Abstract
The extraction conditions and antioxidant activities of rutin from Sophora japonica bud by deep eutectic solvents were investigated. Box–Behnken design was used to optimize the extraction conditions and the scavenging activities of DPPH, O2− and ·OH of purified rutin were evaluated. The highest yield of 279.8 mg/g was achieved in the extraction medium of choline chloride/triethlene glycol (1/4) under the optimum conditions: water content of the DES 18.1%, extraction time 28.3 min, extraction temperature 70 °C and liquid–solid ratio 10 mg/1 g. The highest extraction amount was slightly different from the predicted value of the established second-order polynomial equation. In addition, The EC50 of DPPH scavenging, O2− scavenging and ·OH scavenging of rutin were 5.68 µg/mL, 0.19 and 0.28 mg/mL, respectively. The above results indicate rutin extracted by the choline chloride/triethylene glycol has excellent antioxidant activity and was an admirable free radical scavenger.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3151-9) contains supplementary material, which is available to authorized users.
Keywords: Rutin, Choline chloride/triethylene glycol, Sophora japonica bud, Antioxidant activity
Introduction
The external stimuli can give rise to an improvement of free radicals in the human body (Osińska-Jaroszuk et al. 2015; Ye et al. 2016), resulting in the oxidative stress increase (Adibhatla and Hatcher 2010) and high risks of many diseases, such as dementia, diabetes mellitus, dyslipidemia, hypertension (Xu et al. 2014; Wu et al. 2015). The uptake of antioxidant is in certain degree helpful to get rid of the oxidant in body and keep healthy.
Rutin is a crucial bioactive substance (Li et al. 2012; Nam et al. 2015). Numbers of excellent pharmacological properties are set in the molecular, such as anti-inflammatory, antithrombotic, anti-tumor and antidiabetic (Xu et al. 2014; Chua 2013; Niture et al. 2014; Ren et al. 2003). According to Dietary Supplement Label Database, over 860 products containing rutin are selling in America (Gullón et al. 2017). In the recent, Chinese herbal medicine has attracted considerable interest (Chua 2013) because of the existence of high bioactive compounds and low by-effect for health (Huang et al. 2016; Niture et al. 2014). Sophora japonica (S. japonica) as a conventional herb is officially listed in the Chinese Pharmacopoeia and widely planted in China, Japan, and Korea (Qi et al. 2007; Wang et al. 2003; Kim and Yun-Choi 2008). Its buds and fruits possess the stypticity (Qi et al. 2007). Also, its buds are rich in rutin. The intake amount over 50 and 500 mg of rutin dose per day would be a benefit for preventive and curative effects on the human body, respectively (Brunori et al. 2009). The disadvantages of low concentration in the raw and processing instability limit the effective recovery of rutin in the final products (Brunori et al. 2009; Cho and Lee 2015).
Among the conventional methods on the rutin extraction, abundant organic solvents, such as methanol and ethanol, are often used because of poor solubility in aqueous phase (Xi and Luo 2015). Organic solvents are generally toxic, volatile and environmentally unfriendly. To overcome the shortcomings, deep eutectic solvents (DESs) are developing through hydrogen bond interaction among two or three cheap and safe components (Flores-Ferrándiz and Chinchilla 2017; Lu et al. 2016; Zhang et al. 2012). Many delightful properties are set in the solvents, for instance, low melting point, non-toxic, negligible volatility and alternatively designed. Certain publications described that rutin can be effectively extracted by DESs (Cho and Lee 2015; Faggian et al. 2016). However, the information on rutin extracted from S. japonica bud by DESs is still scarce. In the present work, we optimized the extraction condition of rutin by choline chloride/triethylene glycol (ChCl/TEG) and further focused on the evaluation of antioxidant activities of the purified rutin.
Materials and method
Materials
Sophora japonica bud was purchased from Zhanjiang Yizhou Medicines Co., Ltd. (Guangdong, China). The standard rutin was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). All other chemicals were from commercial sources and of analytical grade.
Rutin extraction
In the typical single factor experiment, powdered S. japonica bud (1.00g, mesh less than 60) was mixed with certain volume of DESs-containing appropriate water in a flask. Then, the flask was thermostatically heated in a water bath with continuous stirring at 240 rpm for relevant time. Next, the mixture was centrifuged at 12,000g for 10 min to achieve the supernatant. The supernatant was further used for determining the concentration of rutin by high-performance liquid chromatography (HPLC).
