Abstract
Response surface methodology was used to optimize processing variable for ultrasound-assisted modification of whey protein. The process was optimized employing Box–Behnken Design with three independent variables i.e. amplitude (20–40%), time (10–20 min) and concentration (10–15%). A second order model was employed to generate response surfaces. Experimental results revealed that analyzed model solutions exhibited the significant influence on various responses signified that the applied statistical model fitted well. The optimized independent variables were found to be 19.77 min time, 20.02% amplitude and 12.78% concentration of feed. The modified whey protein had the solubility, 78.52%; heat stability, 1076.19 s; water solubility index, 92.30%; water holding capacity, 0.469; oil absorption capacity, 1.709; foaming capacity 92.27; foam stability, 27.71 and firmness, 1692.09 g. Analytical response revealed that solubility of modified whey protein exhibited significant positive correlation with water solubility index, emulsion stability index and firmness.
Keywords: Ultrasound, Whey protein, Modification, Solubility, Response surface methodology
Introduction
Whey protein is a group of milk protein, secluded from whey (well-known by-product of dairy industry), having globular conformations and is well recognized for its nutritional, functional and therapeutic properties (Pihlanto and Korhonen 2003). It has a wide range of functional applications in pharmaceutical and food industries, predominantly owing to its surface properties, amphiphilic nature and molecular conformations, and abilities to form gel, foam and emulsion (Kinsella et al. 1994; Zayas 1997a, b). Moreover, interactions of protein with fat, carbohydrate and protein at the interface also lead to various functional properties. Whey protein also provides an excellent substrate of choice, for combating the protein deficiency, as a promising functional food ingredient. However, technological challenges like heat sensitivity and reduced solubility jeopardise functional properties of whey protein like water binding, foaming, gelation and emulsification, limiting its potential application in food industry with sustained health benefits. Additionally, lack of textural and sensory properties and increased hardness over time in high protein products adds to challenges of its utilization. Therefore, food scientists and concerned industry consider it immense area of interest and investigating novel methodologies for modification and improvement of the functional properties of whey proteins for more practical applications. Additionally, increasing consumers demand and awareness for high quality food also put pressure on academia and industry for the development of new, safe and effective methods of food processing and preservation.
In recent years, ultrasound has been evaluated to tailor the functional properties of food molecules and are generally considered as safe, non toxic, and environment friendly technique (Jambrak et al. 2008, 2009, 2010, 2014; Kentish and Ashokkumar 2011; Arzeni et al. 2012; O’Sullivan et al. 2014; Gani et al. 2016; Ren et al. 2016; Zou et al. 2017, Singh et al. 2017, Tavares et al. 2017). The principle for tailoring molecules involves cavitation, heating, dynamic agitation, shear stress and turbulence. It is produced by enhanced localized pressure (up to 50 MPa) and elevated temperature (up to 5000 °C) along with high shear forces and turbulence, occurring in very short periods of time (O’Donnell et al. 2010; Soria and Villamiel 2010).
Response surface methodology (RSM) is a good statistical and mathematical tool for optimization of product and processing parameters in food and dairy industry. It also helps academia and industry for designing the model and for analyzing the interaction of independent and dependent variables. Several researchers have used RSM to forecast optimum conditions (Pires and da Silva Pena 2017; Shirzad et al. 2017; Thakur et al. 2017; Wagh and Chatli 2017) but, to the best of author’s knowledge the process optimization for ultrasound assisted modification of whey protein using RSM has not been so much explored. Thus, present study was carried out for optimization of processing time, amplitude and concentration for ultrasound assisted modification of whey protein and to evaluate the effect of ultrasonication on functional properties of whey protein.
Materials and methods
Raw materials
Whey protein concentrate (WPC-80 from Fontera, New Zealand) was supplied by Sindhwani Traders India, New Delhi. All chemicals were of analytical grade and purchased from Sisco Research Laboratories Pvt. Ltd. (SRL), Maharashtra, India. Millipore (Milli-Q) water was used for sample preparation and further analysis.
Preparation of whey protein dispersion
The whey protein concentrate (WPC) dispersion was prepared in luke warm Milli-Q water. The dispersion was properly dispersed with glass rod with gentle stirring. After the mixture was thoroughly hydrated, the dispersion was allowed to stand at room temperature for 30 min. Just before ultrasound treatment, the dispersion was treated with T 25 digital ultra turrax (IKA India Private Limited, Kengeri, Bengaluru, Karnataka, India) for 5 min at medium velocity (5000 rpm) in chilled water bath to control the temperature of the dispersion.
Ultrasonication
WPC dispersions were sonicated for desired time using Sonics ultrasonic processor (Sonics: model VCX 750, Vibra Cell Sonics, Sonics & Materials Inc, USA) at a frequency of 20 kHz and at variable amplitudes of 20–40%, as per the designed model. A 13 mm (1/2 inch) high-grade titanium alloy probe was used to sonicate 200 ml of solution. Samples contained into glass beaker were immersed into ice water bath to kept temperature below 29 °C during processing. Ultrasonicated whey protein dispersion were spray dried after processing without further storage.
Spray drying
Sonicated whey protein solution was then spray dried to get 4–5% moisture in dried product using a mini spray dryer B-290 (BUCHI India Pvt Ltd., Mumbai, India) having 0.6 kg/h feed rate and 1 kg/h water evaporation capacity. The inlet and outlet air temperature was kept 185 ± 2 °C and 85 ± 2 °C, respectively, for spray drying process. Dried modified whey protein concentrate (m-WPC) was stored at − 20 °C for further analysis.
