Abstract
The effect of five drying methods including shade drying (SHD), solar drying (SOD), and oven drying at 30 (OD30), 40 (OD40) and 50 °C (OD50) on the phytochemical composition and antioxidant potential of C. dactylon leaf and rhizome was assessed. Among drying methods, OD50 resulted in the shortest drying time (18.3 and 12 h for rhizome and leaf, respectively), when compared with SHD and SOD. Based on GC–MS analyses, 15 and 17 constituents were identified in leaf and rhizome extracts, respectively, accounting for ~ 99% of all components. Fatty acids (palmitic acid and linoleic acid) along with their methyl esters (ethyl palmitate, ethyl linoleate and ethyl oleate) and other derivatives (dihomo-γ-linoleic acid) were the main identified constituents shortly after drying procedures; however, other components such as 5-hydroxymethylfurfural, maltol, retinol and phytol were also traced. Some of C. dactylon phytochemicals including 5-hydroxymethylfurfural and ethyl linoleate were sensitive to high drying temperatures. Besides, higher drying temperatures lead to the production or increasing the level of substances such as 2,3-dihydrobenzofuran, tricyclopentadeca-3,7-dien and 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one and diacetin. Based on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay, the IC50 values were generally higher (significance level of 0.05) for oven-dried rhizome compared with shade-dried leaves and rhizomes that quenched more than 84% of the DPPH at the concentration of 400 mg/ml (IC50 59.12). Our findings suggest that OD30 is a versatile drying method not only to reduce drying time but also to preserve the main phytochemicals and antioxidant activity of C. dactylon during dehydration.
Keywords: Medicinal plants; Drying, C. dactylon; Fatty acid ethyl esters; Gas chromatography–mass spectrometry
Introduction
Over the past few decades, medicinal plants and their-derivatives have been extensively used as valuable alternatives to chemically synthetic drugs (Cordell 2014; Mozafari et al. 2015). Compared to chemical drugs that their-action is associated with undesired adverse and occasionally mental side effects, herbal medicine has shown to be more safety having minimal side-effects (Wang et al. 2011). However, remedial efficiency of many natural products is still burdened by the limited potency due to their weak selectivity in targeted cells, there have been increasing efforts to develop natural compounds with improved efficacy and selectivity (Cordell 2014).
Cynodon dactylon (L.) pers. (Poaceae) or Bermuda grass (also called Harez in Iran) is an important warm-season perennial grass mainly used as forage and turf. It traditionally has been used in the folk remedies as a potential source of medicinal bioactive compounds with many human health benefits. C. dactylon is claimed to have various medicinal properties including anti-arrhythmic (Najafi et al. 2008); anti-arthritic (Bhangale and Acharya 2014); anti-cancer (Khlifi et al. 2013); anti-diarrheal (Babu et al. 2012); anti-diabetic (Annapurna et al. 2013; Karthik and Ravikumar 2011); anti-diuretic (Sadki et al. 2010); anti-malarial (Khlifi et al. 2013); cardio-protective (Garjani et al. 2009); immunomodulatory (Mangathayaru et al. 2009) and gastro-protective (Babu et al. 2012) effects.
Quantity and quality of phytochemical ingredients in medicinal plant’s essential oils or extracts are usually influenced by several internal and external factors such as plant genetic make-up (Rahimmalek et al. 2013), phonological stage (Nejad Ebrahimi et al. 2008), nutrition (Mafakheri et al. 2016) and processing method (Chen and Mujumdar 2015). Drying is a crucial step for post-harvest handling and pre-processing of many aromatic and medicinal plants (Chen and Mujumdar 2015). Drying method and temprature can determine quantity and quality of phytochemical compounds as well as biological properties (e.g. antioxidant capacity) of the final plant product (Sellami et al. 2011).
Different drying procedures have been developed for drying of herbs and medicinal plants. However, due to importance of economic and ecological criteria, shade and solar drying are still among the most extensively used methods, new drying methods including oven, microwave and freeze-drying have been recently proposed as effective alternatives procedures for many medicinal and essential oil-bearing plants (Chen and Mujumdar 2015; Eswara and Ramakrishnarao 2013). Information regarding drying optimization of many important herb, aromatic and medicinal plants including plantain (Zubair et al. 2011), sage (Sellami et al. 2011), Savory (Ghasemi Pirbalouti et al. 2013), thyme (Rodríguez et al. 2013) and lemon balm (Argyropoulos and Müller 2014) are available in the literatures. In this respect and to the best of our knowledge, no research has been conducted to optimize drying conditions of C. dactylon leaf and rhizome and also to determine their in vitro antioxidant capacity. Therefore, the aims of the present research were (1) investigation the effect of five drying methods including three oven temperatures on phytochemical composition of C. dactylon hydro-alcoholic extracts and, (2) comparative analysis of these drying methods on C. dactylon leaf and rhizome’s in vitro antioxidant capacity.
Materials and methods
Plant material
Leaves and rhizomes of C. dactylon were collected at mature vegetative stage in the middle of June (2014) from uniform well-grown plants grown in the research field of Horticulture department, University of Kurdistan located at 1420 m altitude, 1 km west of Sanandaj, Iran (35°16′51.4″N 46°59′46.5″E). Sampling done in the morning after dew evaporation. Prior to any experimentation, C. dactylon plants taxonomically verified by comparison to voucher specimen (voucher number 5151) at the agriculture and natural resources research center of Sanandaj, Iran. The homogenous leaf and rhizome samples of C. dactylon with mean length of 5–6 cm were placed in polypropylene ziplock bags and immediately transferred to medicinal plant research lab at university of Kurdistan for further experiments.
Drying experiments
To determine the initial moisture content of C. dactylon leaf and rhizome, 100 g of each sample was oven dried at 100 °C for 24 h in four replicates. The initial mean moisture content of leaf samples were 58% (on a wet weight basis) and 1.38% (on a dry weight basis) respectively, while the equilibrium moisture rates were 52 and 1.2% based on dry and wet weights, respectively. The moisture content of dried samples was measured in triplicate using a laboratory oven at 105 °C.
Shade drying (SHD)
For shade drying method, 100 g of leaf and rhizome samples was loaded on the trays with loading density of 2.5 kg/m2, giving uniform thin layer of samples with 3 cm thickness. Shade drying experiment was performed in a room with controlled relative humidity (65% ± 11%) and temperature (23.4 ± 1.8 °C).
