Abstract
Enzymatic liquefaction (EL) ensures fast extraction and enhanced recovery of bioactives from red capsicum along with reduced degradation of these compounds remained in the pomace. Hence, red capsicum pomace obtained as byproduct after EL was freeze dried to produce capsicum pomace powder (CP). CP had almost 80% of bioactives (total carotenoids 91.23 ± 2.35 mg/100 g) and antioxidant activity (DPPH 1.61 ± 0.12 µmol TE/g) compared to fresh pomace. CP was further used to develop muffins. Different concentrations (2–10%) of CP were mixed in wheat flour for preparation of muffins. Wheat flour was fortified with CP at different levels (2–10%) and subsequently, effect of CP fortification on height, texture, bioactives and sensory quality of muffins was observed. CP fortification improved the quality in terms of color, flavor and texture. During storage, hardness values (34.42–32.56 N) showed decreasing trend with increase in CP content. Overall, 6% CP fortification was found most acceptable without causing significant change in porosity and crust uniformity of fortified muffins. Thus, present study demonstrated that fortification of muffins with 6% CP as functional ingredient offers an opportunity to develop quality muffins with enhanced antioxidant activity (DPPH 13.04 ± 0.02 µmol TE/g) and total carotenoids (3.46 ± 2.41 mg/100 g).
Keywords: Red capsicum, Pomace powder, Functional ingredient, Muffins, Fortification, Antioxidant, Rheology
Introduction
Free radical induced damage and oxidative stress are confirmed contributors to the pathogenesis and pathophysiology of many chronic health problems including neuro-degenerative conditions (Parkinson, Alzheimer, Huntington’s disease and amyotrophic lateral sclerosis), cataracts, cancer, cardiovascular and inflammatory diseases (Arathia et al. 2015). Epidemiological data has confirmed that antioxidant rich diet and therapeutic nutrition can help alleviate the risk of lifestyle and degenerative diseases. The market for such food products is growing continuously to tap the advantages of antioxidant rich crops and to utilize them as functional ingredients. One good example of such crops is red capsicum. Red capsicum (Capsicum annuum L.) is a nutritious vegetable and a promising source of capsanthin, β-carotene, capsorubin, lutein, zeaxanthin and ascorbic acid (Kim et al. 2016). In addition, it is rich with phenolics and flavonoids (Howard et al. 2000). Fresh red capsicum constitutes high levels of ascorbic acid and a 100 g serving supplies 100% of the current recommended daily allowance (RDA) (60 mg/100 g) (Nath et al. 2016). The total carotenoid content of red capsicum (30.37 mg/100 g of fresh weight) is more than that found in carrots (8.0–10.0 mg/100 g) and tomatoes (4–8 mg/100 g) (James et al. 2015). Thus, unique nutritional antioxidant composition of red capsicum deserves high attention for development of functional ingredients through processing and valorization.
Byproducts from red capsicum processing industry is a potential source of dietary fibres, various bioactive compounds such as phenolics, carotenoids and pectin (Navarro-Gonzalez et al. 2011). Apart from this, red capsicum has an excellent coloring ability which blends very well with dry or liquid food products without imparting any pungency (Nath et al. 2016). Despite of the nutritional importance and its potential of being incorporated in novel food uses, utilization of red capsicum is very limited. Presently in India, red capsicum is considered to be an exotic vegetable, eaten raw mainly in salads or used in continental culinary purposes.
Considering the rising importance for health, it is essential to include some functional ingredients in regular daily food items to form an easy delivery mechanism for enhanced nutrition. In this respect, many studies have been conducted in past on development of bakery food products with added dietary fibre from plant sources like fruits, vegetables and their byproducts (Rosales-Soto et al. 2012). Various food products and their byproducts have been explored as a functional ingredient in manufacture of baked goods such as breads, muffins, sweet bread, buns etc. A potential application of processing byproduct such as pomace is its fortification in enrichment of baked products e.g. muffins. Bakery products form an important category of consumed meals and are gaining importance in all age groups due to their taste, flavour, appearance and soft textures. Like other bakery products, muffins are preferred due to their compact size, shape and easy to carry property during travelling. Muffin is a sweet, high calorie bakery product (Rosales-Soto et al. 2012). Muffins are one of such baked products where vegetable pomace enrichment could be considered as a valuable source of antioxidants and other bioactive compounds. In the last decade the food sector noted a large increase in the number of newly available products containing cereal by-products, hence a similar situation might be observed in the future for baked products enriched with byproduct pomace, due to presence of functional ingredients (Bajerska et al. 2016).
Documented studies on development of functional ingredient from tomato pomace are available in literature (Navarro-Gonzalez et al. 2011). However, no such study pertains to utilization of byproduct from red capsicum and its fortification in bakery goods is reported. Red capsicum byproduct rich in dietary fibre, ascorbic acid and color imparting carotenoid pigment has the potential to serve as a functional ingredient (Nath et al. 2016). Therefore, fortification of red capsicum pomace powder (CP) into muffins offers a unique opportunity to develop innovative fusion products with enhanced antioxidant activity and carotenoid content. To the best of our knowledge, there is little background information on valorization of red capsicum pomace for enhancing antioxidant quality and color of muffins is available. Hence, the present study was conducted to evaluate the stability of carotenes, antioxidant activity and sensory quality of muffins incorporated with red capsicum pomace powder obtained after enzymatic liquefaction of red capsicum.
Materials and methods
Preparation of red capsicum pomace powder (CP)
Mature fruits of red capsicum (Capsicum annuum L.), cultivar “Bomby” [soluble solids 6.5–7%; moisture content 90–91% (wb) at the red ripe stage] grown under greenhouse at ICAR-Indian Agricultural Research Institute, New Delhi were selected for enzymatic liquefaction (EL). During EL process, fruits were washed, cut into pieces and blanched at 90 °C for 2 min in a thermostatically controlled water bath (Innova 42, Eppendorf, New Brunswick Scientific Co, Inc., Germany). Blanched fruit pieces were crushed in a domestic blender (Inalsa, India) and the macerate was heated at 90 °C for 1 min. The crushed macerate was poured into amber colored bottles secured with lids to prevent evaporation loss. The macerate then was mixed with viscozyme (from Aspergillus aculeatus, V 2010, Sigma Aldrich, FBU/g ≥ 100) and placed in a thermostatically controlled incubator (Innova 42, Eppendorf, New Brunswick Scientific Co, Inc., Germany) for 1 h at 60 °C at a speed of 150 rpm. After incubation, the liquid extract was separated from solid mass. Red capsicum pomace (solid mass) left after EL process was freeze dried to produce capsicum powder (CP). Freeze drying was achieved in a pilot scale freeze dryer (Armfield, FT 33 Vacuum Freeze Drier, England). The freeze dried red capsicum flakes were pulverized in a domestic blender (Inalsa, India) to yield homogenous powder which was subsequently vacuum packed (VAC-STAR S220 SP, Verpackungsmachininen AG, Sugiez, Switzerland) in HDPE pouches (75 µm thickness and 250 g pack size) and stored at ambient conditions till further use.