Analytical methods
The solubility of m-WPC samples were analyzed as per the method described by Jambrak et al. (2008). Heat stability mentioned as heat coagulation time (HCT) of m-WPC was analyzed as per the procedure described by Khatkar et al. (2014) using oil bath (MW-276 TAF, Macro Scientific Works Pvt. Ltd., Delhi, India). Water holding capacity (WHC) and water solubility index (WSI) of m-WPC were determined as described by Beuchat (1977). Oil holding capacity (OHC) of m-WPC was measured as volume of edible oil held by 1000 mg of WPC as described by Haque and Mozaffar (1992). Emulsion stability index (ESI) of m-WPC were determined according to the method of Tang et al. (2005). Foaming capacity (FC) and foaming stability (FS) were determined according to the method of Coffmann and Garcia (1977).
For the estimation of firmness of heat set gel of m-WPC, the gel was prepared using a method described by Onwulata and Tomasula (2004) and firmness (g) was measured using texture analyzer (TA-XT2 plus, Stable Micro Systems Ltd., Surrey UK) at room temperature. The gel (2.8 cm in height and 4 cm in diameters) was carefully removed from the glass beaker and compressed (to 50% strain) by SMS P/75 probe with the crosshead speed of 1 mm/s. The obtained force–time curves were analyzed by the software supplied with instrument.
Experimental design
RSM was used to optimize the processing parameters for ultrasound-assisted modification of WPC and to examine the effect and correlation of the independent variables on dependent responses. The Box–Behnken design by applying quadratic model with three independent variables i.e. processing time (X1: 10–20 min), amplitude (X2: 20–40%) and concentration of solution (X3: 10–15%) was employed. The solubility, heat stability, water solubility index, water holding capacity, oil holding capacity, emulsion stability index, foaming capacity, foam stability and firmness were the dependent variables/responses.
Numerical optimization of process and statistical analysis
In order to obtain narrower and more effective lower and upper limits of independent variables, preliminary trials were conducted. The experimental data obtained for different responses were evaluated using Design Expert Software and significance of model, lack-of fit, coefficient of determination (R2 and R2 adj) and residual analysis were also evaluated. Second order quadratic polynomial equation (Eq. 1) was fitted to experimental data of all responses for final prediction.
| 1 |
where β0 is a constant coefficient and β1, β2, β3; β12, β13, β23 and β11, β22, β33 are the regression coefficient at linear, interaction and quadratic levels, respectively. X1, X2, X3, were the independent variables and Y was the dependent response. For every response, a polynomial equation was obtained and the variables significant at P < 0.01 and P < 0.05 levels were considered for the construction of final model. The variables effect on individual response was also described at linear, interactive and quadratic level. Analysis of variance (ANOVA) was used to examine the statistical significance of the terms in regression equation for each response. SPSS 20 (SPPSS Inc. Chicago, IL, USA) was used to obtain the Pearson’s correlation matrix of responses in order to examine correlation coefficients between parameters.
Results and discussion
Model fitting and validation
The outline of Box–Behnken design with variables in un-coded forms and mean response values of all the functional properties are presented in Table 1. It was observed that during model evaluation there were no aliases found for Quadratic Model. For model evaluation, degree of freedom were 9 for model, 7 for residuals, 3 for lack of fit, 4 for pure error and 16 Corr Total. The degree of freedom for evaluation of lack of fit is recommended minimum 3 and 4 for pure error and in our selected model, values are much relevant to the recommended values signified that this ensures a valid lack of fit test because fewer df leads to a test that may not detect lack of fit.
Table 1.
Outline of Box–Behnken experimental design with variables in their un-coded forms and dependent responses
