Abstract
In this study, the efficiencies of conventional hydro-distillation and novel microwave hydro-distillation methods in extraction of essential oil from Rosemary officinalis leaves have been compared. In order to attain the best yield and also highest quality of the essential oil in the microwave assisted method, the optimal values of operating parameters such as extraction time, microwave irradiation power and water volume to plant mass ratio were investigated using central composite design under response surface methodology. Optimal conditions for obtaining the maximum extraction yield in the microwave assisted method were predicted as follows: extraction time of 85 min, microwave power of 888 W, and water volume to plant mass ratio of 0.5 ml/g. The extraction yield at these predicted conditions was computed as 0.7756%. The qualities of the obtained essential oils under designed experiments were optimized based on total contents of four major compounds (α-pinene, 1,8-cineole, camphor and verbenone) which determined by gas chromatography equipped with mass spectroscopy (GC–MS). The highest essential oil quality (55.87%) was obtained at extraction time of 68 min; microwave irradiation power of 700 W; and water volume to plant mass ratio of zero.
Keywords: Rosemary officinalis L., Quality assessment of essential oil, Gas chromatography–mass spectroscopy, Response surface methodology, Central composite design
Introduction
Rosemary. officinalis L. (Rosemary) is known as a perennial herb that belongs to the Lamiaceae family. It is widely grown in Western Mediterranean regions (Bellumori et al. 2016).
The extract and essential oil of the Rosemary have been used in wide ranges of applications, including cosmetics, as additive for shampoo, soap, cream; food industry processing, as flavourig agent, and medicinal industry, because of its antibacterial, antioxidant and anti-mutagenic properties. The potential antibacterial, antioxidant and anti-mutagenic properties of this plant was proved in many reported researches in the literature (Babuskin et al. 2015; González-Rivera et al. 2016; Karakaya et al. 2014; Ranjbar Nedamani et al. 2015).
There are many methods for extraction of essential oils from natural plants such as traditional hydro-distillation, steam distillation and organic solvent extraction (Zermane et al. 2016). The main disadvantages of the above methods are degradation of some volatile compounds due to long extraction times, degradation of unsaturated or esteric compounds through thermal or hydrolytic effects (Zermane et al. 2016). Furthermore, using large volumes of organic solvent in some extraction methods, has limited their applications due to environmental considerations (Li et al. 2016). Thus there is growing need to develop the safe and environmentally being method for extraction of essential oil from plants. In recent decades, microwave assisted extraction method is introduced as a safer, faster, more efficient and cost-effective alternative (Bustamante et al. 2016).
In this study, yield and quality of the essential oil extracted from R. officinalis L using conventional hydro-distillation and microwave assisted hydro-distillation methods were compared. Furthermore, the effects of extraction time, microwave irradiation power, and water volume to plant mass ratio on the extraction yield of R. officinalis L under microwave assisted hydro-distillation method were investigated by applying the response surface methodology (RSM).
Biological activity of the Rosemary essential oil has been attributed to the presence of major compounds such as: α-piene, 1,8-cineole, camphor and verbenone (Karakaya et al. 2014; Mezza et al. 2018). Therefore, the quality of essential oils, which were obtained from microwave assisted distillation method under different designed experiments, was optimized based on amount of these four compounds using GC–MS analysis.
Materials and methods
Plant and reagents
Fresh leaves of the Rosmarinus officinalis L. were collected from Ghamsar, Iran and washed to remove debris and other contaminated contents. The leaves were shade dried at room temperature (25 °C) for 10 days. Finally, the dried leaves divided to samples of 50 g and stored in the moisture-free atmosphere for further experiments.
All chemicals, including hexane, sodium sulphate and acetone were analytical grade and purchased from Merck Company.
Microwave assisted hydro-distillation
The accelerated extraction of the essential oil was performed using a microwave oven (DRY DIST, MILESTONE, Italy).
To provide initial humidity, an aliquot of 50 g dried powder of plant leaves were wetted before extraction by soaking in 175 ml distilled water at room temperature for 1 h and then, additional water was removed. The extraction operations were performed under various microwave powers for different irradiation times. The volume of distilled water added to the flat flask containing dried powder of plant was calculated based on various water volumes to mass of plant ratios without considering the constant mass loading of plant (50 g) as follows:
| 1 |
For each predetermined condition, the extraction process started at room temperature (25 °C) and heated up for 5 min until 92 °C was achieved. Then, the mixtures were boiled at this temperature for remaining extraction time (Hashemimoghadam et al. 2013).
To collect the oil, obtained from each run, 1 ml n-pentane was added to the collector tube and the organic phase was collected in amber vial, 0.2 g anhydrous sodium sulfate was added and the sample preserved at 4 °C overnight, far from light. Finally, the sodium sulfate was filtered and the solvent was removed in room temperature. Obtained pure essential oil was maintained at − 20 °C until being analyzed (Akhbari et al. 2018).