Raw material for muffin preparation
Freeze dried CP was used as a functional ingredient for fortification of muffins. Other raw materials like wheat flour (Rapid Flour Mills, Delhi), white sugar (Mawana, Delhi), vanilla essence (Crown, Delhi), baking powder (Fun and Foods, Delhi), fresh white eggs and butter (Amul, India) were procured from local market.
Moisture content, crude lipids and crude proteins of CP and wheat flour
The moisture content, crude lipids and crude protein (N x 6.25) of the CP and wheat flour was analyzed using methods of AOAC (2000). Dietary fiber content was determined in AACC (2000) and water activity (aw) was measured at 25 °C using Rotronic Hygrolab C1, USA. During analysis, muffin sample was crumbled, mixed uniformly in a blender and portion of the homogenous mixed muffin material was taken to represent the contents of whole muffin. Five muffin samples were used as replicate each time.
Muffins preparation
Straight dough method was used for preparation of CP fortified muffins. CP blends of 0% (termed as ‘control’), 2, 4, 6, 8 and 10% were prepared by replacing equivalent amount of wheat flour. Whereas, sugar (100 g), eggs (120 g), shortening (65 g), baking powder (0.5 g) and salt (1.5 g) were added in same amount in all the treatments. Sugar was mixed with butter for around 3–4 min at 200 rpm speed with the help of an electric hand blender (Philips, India). Eggs were whisked separately in a bowl with the hand whisker for 1.5 min and were added slowly to sugar-butter mixture. Wheat flour and baking powder were sifted together for incorporation of air. Mixture of sugar, butter and eggs were added slowly to wheat flour with continuous stirring for 4 min in one direction to avoid loss of air from the batter mixture. Meanwhile muffin molds were greased with vegetable oil for easy and clean removal of muffins after baking followed by filling of muffin pans with batter formulations (60–65 g) each. Prepared batter was poured in greased molds followed by baking in oven at 180–200 °C for around 18–20 min or until done in a preheated oven (Widsons Scientific Works, India). Muffins were removed from the oven on appearance of golden brown color and were allowed to cool at room temperature on wire racks for 2 h followed by packing in polypropylene zip lock bags. Samples were used for physico-chemical analysis within 1 h of preparation. Five muffins were produced from each formulation. Schematic presentation of muffin preparation is given in Fig. 2. The muffins were then analyzed for various quality attributes.
Fig. 2.
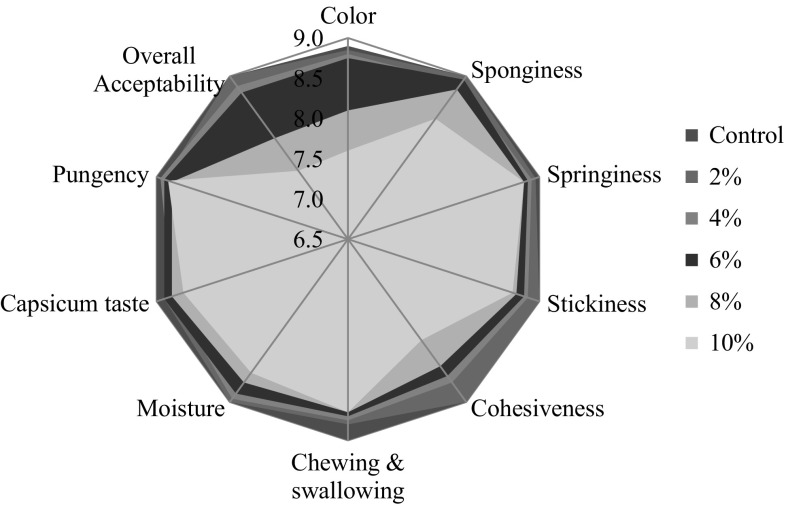
Sensory scores of capsicum pomace powder fortified (2–10%) muffins
Batter properties
Rheology of batter
The rheological properties of muffin batter were measured using Anton Paar dynamic rheometer (MCR-52, Anton Paar, Germany), fitted with plate–plate measuring system (40 mm diameter) at 25 °C. Gap between the plates was 1 mm which was decided on the basis of the size of the starch granule (maximum size 33.5 mm). The rheometre was also connected to the thermostat to maintain the temperature during test runs. Before rheological measurements the samples were maintained with the identical thermo-mechanical history. Accordingly, the batters before the rheological test were held at 25 °C for 60 min after batter preparation. The samples were allowed to rest in the measurement cell for a 25 min equilibration time. A continuous ramp was applied and apparent viscosity was measured as a function of shear rate over the 0.01–100 s−1 range for 5 min; 100 points with a logarithmic distribution at 25 °C were recorded. All curves were adjusted to the Ostwald model following the power-law equation (Eq. 1) (Ferguson and Kembloski 1991):
| 1 |
where n is the apparent viscosity, K is the consistency index, is the shear rate and n is the flow index.
Specific gravity of batter
The specific gravity (Sg) of the raw batter was determined according to Ashwini et al. (2009) with slight modifications. The Sg was calculated gravimetrically as the ratio of the weight of a known volume of batter to that of the weight of an equal volume of water at 28 ± 2 °C using a standard container of known volume. The measurements were made in triplicate for different batches of each formulation.
Physical properties of CP muffins
Height and weight of CP muffins
The muffin height (mm) was measured from the highest point of the baked muffins to the bottom of the muffin mold. It was determined with the help of a vernier caliper (Model No. CD-800CSX, Mitutoyo Corp. Kawasaki, Japan, least count of 0.01 mm). Also, percentages of increase and decrease in muffin height were determined from initial and final heights. Three replicates from each batch were measured. Measurements for initial and final weights of muffins (before and after baking) were performed on weighing balance (Mettler, Toledo, India) and percent loss in moisture content of baked muffins was measured from muffin batter weight and baked muffins weight. Five replicates for each parameter from each batch were measured.
Color attributes
The crumb color of muffin samples was determined in terms of , and values using HunterLab miniScan XE Plus colorimeter (Model No. LX16244, Hunter associates Laboratory, Virginia). From each batch, five muffin samples were taken and the color values of the crumb of muffin samples were recorded at two different points. To measure the color of muffin crumb, the crust was carefully removed with the help of a sharp knife. From the , and values total color difference was calculated and reported.
Texture profile analysis of muffins
The texture profile analysis (TPA) of the fortified muffins was done using Texture analyzer (Model TA + Di, Stable Micro Systems, UK). Each formulation was prepared thrice, on different days, and three muffins from each batch (nine determinations) were measured for each of the storage times. The muffins were cut horizontally at the height of the mold and the lower half (2.5 cm) was considered for texture measurements while the upper half was discarded. A two cycle compression (TPA2) test was performed using 75 mm diameter flat-ended cylindrical probe (P/75), to a height of 1.25 cm (50% compression), at a speed of 1 mm/s with a 5 s waiting time between the two cycles. The parameters obtained from the curves were hardness, cohesiveness, springiness, chewiness and resilience. From each formulation, five muffins were measured for TPA test.