| Std. | Run | Time (min) | Amplitude (%) | Concentration (%) | Solubility (%) | Heat stability (s) | Water solubility index (%) | Water holding capacity (g/g) | Oil holding capacity (g/g) | Emulsion stability index (min) | Foaming capacity (%) | Foam stability (%) | Firmness (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 1 | 15 | 30 | 12.5 | 72.99 | 1028.6 | 90.23 | 0.482 | 1.737 | 22.789 | 112 | 40 | 1478 |
| 14 | 2 | 15 | 30 | 12.5 | 73.54 | 1029.6 | 90.17 | 0.479 | 1.739 | 22.875 | 114 | 34 | 1474 |
| 7 | 3 | 10 | 30 | 15 | 77.02 | 961.2 | 90.66 | 0.569 | 1.676 | 66.501 | 104 | 18 | 1584 |
| 12 | 4 | 15 | 40 | 15 | 78.56 | 1101.3 | 89.88 | 0.540 | 1.495 | 47.058 | 116 | 38 | 1588 |
| 3 | 5 | 10 | 40 | 12.5 | 79.03 | 1056.4 | 91.16 | 0.450 | 1.608 | 33.684 | 94 | 19 | 1501 |
| 6 | 6 | 20 | 30 | 10 | 79.09 | 944.0 | 90.81 | 0.466 | 1.760 | 45.192 | 110 | 18 | 1656 |
| 15 | 7 | 15 | 30 | 12.5 | 72.88 | 1031.6 | 90.22 | 0.488 | 1.736 | 22.881 | 110 | 34 | 1476 |
| 8 | 8 | 20 | 30 | 15 | 78.45 | 1046.0 | 91.62 | 0.496 | 1.599 | 16.535 | 100 | 40 | 1716 |
| 10 | 9 | 15 | 40 | 10 | 74.52 | 1053.2 | 89.92 | 0.491 | 1.613 | 17.979 | 112 | 30 | 1569 |
| 13 | 10 | 15 | 30 | 12.5 | 73.54 | 1027.4 | 90.13 | 0.478 | 1.732 | 22.772 | 112 | 36 | 1471 |
| 1 | 11 | 10 | 20 | 12.5 | 78.61 | 1039.0 | 91.05 | 0.548 | 1.677 | 26.557 | 84 | 10 | 1608 |
| 16 | 12 | 15 | 30 | 12.5 | 72.75 | 1029.9 | 90.18 | 0.484 | 1.740 | 22.898 | 108 | 38 | 1451 |
| 11 | 13 | 15 | 20 | 15 | 71.80 | 1089.6 | 91.10 | 0.575 | 1.651 | 20.171 | 100 | 40 | 1645 |
| 2 | 14 | 20 | 20 | 12.5 | 79.35 | 1068.0 | 92.31 | 0.471 | 1.715 | 14.851 | 92 | 24 | 1703 |
| 5 | 15 | 10 | 30 | 10 | 78.06 | 910.0 | 90.74 | 0.484 | 1.667 | 16.814 | 90 | 26 | 1517 |
| 4 | 16 | 20 | 40 | 12.5 | 78.97 | 1146.8 | 91.00 | 0.452 | 1.590 | 23.753 | 102 | 16 | 1719 |
| 9 | 17 | 15 | 20 | 10 | 78.94 | 968.0 | 90.33 | 0.542 | 1.658 | 28.197 | 110 | 37 | 1612 |
After evaluation of signal/noise ratio, it was observed that the values for standard errors were found similar within type of coefficient (for A, B, C—0.35; for AB, AC, BC—0.40 and for AA, BB, CC—0.49) and even less than or equal to 0.50 meant that the selected model is better. Similarly, values for VIF (variance inflation) was one or very close to one (1.01), which depicted that selection was excellent, and also indicating that coefficients were affluently estimated due to nil multicollinearity. Additionally, the value for Ri-squared was 0.0 for each coefficient and fall under ideal conditions, because high Ri-squared means terms are correlated with each other, possibly leading to poor models.
Furthermore, the model was also evaluated using precision-based metrics via fraction of design space (FDS) statistics and it was observed that FDS score was greater than recommended score (at least 0.8 or 80%) meant that the selected model was good for exploration and optimization. The FDS score for applied model was observed 100% (meant Score 1), which is highly desirable for stability and robustness testing such as demonstrating the design space for quality by design work.
For final mathematical confirmation of design’ fitting and validation, the leverage; condition number of coefficient matrix; maximum variance mean; average variance mean; minimum variance mean; G efficiency; Scaled D-optimality criterion; the determinant of (X’X)−1; trace of (X’X)−1 and I cuboidal of the experimental design were also calculated and observed to be 0.5882; 1.176; 1.363; 0.342; 0.191; 43.2%; 2.939; 2.384E−8; 2.038 and 0.34250, respectively. All the observed values were found to be within excellent zone, hence confirmed the fitting and validation of design.
The predictive regression models designed for independent (X) and dependent (Y) variables in terms of functional properties of m-WPC are presented in Eqs. 2–10.
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