Solar drying (SOD)
As a conventional method used in natural drying process, solar drying was carried out by scattering 100 g of leaf and rhizome samples over 1 m2 trays resulting a similar density as SHD. Samples were subjected to the direct sunlight irradiation at temperatures between 20–25.9 °C in days and 13.5–15 °C in nights at June in Sanandaj, Iran. During solar drying, the mean velocity of wind were 3 ± 0.7 m/s and the relative humidity was 60 ± 23%.
Oven drying (OD)
Like solar and shade drying methods, 100 g leaf and rhizome samples were uniformly placed on oven trays with density of 2.5 kg/m2 and were then dried in a programmable oven with circulating air (UFP800, Memmert, Germany) at 30, 40 and 50 °C representing OD30, OD40 and OD50 drying methods, respectively. Since moderate air-drying temperatures are normally suggested for medicinal and oil-bearing plants, a constant airflow at 0.3 m/s was maintained over the sample. Besides, to achieve a steady-state condition, the ventilated ovens were set to the drying temperatures and the sample’s temperatures were allowed to equilibrate with room temperature before drying steps. During drying process, SOD and SHD samples were weighed in 5 h intervals while for oven drying methods, hourly weighing was continued until the final moisture content of 10% for both leaf and rhizome samples. The moisture ratio of C. dactylon rhizome and leaf samples during drying experiments were estimated using the following equation: MR = Mt − Me/M0 − Me, where M0 = Initial moisture content (kg water/kg dry matter), me = Equilibrium moisture content (kg water/kg dry matter) and Mt = Moisture content at any time (kg water/kg dry matter). All experiments were repeated at least three times for each drying condition.
Preparation of extract
Dried leaf and rhizome materials were finely pulverized using an electric mill (model A11B, IKA, Germany). The powders produced by different drying methods were extracted by maceration in water/ethanol solution (30:70 W/W) for 72 h at room temperature. Extracts were filtered and concentrated under vacuum condition to yield 10 ml of concentrated extract. The resulted extracts were finally maintained in amber bottles at 4 °C refrigerator until GC–MS analysis.
Gas chromatography–mass spectrum analysis (GC–MS)
Phytocomponents present in the hydro/ethanolic extracts of C. dactylon were identified by gas chromatography–mass spectrometry (GC–MS) using a Thermoquest-Finnigan Trace GC–MS instrument (Thermo Finnigan, San Jose, CA) equipped with a DB-17MS column (30 m × 0.32 mm i.d. × film thickness 0.25 µm) (Agilent Technologies, CA, USA). GC–MS conditions were as follows: Column temperature, 40–290 °C at 5 °C; injector and detector temperatures 250 and 290 °C; volume injection, 0.1 μl; split ratio, 1:50; carrier gas, helium (%99.99) with flow rate 1.2 ml/min; ionization potential, 70 eV; ionization current, 150 μA; mass range, 35–465 mui.
Radical scavenging activity using DPPH method
The in vitro activity of free radical scavenging of C. dactylon leaf and rhizome extracts was evaluated spectrophotometrically using DPPH assay as described by Blois (1958). The crude plant extracts were manually diluted with EtOH to prepare sample solutions equivalent to 25, 50, 100, 200 and 400 mg of dried extract per ml solution, respectively. The 1 ml of each sample was added to 1 ml of 0.025 mM DPPH (2,2-diphenyl-1-picrylhydrazyl) solution. After 5 min vigorous shaking, the resulting mixture was allowed to stand in the dark at room temperature for 60 min. To determine the reduction of the DPPH radicals, absorbance of the prepared samples were measured at fixed wavelength of 517 nm. Each sample was analyzed in triplicate. Antiradical activity of extracts was then calculated as inhibition percentage (%) using the following equation: % Inhibition = [(A0 − As/A0) × 100], where A0 and As are absorbances of control and sample, respectively, at 517 nm. Finally, the IC50 (concentration required to cause 50% of DPPH inhibition) was calculated from the initial inhibition data.
Identification of phytochemical components and statistical analysis
Leaf and rhizome extract’s constituents were identified based on their retention times and calculated retention indices where they matched with those of the internal reliable mass spectral fragmentation patterns (Wiley 275. L library). The experimental treatments were conducted as factorial based on randomized complete design with three replications. Mean values of the various treatments were statistically analyzed using analysis of variance (ANOVA) and were compared by Duncan’s multiple-range test (DMRT) at the 5% probability level using SAS software (Version 9.1 SAS Institute, Cary, NC, USA). Dendrogram of the drying methods regarding their influence on phytochemical profiles was drawn based on the Euclidean distance using cluster analysis implemented in SPSS version 16.0.
Result and discussion
Effect of drying method on dehydration time
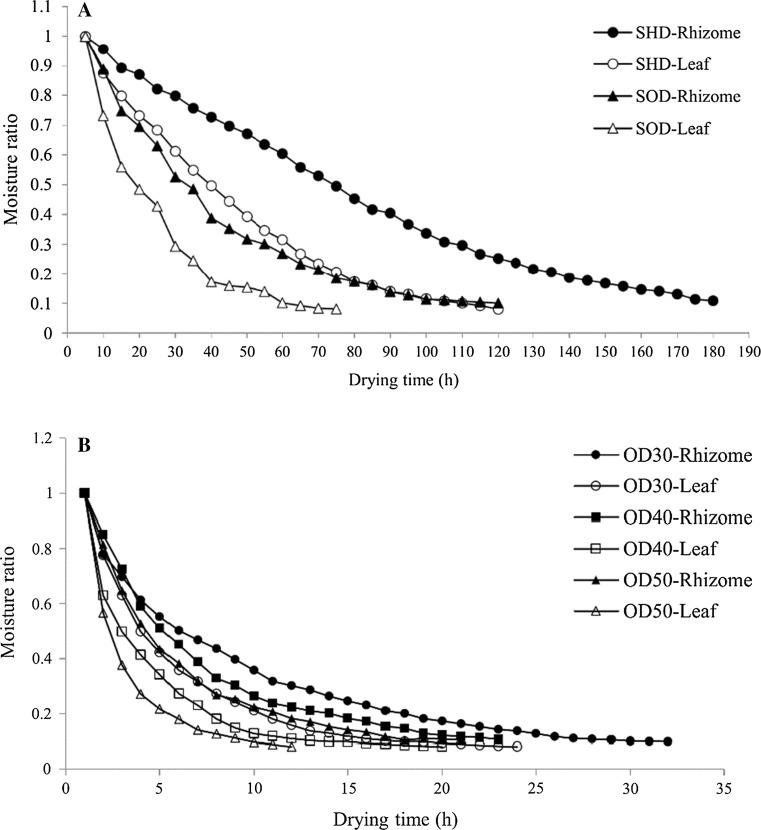
Table 1 represents mean drying time to decrease the C. dactylon leaf and rhizome’s moisture content from its initial moisture content to the final moisture content of 10%. The drying time was significantly influenced by drying methods so that the moisture content of leaf and rhizome samples was continuously reduced with increasing temperature. As it shown in drying curves (Fig. 1), drying with oven was more effective in terms of drying time and therefore weight loss of samples was higher than the SHD and SOD procedures. In the SOD method, the time taken to reach 10% moisture content of leaf and rhizome was 120 and 75 h, respectively, while the required time for shade drying procedure was 168 for rhizome and 120 h for leaf samples to reach same moisture content. Based on the results, C. dactylon samples dried with OD50 method were expectedly had the shortest drying times (18.3 and 12 h for rhizome and leaf, respectively) (Table 1 and Fig. 1). Low and variable temperatures are the main reasons for longer drying period of SHD and SOD methods (Eswara and Ramakrishnarao 2013). On the other side, high-driving force of heat transfer caused by higher temperatures in oven-assisted drying methods is responsible for enhancement in drying rate of plant samples (Chen and Mujumdar 2015; Janjai and Bala 2011). Despite better drying efficiency at higher temperatures imposed by OD 40 and OD50 procedures, many studies have suggested the use of shade or solar drying systems (Ghasemi Pirbalouti et al. 2013; Mediani et al. 2015; Sellami et al. 2011), as they provide more efficient preservation of nutrients and bioactive compounds.