Hedonic sensory evaluation of muffins
The sensory characteristics of muffins were evaluated according to Sanz et al. (2009) with slight modifications. The test was performed by a semi-trained panel comprising of 20 people, aged between 25 and 35 years from the faculty members of the Division of Food Science and Postharvest Technology, IARI, New Delhi. The muffins were presented in a randomized order in their molds on plastic trays, with randomly assigned three-digit codes, and each subject assessed four samples per session. Consistent results (as measured by a two-way ANOVA with assessor and product as independent treatments) were obtained after seven 20-min sessions. At the initial sessions, the control muffin (0% CP) and the muffin with the highest flour replacement (10% CP) were used to fix the ends of the rating scales. The score card was prepared keeping in view the quality characteristics of the bakery products. Sensory profile included descriptors namely; appearance (height, color, sponginess), manual texture (springiness, hardness, cohesiveness and stickiness), oral texture (ease in chewing and swallowing and moisture), flavor (capsicum taste/pungency) and overall acceptability where all attributes are taken into consideration. A nine point hedonic scale was used to rate the preferences with ‘0’ as not acceptable and ‘9’ as most acceptable (BIS 1971).
Shelf life study of acceptable muffin formulation
Muffin samples containing CP in the level of 6% of the flour blend that exhibited the highest overall acceptability were considered for shelf life study at ambient (about 25 ± 2 °C) and refrigerated temperature (4 ± 1 °C) for 15 days along with control samples. The muffins were packaged in LDPE pouches (75 μ thickness and 100 g pack size). During storage, the samples were taken out at 3 days interval (3, 6, 9, 12 and 15 days) and evaluated for change in textural attributes as explained in above section.
Total phenolic content
For estimation of total phenolics, 2 g of sample (CP and muffin samples) was extracted twice with 30 mL of ethanol (80%), by stirring and sonicating for 30 min in the dark. The homogenate was then centrifuged (Eppendorf, Westbury, U.S.A.) for 15 min at 10,000×g at 4 °C. The supernatant was then vacuum concentrated at 40 °C in a rota-evaporator (Genevac Sp scientific Chennai, India) and stored at − 20 °C. The concentrated sample was used as sample extract for estimation of total phenolics and antioxidant activity. Total phenolics were estimated spectrophotometrically using Folin–Ciocalteu reagent (Singleton et al. 1999). Results were expressed as gallic acid equivalent (mg GAE/100 mL). The absorbance of the solution was measured using UV–Vis spectrophotometer (Varian Cary 50, Agilent Technologies).
Total antioxidant activity (AOX)
AOX in CP and muffin extracts was evaluated using four in vitro assays namely; 2,2-diphenyl picryl hydrazyl (DPPH), ferric reducing antioxidant power (FRAP), copper reducing antioxidant capacity (CUPRAC) and trolox equivalent antioxidant capacity (TEAC). Ferric reducing antioxidant power (FRAP) assay was performed according to the procedure described by Benzie and Strain (1996). Reduction of the ferric tripyridyltriazine to the ferrous complex formed an intense blue color which was measured in a UV–Vis spectrophotometer (Varian Cary 50, Agilent Technologies) at 593 nm at the end of 4 min. 2,2-diphenyl picrylhydrazyl (DPPH) assay was carried out on the basis of scavenging ability of antioxidants towards the stable radical DPPH (Brand et al. 1995). The antioxidant activity was expressed as percentage inhibition of the free radical calculated using the equation (Eq. 2):
| 2 |
where; Abst=0 min, was the absorbance of DPPH radical solution at 0 min and Abst=30 min, was the absorbance of sample after 30 min. Values were calibrated against standard trolox (100–1000 μmol/L). Methanol (95%) served as a blank. Change in the absorbance of the sample extract was measured at 515 nm for 30 min. Cupric reducing antioxidant capacity (CUPRAC) assay in red capsicum extract was determined according to the method of Apak et al. (2008). After 30 min, the absorbance was recorded at 450 nm against reagent blank. Standard curve was prepared using different concentrations of Trolox (100–1000 µM). The results were expressed as µmol TE/100 mL, using molar absorptivity of Trolox as 1.67 × 104 mol−1/cm. Trolox equivalent antioxidant capacity (TEAC) assay was measured using ABTS decoloration method using radical ABTS (2,2-azino-bis-3-ethylbenzthiazoline-sulphonic acid) (Re et al. 1999). The percentage inhibition of ABTS+· of the test sample and known solutions of trolox were calculated by the following formula (Eq. 3):
| 3 |
where; Abst = 0 min, was the absorbance of ABTS solution at 0 min and Abst = 10 min was the final absorbance of sample after 10 min. The calibration curve between % inhibition and known solutions of trolox (100–1000 μmol/L) was then established. Absorbance was taken at 734 nm using UV–Vis spectrophotometer (Varian Cary 50, Agilent Technologies).
Ascorbic acid
Ascorbic acid in CP and muffin samples was measured as described by Klopotek et al. (2005). Concentration of L-AA was calculated using standard curve. Measurement was done at 520 nm using UV–Vis spectrophotometer (Varian Cary 50, Agilent Technologies).
Total carotenoid content
Total carotenoid content in CP and muffin samples was estimated by extracting the sample twice with acetone until the extract was colorless. The homogenate was filtered with suction through a Buchner funnel containing Whatman filter paper no. 1 (Fisher Scientific, Pittsburg, PA). The filtrates were combined, transferred to separating funnel containing 50 mL of 4% aqueous NaCl and 100 mL of petroleum ether (BP 40–60 °C). Absorption of the petroleum ether layer was measured at 470 nm in dim light using UV–Vis spectrophotometer (Varian Cary 50, Agilent Technologies). A calibration curve was prepared for each group of samples using 99% pure β-carotene in the range of 10–100 μg/mL (Lee 2001). The results were expressed as mg/100 mL.
Statistical analysis
All the physico-chemical parameters were measured in triplicate and means were reported. Duncan’s multiple range test (DMRT) and ANOVA was performed to test the statistical differences in these parameters as affected by different treatments. SPSS software (version 16.0) was used to conduct the tests. The significance was accepted at 5% levels of significance (p < 0.05). Correlations among different variables were determined using Addin software XLSTAT (version 2014.5.03). The significance was accepted at 5% levels of significance (p < 0.05).
Results and discussion
Five formulations of muffins were prepared with varying levels of CP (2–10%). Wheat flour muffins without CP served as control.
Moisture content, crude lipids and crude proteins of CP and wheat flour
Results showed that significant (p < 0.05) differences were observed among all the corresponding estimated parameters of CP and wheat flour. Moisture content of CP was 6.01% (wb) whereas that of wheat flour was observed as 14.02%. Water activity (aw) of CP was found as 0.29. Crude lipid, crude protein and fibre contents of freeze dried CP was determined as 1.80, 4.08, 4.05%, respectively. The crude protein content of wheat flour (12.83%) was significantly (p < 0.05) higher than that of CP (4.08), whereas CP had higher levels of dietary fibre and lipid contents. The crude protein was higher in wheat flour than CP, however dietary fibre was 2 folds higher in CP than wheat flour. Thus, CP was found to be a wealthy source of dietary fibres. Therefore, CP, if used as a functional ingredient in muffins would ultimately enhance the nutritional quality of the traditional wheat flour muffins.