According to the single-factor experiments, three factors (water concentration of DES, extraction time, extraction temperature) were considered as the main factors on the extraction efficiency. The levels of two other factors were decided by the single-factor experiments. The optimization of the three main factors was further conducted in the Box–Behnken design (BBD). A 17-run, 3-factor, 3-level BBD was applied to establish polynomial models for the optimization process in Design-Expert soft.
Purification and structure analysis of rutin
The rutin extracts were further purified with AB-8 macroporous resin eluted with adequate distilled water, then 70% ethanol. The eluted fractions of ethanol were collected and concentrated by a rotary evaporator. The concentrated supernatant was acidized to pH 3.0 by the diluted hydrochloric acid, and the mixture was maintained at 4 °C for 18 h. The precipitate from the last step was collected by filtration, washed with distilled water to receive crude rutin. The rough rutin was heatedly dissolved in calcium hydroxide solution and filtrated. Acid solution was added into the filtrate, and the mixture was maintained at a low temperature, crystalized again, filtrated, washed with distill water and finally dried to achieve the purified rutin.
After drying under vacuum at 40 °C for 48 h, the structure determination of rutin was conducted with an Avance digital 400 MHz Bruker Spectrometer made in Germany. In addition, the purity of rutin was determined by HPLC.
Antioxidant activity of rutin
DPPH radical scavenging assay
The method determining the DPPH radical scavenging activity of rutin was referenced with previous publication (Mandade et al. 2011) with a few modifications. The rutin dissolved in methanol at different concentrations was injected into 100 mM DPPH solution (dissolved in ethanol) at equal volume. The mixture was darkly maintained at room temperature for 30 min and was measured to the absorbance at 517 nm. Vitamin C was used as the positive control. The DPPH radical scavenging activity (RSA) was calculated using the following formula:
where A1 was the absorbance of DPPH in the mixture consisted of DPPH solution and various concentrations of rutin extracts at equal volume, A2 was the absorbance of DPPH in the mixture consisted of various concentrations of rutin extracts and distilled water at equal volume, and A3 was the absorbance of the mixture of DPPH solution and distilled water at equal volume. The effective concentration of rutin and Vc getting to RSA 50% means EC50.
Superoxide radical scavenging assay
Series concentrations of rutin-methanol solutions, Tris–HCl buffer solution (pH 8.2), nitroblue tetrazolium (NBT) solution (0.98 mM), pyrogallol solution (10 mM) and HCl solution (8 mol/L) were prepared previously. A 2.5 mL of Tris–HCl buffer was formerly hearted at a water-shaker (25 °C) for 20 min. The rutin-methanol solution (0.2 mL), NBT solution (0.6 mL) and pyrogallol solution (0.3 mL) were added into the previous heated Tris–HCl buffer solution. The above reagent was mixed and maintained at 25 °C for 4 min, then adding HCl solution (0.1 ml) to stop the reaction and measuring the absorbance at 530 nm. Moreover, the control group was conducted by the substitution of distilled water for rutin-methanol solution. Similarly, ascorbic acid was used as the positive control. The calculated formula was as following:
where A1 was the absorbance value of positive group, A0 was the absorbance value of control group. The effective concentration of rutin and Vc getting to RSA 50% means EC50.
Hydroxyl radical scavenging assay
The reaction was conducted in the system by the addition of safranine T solution (520 ug/mL, 0.2 mL), EDTA–Na–Fe(II) solution (2 mM, 0.7 mL), different concentrations of rutin solution (1.0 mL) and H2O2 (6%, 0.4 mL) to the phosphate buffer (pH 7.4, 2 mL), respectively. After mixing at 37 °C for 30 min, the absorbance was determined at 520 mm. Moreover, the control group was conducted by the substitution of distilled water for rutin solution. Similarly, ascorbic acid was used as the positive control. The calculated formula was as following:
where A1 was the absorbance value of positive group, A0 was the absorbance value of control group. The effective concentration of rutin and Vc getting to RSA 50% means EC50.