The extraction yield of essential oil in each experimental run was calculated as follows:
| 2 |
Extraction of essential oils by thermal hydro-distillation method
50 g of the dried powder of Rosemarinus officilanis leaves were poured to 1000 ml round bottom flask of conventional Clevenger apparatus. Then, 300 ml distilled water was added into the flask containing plant powder. The hydro-distillation was continued for 5.5 h until no more essential oil obtained (Kusuma and Mahfud 2017). The samples for analyzing were prepared as mentioned above (“Microwave assisted hydro-distillation” section).
Optimization of the extraction process using central composite design (CCD) method
Design of experiments (DOE) was conducted for optimization of the selected process parameters with the help of the CCD method. A three factor-five level CCD followed by response analysis was carried out to evaluate the optimum conditions of the microwave assisted extraction process for obtaining the maximum extraction yield and also the best quality of essential oil. In this study, the key factors selected for optimization purpose were: water volume to mass of plant ratio, microwave power, and extraction time. These factors were considered with five levels as shown in Table 1. Different extraction times (25, 40, 55, 70 and 85 min) and microwave powers (550, 700, 850, 1000 and 1150 W), as well as various water volumes to mass of plant ratios (0, 0.5, 1.5, 2.5 and 3) were selected for codes of − 1.68, − 1, 0, + 1 and + 1.68, respectively (Table 1). As shown in Table 1, seventeen combinations with three replicates at center point were designed to fit the full second order polynomial equation model (Eq. 3).
| 3 |
where Y represents the predicted response variable, is the model intercept; , , and are the regression coefficients of linear, square, and interaction terms, respectively. and indicate the coded independent variables.
Table 1.
The structure of design matrix and obtained response as an extraction yield from each experiment
| Run number | X1: time (min) | X2: power (W) | X3: water volume to mass of plant ratio (ml/g) | Yield (%) |
|---|---|---|---|---|
| 1 | + 1 | + 1 | + 1 | 0.5503 |
| 2 | + 1 | + 1 | − 1 | 0.6881 |
| 3 | + 1 | − 1 | + 1 | 0.4807 |
| 4 | + 1 | − 1 | − 1 | 0.6133 |
| 5 | − 1 | + 1 | + 1 | 0.2399 |
| 6 | − 1 | + 1 | − 1 | 0.2416 |
| 7 | − 1 | − 1 | + 1 | 0.1545 |
| 8 | − 1 | − 1 | − 1 | 0.2230 |
| 9 | + 1.68 | 0 | 0 | 0.5623 |
| 10 | − 1.68 | 0 | 0 | 0.0501 |
| 11 | 0 | + 1.68 | 0 | 0.2637 |
| 12 | 0 | − 1.68 | 0 | 0.1495 |
| 13 | 0 | 0 | + 1.68 | 0.1517 |
| 14 | 0 | 0 | − 1.68 | 0.5275 |
| 15 | 0 | 0 | 0 | 0.4114 |
| 16 | 0 | 0 | 0 | 0.4275 |
| 17 | 0 | 0 | 0 | 0.4293 |
Statistical analysis
Statistical analysis of the experimental data obtained from CCD was performed using MINITAB® (Minitab Inc. Release 16.0).The optimum condition for extraction of essential oil from R. officinalis leaves was determined using analysis of variance (ANOVA). ANOVA was performed to find the effect of each parameter on the responses. The significance of each term in the second order polynomial model (linear, quadratic and interactions) was tested by the associated ANOVA in the central composite design analysis, as well as the accuracy of the regression model.
Gas chromatography–mass spectroscopy (GC–MS) analysis
Identification of the chemical constitutions of the essential oil was carried out using a gas chromatograph coupled with a mass selective detector (Model HP-5973, Agilent, USA) operating in electron ionization mode (70 eV). The gas chromatograph was equipped with an HP-INNOWAX capillary column (60 m × 0.32 mm, film thickness 0.5 mm).
The operational conditions for GC–MS analyses were as follows: carrier gas (helium) at a flow of 1.5 ml/min; injection volume of 0.1 µl; injection temperature of 250 °C; ionization energy of 70 eV. The oven temperature was increased from 80 to 320 °C at a rate of 3 °C/min and kept for 5 min at 320 °C. Then heated to 250 °C and finally, held for 3 min at 250 °C (Akhbari et al. 2017).
The components of the essential oils were identified based on comparing their GC retention indices on the HP-INNOWAX capillary column and reported literature data, as well as, their mass spectral fragmentation patterns with those of similar compounds in a GC–MS library. To investigate the retention indices, C8–C23 n-alkanes were injected as standards to GC–MS analyzer.
Results and discussion
Optimization of the extraction process
Central composite design and fitting the model
Three parameters of extraction time, microwave irradiation power, and water volume to mass of plant ratio were selected based on their effects on microwave assisted extraction yield and constituents composition evaluated by central composite design (CCD). Different extraction times (X1: 25, 40, 55, 70 and 85 min) and microwave powers (X2: 550, 700, 850, 1000 and 1150 W), as well as various water volumes to mass of plant ratios (X3: 0, 0.5, 1.5, 2.5 and 3) were selected for codes of − 1.68, − 1, 0, + 1 and + 1.68, respectively (Table 1).
