling run, the sensors were purged with fresh, dry air. Data were analyzed by the data acquisition system (WinMuster). Each adulterated oil was measured 5 times using 5 independent samples.
E-nose measurements were analyzed by PCA and LDA to determine whether samples of peony seed oil contained adulterants. PCA is a multivariate statistical method that transforms the original sensor signals into variables. The number of variables is then reduced to eliminate redundancy (Nurjuliana et al. 2011; Santonico et al. 2008; Zhang et al. 2006). LDA, another widely used statistical method, is a probabilistic parametric classification technique that projects high-dimensional data onto a low-dimensional space (Hai and Wang 2006a).
Data analysis
SAS V8 (SAS Institute, Cary, NC, USA) and WinMuster (Airsense Analytics) were used for statistical calculations. Variance was calculated using a one-way analysis (ANOVA), and differences between means were compared by Duncan’s multiple range tests, with a threshold for statistical significance of P < 0.05. Figures were plotted using Origin 9.0.
Results and discussion
Composition of fatty acids in edible oils
As shown in Table 1, the unsaturated fatty acid content of peony seed oil was as high as 91.09%, exceeding the content measured for soybean, corn and sunflower oils, but slightly lower than for rapeseed oil (92.12%). The ALA content of peony seed oil was 157.291 mg g−1, accounting for 36.57% of the total fatty acids. In contrast, ALA in the other four oils represents less than 10% of the total. The ALA fraction in some peony seed oils is as much as 67% of total fatty acids, far higher than in other common vegetable oils (Zhu et al. 2014). Because the ALA content in peony seed oil is relatively high, adulteration by other edible oils will result in a mixture with decreased ALA content. GC–MS has been used to measure changes in oleic acid and linoleic acid content to detect contamination of camellia seed oil by soybean oil. The detection limit for the adulterant approached 5% (Xie et al. 2013). Peony seed oil adulteration should also be detectable by measuring ALA content using GC–MS.
Table 1.
Fatty acid composition of five edible oils (%)
| Peony seed oil | Soybean oil | Corn oil | Sunflower oil | Rapeseed oil | |
|---|---|---|---|---|---|
| c14:0 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.08 ± 0.00 | ||
| c14:1 | 0.01 ± 0.00 | ||||
| c15:0 | 0.13 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| c16:0 | 6.82 ± 0.54 | 12.05 ± 0.08 | 13.92 ± 0.96 | 7.33 ± 0.31 | 5.36 ± 0.32 |