Coefficient of equation and the results of ANOVA performed on the models to assess the significance at linear, interaction and quadratic level of the independent variables on dependent responses are given in Tables 2. The “Model F-value” of all the responses implies that the model is highly significant (P < 0.01) and the “Lack of Fit F-value” of responses implies that the lack of fit is not significant relative to the pure error, which is desirable (Table 2). The “Pred R-Squared” of responses are in reasonable agreement with the “Adj R-Squared”. On the basis of analysis of variance, the conclusion is that the selected model can be used to navigate the design space.
Table 2.
Estimated regression coefficients for response variables
| Particulars | Solubility | Heat stability | Water solubility index | Water holding capacity | Oil holding capacity | Emulsion stability index | Foaming capacity | Foam stability | Firmness |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 73.141 | 1029.42 | 90.189 | 0.482 | 1.737 | 22.843 | 111.200 | 36.400 | 1470 |
| Linear | |||||||||
| Time: A | 0.391NS | 29.775** | 0.266** | − 0.021** | 0.004* | − 5.403** | 4** | 3.125** | 73** |
| Amplitude: B | 0.299NS | 24.137** | − 0.352** | − 0.025** | − 0.049** | 4.087** | 4.75** | − 1.000NS | − 23.875** |
| Concentration: C | − 0.597* | 40.363** | 0.181** | 0.025** | − 0.035** | 5.260** | − 0.25NS | 3.125** | 22.375** |
| Interaction | |||||||||
| AB | − 0.198NS | 15.35** | − 0.355** | 0.02** | − 0.014** | 0.444** | 0.000NS | − 4.250** | 30.750** |
| AC | 0.1NS | 12.7** | 0.224** | − 0.014** | − 0.042** | − 19.586** | − 6.0** | 7.500** | − 1.750NS |
| BC | 2.796** | − 18.375** | − 0.206** | 0.004NS | − 0.028** | 9.276** | 3.5* | 1.250NS | − 3.500NS |
| Quadratic | |||||||||
| A2 | 4.026** | − 19.798** | 0.921** | − 0.018** | − 0.009** | 4.889** | − 13.350** | − 14.950** | 88.75** |
| B2 | 1.825** | 67.927** | 0.270** | 0.015** | − 0.080** | − 3.021** | − 4.850** | − 4.200** | 74** |
| C2 | 0.988** | − 44.323** | − 0.151** | 0.039** | − 0.052** | 8.529** | 3.150* | 4.050* | 59.500** |
| R2 | 0.986 | 0.999 | 0.995 | 0.985 | 0.998 | 1.000 | 0.976 | 0.974 | 0.990 |
| Model F value | 53.87** | 984.94** | 163.133** | 51.902** | 479.554** | 34,837.840** | 32.261** | 28.767** | 73.95** |
| Lack of fit F value | 3.15NS | 4.66NS | 4.615NS | 5.774NS | 3.752NS | 5.073NS | 0.833NS | 0.723NS | 2.60NS |
| Adj R− squared | 0.967 | 0.998 | 0.989 | 0.966 | 0.996 | 0.9999 | 0.946 | 0.940 | 0.976 |
| Pred R-squared | 0.833 | 0.990 | 0.939 | 0.804 | 0.980 | 0.9997 | 0.832 | 0.825 | 0.884 |
| Adiquate precision | 18.23 | 124.39 | 46.664 | 23.251 | 79.145 | 699.373 | 19.729 | 15.312 | 22.28 |
A Time, B Amplitude, C Concentration
* and ** significant at 5 and 1% level of probability, respectively
Solubility
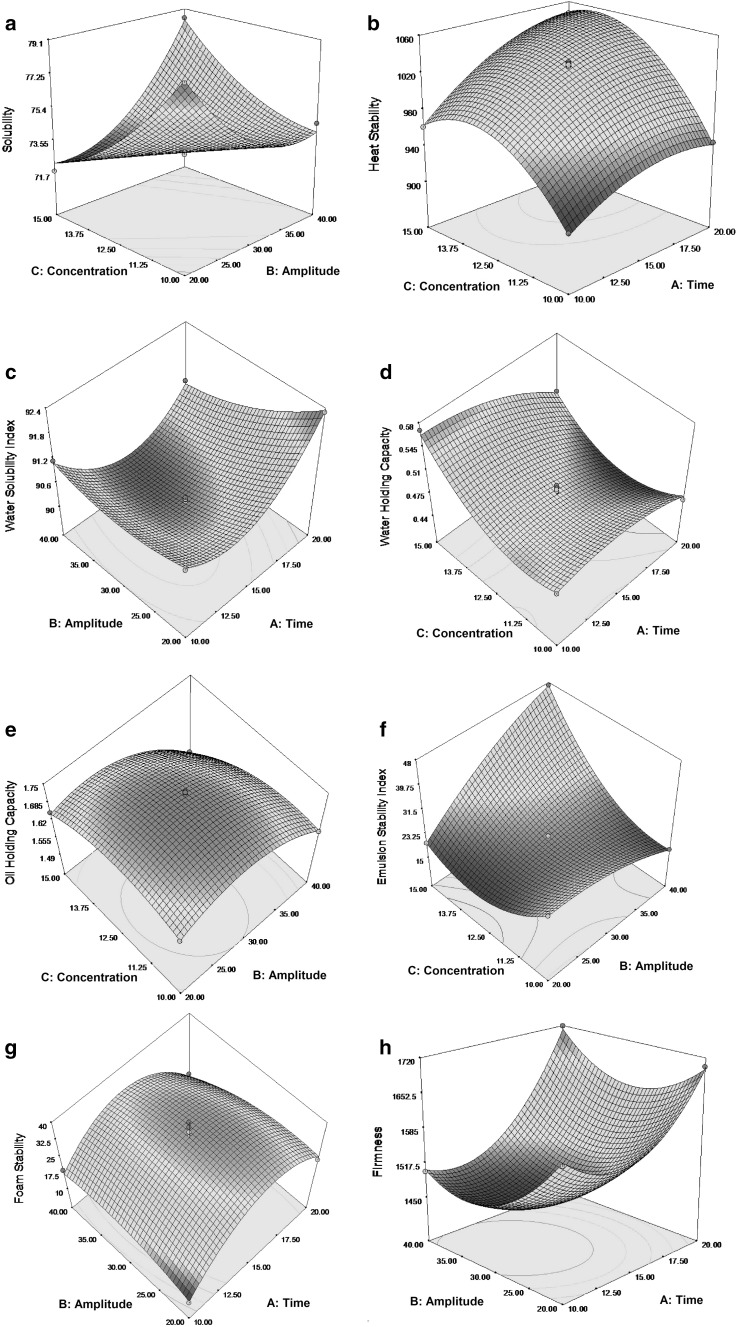
Solubility of a food protein is the most prominant functional property that directly related to protein denaturation and aggregation and thus, an excellent key for improved protein functionality (Arzeni et al. 2012). The solubility of m-WPC varied between 71.79 to 79.35% (Table 1). In this case C, BC, A2, B2, C2 are significant model terms. The interaction of amplitude with concentration showed highly significant (P < 0.01) positive effect on the solubility of m-WPC and their response surface curve (Fig. 1a) also exhibited that increasing level of both these variables resulted in increasing of solubility score. Time, amplitude and concentration at quadratic level had highly significant (P < 0.01) positive correlation with solubility of m-WPC signified that by either increasing or decreasing their levels, the solubility of m-WPC increased. The linear levels of concentration had a significant (P < 0.05) negative correlation with solubility (Table 2), thus decreased the solubility of m-WPC as concentration level was increased (Fig. 1a). The ultrasonic treatment improves protein solubility by modifying protein confirmation and structure so that hidden hydrophillic portions of amino acids are exposed toward water (Jambrak et al. 2009). It could be due to massive amount of cavitation bubbles which resulted in higher localized pressure and temperature that ultimately decreased the particle size and molecular weight of protein (Tang et al. 2009). Overall, this increases the interaction of protein to water which improves solubility of protein (Arzeni et al. 2012; Hu et al. 2013).