After elimination of the two run experiments (13 and 14) as outliers, the statistical analysis of the studied factors was performed. Model terms with P < 0.05 were considered as significant, while the others were non-significant (Lopresto et al. 2014). It was found that three linear terms of extraction time (X1, P < 0.0001), irradiation power (X2, P < 0.05), and water volume to mass of plant ratio (X3, P < 0.05), as well as, quadratic terms of them (X21, P < 0.05), (X22, P < 0.0001), and (X23, P < 0.05) had a significant effect on the yield of essential oil, while three interaction terms of X1X2, X1X3, and X2X3 were not significant (P value > 0.05).
Non-significant “lack of fit” (P > 0.05) indicates that the proposed model fit the experimental data well. The values of the coefficient of determinations (R2), adjusted R2 (R2adj) and predicted R2 (R2pred) indicate the response model accuracy and the high values of these coefficients imply the higher correlation between the experimental data and the predicted values by the model.
Term with a higher F-value and a lower P value has more effect on extraction yield as a response variable. The calculated F-value of linear terms was higher than other quadratic and interaction terms. This suggested that these terms are more effective factors on the yield of essential oil. Among all significant terms, the linear term of extraction time (X1) and quadratic term of water volume to mass of plant ratio (X23) showed the largest effect on the extraction yield.
The second-order mathematical model based on experimental data of extraction yield was proposed as follows:
| 4 |
As indicated in Eq. 4, the extraction time plays the most important role in essential oil yield. In contrast, the irradiation power shows the minimal influence on extraction yield. The linear effects of extraction time and microwave power are significant and positive, which means that by increasing them it is possible to increase the extraction yield.
The reduced quadratic polynomial model was reconstructed without the X1X2 and X2X3 terms (Eq. 5).
| 5 |
The coefficient of determinations (R2), adjusted R2 (R2adj) and predicted R2 (R2pred) values are 0.9905, 0.9810 and 0.9314%, respectively, which are statistically acceptable.
The optimum values were obtained based on the coded values through the calculating the first-order derivatives of the response function (Y) with respect to dependent variables. Maximum extraction yield of 0.7756% is obtained under the optimum values of X1 (+ 1.68), X2 (+ 0.2206) and X3 (− 1) that are respectively corresponded to the extraction time of 85 min, irradiation power of 888 W, and water volume to mass of plant ratio of 0.5 ml/g.
Analysis of response surface
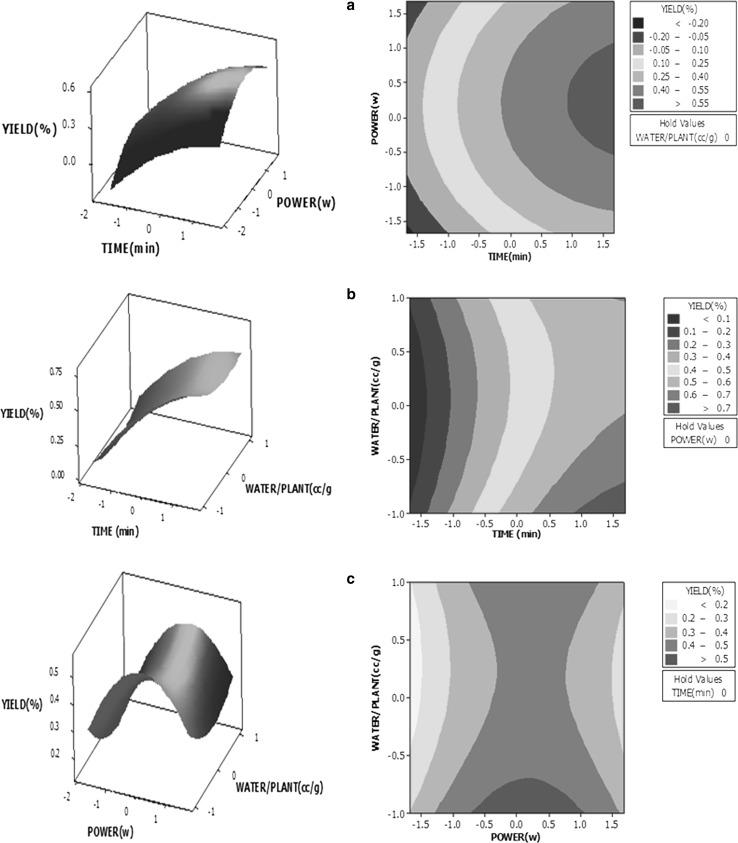
The three-dimensional response surface and two-dimensional contour plots are very useful tools to visualize the effects of mutual interactions of the independent variables on the response and also, for identifying the optimum values of the independent variables for obtaining the maximum yield as a response. Three parameters were maintained at their respective central levels, which coded by zero, as shown in Table 1. RSM was used to determine the interactive effects of three operational parameters on extraction yield from R. officinalis plant leaves (Fig. 1a–c).