| c16:1 | 0.14 ± 0.02 | 0.12 ± 0.00 | 0.13 ± 0.01 | 0.12 ± 0.00 | 0.30 ± 0.02 |
| c17:1 | 0.09 ± 0.01 | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| c18:0 | 1.97 ± 0.32 | 3.97 ± 0.03 | 1.71 ± 0.12 | 3.67 ± 0.16 | 1.90 ± 0.11 |
| c18:1 n9c | 23.86 ± 0.73 | 20.10 ± 0.14 | 28.32 ± 1.62 | 24.49 ± 0.79 | 56.11 ± 0.31 |
| c18:2 n6c | 29.86 ± 0.81 | 54.02 ± 0.40 | 54.40 ± 0.82 | 63.30 ± 1.17 | 24.14 ± 1.26 |
| c20:0 | 0.09 ± 0.01 | 0.23 ± 0.00 | 0.28 ± 0.03 | 0.18 ± 0.01 | 0.42 ± 0.04 |
| c18:3 n6 | 0.38 ± 0.06 | 0.77 ± 0.01 | 0.18 ± 0.01 | 1.31 ± 0.07 | |
| c20:1 | 0.17 ± 0.03 | 0.13 ± 0.00 | 0.16 ± 0.03 | 0.09 ± 0.01 | 0.94 ± 0.07 |
| c18:3 n3(ALA) | 36.57 ± 0.65 | 8.19 ± 0.05 | 0.69 ± 0.05 | 0.13 ± 0.01 | 9.20 ± 0.50 |
| c22:0 | 0.20 ± 0.00 | 0.06 ± 0.00 | 0.42 ± 0.02 | 0.17 ± 0.02 | |
| c22:1 | 0.06 ± 0.00 | ||||
| c22:2 | 0.02 ± 0.00 | ||||
| c24:0 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.12 ± 0.00 | ||
| UFA/TFA | 91.09 | 83.40 | 83.92 | 88.17 | 92.13 |
Iodine values for peony seed oil blended with other oils
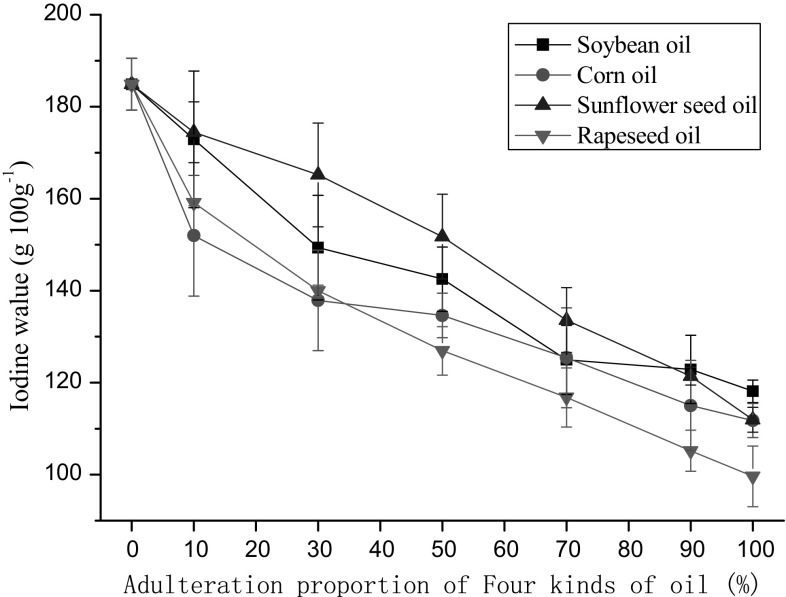
The iodine value reflects the degree of unsaturation of an oil, with higher degrees of unsaturation yielding higher iodine values (Mahapatra et al. 2011). The five edible oils listed in Table 1 are composed mainly of oleic acid and linoleic acid, which contain one and two unsaturated double bonds. Peony seed oil contains ALA in high concentration, and ALA has three unsaturated double bonds. Iodine values for soybean oil, corn oil, sunflower seed oil, and rapeseed oil were 118.12, 111.74, 111.95, and 99.62 g I 100 g−1, respectively (Fig. 1). The iodine value of peony seed oil was 184.89 g I 100 g−1. This value declines as peony oil is diluted with any of the four alternative oils. 10% adulteration by soybean oil or rapeseed oil decreases the iodine value of peony oil significantly. However, higher proportions of corn oil (30%) and sunflower seed oil (50%) were required to shift the iodine value (P < 0.05). Therefore, the iodine value was able to reveal if peony seed oil has been adulterated by soybean oil and rapeseed oil, but was not as effective if corn oil or sunflower oil had been used as adulterants at the 10% level. More generally, the iodine value technique is of limited use because it requires complex sample preparation, has low sensitivity and accuracy, and provides only a rough estimate of adulteration (Xie and Huang 2014). At best, it plays a supplementary role in the detection of adulterants.
Fig. 1.