Results and discussion
Single-factor experiment
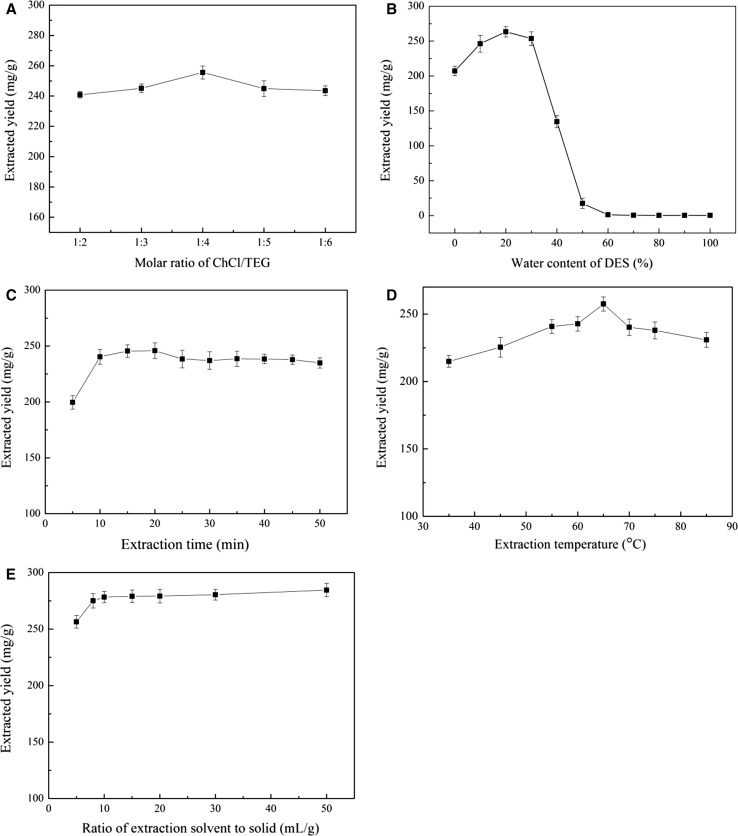
At first, the impact of the molar ratio of ChCl–TEG on the rutin extraction was investigated (Fig. 1a). A slight difference in the yield was observed at the different molar ratio tested. The optimum molar ratio of ChCl to TEG is 1/4, likely due to the appropriate hydrogen bond, which is the interaction forces between DESs and compounds (Dai et al. 2013a). As depicted in Fig. 1b, an increase in the extraction yield was shown at the water concentration from 0 to 20% because of the decline of viscosity and the change in the polarity of extraction solvent. For instance, Huang et al. (2017) reported that the adding water to DESs contributed to the increase of polarity of extraction system so as to influence the solubilizing ability. Further increasing water content leaded to enormous decline in extraction yield, likely due to the progressive rupture of hydrogen bond in DES (Gutiérrez et al. 2010). The results by Dai et al. (2013b) also suggest extraction capacity of DESs was obviously decided by water content. The effect of extraction time on the yield is shown in Fig. 1c. The extraction amount significantly improves with the increase of time from 5 to 10 min. The prolongation of extraction time leads to slight impact in the yield, even achieving slight low yield. The result suggests that overmuch heating leads to mild loss of rutin. Increasing temperature results in a decrease of viscosity of DESs to improve the mass transfer. As shown in Fig. 1d, the extraction amount of rutin increased with the improvement of temperature, up to the maximum at 65 °C, and further increase leaded to slight decrease of yield. Figure 1e shows 10 mL/1 g of ratio of liquid to rutin can achieve the ideal yield in rutin extraction among various ratios examined.
Fig. 1.
Effects of various factors on the extraction yield of rutin. a molar ratio of ChCl/TEG; b water content of DES (v/v); c extraction time; d extraction temperature; e ratio of extraction to solid. The values were expressed as the means ± standard deviations (n = 3)
After the single factor experiment, the favorable extraction conditions were achieved as follows: molar ratio of ChCl/TEG 1/4, water content of ChCl/TEG 20% (v/v), extraction time 20 min, extraction temperature 65 °C, and ratio of liquid to solid 10 mL/1 g. Under the optimum extraction condition, a markedly improvement in the extraction yield (270.3 mg/g) was obtained compared with the methanol–water solution or ethanol–water solution (< 150 mg/g) (Zhao et al. 2015). This adequately proves that the superior advantages using ChCl/TEG as extraction solvent for the extraction of rutin from S. japonica bud compared to traditional organic solvent.
Box–Behnken design
Three main factors (water content of DES, extraction time and extraction temperature) based on the single-factor experiments, were selected as the independent variables to establish the model of rutin extraction. The other factors were at the best value in the single factor experiments.
17 runs BBD experiment was conducted to further optimize the extraction conditions (shown in Table 1). By using Design-Expert soft on the simulation of experiment data, a second-order polynomial equation was obtained as follows.