Bioactive composition and antioxidant activity of freeze dried CP
CP was observed as an excellent source of bioactive compounds and AOX. The content of carotenoids, phenolics and ascorbic acid in freeze dried CP was observed as 91.23 mg/100 g, 166.24 mg GAE/100 g and 255.58 mg/100 g, respectively. AOX ranged from 1.64 to 8.55 µmol TE/g among different assays. The AOX was 1.64, 2.43, 5.01 and 8.49 µmol TE/g in DPPH, FRAP, CUPRAC and TEAC. It was observed that AOX was positively correlated (r > 0.85) with bioactive compounds. Being rich in carotenoids, phenolics and ascorbic acid, this powder concentrate would be suitable for product development where it would not only enhance color and appearance but would also improve bioactivity of the fortified product (Nath et al. 2016). Our results are in line with the observations made by Kamiloglu et al. (2017), where black carrot pomace was used as a source of polyphenols for enhancing the nutritional value of cake.
Batter properties
Rhelogy of CP fortified batter
Rheological behaviour of batter samples was determined through flow parameters. Batter viscosity is an important physical attribute in baking cake or muffins. During baking, the velocity gradient in the batter induces convection current depending on its viscosity, with low batter viscosity resulting in more convection flow. Retention of air and leavening gases by batters is clearly reflected in the specific gravity values (Table 1) of the batters and is also a function of batter viscosity (Bath et al. 1992). The viscosity values, as a function of the shear rate for the control formulation and the batters fortified with CP showed that within the shear rate range studied the flow curves occur in the pseudoplastic zone; in other words, viscosity decreases with increased shear rate (data not presented). To study the behavioural pattern of the batters the experimental values were adjusted to the Ostwald-De Waale rheological model. This model is suitable for characterizing rheological behaviour in the pseudoplastic zone, that is, for shear rate intervals which are neither very high nor very low, and presented good determination coefficients (R2 > 0.97). The addition of fibre significantly (p < 0.05) increased the consistency value (K) (Table 1). Identical observations have been reported by several workers in cocoa fiber chocolate muffins, apple pomace incorporated cake and corn dextrin cake (Martinez-Cervera et al. 2011; Masoodi et al. 2002). CP brought about a non-significant lowering of the flow index (n) indicating slight resistance to the batter flow and hence existence of a complex structure. The increasing viscosity of the batter is an unfavourable factor for the final baked product. Moreover, an increase in viscosity lead to problems in handling the batter, in mould filling (metering) and in cleaning the machinery, or to greater energy expenditure on pumping.
Table 1.
Flow parameters, specific gravity and physical properties of muffin batter and capsicum pomace powder fortified muffins
| Sample (% CP) | Batter properties | Baked muffins properties | |||||
|---|---|---|---|---|---|---|---|
| Flow parameters | Specific gravity (g/L) | Height (mm) | Increase in height (%) | Moisture (g/100 g) | Percent weight loss | ||
| K (Pa s) | n | ||||||
| Control | 19.24 ± 0.03a | 0.58 ± 0.42c | 1.031 ± 0.02a | 47.90 ± 1.24a | 112.12b | 42.01 ± 1.74c | 14.2b |
| 2% | 21.23 ± 0.04b | 0.49 ± 0.23b | 1.041 ± 0.01b | 47.85 ± 1.23a | 110.15a | 41.21 ± 1.12b | 13.8a |
| 4% | 22.54 ± 0.21b | 0.45 ± 0.04b | 1.051 ± 0.15c | 47.87 ± 1.34a | 110.10a | 41.55 ± 1.12b | 13.9a |
| 6% | 23.67 ± 0.05c | 0.39 ± 0.05ab | 1.054 ± 0.21c | 47.70 ± 1.65a | 110.12a | 42.12 ± 1.43c | 14.3b |
| 8% | 24.78 ± 0.06c | 0.36 ± 0.21a | 1.060 ± 0.02d | 47.88 ± 1.42a | 110.15a | 41.62 ± 1.45b | 13.9a |
| 10% | 25.99 ± 0.01d | 0.32 ± 0.31a | 1.065 ± 0.04e | 47.89 ± 1.56a | 110.15a | 40.21 ± 1.21a | 13.7a |
Values are mean of triplicate determinations ± standard deviation
Different letters within the same column are statistically different (p < 0.05)
Specific gravity
The specific gravity values of the samples with flour fortified by CP were slightly and significantly (p < 0.05) higher than those of the control (Table 1). Higher specific gravity in the baked products is associated with less aeration indicating lower capacity of the batter to retain air during beating operation and consequently lower height and volume in the finished baked product. Principally, low values of specific gravity shows greater number of gas nuclei in the batter which consequently results in the development of large air bubbles during baking and therefore provides greater height and volume to the final finished product. However, retention of air bubbles and leavening gases by batters during baking is also a function of batter viscosity (Bath et al. 1992). A number of interrelated factors account for final volume in baking: rheological attributes which are affected by the type and variety of ingredients and raw material, the level of air incorporation during mixing of raw materials, homogenization time and speed. In fact, in the present study slight (non-significant at p < 0.05) difference was observed between heights of fortified and control muffins. However, significant (p < 0.05) decrease in volume of the muffins with addition of CP was observed. It might be attributed to the restricted expansion of muffins during baking due to addition of CP. Similar results have been reported by Grigelmo-Miguel et al. (2001) and Martinez-Cervera et al. (2011) in muffins enriched with peach dietary fiber and chocolate muffin with cocoa fiber.
Physical properties of CP fortified muffins
Height and weight
All the CP fortified muffin samples were smaller than the control samples. Values of the heights of muffin samples were 47.90, 47.85, 47.87, 47.70, 47.88 and 47.89 mm for control, 2, 4, 6, 8 and 10% CP fortified samples, respectively (Table 1). Although, no significant (p < 0.05), slight decrease in the heights of muffins was observed after CP fortification. Loss in height of muffin samples is a reflection of the change in each formulation. In general, addition of any type of fibre to the ingredients of a bakery product causes a reduction in both the volume and the height of the final product. Grigelmo-Miguel et al. (2001) reported similar findings when peach dietary fibre replaced part of oil in the formulation. They found that height of the muffins remained the same as the control (without fibre) when the content in peach fibre was lower than 4/100 g batter, while its higher concentrations produced smaller sized muffins. Baixauli et al. (2008) also found a decrease in muffin height when increasing proportions of the flour were replaced by resistant starch. Gomez et al. (2010) also encountered less volume in layered cakes to which various fibres of different granulometries were added for flour replacement.
It is evident from Table 1 that significant (p < 0.05) differences were observed in the values of weight loss in muffin samples upon baking (after 24 h cooling). However, the weight loss was approximately close to 14 g/100 g for all the formulations (Table 1). Twenty-four hours after baking, the moisture content values of the CP fortified samples (except 6% CP) were significantly (p < 0.05) lower than those for control sample. The moisture content in control, 2, 4, 6, 8 and 10% CP fortified muffins was recorded as 14.2, 13.8, 13.9, 14.3, 13.9 and 13.7%, respectively. The study showed that the addition of CP did not contribute any moisture.
Color attributes
Effect of CP fortification on color attributes of muffins is presented in Table 2. Fortification significantly (p < 0.05) influenced color attributes (lightness, redness, yellowness and total color change) of muffins as depicted in Fig. 1. Addition of CP having = 36.02, = 40.23 and = 21.61 gave muffins (both crust and crumb) reddish brown shade. On the contrary, control sample without any CP showed natural golden brown color after baking.