Fig. 1.
Three-dimensional response surface and the two-dimensional contour plots for extraction yield of essential oil as a function of a extraction time and microwave power; b extraction time and water volume to mass of plant ratio; c microwave power and water volume to mass of plant ratio
Figure 1a implies the interactive effects of extraction time and microwave power on extraction yield of essential oil. As shown in this figure increasing the extraction time showed the higher effect on response variable (extraction yield) in compare to increasing the microwave power level. This is in agreement with the result of Eq. 4. Increasing the extraction time from 25 min (Coded = − 1.68) to 85 min (Coded = + 1.68) and microwave power from 550 W (Coded = − 1.68) to 936 W (Coded = 0.5) led to extraction yield enhancement of the essential oils, while the further increase in microwave power caused to reduce the extraction yield. The two dimensional contour plot indicated that the extraction yield increased up to 55% under the following conditions: microwave power between 850 (Coded = 0) and 936 W (Coded = 0.5) and extraction time of 85 min (Coded = + 1.68). This verified the results of process optimization, which calculated the optimum values for microwave power and extraction time at two coded levels of + 0.2206 and + 1.68, respectively.
The interaction effects of extraction time and water volume to mass of plant ratio on the essential oil yield are presented in Fig. 1b. The sensitivity of the extraction yield to extraction time increment was considerably higher than its sensitivity to increasing the water volume to mass of plant ratio. Results indicated that the extraction yield shifted to higher than 70%, when time increment from 70 min (Coded = + 1) to 85 min (Coded = + 1.68), and increasing the water volume to mass of plant ratio from 0.8 (Coded = − 0.75) to 0.5 ml/g (Coded = − 1) were occurred.
The enhancement of yield with increasing extraction time is related to the increase in interfacial area between the plant matrix and solvent that causes to release of essential oil from the disrupted cells of the plant (Chen et al. 2018).
Response surface plot of extraction yield as a function of microwave power and water volume to mass of plant ratio (Fig. 1c) indicates that the yield considerably is affected by increasing the microwave power until 936 W (Coded = 0.5) and then decreased by an increase in the microwave power. Thus microwave irradiation power showed both negative and positive effects on extraction yield, while the extraction yield was not further changed with increasing the water volume to mass of plant ratio.
The positive influence of the microwave irradiation power on extraction yield can be attributed to the accelerated destruction of the plant cells followed by rapid diffusion rate of intracellular constituents to liquid solution. On the other hands, fast variation of temperature as a consequence of excessive microwave irradiation power could cause to partial thermal decomposition of essential oil and leading to yield decrement (Chen et al. 2016; Qi et al. 2014).
Contour plot revealed that it is possible to obtain the higher essential oil extraction yield (more than 50%) at microwave power ranges of 850 (Coded = 0) − 936 W (Coded = 0.5), water volume to mass of plant ratio ranges of 0.8 (Coded = − 0.75) − 2.5 ml/g (Coded = + 1).
All above results verified that a nominal change in extraction time could affect the response at a greater extent. Based on three dimensional RSM analyses, model terms of extraction time (X1), and microwave irradiation power (X2) showed the significant influence on the essential oil extraction yield. These findings are in agreement with quadratic model and statistical analysis.
Comparison of two distillation methods
The extraction yield obtained from hydro-distillation method during 5.5 h was measured as 1.2404. Decreasing the extraction time in the microwave assisted method in compared with common hydro-distillation method could reduce the probability of unwanted hydrolyzation and oxidation. The mechanism of heat transfer in hydro-distillation method is based on thermal conductivity from the outside to the inside of the sample, whereas the mechanism of heat transfer in the microwave assisted method, dominantly occurred via convection from samples center to the outside. It was also reported that synergistic combination of the unidirectional mass and heat transfer and the fast internal heat transfer which depends on microwave power resulted in acceleration of the extraction rates under microwave assisted extraction (Bousbia et al. 2009; Gavahian et al. 2015). These might be the main reason of the difference in extraction yield between two extraction methods.
Optimization of the quality of the essential oil from Rosemary
Central composite design and fitting the model
In order to optimize the quality of the essential oil, quantification of essential oil constitutes as the key quality indices was performed. To facilitate the data analysis, the quantities of four major identified components of rosemary were selected as criteria of essential oil quality. The volatile compounds detected in the R. officinalis essential oils were mainly α-pinene, verbenone, 1,8-cineole and camphor. Essential oils resulted from microwave-assisted hydro-distillation (under seventeen run experiments) and hydro-distillation extraction methods (using Clevenger) were quantified using GC–MS, based on sum of these four components (Table 2).
Table 2.