Iodine value determination for four edible oils combined in different proportions with peony seed oil. 0% represents pure peony seed oil. Values represent means and error bars represent standard deviations for three measurements
E-nose response to oil samples
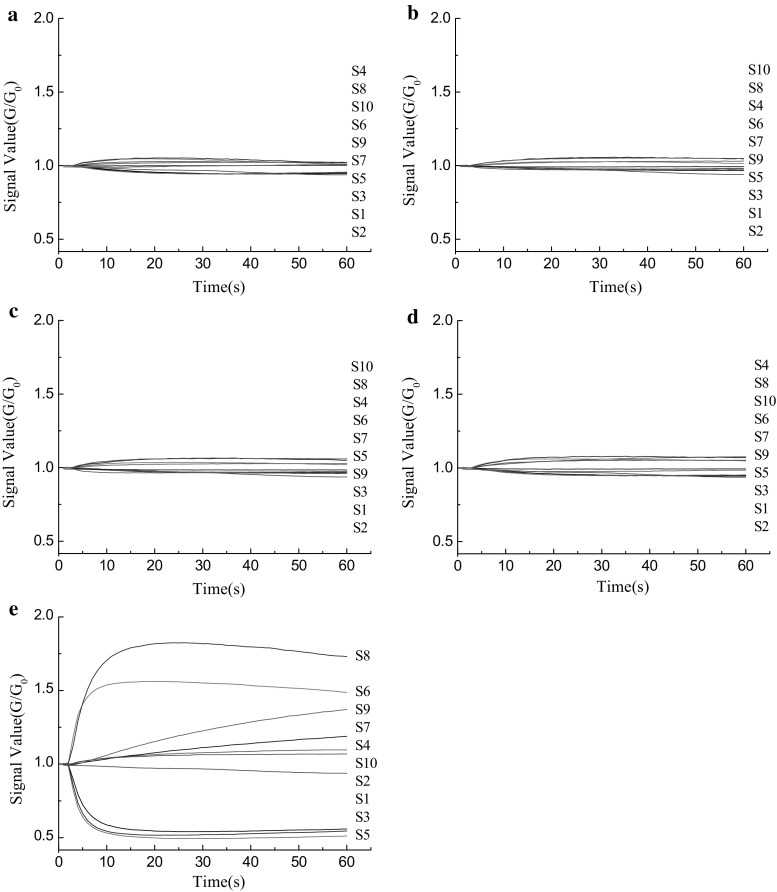
The E-nose transforms chemical signals into electrical signals (Zheng and Lin 2012). Figure 2 shows the transient sensor response curve for each oil. The abscissa is the measurement time and the ordinate is the sensor response signal value, G/G0. G and G0 are the values reported by the sensors after exposure to the sample gas and the zero (control) gas, respectively. The sensor array is composed of 10 sensors that are exposed to the headspace of the oil samples. During measurement, data are recorded every second for 60 s, for a total of 600 records per sample, to allow the sensors to reach stable signal values.
Fig. 2.
Response curves generated by the E-nose for five edible oils. a Soybean oil; b corn oil; c sunflower seed oil; d rapeseed oil; e peony seed oil
The response values to peony seed oil range from 0.5 to 1.8 (Fig. 2e). Values from sensors S8, S6, S5, S3, and S1 deviate considerably from 1, reflecting the high broad alcohol, broad methane, aromatic aliphatics, and aromatic content in peony seed oil. In contrast, the response values for the other four edible oils are quite similar and range from 0.9 to 1.1. This result indicates that the volatile content in these four oils is lower than in peony seed oil, and suggests that the E-nose can be used to distinguish peony seed oil from the other oils. Sensor response signal values consistently reached stable equilibrium during the measurement period. A gradually stabilized sensor response signal is desirable when the electronic nose is used for detection (Tian et al. 2013). Using Fig. 2 as a guide, 55 s was selected for our analysis protocol.
Separation and classification analysis
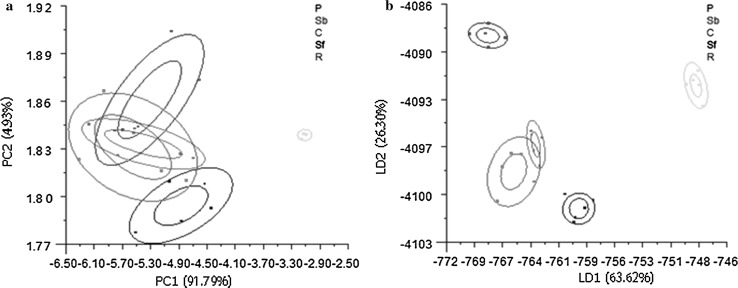
PCA and LDA were used to separate and classify the results obtained from the E-nose for the five oils (Fig. 3). In PCA, the first component explains 91.79% of variance and the second explains 4.93% (Fig. 3a). The results are reliable since the first two principal components together contribute far more than 85% to the cumulative variance (Rizelio et al. 2012). The corn oil data overlaps substantially with rapeseed oil, and these overlap partially with sunflower oil and soybean oil. However, all four oils are separated completely from peony seed oil.