Table 1.
Effect of drying methods on mean drying time to decrease the C. dactylon leaf and rhizome’s moisture content from its initial moisture to the final moisture of 10%
| Drying treatment | Mean time to each 10% moisture content (h) | |
|---|---|---|
| Rhizome | Leaf | |
| SHD | 168 ± 14.8g | 120 ± 12.3f |
| SOD | 120 ± 11.6f | 75 ± 9.5e |
| OD30 | 32 ± 4.4d | 24 ± 3.1c |
| OD40 | 26 ± 5.5bcd | 20 ± 3.2bc |
| OD50 | 18 ± 3.1abc | 12 ± 3.6a |
Means with the same letter for each parameter are not significantly different at the 5% level according to DMRT
Fig. 1.
Plot of moisture ratio with respect to drying time for a Solar drying (SOD) and shade drying (SHD) methods and, b 30, 40 and 50 oven drying temperatures for both rhizome and leaf C. dactylon -parts
C. dactylon ethanolic extract’s components and effect of drying method
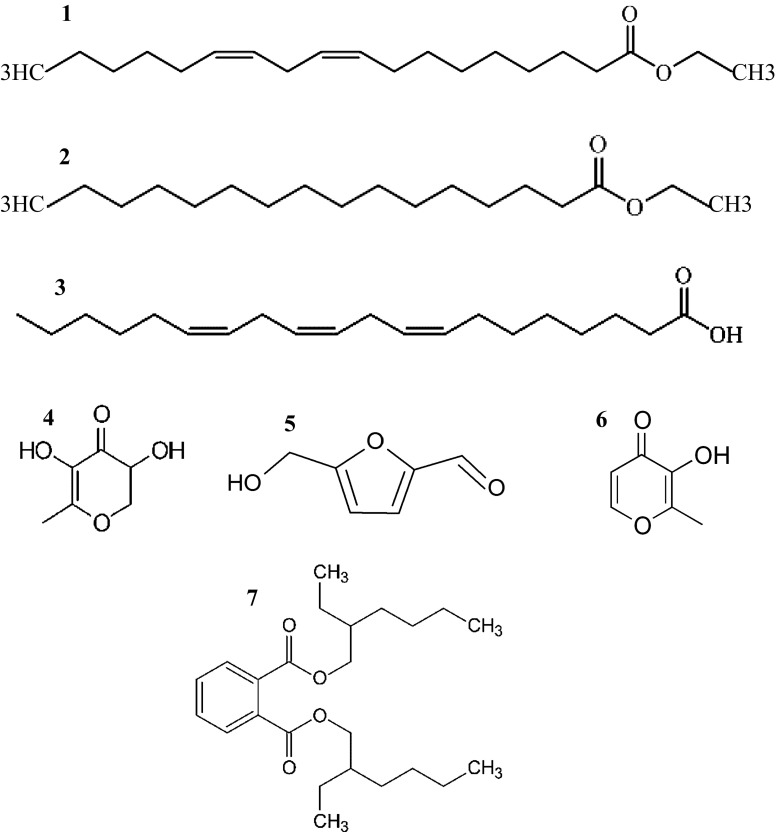
To our knowledge, no previous attempt has been made to investigate the impact of drying procedure on C. dactylon phytochemical profile as many volatile components are subjected to various alterations imposed by dehydration method. The present study identifies the composition of hydro-alcoholic extracts of both aerial and root-rhizome parts dried under different conditions. Based on the GC–MS results, 15 and 17 constituents were identified in C. dactylon leaf and rhizome extracts, respectively. These represent totally 22 constituents accounting for ~ 99% of all identified components. The component profiles of the C. dactylon hydro-alcoholic extracts under different drying procedures are shown in Table 2 and chemical structures of some important identified compounds are also represented in Fig. 2. Regardless of drying conditions, the main identified constituents were palmitic acid, ethyl linoleate, ethyl palmitate, dihomo-γ-linoleic acid (DGLA), linoleic acid, ethyl and 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP), respectively (Fig. 2).
Table 2.