Y: predicted extraction yield of rutin (mg/g), A: code of water concentration in DES (− 1, 0, 1), B: code of extraction time (− 1, 0, 1), C: code of extraction temperature (− 1, 0, 1).
Table 1.
The design and results of the BBD experiments
| Entry | Factors | Extracted yield (mg/g) | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | 1 (30) | 0 (20) | − 1 (55) | 249.66 |
| 2 | 0 (20) | − 1 (10) | 1 (75) | 246.92 |
| 3 | 0 | 1 (30) | 1 | 263.88 |
| 4 | 1 | 1 | 0 (65) | 251.01 |
| 5 | 0 | − 1 | − 1 | 242.30 |
| 6 | − 1 (10) | 0 | − 1 | 206.04 |
| 7 | 1 | 0 | 1 | 239.93 |
| 8 | − 1 | 1 | 0 | 254.58 |
| 9 | − 1 | 0 | 1 | 255.89 |
| 10 | 1 | − 1 | 0 | 246.65 |
| 11 | 0 | 1 | − 1 | 247.52 |
| 12 | 1 | − 1 | 0 | 229.35 |
| 13a | 0 | 0 | 0 | 268.89 |
| 14a | 0 | 0 | 0 | 268.23 |
| 15a | 0 | 0 | 0 | 267.88 |
| 16a | 0 | 0 | 0 | 268.43 |
| 17a | 0 | 0 | 0 | 263.56 |
A Water content (v/v, %), B time (min), C temperature (°C)
aCentral point
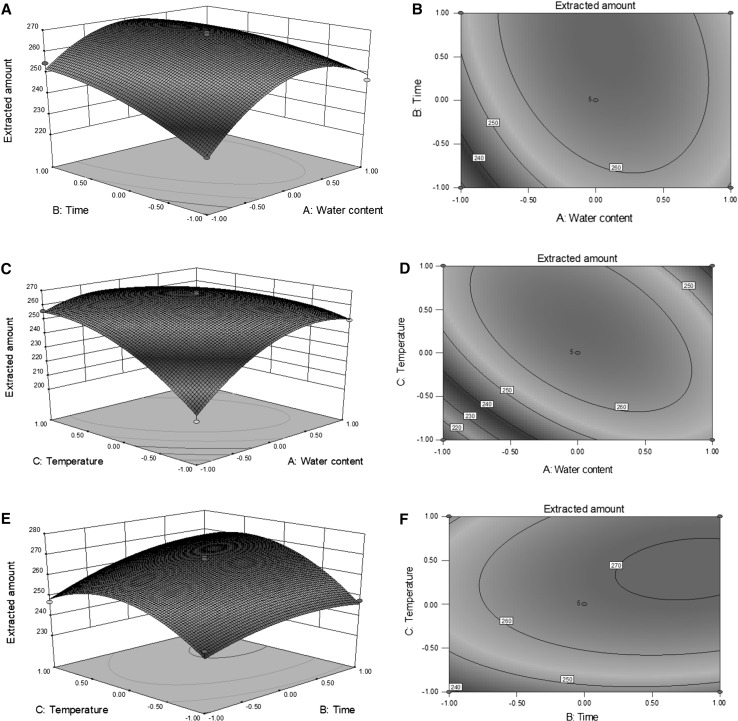
Next, one-way analysis of variance by Design-Expert soft was used for significance analysis of the model above. Model F-value of 43.89 and the P value less 0.001 shown in Table 2 indicate the model achieved is noteworthy. Moreover, all items examined on linear coefficients, interaction term coefficients and quadratic term coefficients, have significant effects on the extraction yield of rutin (all P-values less 0.05). The high determination coefficient (R2 = 0.9634) implies most of the total variations can be interpreted by the achieved model. As depicted in Fig. 2, the change in the extraction amount of rutin was investigated as two varieties was in experimental range and other variety was at zero. All contour plots showing ellipticity suggest the mutual interactions between the varieties are significant. Furthermore, the contour line in the Fig. 2d was denser than those in Fig. 2b, f, indicating that the mutual interaction between water content and extraction temperature was more important. The optimal extraction conditions were given by the resolution of regression equation as follows: water content of ChCl/TEG (1:4, molar ratio) 18.1% (v/v), extraction time 28.3 min, extraction temperature 70 °C, ratio of liquid to solid 10 ml/1 g. There is slight difference between the predicted extraction yield (272.5 mg/g) and the actual extraction yield (279.8 mg/g) under the above conditions, suggesting the model is perfect for the predication of rutin extraction from S. japonica bud.