Fig. 3.
Principal component analysis (PCA) (a) and linear discriminant analysis (LDA) (b) for five oils. (P) peony seed oil; (Sb) soybean oil; (C) corn oil; (Sf) sunflower seed oil; (R) rapeseed oil
The same data set was analyzed by LDA, a supervised pattern recognition method that also can discriminate samples effectively. Although corn oil and rapeseed oil overlapped partially, the other oils are easily distinguished from one another (Fig. 3b).
These results indicate that PCA and LDA both offer the ability to distinguish peony seed oil from four likely adulterants. Compared to PCA, LDA more effectively clusters the data and allows superior discrimination. In earlier studies on methods for testing the degree of oxidation in edible oils and beef freshness, LDA also demonstrated superior classification characteristics (Hong et al. 2012; Xu et al. 2016). Therefore, LDA was used in the subsequent adulteration tests.
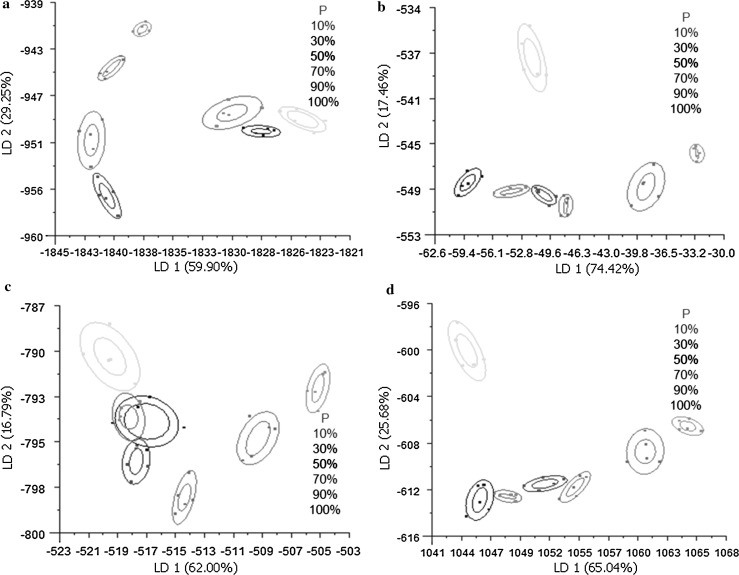
Discrimination and classification of adulterated peony seed oil
The classification of E-nose results based on the first linear discriminant (LD1) and the second linear discriminant (LD2) are shown in Fig. 4a–d as score plots. In the peony seed oil and soybean oil adulteration experiment (Fig. 4a), pure peony seed oil can be distinguished from any of the peony seed oil/soybean oil mixtures. Compositions incorporating more than 50% soybean oil were more easily discriminated from pure peony seed oil than those with less than 30%.
Fig. 4.
Results of linear discriminant analysis (LDA) for adulterated peony seed oil. a–d Peony seed oil mixed with soybean oil, corn oil, sunflower oil, or rapeseed oil, respectively. P, pure peony seed oil; 10, 30, 50, 70, 90, 100%, concentration of adulterating oil
Analyses in which peony seed oil was adulterated with corn oil or rapeseed oil yielded similar results (Fig. 4b, d). Peony seed oil adulterated with any percentage of corn oil or rapeseed oil can be easily detected. In these cases, LD1 was sufficient to discriminate between mixed components.
Figure 4c shows the analysis of peony seed oil adulterated with sunflower oil. Because the components generated by 10 and 30% sunflower oil overlap, it is not easy to distinguish these mixtures. They also lie close to the component that represents 50% adulteration. However, pure peony seed oil can be distinguished even from a mixture containing only 10% sunflower oil. Therefore LDA, in combination with data obtained from an E-nose, offers a highly effective method for detecting peony seed oil adulteration.