Comparison of major chemical composition of R. officinalis essential oil obtained by microwave-assisted (under different operational condition) and Clevenger extraction methods
| Run number | Component | RIa | RIb | Content (%w/w) | Sum of four main contents of essential oil | Ratio of oxygenated compoundsc |
|---|---|---|---|---|---|---|
| 1 | α-pinene | 940 | 933 | 7.58 | 52.83 | 0.856 |
| 1, 8-Cineole | 1024 | 1030 | 15.56 | |||
| Camphor | 1159 | 1147 | 9.40 | |||
| Verbenone | 1230 | 1204 | 20.29 | |||
| 2 | α-Pinene | 938 | 933 | 5.14 | 46.72 | 0.890 |
| 1, 8-Cineole | 1040 | 1030 | 12.51 | |||
| Camphor | 1157 | 1147 | 8.18 | |||
| Verbenone | 12,227 | 1204 | 20.89 | |||
| 3 | α-pinene | 937 | 933 | 6.76 | 53.29 | 0.873 |
| 1, 8-Cineole | 1038 | 1030 | 15.12 | |||
| Camphor | 1156 | 1147 | 10.24 | |||
| Verbenone | 1227 | 1204 | 21.17 | |||
| 4 | α-pinene | 937 | 933 | 8.08 | 55.46 | 0.584 |
| 1, 8-Cineole | 1039 | 1030 | 16.30 | |||
| Camphor | 1156 | 1147 | 9.46 | |||
| Verbenone | 1229 | 1204 | 21.62 | |||
| 5 | α-pinene | 936 | 933 | 3.92 | 49.71 | 0.923 |
| 1, 8-Cineole | 1037 | 1030 | 12.05 | |||
| Camphor | 1154 | 1147 | 9.90 | |||
| Verbenone | 1225 | 1204 | 23.84 | |||
| 6 | α-pinene | 936 | 933 | 4.15 | 49.28 | 0.916 |
| 1, 8-Cineole | 1037 | 1030 | 11.41 | |||
| Camphor | 1155 | 1147 | 10.00 | |||
| Verbenone | 1225 | 1204 | 23.72 | |||
| 7 | α-pinene | 936 | 933 | 4.70 | 40.63 | 0.884 |
| 1, 8-Cineole | 1037 | 1030 | 11.22 | |||
| Camphor | 1155 | 1147 | 10.55 | |||
| Verbenone | 1225 | 1204 | 14.16 | |||
| 8 | α-pinene | 936 | 933 | 5.65 | 49.91 | 0.887 |
| 1, 8-Cineole | 1037 | 1030 | 11.97 | |||
| Camphor | 1154 | 1147 | 10.25 | |||
| Verbenone | 1226 | 1204 | 22.04 | |||
| 9 | α-pinene | 937 | 933 | 5.92 | 52.41 | 0.887 |
| 1, 8-Cineole | 1039 | 1030 | 14.89 | |||
| Camphor | 1156 | 1147 | 9.35 | |||
| Verbenone | 1229 | 1204 | 22.25 | |||
| 10 | α-pinene | 936 | 933 | 2.22 | 32.12 | 0.931 |
| 1, 8-Cineole | 1037 | 1030 | 7.45 | |||
| Camphor | 1155 | 1147 | 8.97 | |||
| Verbenone | 1228 | 1204 | 13.48 | |||
| 11 | α-pinene | 936 | 933 | 4.94 | 48.58 | 0.898 |
| 1, 8-Cineole | 1037 | 1030 | 11.64 | |||
| Camphor | 1155 | 1147 | 9.28 | |||
| Verbenone | 1229 | 1204 | 22.72 | |||
| 12 | α-pinene | 936 | 933 | 5.22 | 35.38 | 0.852 |
| 1, 8-Cineole | 1037 | 1030 | 8.40 | |||
| Camphor | 1155 | 1147 | 9.60 | |||
| Verbenone | 1227 | 1204 | 12.16 | |||
| 13 | α-pinene | 936 | 933 | 3.82 | 49.96 | 0.924 |
| 1, 8-Cineole | 1038 | 1030 | 11.41 | |||
| Camphor | 1155 | 1147 | 9.79 | |||
| Verbenone | 1229 | 1204 | 24.94 | |||
| 14 | α-pinene | 941 | 933 | 7.87 | 52.15 | 0.849 |
| 1, 8-Cineole | 1042 | 1030 | 14.24 | |||
| Camphor | 1155 | 1147 | 9.20 | |||
| Verbenone | 1229 | 1204 | 20.48 | |||
| 15 | α-pinene | 937 | 933 | 7.02 | 52.36 | 0.866 |
| 1, 8-Cineole | 1038 | 1030 | 14.54 | |||
| Camphor | 1156 | 1147 | 9.59 | |||
| Verbenone | 1226 | 1204 | 21.21 | |||
| 16 | α-pinene | 936 | 933 | 6.94 | 49.42 | 0.860 |
| 1, 8-Cineole | 1037 | 1030 | 13.79 | |||
| Camphor | 1155 | 1147 | 9.12 | |||
| Verbenone | 1226 | 1204 | 19.57 | |||
| 17 | α-pinene | 936 | 933 | 5.89 | 51.96 | 0.887 |
| 1, 8-Cineole | 1038 | 1030 | 13.60 | |||
| Camphor | 1155 | 1147 | 9.74 | |||
| Verbenone | 1226 | 1204 | 22.73 | |||
| Clevenger | α-pinene | 937 | 933 | 28.48 | 62.72 | 0.530 |
| 1, 8-Cineole | 1040 | 1030 | 14.83 | |||
| Camphor | 1156 | 1147 | 5.37 | |||
| Verbenone | 1227 | 1204 | 14.04 |
aRetention indices relative to C8-C23n-alkanes on HP-INNOWAX capillary column
bRetention indices obtained from literature review
cRatio of three oxygenated content (1, 8-Cineole, Camphor, and Verbenone) to total content