Fig. 1.
The 3D response surface curve relating to a solubility influenced by concentration and amplitude; b heat stability influenced by concentration and time; c water solubility index influenced by amplitude and time; d water holding capacity influenced by concentration and time. e Oil holding capacity influenced by concentration and amplitude; f emulsion stability index influenced by concentration and amplitude; g foam stability influenced by amplitude and time; h firmness influenced by amplitude and time
Jambrak et al. (2009) and Arzeni et al. (2012) obtained similar results for solubility of WPC and soy protein concentrate, respectively, and observed that ultrasonication using 20 kHz probe resulted in the largest increase in protein solubility (P < 0.01) of whey and soy protein model system.
Heat stability
The heat stability of m-WPC varied between 910 to 1146 s (Table 1). In this case A, B, C, AB, AC, BC, A2, B2, C2 are significant (P < 0.01) model terms (Table 2). The partial coefficient of regression model exhibited that the linear levels of time, amplitude and concentration had a highly significant (P < 0.01) positive correlation with heat stability (Table 2) of m-WPC and increased the heat stability of m-WPC as their level was increased. The quadratic effect of amplitude had highly significant (P < 0.01) positive correlation signified that by either increasing or decreasing the stage of amplitude, the heat stability of m-WPC increased (Table 2).
The interaction of time with amplitude (Table 2) and time with concentration (Fig. 1b) exhibited highly significant (P < 0.01) positive effect on the heat stability of m-WPC depicted that with increasing levels of these variables, the heat stability improved. On the other side, the interaction of amplitude with concentration imparted highly significant (P < 0.01) negative correlation signified that increasing level of both these variables resulted in lowering of heat stability. At quadratic level, the time and concentration affected highly significant (P < 0.01) the heat stability of WPC, but the negative sign revealed that a low or high level of time and concentration decreased the heat stability of m-WPC (Table 2). Ultrasound treated protein mostly do not have active site like free thiol groups to propagate aggregation, consequently it delay heat coagulation. On the other hand, localized elevated pressure also has disruptive action on intermolecular hydrophobic and electrostatics bonding and ultimately proceeds to realignment of intra as well as inter molecular bonds within the modified protein (Bouaouina et al. 2006). Therefore, ultrasonication may have directly or indirectly decreased the availability of reactive sites, those responsible for enhanced coagulations, and consequently increasing the heat stability.
Water solubility index (WSI)
The WSI of m-WPC varied between 89.97 to 92.3 (Table 1). In this case A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model exhibited that the linear levels of time and concentration; the quadratic effect of time and amplitude; and the interaction of time with concentration had highly significant (P < 0.01) positive correlation with WSI (Table 2) signified that by either increasing or decreasing their levels, the WSI of m-WPC increased. On other side, linear coefficient of amplitude; quadratic terms of concentration; and the interaction of time with amplitude (Fig. 1c), and amplitude with concentration (Table 2) exhibited highly significant (P < 0.01) negative effect on the WSI of m-WPC, signified that by increasing the level of these variables, WSI decreased.
Water holding capacity (WHC)
The WHC of m-WPC varied between 0.449 to 0.575 (Table 1). In this case A, B, C, AB, AC, A2, B2, C2 are significant model terms (Table 2). The model pointed out that the concentration exerted the highly significant positive effect (P < 0.01) on WHC of m-WPC at the linear level (Table 2). The quadratic effect of amplitude and concentration; and interaction of time with amplitude had highly significant (P < 0.01) positive correlation with WHC of m-WPC signified that by either increasing or decreasing their levels, the WHC of m-WPC increased. The interaction of time and concentration showed highly significant (P < 0.01) negative effect on WHC of m-WPC. The response surface curve (Fig. 1d) revealed that by increasing the level of these variables, the WHC of m-WPC decreased. The linear coefficient of time and amplitude; and time alone at quadratic level had highly significant (P < 0.01) negative correlation with WHC of m-WPC (Table 2). Riener et al. (2009) also stated that sonication increased the WHC almost twice in yoghurt model.
Oil holding capacity (OHC)
The OHC of m-WPC varied between 1.49 to 1.76 (Table 1). In this case A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model exhibited that at linear levels, the time had significant (P < 0.05) positive effect on OHC of m-WPC (Table 2, Fig. 1e) and increased the OHC of m-WPC as their level was increased, while the linear coefficient of amplitude and concentration had highly significant (P < 0.01) negative correlation with OHC of m-WPC. The quadratic response of time, amplitude and concentration affected the OHC of m-WPC significantly at higher level (P < 0.01) with negative response signified that with increasing and decreasing these variables, the OHC of m-WPC decreased. The interaction of all the variables exhibited highly significant (P < 0.01) negative effect on OHC of m-WPC (Table 2). Similar results for OHC of m-WPC was also reported by Liu et al. (2012).