Conclusion
A comparison of fatty acid content by GC–MS shows that the ALA content in peony seed oil is far higher than in four other common food oils. Iodine value testing offers one approach to detect adulteration of peony seed oil by other oils, but it is not suitable when the adulterants are present at relatively low levels. In contrast, the E-nose combined with LDA can successfully discriminate among peony seed oil and four other oils, even at very low (10%) levels. The results suggest that the E-nose can be used as a rapid method to detect adulterants in peony seed oil.
Acknowledgements
This study was funded by the National Science Foundation of China (31671903), the Nature Science Foundation of Zhejiang Province (No. LR15C200002) and the K.C. Wong Magna Fund in Ningbo University.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Berna A. Metal oxide sensors for electronic noses and their application to food analysis. Sensors. 2010;10:3882–3910. doi: 10.3390/s100403882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor WE. Alpha-linolenic acid in health and disease. Am J Clin Nutr. 1999;69:827–828. doi: 10.1093/ajcn/69.5.827. [DOI] [PubMed] [Google Scholar]
- Cosio MS, Ballabio D, Benedetti S, Gigliotti C. Geographical origin and authentication of extra virgin olive oils by an electronic nose in combination with artificial neural networks. Anal Chem Acta. 2006;567:202–210. doi: 10.1016/j.aca.2006.03.035. [DOI] [Google Scholar]
- Dourtoglou VG, Dourtoglou T, Antonopoulos A, Stefanou E, Lalas S, Poulos C. Detection of olive oil adulteration using principal component analysis applied on total and regio FA content. J Am Oil Chem Soc. 2003;80:203–208. doi: 10.1007/s11746-003-0677-1. [DOI] [Google Scholar]
- Ge F, Chen CY, Liu DQ, Zhao SL. Rapid quantitative determination of walnut oil adulteration with sunflower oil using fluorescence spectroscopy. Food Anal Methods. 2014;7:146–150. doi: 10.1007/s12161-013-9610-z. [DOI] [Google Scholar]
- Gurdeniz G, Ozen B. Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. FoodChem. 2009;116:519–525. [Google Scholar]
- Hai Z, Wang J. Detection of adulteration in camellia seed oil and sesame oil using an electronic nose. Eur J Lipid Sci Technol. 2006;108:116–124. doi: 10.1002/ejlt.200501224. [DOI] [Google Scholar]
- Hai Z, Wang J. Electronic nose and data analysis for detection of maize oil adulteration in sesame oil. Sens Actuators B Chem. 2006;119:449–455. doi: 10.1016/j.snb.2006.01.001. [DOI] [Google Scholar]
- Han JG, Liu ZQ, Li XQ, Li J, Hu YH. Diversity in seed oil content and fatty acid composition in three tree peony species with potential as sources of omega-3 fatty acids. J Hortic Sci Biotechnol. 2016;91:175–179. doi: 10.1080/14620316.2015.1133538. [DOI] [Google Scholar]
- Hong XZ, Wang J, Hai Z. Discrimination and prediction of multiple beef freshness indexes based on electronic nose. Sens Actuators B Chem. 2012;161:381–389. doi: 10.1016/j.snb.2011.10.048. [DOI] [Google Scholar]
- Kunz MR, Ottaway J, Kalivas JH, Georgiou CA, Mousdis GA. Updating a synchronous fluorescence spectroscopic virgin olive oil adulteration calibration to a new geographical region. J Agric Food Chem. 2011;59:1051–1057. doi: 10.1021/jf1038053. [DOI] [PubMed] [Google Scholar]
- Kuriakose S, Joe IH. Feasibility of using near infrared spectroscopy to detect and quantify an adulterant in high quality sandalwood oil. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:568–573. doi: 10.1016/j.saa.2013.06.076. [DOI] [PubMed] [Google Scholar]
- Lerma MN, Bellincontro A, García-Martínez T, Mencarelli F, Moreno JJ. Feasibility of an electronic nose to differentiate commercial Spanish wines elaborated from the same grape variety. Food Res Int. 2013;51:790–796. doi: 10.1016/j.foodres.2013.01.036. [DOI] [Google Scholar]