Table 2.
Analysis of variance (ANOVA) for the experimental result of BBD
| Source | Sum of squares | df | Mean square | F value | P value* |
|---|---|---|---|---|---|
| Model | 4372.52 | 9 | 485.84 | 43.89 | < 0.0001 |
| A | 214.14 | 1 | 214.14 | 19.35 | 0.0032 |
| B | 335.02 | 1 | 335.02 | 30.27 | 0.0009 |
| C | 466.65 | 1 | 466.65 | 42.16 | 0.0003 |
| AB | 108.89 | 1 | 108.89 | 9.84 | 0.0165 |
| AC | 887.44 | 1 | 887.44 | 80.18 | < 0.0001 |
| BC | 34.46 | 1 | 34.46 | 3.11 | 0.0210 |
| A2 | 1309.85 | 1 | 1309.85 | 118.34 | < 0.0001 |
| B2 | 121.09 | 1 | 121.09 | 10.94 | 0.0130 |
| C2 | 698.53 | 1 | 698.53 | 63.11 | < 0.0001 |
| Residual | 77.48 | 7 | 11.07 | ||
| Lack of fit | 76.91 | 3 | 25.64 | 181.22 | 0.1139 |
| Pure error | 0.57 | 4 | 0.14 | ||
| Cor total | 4450.00 | 16 | |||
| R2 = 0.9634 | R2adj = 0.9602 | ||||
A Water content (v/v, %), B time (min), C temperature (°C)
*Significant (P < 0.05)
Fig. 2.
Response surface plots (a, c, e) and Contour plots (b, d, f) showing the effects of variables and their mutual effects on the extraction yield of rutin. Code number: − 1, 0, 1
Purification and structure determination
The yellow rutin extracted powder (Fig. S1A) was obtained at the yield of 62.7% after the purification process. The purity of purified rutin determined by HPLC was more than 95% (Fig. S1B). Moreover, the purified compound structure was characterized by nuclear magnetic resonance (NMR) spectroscopy (Fig. S1C). The master data given in Hz was shown as follows, and the data suggests the extract is rutin.
Rutin: δ1.00 (3H, d, CH3), 3.04-3.31 (9H, m, H2″-H6″, OH2‴-H5‴), 4.42 (1H, s, H1‴), 5.34 (1H, d, H1″), 6.20 (1H, s, H6), 6.39 (1H, s, H8), 6.85 (1H, d, H5′), 7.53-7.55 (2H, m, H2′ and H6′), 12.60 (1H, s, 5-OH).
Free radical scavenging capacities
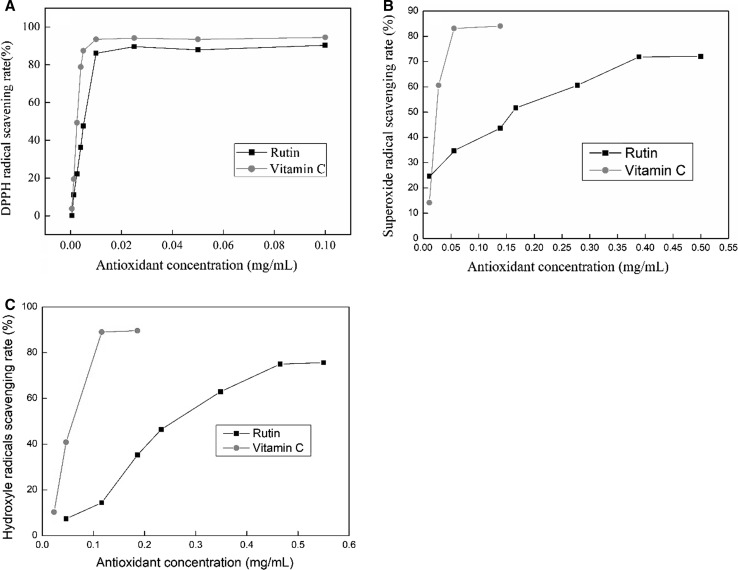
The excellent antioxidant capacities of rutin from S. japonica bud was shown in Fig. 3. DPPH-RSA of rutin and ascorbic acid at different concentrations was shown in Fig. 3a. At the tested concentrations of antioxidants, the highest DPPH-RSA was achieved at 10 μg/mL for rutin and ascorbic acid, reached up to 86.1 and 93.5%, respectively. This suggests that rutin has excellent DPPH radical scavenging ability, slightly below than that of ascorbic acid. Furthermore, DPPH radical EC50 values of rutin and ascorbic acid were 5.68 and 2.76 μg/mL, respectively. Compared to the conventional solvent, such as ethanol (Wu et al. 2015), the method used by us has noteworthy better effect on the DPPH radical scavenging activity (EC50, 5.68 vs. 10.97 μg/mL).