Emulsion stability index (ESI)
The ESI of m-WPC varied between 14.85 to 66.50 (Table 1). In this case A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model signified that the amplitude and concentration imparted the most significant (P < 0.01) positive effect on ESI of m-WPC at the linear level (Table 2), while the linear effect of time exhibited significant (P < 0.01) negative response. The highly significant (P < 0.01) positive interaction behaviour of amplitude with time, and amplitude with concentration (Fig. 1f) signified that the increasing level of these variables resulted in increasing of ESI of m-WPC. While, the interaction of time with concentration imparted highly significant (P < 0.01) negative effect and overall decreased the ESI of m-WPC with increasing their levels. The quadratic effect of time and concentration also had highly significant (P < 0.01) positive correlation with ESI of m-WPC but, the quadratic effect of amplitude influenced highly significant (P < 0.01) the ESI of m-WPC with negative response (Table 2) meant that the low or high level of amplitude decreased the ESI of m-WPC. Ultrasonication increased emulsion properties by scattering of micro-aggregates and exposing concealed hydrophobic groups, thus making them available towards oil medium. Furthermore, the soluble aggregates generated by ultrasonication are likely to be adsorbed with greater potential at the oil–water interface and results in better adsorption capability of modified protein. Finally, it could be justified by improved proteins orientation due to turbulent behaviour and incorporation of oil bubbles in oil and modified protein emulsion (Jambrak et al. 2009).
Foaming capacity (FC)
FC of m-WPC varied between 84 to 116 (Table 1). In this case A, B, AC, BC, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model signified that time and amplitude at linear levels had a highly significant (P < 0.01) positive effect on FC of m-WPC (Table 2). The quadratic effect of concentration had significant (P < 0.05) positive correlation with FC of m-WPC, while time and amplitude at quadratic level exhibited negative response significantly (P < 0.01) for FC of m-WPC, meant that time and amplitude at low or high level, decreased the FC of m-WPC (Table 2). The interaction of amplitude and concentration also exhibited significant (P < 0.05) positive effect on FC of m-WPC (Table 2) signified that increasing level of these variables resulted in higher FC but, the interaction of time and concentration showed highly significant (P < 0.01) negative effect on FC of m-WPC.
Foaming stability (FS)
The FS of m-WPC varied between 10 to 40 (Table 1). In this case A, C, AB, AC, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model signified that time and concentration (P < 0.01) at linear levels had a significant positive effect on FS of m-WPC. The interaction of time and concentration also showed highly significant (P < 0.01) positive effect on FS of m-WPC, while the interaction of time and amplitude (Fig. 1g) exhibited highly significant (P < 0.01) negative effect on FS of m-WPC. The quadratic response of concentration had significant (P < 0.05) positive correlation with FS but, the quadratic response of time and amplitude affected the FS of m-WPC significantly at higher level (P < 0.01), but with negative response.
Earlier, Jambrak et al. (2008) also reported that ultrasound treatment for 15 min improved significantly the foaming capacity and stability. The increase in foaming properties might be supported by the fact that ultrasonication imparted microparticulation of whey protein and intensive mechanical homogenization, which usually scatters the protein and fat particles more consistently and ultimately increase the foaming property. Additionally, unfolding in structure of whey protein and exposure of more hydrophilic bonds to water, which enhances adsorption on interface of air/water also tended to increase the foaming properties (Turgeon et al. 1992). Schmidt et al. (1984) also reported that the concentration of protein dispersion is also responsible for the foaming abilities of WPC.
Firmness
The firmness of m-WPC varied between 1451 to 1719 (Table 1). In this case A, B, C, AB, A2, B2, C2 are significant model terms (Table 2). The partial coefficient of regression model indicated that the linear coefficient of time and concentration, and the quadratic response of time, amplitude and concentration exerted the most significant positive effect (P < 0.01) on the firmness of m-WPC (Table 2) signified that by either increasing or decreasing their levels, the firmness of m-WPC increased. The interaction of time with amplitude showed highly significant (P < 0.01) positive effect on the firmness of m-WPC and their response surface curve (Fig. 1h) revealed that by increasing the levels of these variables, the firmness score increased. The linear coefficient of amplitude had highly significant (P < 0.01) negative correlation with firmness score signified that by increasing the level of amplitude alone, the firmness score of m-WPC decreased (Fig. 1h). The similar results and direct correlation of time and concentration of solution on gel strength of sonicated WPC was also reported by Zisu et al. (2011). The improved gelling abilities of m-WPC are believed to be correlated with enhanced solubility as compared to direct manipulation of disulphide interactions (Zisu et al. 2011). The sonication resulted in increased firmness or gel strength with enhanced water holding properties.
Numerical process optimization
The selected responses, which were well fitted based on model adequacy and significance, were optimized. The independent variable’s levels were optimized for an individual dependent response along with their combined responses to obtain realistic optimum levels. For synchronized numerical optimization of independent and dependent variables, the independent variables time, amplitude and concentration and the dependent variables solubility, heat stability, WSI, WHC, OHC, FC, FS and firmness score were all set within range with setting goal importance of 3, which indicated that the each variable is considered equally important. The optimum independent variables were found to be 19.77 min process time, 20.02% amplitude and 12.78% concentration of feed which produced m-WPC with 78.52% solubility, 1076.19 s heat stability, 92.30 WSI, 0.469 WHC, 1.709 OHC, 92.27 FC, 27.71 FS and 1692.09 g firmness, with a desirability level of 1. These results signified that optimum values could be obtained in the ultrasound treatment of WPC that can satisfy the requirements of ultrasound assisted modification of whey protein with improved functional properties.