As indicated in Table 2, higher amounts of oxygenated compounds are present in the essential oils extracted by microwave assisted in comparison with hydro-distillation. This may be due to the fact that microwave assisted distillation method causes less intense thermal and hydrolytic effects than hydro-distillation which uses a high amount of water. Furthermore, oxygenated compounds have more dipolar moment in compared with monoterpene hydrocarbons. This caused to their more interaction with microwaves and therefore their easier extraction comparing to monoterpene hydrocarbons (Filly et al. 2014). Oxygenated compounds of essential oils and especially, these four compounds in rosemary essential oil, pose effective biological features. Hence, higher extraction yield of these valuable compounds using microwave-assisted extraction verify the superiority of this method for essential oil extraction.
The analysis of variance (ANOVA) for resultant experimental data was conducted. It is well-known that the regression coefficients with small P value (P < 0.05) have significant influence on response variable (four major content of essential oil). So, two linear terms of extraction time (X1, P < 0.001), and water volume to mass of plant ratio (X3, P < 0.001), the quadratic terms of (X21, P < 0.05) and (X22, P < 0.05) and three interaction terms of X1X2, X1X3, X2X3 (P < 0.001) were significant for essential oil quality. To include the interaction and quadratic term, term X2 must also be selected; otherwise, the model is not hierarchical.
The higher F-Value and smaller P value (P < 0.05) of the model terms imply a remarkable effect of those model terms on essential oil quality. According to this, in all significant terms, interaction (F-Value = 171.28) and linear (F-Value = 157.41) terms exhibited more influence on response variables.
It can be concluded from the model F-value of 95.45 (P < 0.001) that this predicted model is significant and appropriate for fitting the experimental data. The P value of the lack of fit is found to be 0.401. Also, the coefficient determination (R2) of the model was reported as 0.9954. Insignificant lack of fit and high value of R2 represent further evidences about satisfactory of the model for data fitness.
Based on obtained F-Values, two linear terms of extraction time (X1) and water volume to mass of plant ratio (X3), followed by three interaction terms of X1X2, X1X3 and X2X3 were identified as key terms in controlling the essential oil quality. Also, it was found that the extraction time has the most important effect on essential oil quality.
Decreasing the percentage of the major compounds in essential oil (essential oil quality) as a consequence of water volume to mass of plant ratio increment can be attributed to the presence of high water volumes (content) which may be caused to hydrating or hydrolyzing of the reactive compounds such as carbonyl derivatives.
It was found that the temperature and extraction time did not affect the type of extracted components, but led to increase the yield of some major components, which is in agreement with previous results (Karabegović et al. 2013).
After elimination of the three run experiments (10, 12 and 16) as outliers, the reduced second order polynomial model (Eq. 6) satisfy the empirical relationship between the total major content of essential oil as a response variable and operational independent variables (microwave power, extraction time, and water volume to mass of plant ratio).
| 6 |
Regression coefficients of linear terms in Eq. 6 are evidences of the positive effect of time (X1) and negative effects of microwave power (X2) and water volume to mass of plant ratio (X3) on essential oil quality.
The maximum essential oil quality was predicted as 55.87%. This quality was obtained under following optimum conditions: extraction time of 68 min, microwave irradiation power of 700 w and water volume to mass of plant ratio of zero that are respectively corresponded to the optimum coded values of X1 (+ 0.7867), X2 (− 1) and X3 (− 1.68).