Fig. 3.
Free radical scavenging rate of rutin and vitamin C. a DPPH; b O2−; c ·OH. The values were expressed as means with less than 1% SD (n = 2)
As shown in Fig. 3b, the scavenging ability of rutin in the superoxide anion is obviously lower than vitamin C. The highest scavenging ability for superoxide anion is 72% at 0.39 mg/mL. In addition, a comparative EC50 value (0.19 mg/mL vs. 0.16–1.05 mg/mL) in the method was achieved compared with previous publications (Yang et al. 2008; Zhang et al. 2016). The results indicate that applying the above method to extract rutin can achieve excellent superoxide anion scavenging activity.
The highest hydroxyl radical scavenging activities of rutin and ascorbic acid were 75.0 and 89.0%, respectively (Fig. 3c). Furthermore, ascorbic acid displayed a prominent scavenging activity with EC50 value of 0.06 mg/mL; nevertheless, the purified rutin has a relative lower scavenging activity with an EC50 value of 0.28 mg/mL. It was also observed in the other study that ascorbic acid had a better hydroxyl radical scavenging activity than rutin (Wu et al. 2015).
Conclusion
We have established a method for the efficient extraction of rutin by ChCl/TEG (1/4) and the antioxidant activities were also evaluated. A highest yield of 279.8 mg/g was achieved for the rutin extraction under the optimum conditions. The anti-oxidative activity of rutin proved that rutin has excellent oxidation resistance and the extraction method established has a little effect on the antioxidant activity of rutin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to thank the National Natural Science Foundation of China (21676104; 21336002; 21376096), the Open Funding Project of the State Key Laboratory of Bioreactor Engineering, and the Program of State Key Laboratory of Pulp and Paper Engineering (2017ZD05) for partially funding this work.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3151-9) contains supplementary material, which is available to authorized users.
References
- Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Sign. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- Brunori A, Sándor GERGŐ, Baviello G, Zannettino C, Corsini G, Végvári G. The use of tartary buckwheat whole flour to introduce rutin in preventive amounts in bread typical of the region of Tuscany (Central Italy) Ann Univ Dunarea de Jos of Galati-Fascicle VI: Food Technol. 2009;33:46–49. [Google Scholar]
- Cho YJ, Lee S. Extraction of rutin from Tartary buckwheat milling fractions and evaluation of its thermal stability in an instant fried noodle system. Food Chem. 2015;176:40–44. doi: 10.1016/j.foodchem.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Chua LS. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol. 2013;150(3):805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Dai Y, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal Chem. 2013;85(13):6272–6278. doi: 10.1021/ac400432p. [DOI] [PubMed] [Google Scholar]
- Faggian M, Sut S, Perissutti B, Baldan V, Grabnar I, Dall’Acqua S. Natural deep eutectic solvents (NDESs) as a tool for bioavailability improvement: pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: possible application in nutraceuticals. Molecules. 2016;21(11):1531. doi: 10.3390/molecules21111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Ferrándiz J, Chinchilla R. Organocatalytic enantioselective conjugate addition of aldehydes to maleimides in deep eutectic solvents. Tetrahedron-Asymmetry. 2017;28(2):302–306. doi: 10.1016/j.tetasy.2016.12.009. [DOI] [Google Scholar]
- Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Tech. 2017;67:220–235. doi: 10.1016/j.tifs.2017.07.008. [DOI] [Google Scholar]
- Gutiérrez MC, Ferrer ML, Yuste L, Rojo F, del Monte F. Bacteria incorporation in deep-eutectic solvents through freeze-drying. Angew Chem Int Edit. 2010;49(12):2158–2162. doi: 10.1002/anie.200905212. [DOI] [PubMed] [Google Scholar]
- Huang Y, Qi A, Han BH. Extraction of rutin and rhoifolin by inorganic borate functionalized magnetic particles. Chin J Chem. 2016;34(8):823–829. doi: 10.1002/cjoc.201600215. [DOI] [Google Scholar]