The phytochemical components isolated from hydro-alcoholic extract of rhizome and leaf of C. dactylon submitted to different drying methods
| Compound | Formula | RIa | SHD | SOD | OD30 | OD40 | OD50 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | |||
| 2,3-Dihydrobenzofuran | C8H8O | 396 | – | 0.24 | – | – | 1.2 | 0.8 | – | tr. | 13.44 | tr. |
| 5-Hydroxymethylfurfural (5-HMF) | C6H6O3 | 467 | 27.9 | 1.37 | 18.0 | 2.08 | 9.07 | 1.11 | 7.28 | 1.05 | 5.12 | 0.85 |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) | C6H8O4 | 600 | 3.09 | 0.58 | – | 2.93 | 0.82 | 1.26 | 4.68 | 2.45 | 4.31 | 3.78 |
| Ethyl pentadecanoic acid | C17H34O2 | 814 | 0.63 | 0.64 | – | – | – | – | – | 1.38 | – | – |
| Ethyl palmitate | C18H36O2 | 838 | 22.1 | 31.99 | 26.28 | 29.88 | 34.48 | 31.69 | 39.48 | 31.5 | 31.29 | 31.4 |
| Ethyl linoleate | C20H36O2 | 857 | 22.57 | 39.78 | 20.17 | 38.15 | 22.6 | 34.8 | 21.17 | 24.74 | 19.58 | 19.52 |
| Palmitic acid | C16H32O2 | 873 | 2.68 | 1.89 | 1.61 | 3.07 | 6.02 | 2.99 | 2.73 | 1.47 | – | 1.46 |
| Furfural | C5H40O2 | 899 | 1.42 | – | – | – | – | – | – | – | – | – |
| Phytol | C20H40O | 974 | – | 3.42 | – | 1.67 | – | 2.88 | – | tr. | – | 2.14 |
| Dihydroxyacetone | C3H6O3 | 985 | tr. | – | 9.07 | – | tr. | – | – | – | – | – |
| 1,4-Butanediol | C4H10O2 | 1018 | – | tr. | – | 2.99 | – | – | – | tr. | – | 4.42 |
| Ethyl oleate | C20H38O2 | 1139 | 5.48 | 3.83 | 5.33 | 2.36 | 5.97 | 3.87 | 4.8 | 3.9 | 4.47 | 3.42 |
| Maltol | C6H6O3 | 1153 | 3.16 | – | 6.15 | 0.0 | 2.26 | – | 3.16 | 0.6 | 3.08 | – |
| Linoleic acid | C18H32O2 | 1191 | 5.09 | 4.16 | 6.73 | 2.9 | 7.68 | 4.6 | 5.11 | 2.45 | 4.62 | 1.47 |
| Diacetin | C7H12O5 | 1365 | tr. | – | tr. | – | 2.57 | – | 4.38 | – | 8.0 | – |
| 2-Methoxy-4-vinylphenol | C9H10O2 | 1387 | tr. | – | – | – | 0.73 | 0.67 | – | – | tr. | – |
| Dihomo-γ-linoleic acid | C20H34O2 | 1405 | 2.35 | 10.63 | 2.0 | 12.61 | 2.57 | 13.13 | 2.0 | 10.61 | 1.8 | 9.65 |
| Ethyl stearate | C20H40O2 | 1566 | 0.65 | 0.56 | 1.51 | 0.69 | 1.09 | 0.71 | 1.85 | tr. | tr. | 0.63 |
| Tricyclopentadeca-3,7-dien | C15H22 | 1641 | – | – | – | – | – | – | – | 3.58 | – | 9.92 |
| Bis (2-ethylhexyl) phthalate | C24H38O4 | 1905 | 0.65 | 0.8 | 1.47 | 0.67 | 1.37 | 1.54 | 1.7 | 3.07 | 3.39 | 1.49 |
| Ethyl 3-(4-hydroxyphenyl)-propenoate | C11H14O3 | 2033 | 1.54 | – | 1.23 | – | 0.84 | – | tr. | – | tr. | – |
| Retinol | C20H30O | 2368 | – | – | – | – | tr. | – | – | 6.28 | – | 8.03 |
| Total | 99.31 | 99.89 | 99.55 | 100 | 99.27 | 100 | 98.34 | 93.08 | 99.1 | 98.18 | ||
aRI, retention indices determined on DB-17MS capillary column. Components are listed in order of elution in DB-17MS column. – not detected, tr: traces amounts were detected
Fig. 2.
Structures of some abundant compounds identified in C. dactylon leaf and rhizome dried under different conditions. (1) Ethyl linoleate (2) Ethyl palmitate (3) Dihomo-γ-linoleic acid (4) 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) (5) 5-Hydroxymethylfurfural (6) Maltol (7) Bis (2-ethylhexyl) phthalate
According to the previous studies, the most important phytochemical components which have been identified in different morphological parts of the C. dactylon, classify under different categories, including tannins (Garjani et al. 2009; Khlifi et al. 2013), saponins (Najafi et al. 2008), flavonoids (Annapurna et al. 2013; Garjani et al. 2009; Karthik and Ravikumar 2011), alkaloids (Bhangale and Acharya 2014), sterols (Annapurna et al. 2013; Garjani et al. 2009) and fatty acids (Annapurna et al. 2013; Mohamed Shabi et al. 2010). In this regard, main components identified by Annapurna et al. (2013) from C. dactylon leaf organic (hexane) extracts were linolenic acid, docosanoic acid ethyl ester, palmitic acid ethyl ester, ethyl eicosanoate and 2,6-dimethoxy phenol. In another report, hydro-alcoholic extract of C. dactlyon was analyzed and 22 compounds were totally identified and ethyl palmitate, d-mannose and ethyl linoleate were the most abundant consitituents. Besides, hydroquinone, furfural and levoglucosenone were found to be the richest constituents among the 20 characterized phytochemicals from phenolic extracts (Mohamed Shabi et al. 2010) of C. dactylon.
Our hydro-ethanolic extracts didn’t include some of above-docking constituents as molecular entities. The range of phytochemical components that could be isolated from each plant material is usually affected by the nature of solvents and their potential to break up the material matrix (Mediani et al. 2015). Alcoholic extracts has been reported to predominantly compose oxygenated hydrocarbons (Mohamed Shabi et al. 2010), while the aqueous phenolic fraction mainly contains flavonoids, glycosides and phenolic hydrocarbons. Beside drying and solvent extraction effects, observed variations between the present study with those reported by different authors may also be attributed to the influence of environmental factors, genetic background and their interactions, which is beyond the scope of this work.