- Li JJ. Tree peony of China. Beijing: Encyclopedia of China Publishing House; 2011. p. 63. [Google Scholar]
- Li MC, Zhao MM, Wu HY, Wu W, Xu YN. Cloning, characterization and functional analysis of two type 1 diacylglycerol acyltransferases (DGAT1s) from tetraena mongolica. J Integr Plant Biol. 2013;55:490–503. doi: 10.1111/jipb.12046. [DOI] [PubMed] [Google Scholar]
- Li SS, Yuan RY, Chen LG, Wang LS, Hao XH, Wang LJ, Zheng XC, Du H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015;173:133–140. doi: 10.1016/j.foodchem.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Mabood F, Boqué R, Folcarelli R, Busto O, Jabeen F, Al-Harrasi A, Hussain J. The effect of thermal treatment on the enhancement of detection of adulteration in extra virgin olive oils by synchronous fluorescence spectroscopy and chemometric analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2016;161:83–87. doi: 10.1016/j.saa.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Mahapatra PK, Singh M, Pandey L, Singla ML. Electro-chemical system for the determination of degree of unsaturation of edible oils. Food Chem. 2011;126:1505–1507. doi: 10.1016/j.foodchem.2010.11.109. [DOI] [Google Scholar]
- Melucci D, Bendini A, Tesini F, Barbieri S, Zappi A, Vichi S, Conte L, Toschi TG. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016;204:263–273. doi: 10.1016/j.foodchem.2016.02.131. [DOI] [PubMed] [Google Scholar]
- Nurjuliana M, Che MY, Mat HD, Mohamed AK. Rapid identification of pork for halal authentication using the electronic nose and gas chromatography mass spectrometer with headspace analyzer. Meat Sci. 2011;88:638–644. doi: 10.1016/j.meatsci.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Özdemir D, Öztürk B. Near infrared spectroscopic determination of olive oil adulteration with sunflower and corn oil. J Food Drug Anal. 2007;15:40–47. [Google Scholar]
- Rincóncervera MÁ, Valenzuela R, Hernandezrodas MC, Barrera C, Espinosa A, Marambio M, Valenzuela A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot Essent Fatty Acids. 2016;111:25–35. doi: 10.1016/j.plefa.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Rizelio VM, Gonzaga LV, Borges GDSC, Maltez HF, Costa ACO, Fett R. Fast determination of cations in honey by capillary electrophoresis: a possible method for geographic origin discrimination. Talanta. 2012;99:450–456. doi: 10.1016/j.talanta.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Rizzolo A, Bianchi G, Vanoli M, Lurie S, Spinelli L, Torricelli A. Electronic nose to detect volatile compound profile and quality changes in ‘Spring Belle’ peach (Prunus persica L.) during cold storage in relation to fruit optical properties measured by time-resolved reflectance spectroscopy. J Agric Food Chem. 2013;61:1671–1685. doi: 10.1021/jf302808g. [DOI] [PubMed] [Google Scholar]
- Salghi R, Armbruster W, Schwack W. Detection of argan oil adulteration with vegetable oils by high-performance liquid chromatography-evaporative light scattering detection. Food Chem. 2014;153:387–392. doi: 10.1016/j.foodchem.2013.12.084. [DOI] [PubMed] [Google Scholar]
- Samman S, Chow JWY, Foster MJ, Ahmad ZI, Phuyal JL, Petocz P. Fatty acid composition of edible oils derived from certified organic and conventional agricultural methods. Food Chem. 2008;109:670–674. doi: 10.1016/j.foodchem.2007.12.067. [DOI] [Google Scholar]
- Santonico M, Pittia P, Pennazza G, Martinelli E, Bernabei M, Paolesse R, D’Amico A, Compagnone D, Natale CD. Study of the aroma of artificially flavoured custards by chemical sensor array fingerprinting. Sens Actuators B Chem. 2008;133:345–351. doi: 10.1016/j.snb.2008.02.053. [DOI] [Google Scholar]