Correlation between the responses
Statistical analysis revealed that the solubility exhibited significant (P < 0.05) positive correlation with water solubility index and emulsion stability index, which is a direct indication of improved functional properties of m-WPC. Water solubility index was negatively correlated with water holding capacity (P < 0.05). Oil absorption capacity imparted significant (P < 0.05) negative correlation with solubility, heat stability, WHC and firmness. Firmness exhibited highly significant (P < 0.01) positive correlation with solubility, WSI, WHC and ESI, while firmness exhibited highly significant (P < 0.01) negative correlation with FS. FC exhibited highly significant (P < 0.01) positive correlation with ESI and FS, while exhibited significant (P < 0.05) negative correlation with solubility, WSI, WHC and firmness. FS showed significant (P < 0.01) positive correlation with WHC, while exhibited significant (P < 0.01) negative correlation with solubility (Table 3).
Table 3.
Correlation coefficient between dependent variables
| Solubility | Heat stability | Water solubility index | Water holding capacity | Oil holding capacity | Emulsion stability index | Firmness | Foaming capacity | Foam stability | |
|---|---|---|---|---|---|---|---|---|---|
| Solubility | 1 | − 0.093 | 0.500*a | − 0.094 | − 0.435*b | 0.276*a | 0.609**b | − 0.452*b | − 0.591**b |
| Heat stability | 1 | 0.142 | − 0.080 | − 0.480*b | − 0.248 | 0.283 | 0.041 | 0.094 | |
| Water solubility index | 1 | − 0.107*a | − 0.025 | − 0.208 | 0.668**b | − 0.746**b | − 0.380 | ||
| Water holding capacity | 1 | − 0.236*b | 0.382 | 0.164**b | − 0.049*b | 0.132**b | |||
| Oil holding capacity | 1 | − 0.151 | − 0.400*a | 0.073*a | 0.000 | ||||
| Emulsion stability index | 1 | 0.024**b | 0.215**b | − 0.337 | |||||
| Firmness | 1 | − 0.334*b | − 0.299**a | ||||||
| Foaming capacity | 1 | 0.580**a | |||||||
| Foam stability | 1 |
*Correlation is significant at P < 0.05; **Correlation is significant at P < 0.01
aCorrelation discussed within row; bcorrelation discussed within column
Future recommendations
It is expected that m-WPC with enhanced functional properties can exhibit wide range of food applications like for heat processed foods, beverages (heat stability); high protein foods, protein isolates and concentrate (solubility); baked goods, certain confectionaries, soufflés (foaming ability); mayonnaise, salad/cream dressing, sauces, custards, puddings, ice creams (emulsification); and cakes, creams, confectionary and sauces (gelation). For optimum results, few preventative measures that need to be considered carefully when applying ultrasound is to control temperature during process because, whey protein is highly sensitive to temperature and to select appropriate processing conditions that can impart desired structural and functional modifications.
Acknowledgements
Authors are highly thankful to Department of Food Science and Technology, Punjab Agricultural University, Ludhiana for providing research oriented environment and great opportunity for successful completion of this work.
References
- Arzeni C, Martinez K, Zema P, Arias A, Pérez OE, Pilosof AMR. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. 2012;108:463–472. doi: 10.1016/j.jfoodeng.2011.08.018. [DOI] [Google Scholar]
- Beuchat LR. Functional and electrophoretic characteristics of succinylated peanut flour protein. J Agric Food Chem. 1977;25:258–261. doi: 10.1021/jf60210a044. [DOI] [Google Scholar]
- Bouaouina H, Desrumaux A, Loisel C, Legrand J. Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int Dairy J. 2006;16:275–284. doi: 10.1016/j.idairyj.2005.05.004. [DOI] [Google Scholar]
- Coffmann CW, Garcia VV. Functional properties and amino acid content of a protein isolate from mung bean flour. J Food Technol. 1977;12:473–484. doi: 10.1111/j.1365-2621.1977.tb00132.x. [DOI] [Google Scholar]
- Gani A, Baba WN, Ahmad M, Shah U, Khan AA, Wani IA, Masoodi FA, Gani A. Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT - Food Sci Technol. 2016;66:496–502. doi: 10.1016/j.lwt.2015.10.067. [DOI] [Google Scholar]
- Haque ZU, Mozaffar Z. Casein hydrolysate. II. Functional properties of peptides. Food Hydrocoll. 1992;5:559–571. doi: 10.1016/S0268-005X(09)80125-2. [DOI] [Google Scholar]
- Hu H, Wu J, Li-Chan ECY, Zhu L, Zhang F, Xu X, Fan G, Wang L, Huang X, Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J Food Eng. 2008;86(2):281–287. doi: 10.1016/j.jfoodeng.2007.10.004. [DOI] [Google Scholar]
- Jambrak AR, Lelas V, Mason TJ, Krešić G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng. 2009;93:386–393. doi: 10.1016/j.jfoodeng.2009.02.001. [DOI] [Google Scholar]
- Jambrak AR, Mason TJ, Lelas V, Krešić G. Ultrasonic effect on physicochemical and functional properties of α-lactalbumin. LWT - Food Sci Technol. 2010;43:254–262. doi: 10.1016/j.lwt.2009.09.001. [DOI] [Google Scholar]
- Jambrak AR, Mason TJ, Lelas V, Paniwnyk L, Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J Food Eng. 2014;121:15–23. doi: 10.1016/j.jfoodeng.2013.08.012. [DOI] [Google Scholar]
- Kentish S, Ashokkumar M (2011) The physical and chemical effects of ultrasound BT—ultrasound technologies for food and bioprocessing. In: Feng H, Barbosa-Canovas G, Weiss J (eds) Ultrasound technologies for food and bioprocessing. Springer, New York, p 1–12