Table 2.
Color attributes of the crust and crumb of capsicum pomace powder fortified muffins
| CP (%) | ||||
|---|---|---|---|---|
| Crumb color | ||||
| Control | 68.03 ± 1.44de | 11.65 ± 0.89a | 35.26 ± 1.26a | 0.00 |
| 2% | 64.51 ± 1.35d | 16.96 ± 1.07b | 38.65 ± 1.44b | 7.22 ± 1.44a |
| 4% | 60.31 ± 1.01c | 20.13 ± 1.03c | 40.90 ± 1.02c | 12.78 ± 1.45b |
| 6% | 53.22 ± 0.99b | 29.77 ± 1.01d | 48.61 ± 1.01d | 26.94 ± 2.24c |
| 8% | 49.23 ± 1.65a | 32.91 ± 1.65e | 48.41 ± 1.64d | 31.28 ± 1.65d |
| 10% | 45.24 ± 1.45a | 33.24 ± 0.98e | 48.15 ± 0.97d | 31.24 ± 2.56d |
| Crust color | ||||
| Control | 62.12 ± 1.01e | 20.45 ± 0.97a | 36.41 ± 0.21a | 0.00a |
| 2% | 61.65 ± 1.14e | 21.28 ± 1.11a | 39.87 ± 1.17b | 3.59 ± 1.01b |
| 4% | 56.35 ± 1.20d | 25.68 ± 1.25b | 40.15 ± 1.25b | 8.64 ± 1.14c |
| 6% | 45.15 ± 1.02c | 45.11 ± 1.40c | 42.62 ± 0.58c | 30.57 ± 1.17d |
| 8% | 43.24 ± 0.89b | 47.52 ± 1.54c | 48.87 ± 2.15d | 35.28 ± 1.23e |
| 10% | 40.11 ± 2.11a | 47.99 ± 0.87c | 48.59 ± 2.40d | 37.30 ± 1.71e |
Values are mean of triplicate determinations ± standard deviation
—lightness or darkness, —redness or greenness, —yellowness or blueness
Different letters within the same column are statistically different (p < 0.05)
Fig. 1.

Effect of capsicum pomace powder fortification on whole muffins (control, 6, 8 and 10%) (a) and crust and crumb of muffins (b)
Muffin crumb and crust
Color of muffins is credited to different ingredients in it. Although, darker color is not desirable in many food products, consumers consider darker muffins being healthier than the lighter ones (Walker et al. 2014). The change in crumb color of muffins after CP fortification was quite evident. With increase in percent incorporation of CP in muffins, crumb color became darker and more reddish. CP had a reddish color due to the presence of carotenoid pigments, which darkened the color of baked muffins (decreased of crusts and crumbs) depending on the level of incorporation and also due to the interaction between carotenoids and other constituents (Xu and Diosady 2000).
Lightness (), redness () and yellowness () for muffin crumbs ranged from 49.23 to 68.03, 11.65 to 33.24, 35.26 to 48.61, respectively. As clearly evident from the results, value decreased significantly (p < 0.05) with increase in level of CP from control to 6% CP. Control muffins had the highest brightness compared to CP fortified muffins. No specific trend was observed in the change in value. However, value showed a significant (p < 0.05) increase with increase in the levels of CP. Total color change, also termed as overall color change , ranged from 7.22 to 31.24 after fortification. The values increased significantly (p < 0.05) with percent CP levels in the range of 2–6%, however no significant (p < 0.05) difference was observed beyond 8% CP. Increase in was due to darkening of the muffins caused by CP incorporation.
The variations in the crust color of muffin samples with increase in CP level were similar to the variations in crumb color. The lightest samples (highest values) were those in which no CP was added. The lightness values decreased significantly (p < 0.05) as the percent CP increased. The samples with the highest CP also had the highest corresponding values of (red component) and (yellow component), indicating a significantly brighter and more saturated red-brown color than control samples (Table 2). According to results, was appreciable ( > 3) by the human eye in all the samples fortified with CP (Table 2). Our observations were in line with the reports of Ho et al. (2011) for sausage enriched with tomato peel and bread fortified with Curcuma longa L. Muffin fortification with Curcuma longa L. and tomato peel resulted in decrease in value and increase in value.
Textural attributes of fortified muffins
The addition of CP in muffins gave more tender and crumbly muffins with a more compact, less aerated crumb. CP fortified muffins showed significantly (p < 0.005) lower hardness values than the control (Table 3). The hardness values decreased (by 9.33–13.35%) as the CP content increased from 2 to 10% and the muffin fortified with 10% CP was significantly less harder than the control. Similar results were quoted by Swanson et al. (2002) in brownies when shortening was fortified with polydextrose and lecithin. The springiness value decreased significantly (p < 0.05) in all the CP fortified muffin samples than control sample reflecting a more open crumb structure. Similarly, cohesiveness values were also significantly lower than that in the control samples (p < 0.05). Cohesiveness could be associated with the decreased hardness and would result in a greater crumbliness of the samples with lower fat levels. Our results contradicts with the findings of Grigelmo-Miguel et al. (1999) where flour was replaced by peach fibre, the resulting muffins were harder and chewier; this change in muffin texture was related to greater batter density, lower formation of bubble nuclei and the inability to rise during baking. Baixauli et al. (2008) obtained lower springiness and cohesiveness values than those of the control when resistant starch was substituted for flour.
Table 3.
Textural parameters of capsicum pomace powder fortified muffins
| Sample (% CP) | Fresh muffins | After 30 days | ||||
|---|---|---|---|---|---|---|
| Hardness (N) | Springiness | Cohesiveness | Chewiness (N) | Resilience | Hardness (N) | |
| Control | 21.42 ± 2.23c | 0.82 ± 0.21c | 0.53 ± 0.02c | 10.35 ± 0.54c | 0.18 ± 0.02b | 46.52 ± 2.23d |
| 2% | 19.42 ± 1.97b | 0.80 ± 0.32b | 0.52 ± 0.03bc | 10.02 ± 0.43c | 0.16 ± 0.02ab | 34.42 ± 1.97c |
| 4% | 19.21 ± 2.12b | 0.78 ± 0.12a | 0.51 ± 0.01b | 9.78 ± 0.39b | 0.16 ± 0.01ab | 33.21 ± 2.12b |
| 6% | 18.76 ± 2.37a | 0.79 ± 0.25ab | 0.51 ± 0.04b | 9.44 ± 0.56b | 0.15 ± 0.03a | 33.76 ± 2.37b |
| 8% | 18.88 ± 1.54a | 0.77 ± 0.34a | 0.49 ± 0.03a | 8.98 ± 0.65a | 0.14 ± 0.02a | 32.88 ± 1.54a |
| 10% | 18.56 ± 1.87a | 0.76 ± 0.53a | 0.47 ± 0.01a | 8.66 ± 0.34a | 0.14 ± 0.02a | 32.56 ± 1.87a |
Values are mean of triplicate determinations ± standard deviation
Different letters within the same column are statistically different (p < 0.05)
Chewiness is a secondary texture parameter that is associated with difficulty in chewing the sample and forming a bolus before swallowing. The sequence was similar to that of hardness values: almost all the CP fortified samples except 2% CP showed significantly (p < 0.05) lower chewiness values than the control samples (Table 3) but no significant differences were observed as a result of higher CP levels. These results could be related to the ability of CP to retain moisture making them easy to chew. Resilience reflects the greater or lesser symmetry of the first compression curve and is related to the degree to which the sample recovers when compression ceases; a resilience means that the sample behaves like a spring, returning immediately to its initial height. A dense structure containing little air would take longer to recover. In the present study, all the samples had low resilience values, which are typical of formulations containing sugar and fat, and the CP fortified samples presented non-significant (p < 0.05) lower resilience than the control.