- Servili M, Esposto S, Selvaggini R, Taticchi A, Urbani S, Montedoro GF, Ricco I. Characterization of virgin olive oil aroma. Comparison by three different methods: solid phase microextraction—gas chromatography/mass spectrometry (SPME-GC/MS), electronic nose and proton transfer reaction mass spectrometry (PTR-MS) Acta Hortic. 2008;791:729–734. doi: 10.17660/ActaHortic.2008.791.111. [DOI] [Google Scholar]
- Souza LM, Santana FB, Gontijo LC, Mazivila SJ, Borges NW. Quantification of adulterations in extra virgin flaxseed oil using MIR and PLS. Food Chem. 2015;182:35–40. doi: 10.1016/j.foodchem.2015.02.081. [DOI] [PubMed] [Google Scholar]
- Standardization Administration of China (2008) Animal and vegetable fats and oils-determination of iodine value (GB/T 5532-2008). (in Chinese)
- Su JH, Ma CY, Liu CX, Gao CZ, Nie RJ, Wang HX. Hypolipidemic activity of peony seed oil rich in α-Linolenic, is mediated through inhibition of lipogenesis and upregulation of fatty acid β-oxidation. J Food Sci. 2016;81:H1001–H1009. doi: 10.1111/1750-3841.13252. [DOI] [PubMed] [Google Scholar]
- Sun XM, Zhang LX, Li PW, Xu BC, Ma F, Zhang Q, Zhang W. Fatty acid profiles based adulteration detection for flaxseed oil by gas chromatography mass spectrometry. LWT Food Sci Technol. 2015;63:430–436. doi: 10.1016/j.lwt.2015.02.023. [DOI] [Google Scholar]
- Tian XL, Wang J, Cui SQ. Analysis of pork adulteration in minced mutton using electronic nose of metal oxide sensors. J Food Eng. 2013;119:744–749. doi: 10.1016/j.jfoodeng.2013.07.004. [DOI] [Google Scholar]
- Wang LY, Yuan T. Sequel of Chinese tree peony. Beijing: China forestry publishing house; 2015. p. 105. [Google Scholar]
- Wang CC, Xu L, Wu Q, Zhou ZK, Ren XC, Yang R. The importance of ultrahigh pressure processing over the quality of the extracted oil from peony seeds (Paeonia suffruticosa Andr.) Ind Crops Products. 2015;76:1142–1147. doi: 10.1016/j.indcrop.2015.08.021. [DOI] [Google Scholar]
- Wilson AD, Baietto M. Applications and advances in electronic-nose technologies. Sensors. 2009;9:5099–5148. doi: 10.3390/s90705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie TT, Huang L. Research status of camellia oil adulteration detection technology. Sci Technol Innov. 2014;21:9. [Google Scholar]
- Xie J, Liu TS, Yu YX, Song GX, Hu YM. Rapid detection and quantification by GC–MS of camellia seed oil adulterated with soybean oil. J Am Oil Chem Soc. 2013;90:641–646. doi: 10.1007/s11746-013-2209-0. [DOI] [Google Scholar]
- Xu Z, Morris RH, Bencsik M, Newton MI. Detection of virgin olive oil adulteration using low field unilateral NMR. Sensors. 2014;14:2028–2035. doi: 10.3390/s140202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LR, Yu XZ, Liu L, Zhang R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016;202:229–235. doi: 10.1016/j.foodchem.2016.01.144. [DOI] [PubMed] [Google Scholar]
- Xue JQ, Wang SL, Zhang P, Zhu FY, Ren XX, Liu CJ, Zhang XX. On the role of physiological substances, abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii ‘Feng Dan’) Sci Hortic. 2015;182:92–101. doi: 10.1016/j.scienta.2014.11.021. [DOI] [Google Scholar]
- Zhang QY, Zhang SP, Xie CS, Zeng DW, Fan CQ, Li DF, Bai ZK. Characterization of Chinese vinegars by electronic nose. Sens Actuators B Chem. 2006;119:538–546. doi: 10.1016/j.snb.2006.01.007. [DOI] [Google Scholar]
- Zheng ZZ, Lin XJ. Study on application of medical diagnosis by electronic nose. World Sci Technol. 2012;14:2115–2119. doi: 10.1016/S1876-3553(13)60016-2. [DOI] [Google Scholar]
- Zhu XB, Zhai WT, Dong XX, Xu H. Progress on chemical composition and function of peony seed oil. China Oils Fats. 2014;1:88–91. [Google Scholar]