Response surface analysis
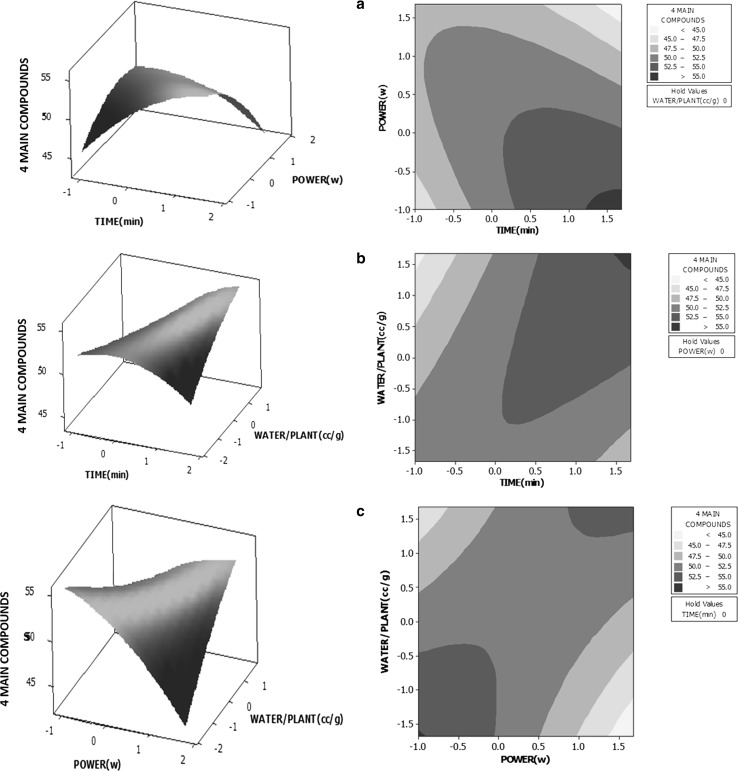
Evaluation of the binary interactive effects of three independent variables of extraction time, microwave power and water volume to mass of plant ratio on resultant essential oil quality (total percentage of four major components) was performed by aiding of the three-dimensional response surface and two dimensional contour plots. Plots are presented in Fig. 2a–c.
Fig. 2.
Three-dimensional response surface and two-dimensional contour plots for essential oil quality (total percentage of four major components of α-pinene, verbenone, 1,8-cineole and camphor) as a function of a extraction time and microwave power; b extraction time and water volume to mass of plant ratio; c microwave power and water volume to mass of plant ratio
The interactive effects of extraction time and microwave power (Fig. 2a) revealed that increasing the extraction time and decreasing the microwave power led to increasing the total percentage of four major compounds of the essential oil. Although increasing the microwave power appeared to favor the extraction yield, but under this condition, hydrolysis of the main constituents of the essential oil, or production of other chemicals and metabolites may be occurred. Thus, the total content of these four major components presented in essential oil of R. officinalis will be decreased.
Investigating the interactions between extraction time and water volume to mass of plant ratio (Fig. 2b) verified that the total content of major constituents in essential oil enhanced as a consequence of increasing the level of both variables of extraction time and water volume to mass of plant ratio.
Figure 2c presented the interactive effects of microwave power and water volume to mass of plant ratio on essential oil quality when the third variable of extraction time was considered constant (at zero level). As indicated in the contour plot, the higher essential oil quality resulted from lower levels of both microwave power and water volume to mass of plant ratio.
Conclusion
The extraction process from rosemary, under microwave assisted hydro-distillation method, was optimized using RSM technique and it was found that the extraction yield and also quality of the essential oil were affected by dependent variables of time (X1), microwave irradiation power (X2) and water volume/mass ratio of the plant (X3). Optimum conditions for extraction yield were found to be 85 min; 888 W; and 0.5 ml/g and the extraction yield under these conditions was measured as 0.7756%. The maximum essential oil quality (55.87%) was obtained under: extraction time of 68 min; microwave irradiation power of 700 W; and water volume to mass of plant ratio of zero. The essential oil extracted from rosemary using microwave assisted hydro-distillation method contained more oxygenated compounds than conventional method. Microwave assisted hydro-distillation method provided important advantages over conventional method such as: accelerated extraction time, reduced energy consumption, and cleaner production.