Effect of storage time on texture profile of CP fortified muffins
The hardness of all the CP fortified samples increased significantly (p < 0.05) over the storage time of 15 days. The hardness of control sample was significantly (p < 0.05) higher (46.52 N), indicating a higher staling rate whereas lower values were attained by all the CP fortified samples over the same storage conditions and time. An analysis of the hardness values of the samples containing CP showed that as the CP percent increased (2–10%), the hardness values showed a decreasing trend (34.42–32.56 N) and the values were significantly lower (p < 0.05) than the control muffin samples. Results also indicated that the hardness values of the control rose greatly towards the end of the storage period, while those of the CP fortified muffin samples remained significantly lower (Table 3). In general, the springiness of all the samples decreased significantly over the storage time. Nonetheless, the variations were small and all the values fell within a fairly narrow range, with few significant differences between them (Table 3). The cohesiveness and resilience values of all the samples fell slightly but significantly over the storage time (Table 3). Lastly, the chewiness values of the CP fortified samples presented no significant differences with storage time, whereas a significant rise, directly related to the increase in hardness, was observed in the controls (Table 3).
Hedonic sensory evaluation of CP fortified muffins
The sensory scores for fresh formulated muffins as judged by the panel members are presented in Fig. 2. Significant (p < 0.05) interactions were observed between the textural parameters and amount of CP added. Sensory scores for control muffin ranged from 8.9 to 9.0 for various descriptors including; height, capsicum red colour, sponginess, springiness, stickiness, cohesiveness, ease of chewing and swallowing, capsicum taste, pungency, sweetness and overall acceptability. All the attributes varied significantly (p < 0.05) with the addition of CP with the exception of sweetness, which was perceived as unchanged due to same formulation used (data not shown). This result also shows that the CP did not affect the perception of sweetness at any level of fortification. The surface stickiness of the crust was imperceptible in all the samples and control. This could be a positive quality factor. Both the sponginess of the crumb, perceived visually, and its springiness, perceived manually, fell as the CP level raised. It might be due to higher moisture content of CP that caused the crumb to stick together when compressed and fail to recover its initial shape with any speed, giving an impression of a denser, less airy crumb with a more closed type of structure. This structure also caused the perception of cohesiveness and difficulty in chewing and swallowing the samples to increase with higher percentages of CP, making them more difficult to chew and more cohesive and difficult to crumble.
Followed by control, 2% CP scored highest owing to crust color (8.85), chewing ability (8.80) and springiness (8.85). Sensory evaluation studies showed that crumb and crust brownness of fortified muffins were acceptable as those of control muffins up to 6% level of fortification. Above this level, the color of the muffins were relatively dark, therefore, less acceptable. Increase in darkness was also reflected in values which is due to inherent color of CP followed by non-enzymatic browning occurring during baking process. Muffins showed relative hardness with the level of fortification. Consequently, fortification > 8% scored lowest as increase in level of CP, progressively imparted low sensory scores for color (8.10–7.60), stickiness and cohesiveness (8.05–8.70) which are undesirable quality traits in bakery goods affecting the muffin taste negatively (Fig. 2).
The lower sensory scores are attributed due to color imparted by the added ingredient owing to higher values (Table 2). Overall liking of 10% CP muffin was lowest. Increase in CP substitution, imparted brownness (Maillard browning and caramelization reactions) and decreased sponginess which are undesirable qualities for muffins (Fig. 2). As expected, CP did not imparted any off notes of pungency and in most of the cases it was not even noticed by the panel members and the scores for pungent taste in muffins ranged from 8.85 to 9.0 for 2–10% CP, respectively. The overall acceptability scores were the lowest for 8% and 10% CP (7.55–8.80) and the highest for 2–6% CP (9.0–8.80). Overall, 6% CP samples with sensory scores ranging from 8.75 to 8.95 for different descriptors was found as most acceptable by the panel of judges. For other sensory characteristics no statistical significant (p < 0.05) difference was observed among all the formulations. The sensory profiling of formulated muffins pointed out that a partial replacement of wheat flour with up to 6% CP gives satisfactory overall consumer acceptability.
Similar observations were made in bread fortified with curcuma longa powder at levels, control, 2, 4, 6 and 8%. Fortification of curcuma longa at control to 4% CP gave highest liking scores whereas, higher substitution levels of 6 and 8% CP gave lowest sensory scores (Ho et al. 2011). Development of vitamin and fibre enriched buns with carrot pomace at different levels showed that overall acceptability scores for 2.5% pomace was higher (7.5) over 10% pomace (5.5) substitution levels (Kumar and Kumar 2012). Kim et al. 2012 studied the sensory characteristics of fibre rich sponge cakes in combination with Opuntia humifusa and overall, addition of 9 g of cheonnyuncho powder/100 g of wheat flour improved the physical quality and taste of sponge cake. Tatjana-Rakcejeva et al. (2011) developed wheat bread incorporated with pumpkin powder. Bread with pumpkin (7.3) scored higher than its control (6.3) bread without pumpkin. In accordance with our results similar acceptability sensory scores were reported by Lin et al. (2009) in bread developed with buckwheat flour with and without husks at substitution level of 15% and Yousif et al. (2012) showed acceptable scores for bakery items substituted with sorghum flour (50%). Sensory analysis for mango fortified muffins showed statistical significant difference (p < 0.05) between control and muffins fortified at 75% (Ramirez-Maganda et al. 2015).
Dietary fiber content of CP fortified muffins
Table 4 presents the dietary fiber contents of muffins samples. The fibre contents of control, 2, 4, 6, 8 and 10% CP fortified muffin samples were observed as 0.53, 1.51, 2.47, 3.51, 4.43, and 5.50 g/100 g, respectively. As expected, the fiber content increased as the level of CP increased from 2 to 10%.
Table 4.