- Khatkar SK, Gupta VK, Khatkar AB. Studies on preparation of medium fat liquid dairy whitener from buffalo milk employing ultrafiltration process. J Food Sci Technol. 2014;51(9):1956–1964. doi: 10.1007/s13197-014-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella JE, Rector DJ, Phillips LG (1994) Physicochemical properties of proteins: texturization via gelation, glass and film formation BT—protein structure-function relationships in foods. In: Yada RY, Jackman RL, Smith JL (eds) Protein structure–functional relationships in foods. Springer, Boston, p 1–21
- Liu Z, Guo B, Su M, Wang Y. Effect of ultrasonic treatment on the functional properties of whey protein isolates. Adv Mat Res. 2012;443–444:660–665. [Google Scholar]
- O’Donnell CP, Tiwari BK, Bourke P, Cullen PJ. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci Technol. 2010;21:358–367. doi: 10.1016/j.tifs.2010.04.007. [DOI] [Google Scholar]
- O’Sullivan J, Arellano M, Pichot R, Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of dairy proteins. Food Hydrocoll. 2014;42:386–396. doi: 10.1016/j.foodhyd.2014.05.011. [DOI] [Google Scholar]
- Onwulata C, Tomasula P. Whey texturization: a way forward. Food Technol. 2004;58:50–54. [Google Scholar]
- Pihlanto A, Korhonen H. Bioactive peptides and proteins. Adv Food Nutr Res. 2003;47:175–276. doi: 10.1016/S1043-4526(03)47004-6. [DOI] [PubMed] [Google Scholar]
- Pires FCS, da Silva Pena R. Optimization of spray drying process parameters for tucupi powder using the response surface methodology. J Food Sci Technol. 2017 doi: 10.1007/s13197-017-2803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Park EY, Kim JY, Lim ST. Enhancing dispersion stability of alpha-tocopherol in aqueous media using maize starch and ultrasonication. LWT - Food Sci Technol. 2016;68:589–594. doi: 10.1016/j.lwt.2016.01.001. [DOI] [Google Scholar]
- Riener J, Noci F, Cronin DA, Morgan DJ, Lyng JG. The effect of thermosonication of milk on selected physicochemical and microstructural properties of yoghurt gels during fermentation. Food Chem. 2009;114:905–911. doi: 10.1016/j.foodchem.2008.10.037. [DOI] [Google Scholar]
- Schmidt RH, Packard VS, Morris H. Effect of processing on whey protein functionality. J Dairy Sci. 1984;67:2723–2733. doi: 10.3168/jds.S0022-0302(84)81630-6. [DOI] [Google Scholar]
- Shirzad H, Niknam V, Taheri M, Ebrahimzadeh H. Ultrasound-assisted extraction process of phenolic antioxidants from olive leaves: a nutraceutical study using RSM and LC–ESI–DAD–MS. J Food Sci Technol. 2017;54:2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh N, Thakur S, Kaur A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol. 2017;54(4):921–932. doi: 10.1007/s13197-016-2356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Tang C, Yang X-Q, Chen Z, Wu H, Peng ZY. Physicochemical and structural characteristics of sodium caseinate biopolymers induced by microbial transglutaminase. J Food Biochem. 2005;29:402–421. doi: 10.1111/j.1745-4514.2005.00038.x. [DOI] [Google Scholar]
- Tang CH, Wang XY, Yang XQ, Li L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J Food Eng. 2009;92:432–437. doi: 10.1016/j.jfoodeng.2008.12.017. [DOI] [Google Scholar]
- Tavares T, Ramos OL, Malcata FX. β-Lactoglobulin microparticles obtained by high intensity ultrasound as a potential delivery system for bioactive peptide concentrate. J Food Sci Technol. 2017;54(13):4387–4396. doi: 10.1007/s13197-017-2912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur R, Saberi B, Pristijono P, Stathopoulos CE, Golding JB, Scarlett CJ, Bowyer M, Vuong QV. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J Food Sci Technol. 2017;54:2270–2278. doi: 10.1007/s13197-017-2664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SL, Gauthier SF, Paquin P. Emulsifying property of whey peptide fractions as a function of pH and ionic strength. J Food Sci. 1992;57:601–604. doi: 10.1111/j.1365-2621.1992.tb08052.x. [DOI] [Google Scholar]
- Wagh RV, Chatli MK. Response surface optimization of extraction protocols to obtain phenolic rich antioxidant from sea buckthorn and their potential application into model meat system. J Food Sci Technol. 2017;54:1565–1576. doi: 10.1007/s13197-017-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas JF (1997a) Emulsifying properties of proteins BT—functionality of proteins in food. In: Zayas JF (ed) Functionality of protein in food. Springer, Berlin, Heidelberg, p 134–227
- Zayas JF (1997b) Solubility of proteins BT—functionality of proteins in food. In: Zayas JF (ed) Functionality of protein in food. Springer, Berlin, p 6–75
- Zisu B, Lee J, Chandrapala J, Bhaskaracharya R, Palmer M, Kentish S, Ashokkumar M. Effect of ultrasound on the physical and functional properties of reconstituted whey protein powders. J Dairy Res. 2011;78:226–232. doi: 10.1017/S0022029911000070. [DOI] [PubMed] [Google Scholar]
- Zou Y, Wang L, Cai P, Li P, Zhang M, Sun Z, Sun C, Xu W, Wang D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int J Biol Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.03.011. [DOI] [PubMed] [Google Scholar]