- Huang Y, Feng F, Jiang J, Qiao Y, Wu T, Voglmeir J, Chen ZG. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017;221:1400–1405. doi: 10.1016/j.foodchem.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Kim JM, Yun-Choi HS. Anti-platelet effects of flavonoids and flavonoid-glycosides from Sophora japonica. Arch Pharm Res. 2008;31(7):886–890. doi: 10.1007/s12272-001-1242-1. [DOI] [PubMed] [Google Scholar]
- Li FJ, Ning SL, Li Y, Yu YJ, Shen CD, Duan GL. Optimisation of infrared-assisted extraction of rutin from crude flos sophorae immaturus using response surface methodology and HPLC analysis. Phytochem Anal. 2012;23(4):292–298. doi: 10.1002/pca.1357. [DOI] [PubMed] [Google Scholar]
- Lu W, Alam MA, Pan Y, Wu J, Wang Z, Yuan Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour Technol. 2016;218:123–128. doi: 10.1016/j.biortech.2016.05.120. [DOI] [PubMed] [Google Scholar]
- Mandade R, Sreenivas SA, Choudhury A. Radical scavenging and antioxidant activity of Carthamus tinctorius extracts. Free Radic Antioxid. 2011;1(3):87–93. doi: 10.5530/ax.2011.3.12. [DOI] [Google Scholar]
- Nam MW, Zhao J, Lee MS, Jeong JH, Lee J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015;17(3):1718–1727. doi: 10.1039/C4GC01556H. [DOI] [Google Scholar]
- Niture NT, Ansari AA, Naik SR. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J Exp Biol. 2014;52:720–727. [PubMed] [Google Scholar]
- Osińska-Jaroszuk M, Jarosz-Wilkołazka A, Jaroszuk-Ściseł J, Szałapata K, Nowak A, Jaszek M, Ozimek E, Majewska M. Extracellular polysaccharides from Ascomycota and Basidiomycota: production conditions, biochemical characteristics, and biological properties. World J Microb Biotechnol. 2015;31(12):1823–1844. doi: 10.1007/s11274-015-1937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YY, Sun A, Liu RM, Meng ZL, Xie HY. Isolation and purification of flavonoid and isoflavonoid compounds from the pericarp of Sophora japonica L. by adsorption chromatography. J Chromatogr A. 2007;1140:219–224. doi: 10.1016/j.chroma.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23(4):519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Wang JH, Lou FC, Wang YL, Tang YP. A flavonol tetraglycoside from Sophora japonica seeds. Phytochemistry. 2003;63:463–465. doi: 10.1016/S0031-9422(02)00757-4. [DOI] [PubMed] [Google Scholar]
- Wu P, Ma G, Li N, Deng Q, Yin Y, Huang R. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015;173:194–202. doi: 10.1016/j.foodchem.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Xi J, Luo S. Pressure-enhanced solid–liquid extraction of rutin from Chinese scholar-tree flower: kinetic modeling of influential factors. Sep Purif Technol. 2015;156:809–816. doi: 10.1016/j.seppur.2015.11.006. [DOI] [Google Scholar]
- Xu PX, Wang SW, Yu XL, Su YJ, Wang T, Zhou WW, Zhang H, Wang YJ, Liu RT. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014;264:173–180. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci Technol. 2008;41(6):1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- Ye Z, Wang W, Yuan Q, Ye H, Sun Y, Zhang H, Zeng X. Box–Behnken design for extraction optimization, characterization and in vitro antioxidant activity of Cicer arietinum L. hull polysaccharides. Carbohyd Polym. 2016;147:354–364. doi: 10.1016/j.carbpol.2016.03.092. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Vigier KDO, Royer S, Jérôme F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41(21):7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Zhang QA, Wang X, Song Y, Fan XH, García Martín JF. Optimization of pyrogallol autoxidation conditions and its application in evaluation of superoxide anion radical scavenging capacity for four antioxidants. J AOAC Int. 2016;99(2):504–511. doi: 10.5740/jaoacint.15-0223. [DOI] [PubMed] [Google Scholar]
- Zhao BY, Xu P, Yang FX, Wu H, Zong MH, Lou WY. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain Chem Eng. 2015;3(11):2746–2755. doi: 10.1021/acssuschemeng.5b00619. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.