The status of phytochemical components in the analyzed C. dactylon extracts was varied depending on the drying methods. In this respect, the comparison of GC–MS profile of hydro-ethanolic extracts obtained from dried materials showed that drying method not only affected appearance and disappearance of C. dactylon phytochemicals but also it changed the level of some major and minor constituents. Beside ethyl linoleate and ethyl palmitate which were two first principal components of all extracts, the main phytochemicals of the SHD rhizome extract were 5-hydroxymethylfurfural (5-HMF) (27.9%), ethyl oleate (5.48%), linoleic acid (5.09%), DDMP (3.09%), maltol (3.16%), palmitic acid (2.68%) and DGLA (2.35%), while the most abundant components in SHD leaf extract were DGLA (10.63%), linoleic acid (4.16%), ethyl oleate (3.83%) and phytol (3.42%). Among these compounds, DGLA (20:3) is an omega-6 polyunsaturated fatty acid (PUFA) derived from linolenic acid having both anti-inflammatory and anti-proliferative properties in human (Wang et al. 2012). 5-HMF is a six-carbon heterocyclic aldehyde consists of a furan ring, carrying both aldehyde and alcohol functional groups (Kowalski et al. 2013). Based on a recent study, 5-HMF in Cornus officinalis L. is able to prevent cardiovascular and diabetes mellitus diseases (Cao et al. 2013). Besides, nematicidal activity of Melia azedarach fruit’s extract against Meloidogyne incognita has been attributed to 5-HMF (Ntalli and Caboni 2012). DDMP has been also proposed as a strong antioxidant present in medicinal plants (da Silva et al. 2000) and Maillard reaction products (Yu et al. 2013).
The major components of solar dried rhizome’s extract (SOD) were 5-HMF (18%), dihydroxyacetone (9.07%), linoleic acid (6.73%), maltol (6.15%) and ethyl oleate (5.33%). Dihydroxyacetone is a high valued 3-carbon ketose carbohydrate resulted from the oxidation of glycerol, which is extensively used in cosmetic industry as one of the main ingredients of sunless tanning formulations (Satirapipatkul et al. 2017). In plant roots, it plays role as part of an osmotic adjustment mechanism under neutral salt stress (Li et al. 2017). On the other hand, the main constituents of SOD leaf’s extract were DGLA (12.61%), palmitic acid (3.07%), 1,4-Butanediol (2.99%) and DDMP (2.93%). When C.dactylon rhizome was dried with OD30, the main components of extract were 5-HMF (9.07%), linoleic acid (7.68%), palmitic acid (6.02%), ethyl oleate (5.97%), diacetin and DGLA (2.57%), while DGLA (13.13%), linoleic acid (4.6%), ethyl oleate (3.87%), palmitic acid (2.99%), phytol (2.88%) were the principal components of OD30 leaf material’s extract.
When drying performed in oven at 40 °C, it was observed that 5-HMF (7.28%), linoleic acid (5.11%), ethyl oleate (4.8%), DDMP (4.68%), diacetin (4.38%) and maltol (3.16%) are more abundant than other components. In same drying condition, DGLA (10.61%), retinol (6.28%), ethyl oleate (3.9%) and tricyclopentadeca-3,7-dien (3.58%) were present at highest amounts in leaf hydro-alcoholic extracts. Diacetin has been found to be present in the nectar of oil secreting plants’ flower acting as signal for pollinator attraction and recognition of the host by the pollinators (Schaffler et al. 2015).
Finally, with the highest variation in quantity and quality of phytochemicals, the main components of OD50 dried rhizome’s extract were 2,3-dihydrobenzofuran (13.44%), diacetin (8%), 5-HMF (5.12%) and linoleic acid (4.62) and the main isolated constituents from OD50 dried leaf’s extract were DGLA (9.65%), tricyclopentadeca-3,7-dien (9.92%), retinol (8.03%), 1,4-Butanediol (4.42%) and DDMP (3.78%). DDMP is a product of Maillard reaction that take place between carbonyl group of sugars and amino group of proteins and other nitrogenized compounds. Bitter taste of C. dactylon and many plant species is due to the presence of DDMP-containing saponins. Maltol that also has been detected in C. dactylon extracts, can be released from these saponins upon heating (Osbourn et al. 2011). As a major plant-derived aromatic substance, maltol is widely used for spice and food additive, which possess promising anti-oxidative and antiinflammatory capacities as well as anti proliferative activity against cancer cell lines.
It seems that some constituents of C. dactylon were sensitive to high drying temperatures, depending on the applied drying method. For example, 5-hydroxymethylfufural and ethyl linoleate were progressively decreased in higher temperatures. Esparza et al. (2015) observed a significant increase in the content of unsaturated free fatty acids of hydro-alcoholic extracts prepared from oven dried maca (Lepidium meyenii) materials. In the case of fatty acid and their esters, they have a weak thermal stability mainly due to peroxide-mediated autoxidation and photo-oxiadation at high temperatures (Irshad et al. 2015), probably resulted in hydrolysis of reserve and membrane lipids (Esparza et al. 2015). Increasing drying temperature lead to formation of new components such as tricyclopentadeca-3,7-dien, retinol and diacetin, which were absent in shade- and solar-dried materials. This could also be the effect of oxidation reactions, hydrolysis of glycosylated forms, or even the formation of substances resulted from cell walls disruption (Sellami et al. 2011; Wang et al. 2013). The above docking changes in quality and quantity of phytochemical compositions under different drying methods, have been also demonstrated in other medicinal plants such as savory (Ghasemi Pirbalouti et al. 2013), thyme (Rodríguez et al. 2013), lemon balm (Argyropoulos and Müller 2014) and sage (Sellami et al. 2011).
Apart from the effect of temperature, UV irradiation could also be considered as a minor influencing factor on phytochemical properties of dried materials. For example, compounds such as dihydroxyacetone, HMF, furfural, phytol and Ethyl 3-(4-hydroxyphenyl)-propenoate were present at higher concentrations in extract prepared with SHD and SOD. It seems that different wavelengths of sunlight have direct and indirect effects on phytochemicals of SHD and especially SOD dried plant parts. In this regard, it has been reported that UV-B as a small fraction of solar radiation is a powerful elicitor of metabolic responses in plants as a reaction to oxidative stress (Dzakovich et al. 2016).
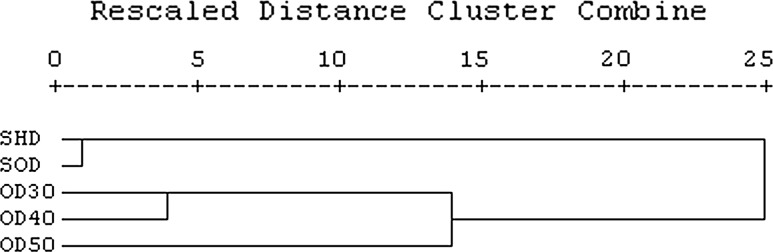
Hierarchical cluster analysis (CA) was carried out to elucidate the relationship among the different drying methods based on their phytochemical profiles (Fig. 3). Obtained dendrogram separated the drying methods into three somewhat well defined groups. First group was represented by SHD and SOD drying methods, which were closed together. Second group was included OD30 and OD40 and finally OD50 founded the last cluster. Result of cluster analysis further confirm that higher temperature of drying significantly affect phytochemical components of C. dactylon, where all oven drying method were closed together showing more similar phytochemical profile different with SOD and SHD drying methods.