Bioactive composition and antioxidant activity of muffins fortified with capsicum pomace powder
| Parameters | Percent capsicum powder | |||||
|---|---|---|---|---|---|---|
| Control | 2% | 4% | 6% | 8% | 10% | |
| Dietary fiber (g/100 g) | 0.53 ± 1.43a | 1.51 ± 1.60 b | 2.47 ± 0.50c | 3.51 ± 2.40d | 4.43 ± 1.20e | 5.50 ± 1.25f |
| Bioactive composition (mg/100 g) | ||||||
| TCC | 0.25 ± 1.40a | 1.58 ± 1.12b | 2.24 ± 1.23b | 3.46 ± 2.41c | 4.12 ± 1.36c | 5.54 ± 2.78d |
| TPC (mgGAE/100 g) | 26.7 ± 1.45a | 31.89 ± 1.45b | 33.21 ± 1.67c | 34.14 ± 1.65d | 35.65 ± 1.27e | 37.36 ± 1.25f |
| AA | 0.00a | 0.56 ± 1.34b | 1.05 ± 1.49c | 1.28 ± 1.86d | 1.86 ± 1.52e | 1.98 ± 1.13f |
| Antioxidant activity (µmolTE/100 g) | ||||||
| DPPH | 5.71 ± 1.05a | 8.00 ± 0.01b | 11.02 ± 0.01c | 13.04 ± 0.02d | 14.23 ± 0.04d | 15.35 ± 0.02de |
| FRAP | 11.60 ± 0.45a | 15.1 ± 0.02b | 20.02 ± 0.03c | 24.02 ± 0.02d | 28.06 ± 0.03e | 31.08 ± 0.04f |
| CUPRAC | 20.21 ± 1.55a | 29.1 ± 0.05b | 33.21 ± 0.03c | 43.01 ± 0.01d | 52.04 ± 0.02e | 65.2 ± 0.05df |
| TEAC | 26.10 ± 1.40a | 35.6 ± 0.04b | 42.51 ± 0.02c | 56.01 ± 0.05d | 62.06 ± 0.02d | 78.03 ± 0.04e |
Values in a row followed by same letter does not differ significantly (p < 0.05)
Values are mean of triplicate determinations ± standard deviation
TCC total carotenoid content, TPC total phenolic content, AA ascorbic acid, FRAP ferric reducing antioxidant power, DPPH-2 2-diphenyl picryl hydrazyl, CUPRAC copper reducing antioxidant capacity, TEAC trolox equivalent antioxidant capacity
The control sample showed the lowest value, which may be explained by the absence of CP. In accordance with European legislation, samples containing > 6% CP can be considered as the “sources of fiber” because these products contains more than 3 g of fiber per 100 g of the product.
Bioactive composition and antioxidant activity of fortified muffins
The results pertaining to bioactive composition and antioxidant activity of fortified muffins is presented in Table 4. Addition of CP significantly (p < 0.05) increased the bioactive composition of fortified muffins in comparison to plain muffins (control). Total carotenoid content increased significantly (p < 0.05) from 0.25 mg/100 g in control to 5.54 mg/100 g in muffins fortified with 10% CP depicting increase by 21.16%. Similar trend was observed with total phenolic content, the value increased from 26.7 mgGAE/100 g in control to a final content of 37.36 mgGAE/100 g in muffins fortified with 10% CP, depicting increase by 40%. Ascorbic acid content ranged from 0.56 to 1.98 mg/100 g in fortified muffins (2–10% CP). 10% CP samples showed 3.5 fold higher ascorbic acid content than 2% CP samples.
Red capsicum is an exceptional source of capsorubin and capsanthin, which are reported to have high AOX. This distinctive quality trait of red capsicum renders it as one of the unique sources for use in development of functional food. The AOX of muffins prepared with different levels of fortification of wheat flour with CP were analyzed using four in vitro assays namely; FRAP, DPPH, CURAC and TEAC. The AOX of muffins increased significantly (p < 0.05) with increase in the level of CP. Muffin extracts showed high AOX ranging from 5.71 to 78.03 µmolTE/100 g, depicting variation of 13 fold among assays. The increase in AOX of MCP10 was around two- to threefold greater over 2% CP. Among all the formulations, 2% CP depicted the lowest AOX (8–35.6 µmolTE/100 g) while 10% CP presented the highest AOX (15.35–78.03 µmolTE/100 g), irrespective of the assay. Increase in bioactive composition of CP fortified muffins further enhanced their AOX. This can be explained from strong positive correlation between bioactives and AOX (r ≥ 0.80). Our results can be validated from the observations made by Ramirez-Maganda et al. 2015, Danza et al. 2014) in utilization of yellow pepper flour, mango processing byproduct, apple skin powder, aronia extracts and apple processing byproducts in preparation of functional muffins.
Conclusion
Muffins fortified with CP (2–10% levels) were prepared in the laboratory and subsequently analyzed for their selected physical characteristics, bioactive components and antioxidant activities. The results indicates that addition of CP significantly (p < 0.05) increased the bioactive profile and AOX of muffins. The results showed that 6% CP could be included in a muffin formulation without any significant interference with the sensory acceptability of the product. Addition of CP (2–10%) into muffins, in most cases, increased the overall acceptability in contrast to control muffins. Overall, CP fortification improved the muffins quality in terms of color, flavor and texture. Thus, utilization of red capsicum byproduct (CP) into product development (fortified muffins) as functional ingredient offers a unique opportunity to develop fortified muffins having enhanced AOX and carotenoids.
Acknowledgements
The authors duly acknowledge the financial support and facilities provided by PG School, Indian Agriculture Research Institute, New Delhi and the Council of Scientific and Industrial Research, New Delhi during the course of study.
References
- AACC International . Approved methods of the American Association of Cereal Chemists (10th ed), methods 14-50 and 44-15A. St. Paul: AACC International; 2000. [Google Scholar]
- Apak R, Guclu K, Ozyurek M, Celik SE. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim Acta. 2008;160:413–419. doi: 10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
- Arathia PB, Sowmyaa PRS, Vijaya Kariyappa, Baskaran Vallikannan, Lakshminarayanaa Rangaswamy. Metabolomics of carotenoids: the challenges and prospects: a review. Trends Food Sci Technol. 2015;45:105–117. doi: 10.1016/j.tifs.2015.06.003. [DOI] [Google Scholar]
- Ashwini A, Jyotsana R, Indrani D. Effect of hydrocolloids and emulsifiers on the rheological, microstructural and quality characteristics of eggless cake. Food Hydrocol. 2009;23:700–707. doi: 10.1016/j.foodhyd.2008.06.002. [DOI] [Google Scholar]
- Association of Official Analytical Chemist (AOAC) Official methods of analysis. 17. Gaithersburg: Association of Official Analytical Chemist (AOAC); 2000. [Google Scholar]
- Baixauli R, Salvador A, Fiszman SM. Textural and colour changes during storage and sensory shelf life of muffins containing resistant starch. Eur Food Res Technol. 2008;226:523–530. doi: 10.1007/s00217-007-0565-4. [DOI] [Google Scholar]
- Bajerska J, Mildner-Szkudlarz S, Gornas P, Seglina D. The effects of muffins enriched with sour cherry pomace on acceptability, glycemic response, satiety and energy intake: a randomized crossover trial. J Sci Food Agric. 2016;96:2486–2493. doi: 10.1002/jsfa.7369. [DOI] [PubMed] [Google Scholar]