Acknowledgements
The authors would like to thank University of Kashan for its financial supports.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Akhbari M, Yaghoobei M, Hamedi S. Composition of the oily compounds, phytochemical screening and biological activity of different aerial parts of Smirnovia turkestana Bunge. Nat Prod Res. 2017 doi: 10.1080/14786419.2017.1374263. [DOI] [PubMed] [Google Scholar]
- Akhbari M, Kord R, Jafari Nodooshan S, Hamedi S. Analysis and evaluation of the antimicrobial and anticancer activities of the essential oil isolated from Foeniculum vulgare from Hamedan, Iran. Nat Prod Res. 2018 doi: 10.1080/14786419.2017.1423310. [DOI] [PubMed] [Google Scholar]
- Babuskin S, et al. Effects of Rosemary extracts on oxidative stability of chikkis fortified with microalgae biomass. J Food Sci Technol. 2015;52:3784–3793. doi: 10.1007/s13197-014-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellumori M, Innocenti M, Binello A, Boffa L, Mulinacci N, Cravotto G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound and microwave-assisted extraction procedures. C R Chim. 2016;19:699–706. doi: 10.1016/j.crci.2015.12.013. [DOI] [Google Scholar]
- Bousbia N, Abert Vian M, Ferhat MA, Petitcolas E, Meklati BY, Chemat F. Comparison of two isolation methods for essential oil from rosemary leaves: hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009;114:355–362. doi: 10.1016/j.foodchem.2008.09.106. [DOI] [Google Scholar]
- Bustamante J, et al. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J Clean Prod. 2016;137:598–605. doi: 10.1016/j.jclepro.2016.07.108. [DOI] [Google Scholar]
- Chen F, Du X, Zu Y, Yang L, Wang F. Microwave-assisted method for distillation and dual extraction in obtaining essential oil, proanthocyanidins and polysaccharides by one-pot process from Cinnamomi Cortex. Sep Purif Technol. 2016;164:1–11. doi: 10.1016/j.seppur.2016.03.018. [DOI] [Google Scholar]
- Chen F, Xu M, Yang X, Liu J, Xiao Y, Yang L. An improved approach for the isolation of essential oil from the leaves of Cinnamomum longepaniculatum using microwave-assisted hydrodistillation concatenated double-column liquid-liquid extraction. Sep Purif Technol. 2018;195:110–120. doi: 10.1016/j.seppur.2017.12.013. [DOI] [Google Scholar]
- Filly A, Fernandez X, Minuti M, Visinoni F, Cravotto G, Chemat F. Solvent-free microwave extraction of essential oil from aromatic herbs: from laboratory to pilot and industrial scale. Food Chem. 2014;150:193–198. doi: 10.1016/j.foodchem.2013.10.139. [DOI] [PubMed] [Google Scholar]
- Gavahian M, Farahnaky A, Farhoosh R, Javidnia K, Shahidi F. Extraction of essential oils from Mentha piperita using advanced techniques: microwave versus ohmic assisted hydrodistillation. Food Bioprod Process. 2015;94:50–58. doi: 10.1016/j.fbp.2015.01.003. [DOI] [Google Scholar]
- González-Rivera J, Duce C, Falconieri D, Ferrari C, Ghezzi L, Piras A, Tine MR. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): chemical composition and thermal analysis. Innov Food Sci Emerg Technol. 2016;33:308–318. doi: 10.1016/j.ifset.2015.12.011. [DOI] [Google Scholar]
- Hashemimoghadam H, Feyzi P, Kamali H, Nematollahi A. Comparison of microwave extraction and hydrodistillation of essential oil from Biebersteinia multifida DC. Conjunction with gas chromatography–mass spectroscopy: optimization via Box-Behnken. JNKUMS. 2013;4:13–21. doi: 10.29252/jnkums.4.5.S5.13. [DOI] [Google Scholar]
- Karabegović IT, Stojičević SS, Veličković DT, Nikolić NČ, Lazić ML. Optimization of microwave-assisted extraction and characterization of phenolic compounds in cherry laurel (Prunus laurocerasus) leaves. Sep Purif Technol. 2013;120:429–436. doi: 10.1016/j.seppur.2013.10.021. [DOI] [Google Scholar]
- Karakaya S, El SN, Karagozlu N, Sahin S, Sumnu G, Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J Food Sci Technol. 2014;51:1056–1065. doi: 10.1007/s13197-011-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma HS, Mahfud M. Comparison of conventional and microwave-assisted distillation of essential oil from Pogostemon cablin leaves: analysis and modelling of heat and mass transfer. JARMAP. 2017;4:55–65. [Google Scholar]
- Li S, et al. Ionic liquid-mediated microwave-assisted simultaneous extraction and distillation of gallic acid, ellagic acid and essential oil from the leaves of Eucalyptus camaldulensis. Sep Purif Technol. 2016;168:8–18. doi: 10.1016/j.seppur.2016.05.013. [DOI] [Google Scholar]
- Lopresto CG, Petrillo F, Casazza AA, Aliakbarian B, Perego P, Calabrò V. A non-conventional method to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction. Sep Purif Technol. 2014;137:13–20. doi: 10.1016/j.seppur.2014.09.015. [DOI] [Google Scholar]
- Mezza GN, Borgarello AV, Grosso NR, Fernandez H, Pramparo MC, Gayol MF. Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chem. 2018;242:9–15. doi: 10.1016/j.foodchem.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Qi X-L, et al. Solvent-free microwave extraction of essential oil from pigeon pea leaves [Cajanus cajan (L.) Millsp.] and evaluation of its antimicrobial activity. Ind Crops Prod. 2014;58:322–328. doi: 10.1016/j.indcrop.2014.04.038. [DOI] [Google Scholar]
- Ranjbar Nedamani E, Sadeghi Mahoonak A, Ghorbani M, Kashaninejad M. Evaluation of antioxidant interactions in combined extracts of green tea (Camellia sinensis), rosemary (Rosmarinus officinalis) and oak fruit (Quercus branti) J Food Sci Technol. 2015;52:4565–4571. doi: 10.1007/s13197-014-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermane A, Larkeche O, Meniai A-H, Crampon C, Badens E. Optimization of Algerian rosemary essential oil extraction yield by supercritical CO2 using response surface methodology. C R Chim. 2016;19:538–543. doi: 10.1016/j.crci.2015.08.011. [DOI] [Google Scholar]