Fig. 3.
Dendrogram obtained by hierarchical cluster analysis of the different drying methods in Cynodon dactylon based on phytochemical compound contents. SHD, shade drying; SOD, solar drying; OD30, oven drying at 30 °C; OD40, oven drying at 40 °C; OD50, oven drying at 50 °C
DPPH radical scavenging activity
The effect of drying methods on the in vitro radical scavenging activity of C. dactylon for leaf and rhizome is presented in Table 3. DPPH radical scavenging activity was significantly affected by both drying method and plant part in a dose dependent manner. Extract prepared from SHD rhizome was significantly superior to all of the other extracts, with quenching more than 84% of the DPPH at the concentration of 400 mg/ml (IC50 = 59.12). This was in agreement with results of Albert-Baskar and Ignacimuthu (2010) who obtained an IC50 value of 63.03 for methanolic extract prepared from shade dried C. dactylon rhizome. Hydro-alcoholic extracts obtained from SOD and OD30 rhizome were also very effective, where they could eliminate 79.73 and 74.07% of the DPPH, respectively. On the other hand, extracts from samples dried with OD40 and OD50 methods exhibited relatively poor radical-scavenging capacity. From the results, we can infer that drying temperature is an influential factor influencing in vitro antioxidant activity of C. dactylon leaf and rhizome’s extract. In this way, drying temperature higher than 30 °C could result in a significant decrease of the antioxidant activity. Negative effects of high drying temperatures on in vitro radical scavenging capacity have also been reported in blueberry (Routray et al. 2014), pepper (Kim et al. 2006) and sage (Sellami et al. 2011). Altogether, our findings allowed to suggest that the decrease in the antioxidant activity that was observed with the higher drying temperatures can be associated with degradation of thermo-sensitive phytochemicals having antioxidant activity like HMF, furfural, phytol, dihydroxyacetone, maltol and ethyl 3-(4-hydroxyphenyl)-propenoate. Probably, convective and mass heat transfer generated in oven equipped with fan are other factors that negatively affected the antioxidant activity of extracts mainly by initial enzymatic degradation of antioxidant compounds and thermal degradation of antioxidant. Drop in antioxidant properties of dried plant material imposed by heat transfer in oven drying methods has also been reported in pomegranate (Fazaeli et al. 2013), Chinese chaste tree (Chong and Lim 2012) and spearmint (Orphanides et al. 2013). As shown in Table 2, higher percentage of 5-HMF, DDMP and maltol was found in the extract of SHD, SOD and OD30 plant material, while increasing drying temperature reduced the percentage of these compounds. Antioxidant capacity of 5-HMF (Zhao et al. 2013), DDMP (Hwang et al. 2013; Yu et al. 2013) and maltol (Han et al. 2015) is well-documented. In general, we should consider that the consequences of drying methods on the final antioxidant capacity of medicinal plants are the results of diverse, and sometimes reverse events which can occur sequentially or simultaneously (Sellami et al. 2011).
Table 3.
Antioxidant activities from hydro-alcoholic extract of rhizome and leaf of C. dactylon submitted to different drying methods measured by the DPPH method
| Concentration (mg/ml) | DPPH inhibition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SHD | SOD | OD30 | OD40 | OD50 | ||||||
| Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | Rhizome | Leaf | |
| 25 | 26.2aE ± 0.65 | 18.35cE ± 0.35 | 21.79bE ± 1.50 | 13.22efE ± 0.65 | 18.33cE ± 0.78 | 14.13deE ± 1.71 | 12.15gE ± 0.65 | 10.04ghE ± 1.08 | 15.41dE ± 0.65 | 10.35ghE ± 1.21 |
| 50 | 47.23aD ± 2.64 | 27.39eD ± 0.52 | 44.96cD ± 1.12 | 25.38efD ± 1.06 | 48.17aD ± 0.35 | 25.29efD ± 0.16 | 34.57dD ± 0.54 | 20.13ghD ± 0.70 | 24.48gD ± 2.51 | 17.83ghD ± 1.30 |
| 100 | 67.53aC ± 0.74 | 42.55eC ± 1.90 | 61.48cC ± 1.09 | 41.93efC ± 0.24 | 65.26aC ± 2.76 | 47.17dC ± 0.98 | 46.19dC ± 3.90 | 33.45gC ± 1.04 | 42.12efC ± 0.30 | 28.58hC ± 2.21 |
| 200 | 78.82aB ± 0.94 | 59.92cB ± 1.84 | 68.17bB ± 1.98 | 57.82cdeB ± 0.92 | 68.28bB ± 2.09 | 58.69cdeB ± 0.55 | 58.96 cdB ± 0.63 | 49.99fB ± 2.51 | 48.40fB ± 0.82 | 31.42gB ± 2.45 |
| 400 | 84.13aA ± 1.24 | 68.17dA ± 1.17 | 79.73bA ± 2.24 | 63.06dA ± 0.99 | 74.07cA ± 0.19 | 67.06dA ± 1.01 | 61.39dA ± 1.01 | 53.21fA ± 0.35 | 50.95fA ± 2.00 | 39.15gA ± 0.76 |
| IC50 | 59.12e ± 0.76 | 143.26 cd ± 2.6 | 76.40de ± 1.87 | 169.73c ± 1.65 | 77.39de ± 0.32 | 148.64c ± 2.82 | 154.17c ± 2.63 | 263.20b ± 6.89 | 267.18b ± 11.67 | 792.88a ± 68.45 |
Means with the same capital letter within the same column are not significantly different at the 5% level according to DMRT. Means with the same letter within the same line are not significantly different at the 5% level according to DMRT
Conclusion
It could be concluded that drying C. dactylon leaf and rhizome at 50 °C in oven to reach 10% moisture was ten-time faster than shade-drying method, however high drying temperatures lead to profound changes in the biochemical constituent’s quality and quantity. In some cases, these changes were in favor of improvement in the medicinal value of extracts e.g., elevated levels of DGLA as an anti-inflammatory and anti-proliferative. The increase in rising drying temperature resulted in production of relatively toxic substances such as 2,3-dihydrobenzofuran. All drying procedures (SHD, SOD, OD30, OD40 and OD50) resulted in the formation of high level of free unsaturated fatty acids (FUFA) and their esters may be attributed to lipids hydrolysis due to the effect of endogenous or microbial enzymes or even through non-enzymatic processes such as auto-oxidation and photo-oxidation. The in vitro antioxidant capacity of hydro-alcoholic extract revealed that extracts prepared from SHD, SOD, and OD30 were more efficient to inhibit DPPH stable radicals. These findings suggested that versatile drying methods such as OD30 could be retained not only in terms of drying time reducing but also for the preservation of main phytochemicals as well as antioxidant activity in C. dactylon plant part’s extracts.