- Bath DE, Shelke K, Hoseney RC. Fat replacers in high-ratio layer cakes. Cereal Foods World. 1992;37(7):495–496. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- BIS . Indian Standard IS: 6273 Part I and Part II. Guide for sensory evaluation of foods. New Delhi: Indian Standard Institution (BIS), Manak Bhawan; 1971. [Google Scholar]
- Brand WW, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Danza A, Mastromatteo M, Cozzolino F, Lecce L, Lampignano V, Laverse J. Processing and characterization of durum wheat bread enriched with antioxidant from yellow pepper flour. LWT Food Sci Technol. 2014;59(1):479–485. doi: 10.1016/j.lwt.2014.06.001. [DOI] [Google Scholar]
- Ferguson J, Kembloski Z. Applied fluid rheology. Cambridge: Elsevier Science Publishers LTD/University Press; 1991. [Google Scholar]
- Francis FJ, Clydesdale FM. Food colorimetry: theory and applications. Westport: The Avi Publishing Company Inc; 1975. [Google Scholar]
- Gomez M, Moraleja A, Oliete B, Ruiz E, Caballero PA. Effect of fibre size on the quality of fibre-enriched layer cakes. LWT Food Sci Technol. 2010;43:33–38. doi: 10.1016/j.lwt.2009.06.026. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Carreras-Boladeras E, Martin-Belloso O. Development of high-fruit-dietary-fibre muffins. Eur Food Res Technol. 1999;210:123–128. doi: 10.1007/s002170050547. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Carreras-Boladeras E, Martin-Belloso O. Influence of the addition of peach dietary fiber in composition, physical properties and acceptability of reduced-fat muffins. Food Sci Technol Int. 2001;7(5):425–431. doi: 10.1106/FLLH-K91M-1G34-Y0EL. [DOI] [Google Scholar]
- Ho SL, So HP, Kashif G, Sung YH, Jiyong P. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011;124:1577–1582. doi: 10.1016/j.foodchem.2010.08.016. [DOI] [Google Scholar]
- Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J Agric Food Chem. 2000;48:1713–1720. doi: 10.1021/jf990916t. [DOI] [PubMed] [Google Scholar]
- James K, Rodriguez-Uribe L, Richard DR, Juan MGA, Jesus V, Mary AO. Correlations of carotenoid content and transcript abundances forfibrillin and carotenogenic enzymes in Capsicum annum fruit pericarp. Plant Sci. 2015;232:57–66. doi: 10.1016/j.plantsci.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Kamiloglu S, Ozkan G, Isik H, Horoz O, Camp JV, Capanoglu E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: an in vitro digestion study with a standardized static model. LWT Food Sci Technol. 2017;77:475–481. doi: 10.1016/j.lwt.2016.12.002. [DOI] [Google Scholar]
- Kim JH, Lee HJ, Lee HS, Lim EJ, Immd JY, Suh HJ. Physical and sensory characteristics of fibre-enriched sponge cakes made with Opuntia humifusa. LWT Food Sci Technol. 2012;47:478–484. doi: 10.1016/j.lwt.2012.02.011. [DOI] [Google Scholar]
- Kim JS, Chul GA, Park JS, Lim YP, Kim S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chem. 2016;201:64–71. doi: 10.1016/j.foodchem.2016.01.041. [DOI] [PubMed] [Google Scholar]
- Klopotek Y, Otto K, Bohm V. Processing strawberries to different products alters contents of vitamin C, total phenolics, total anthocyanins, and antioxidant capacity. J Agric Food Chem. 2005;53:5640–5646. doi: 10.1021/jf047947v. [DOI] [PubMed] [Google Scholar]
- Kumar K, Kumar N. Development of vitamin and dietary fibre enriched carrot pomace and wheat flour based buns. J Pure Appl Sci Technol. 2012;2(1):107–115. [Google Scholar]
- Lee HS. Characterization of carotenoids in juice of red navel orange (Cara Cara) J Agric Food Chem. 2001;49:2563–2568. doi: 10.1021/jf001313g. [DOI] [PubMed] [Google Scholar]
- Lin LY, Liu HM, Yu YW, Lin SD, Mau JL. Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem. 2009;112(4):987–991. doi: 10.1016/j.foodchem.2008.07.022. [DOI] [Google Scholar]
- Martinez-Cervera S, Salvador A, Muguerza B, Moulay L, Fiszman SM. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT Food Sci Technol. 2011;44:729–736. doi: 10.1016/j.lwt.2010.06.035. [DOI] [Google Scholar]
- Masoodi FA, Sharma B, Chauhan GS. Use of apple pomace as a source of dietary fiber in cakes. Plant Foods Hum Nutr. 2002;57:121–128. doi: 10.1023/A:1015264032164. [DOI] [PubMed] [Google Scholar]
- Nath P, Kaur C, Rudra SG, Varghese E, Sharma K. Enzyme assisted extraction of carotenoid rich extract from red capsicum (Capsicum annuum L) Agric Res. 2016 [Google Scholar]
- Navarro-Gonzalez I, Veronica GV, Javier GA, Jesus PM. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res Int. 2011;44:1528–1535. doi: 10.1016/j.foodres.2011.04.005. [DOI] [Google Scholar]
- Rakcejeva T, Galoburda R, Cude L, Strautniece E. Use of dried pumpkins in wheat bread production. Procedia Food Sci. 2011;1:441–447. doi: 10.1016/j.profoo.2011.09.068. [DOI] [Google Scholar]
- Ramirez-Maganda J, Blancas-Benitez FJ, Zamora-Gasga VM, Garcia-Magana ML, Bello-Perez LA, Tovar J, Sayago-Ayerdi SG. Nutritional properties and phenolic content of a bakery product substituted with a mango (Mangifera indica) ‘Ataulfo’ processing by-product. Food Res Int. 2015;73:117–123. doi: 10.1016/j.foodres.2015.03.004. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rosales-Soto MU, Powers JR, Alldredge JR. Effect of mixing time, freeze-drying and baking on phenolics, anthocyanins and antioxidant capacity of raspberry juice during processing of muffins. J Sci Food Agric. 2012;92:1511–1518. doi: 10.1002/jsfa.4735. [DOI] [PubMed] [Google Scholar]
- Sanz T, Salvador A, Baixauli R, Fiszman S. Evaluation of four types of resistant starch in muffins. II. Effects in texture, colour and consumer response. Eur Food Res Technol. 2009;229:197–204. doi: 10.1007/s00217-009-1040-1. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Ranventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Swanson RB, Perry JM, Carden LA. Acceptability of reduced-fat brownies by school-aged children. J Am Diet Assoc. 2002;102(6):856–859. doi: 10.1016/S0002-8223(02)90193-8. [DOI] [PubMed] [Google Scholar]
- Walker R, Tseng A, Cavender G, Ross A, Zhao Y. Physicochemical, nutritional, and sensory qualities of wine grape pomace fortified baked goods. J Food Sci. 2014;79:S1811–S1822. doi: 10.1111/1750-3841.12554. [DOI] [PubMed] [Google Scholar]
- Xu L, Diosady LL. Interactions between canola proteins and phenolic compounds in aqueous media. Food Res Int. 2000;33:725–731. doi: 10.1016/S0963-9969(00)00062-4. [DOI] [Google Scholar]
- Yousif A, Nhepera D, Johnson S. Influence of sorghum flour addition on flat bread in vitro starch digestibility, antioxidant capacity and consumer acceptability. Food Chem. 2012;134(2):880–887. doi: 10.1016/j.foodchem.2012.02.199. [DOI] [PubMed] [Google Scholar]