Abbreviations
- GC–MS
Gas chromatography–mass spectrophotometry
- DGLA
Dihomo-γ-linoleic acid
- 5-HMF
5-Hydroxy methyl furfural
- DDMP
2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one
- PUFA
Poly-unsaturated fatty acid
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
References
- Albert-Baskar A, Ignacimuthu S. Chemopreventive effect of Cynodon dactylon (L.) Pers. extract against DMH-induced colon carcinogenesis in experimental animals. Exp Toxicol Pathol. 2010;62:423–431. doi: 10.1016/j.etp.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Annapurna HV, et al. Isolation and in silico evaluation of antidiabetic molecules of Cynodon dactylon (L.) J Mol Graph Model. 2013;39:87–97. doi: 10.1016/j.jmgm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Argyropoulos D, Müller J. Kinetics of change in colour and rosmarinic acid equivalents during convective drying of lemon balm (Melissa officinalis L.) JARMAP. 2014;1:e15–e22. [Google Scholar]
- Babu KS, Shaker IA, Kumaraswamy D, Saleembasha S, Sailaja I. Indigenous effect of Cynodon dactylon in experimental induced ulcers and gastric secretions. Int Res J Pharm. 2012;3:301–304. [Google Scholar]
- Bhangale J, Acharya S. Antiarthritic activity of Cynodon dactylon (L.) Pers. Indian J Exp Biol. 2014;52:215–222. [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Cao G, Cai H, Cai B, Tu S. Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013;140:273–279. doi: 10.1016/j.foodchem.2012.11.143. [DOI] [PubMed] [Google Scholar]
- Chen G, Mujumdar AS. Drying of herbal medicines and tea. In: Mujumdar A, editor. Handbook of industrial drying. 4. Buca Raton: Taylor & Francis Group; 2015. pp. 566–580. [Google Scholar]
- Chong KL, Lim YY. Effects of drying on the antioxidant properties of herbal tea from selected vitex species. J Food Qual. 2012;35:51–59. doi: 10.1111/j.1745-4557.2011.00422.x. [DOI] [Google Scholar]
- Cordell GA. Phytochemistry and traditional medicine—the revolution continues. Phytochem Lett. 2014;10:28–40. doi: 10.1016/j.phytol.2014.06.002. [DOI] [Google Scholar]
- da Silva AP, Rocha R, Silva CML, Mira L, Duarte MF, Florencio MH. Antioxidants in medicinal plant extracts. A research study of the antioxidant capacity of Crataegus, Hamamelis and Hydrastis. Phytother Res. 2000;14:612–616. doi: 10.1002/1099-1573(200012)14:8<612::AID-PTR677>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dzakovich MP, Ferruzzi MG, Mitchell CA. Manipulating sensory and phytochemical profiles of greenhouse tomatoes using environmentally relevant doses of ultraviolet radiation. J Agric Food Chem. 2016;64:6801–6808. doi: 10.1021/acs.jafc.6b02983. [DOI] [PubMed] [Google Scholar]
- Esparza E, Hadzich A, Kofer W, Mithofer A, Cosio EG. Bioactive maca (Lepidium meyenii) alkamides are a result of traditional Andean postharvest drying practices. Phytochemistry. 2015;116:138–148. doi: 10.1016/j.phytochem.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Eswara AR, Ramakrishnarao M. Solar energy in food processing—a critical appraisal. J Food Sci Technol. 2013;50:209–227. doi: 10.1007/s13197-012-0739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazaeli M, Yousefi S, Emam-Djomeh Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum L.) and black mulberry juices. Food Res Int. 2013;50:568–573. doi: 10.1016/j.foodres.2011.03.043. [DOI] [Google Scholar]
- Garjani A, Afrooziyan A, Nazemiyeh H, Najafi M, Kharazmkia A, Maleki-Dizaji N. Protective effects of hydroalcoholic extract from rhizomes of Cynodon dactylon (L.) Pers. on compensated right heart failure in rats. BMC Complement Altern Med. 2009;9:28. doi: 10.1186/1472-6882-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi Pirbalouti A, Oraie M, Pouriamehr M, Babadi ES. Effects of drying methods on qualitative and quantitative of the essential oil of Bakhtiari savory (Satureja bachtiarica Bunge.) Ind Crops Prod. 2013;46:324–327. doi: 10.1016/j.indcrop.2013.02.014. [DOI] [Google Scholar]
- Han Y, Xu Q, Hu JN, Han XY, Li W, Zhao LC. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients. 2015;7:682–896. doi: 10.3390/nu7010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IG, Kim HY, Woo KS, Lee SH, Lee J, Jeong HS. Isolation and Identification of the antioxidant DDMP from heated pear (Pyrus pyrifolia Nakai) Prev Nutr Food Sci. 2013;18:76–79. doi: 10.3746/pnf.2013.18.1.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad A, Delor-Jestin F, Chalard P, Verney V. Physico-chemical durability criteria of oils and linked bio-based polymers. OCL. 2015;22:D107. doi: 10.1051/ocl/2014048. [DOI] [Google Scholar]
- Janjai S, Bala BK. Solar drying technology Food Eng Rev. 2011;4:16–54. [Google Scholar]
- Karthik D, Ravikumar S. Proteome and phytochemical analysis of Cynodon dactylon leaves extract and its biological activity in diabetic rats. Biomed Prev Nutr. 2011;1:49–56. doi: 10.1016/j.bionut.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Khlifi D, Hayouni EA, Valentin A, Cazaux S, Moukarzel B, Hamdi M, Bouajila J. LC–MS analysis, anticancer, antioxidant and antimalarial activities of Cynodon dactylon L. extracts. Ind Crops Prod. 2013;45:240–247. doi: 10.1016/j.indcrop.2012.12.030. [DOI] [Google Scholar]
- Kim S, Lee KW, Park J, Lee HJ, Hwang IK. Effect of drying in antioxidant activity and changes of ascorbic acid and colour by different drying and storage in Korean red pepper (Capsicum annuum, L.) Int J Food Sci Technol. 2006;41:90–95. doi: 10.1111/j.1365-2621.2006.01349.x. [DOI] [Google Scholar]
- Kowalski S, Lukasiewicz M, Duda-Chodak A, Zięć G. 5-Hydroxymethyl-2-furfural (HMF)—heat-induced formation, occurrence in food and biotransformation—a review. Pol J Food Nutr Sci. 2013;63:207–225. [Google Scholar]
- Li M, Guo R, Jiao Y, Jin X, Zhang H, Shi L. Comparison of salt tolerance in soja based on metabolomics of seedling. Roots Front Plant Sci. 2017;8:1101. doi: 10.3389/fpls.2017.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafakheri S, Hajivand S, Zarrabi MM, Arvane A. Effect of bio and chemical fertilizers on the essential oil content and constituents of Melissa officinalis (Lemon Balm) J Essent Oil Bear Plants. 2016;19:1277–1285. doi: 10.1080/0972060X.2014.983995. [DOI] [Google Scholar]
- Mangathayaru K, Umadevi M, Reddy CU. Evaluation of the immunomodulatory and DNA protective activities of the shoots of Cynodon dactylon. J Ethnopharmacol. 2009;123:181–184. doi: 10.1016/j.jep.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Mediani A, et al. Relationship between metabolites composition and biological activities of Phyllanthus niruri extracts prepared by different drying methods and solvents extraction. Plant Foods Hum Nutr. 2015;70:184–192. doi: 10.1007/s11130-015-0478-5. [DOI] [PubMed] [Google Scholar]
- Mohamed Shabi M, Gayathri K, Venkatalakshmi Sasikala C. Chemical constituents of hydro alcoholic extract and phenolic fraction of Cynodon dactylon. Int J Chem Technol Res. 2010;2:149–154. [Google Scholar]
- Mozafari AA, Vafaee Y, Karami E. In vitro propagation and conservation of Satureja avromanica Maroofi-an indigenous threatened medicinal plant of Iran. Physiol Mol Biol Plants. 2015;21:433–439. doi: 10.1007/s12298-015-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Ghavimi H, Gharakhani A, Garjani A. Effects of hydroalcoholic extract of Cynodon dactylon (L.) pers. on schemia/reperfusion induced arrhythmias. DARU J Pharm Sci. 2008;16:233–238. [Google Scholar]
- Nejad Ebrahimi S, Hadian J, Mirjalili MH, Sonboli A, Yousefzadi M. Essential oil composition and antibacterial activity of Thymus caramanicus at different phenological stages. Food Chem. 2008;110:927–931. doi: 10.1016/j.foodchem.2008.02.083. [DOI] [PubMed] [Google Scholar]
- Ntalli NG, Caboni P. Botanical nematicides in the mediterranean basin. Phytochem Rev. 2012;11:351–359. doi: 10.1007/s11101-012-9254-4. [DOI] [Google Scholar]
- Orphanides A, Goulas V, Gekas V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech J Food Sci. 2013;31:509–513. doi: 10.17221/526/2012-CJFS. [DOI] [Google Scholar]
- Osbourn A, Goss RJ, Field RA. The saponins: polar isoprenoids with important and diverse biological activities. Nat Prod Rep. 2011;28:1261–1268. doi: 10.1039/c1np00015b. [DOI] [PubMed] [Google Scholar]
- Rahimmalek M, Mirzakhani M, Pirbalouti AG. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations collected from different geographical regions in Iran. Ind Crops Prod. 2013;51:328–333. doi: 10.1016/j.indcrop.2013.09.010. [DOI] [Google Scholar]
- Rodríguez J, Ortuño C, Benedito J, Bon J. Optimization of the antioxidant capacity of thyme (Thymus vulgaris L.) extracts: management of the drying process. Ind Crops Prod. 2013;46:258–263. doi: 10.1016/j.indcrop.2013.02.002. [DOI] [Google Scholar]
- Routray W, Orsat V, Gariepy Y. Effect of different drying methods on the microwave extraction of phenolic components and antioxidant activity of highbush blueberry leaves. Drying Technol. 2014;32:1888–1904. doi: 10.1080/07373937.2014.919002. [DOI] [Google Scholar]
- Sadki C, Hacht B, Souliman A, Atmani F. Acute diuretic activity of aqueous Erica multiflora flowers and Cynodon dactylon rhizomes extracts in rats. J Ethnopharmacol. 2010;128:352–356. doi: 10.1016/j.jep.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Satirapipatkul C, Pungrasmi W, Nootong K. Production of 1,3-Dihydroxyacetone by Gluconobacter nephelii in upflow aerated bioreactors with agro-industrial wastes as external nitrogen source. Eng J. 2017;21:81–92. doi: 10.4186/ej.2017.21.5.81. [DOI] [Google Scholar]
- Schaffler I, et al. Diacetin, a reliable cue and private communication channel in a specialized pollination system. Sci Rep. 2015;5:12779. doi: 10.1038/srep12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami IH, Rebey IB, Sriti J, Rahali FZ, Limam F, Marzouk B. Drying sage (Salvia officinalis L.) plants and its effects on content, chemical composition, and radical scavenging activity of the essential oil. Food Bioprocess Technol. 2011;5:2978–2989. doi: 10.1007/s11947-011-0661-0. [DOI] [Google Scholar]
- Wang B, Deng J, Gao Y, Zhu L, He R, Xu Y. The screening toolbox of bioactive substances from natural products: a review. Fitoterapia. 2011;82:1141–1151. doi: 10.1016/j.fitote.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chantreau M, Sibout R, Hawkins S. Plant cell wall lignification and monolignol metabolism. Front Plant Sci. 2013;4:1–14. doi: 10.3389/fpls.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zhao M, Liu F, Zeng S, Hu J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Res Int. 2013;51:397–403. doi: 10.1016/j.foodres.2012.12.044. [DOI] [Google Scholar]
- Zhao L, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61:10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- Zubair M, Nybom H, Lindholm C, Rumpunen K. Major polyphenols in aerial organs of greater plantain (Plantago major L.), and effects of drying temperature on polyphenol contents in the leaves. Sci Hortic. 2011;128:523–529. doi: 10.1016/j.scienta.2011.03.001. [DOI] [Google Scholar]