Abstract
Brassica vegetables, which include broccoli, kale, cauliflower, and Brussel sprouts, are known for their high glucosinolate content. Glucosinolates and their derived forms namely isothiocyanates are of special interest in the pharmaceutical and food industries due to their antimicrobial, neuroprotective, and anticarcinogenic properties. These compounds are water soluble and heat-sensitive and have been proved to be heavily lost during thermal processing. In addition, previous studies suggested that novel non-thermal technologies such as high pressure processing, pulsed electric fields, or ultraviolet irradiation can affect the glucosinolate content of cruciferous vegetables. The objective of this paper was to review current knowledge about the effects of both thermal and non-thermal processing technologies on the content of glucosinolates and their derived forms in brassica vegetables. This paper also highlights the importance of the incorporation of brassica vegetables into our diet for their health-promoting properties beyond their anticarcinogenic activities.
Keywords: Glucosinolates, Crucifers, Thermal processing, Novel technologies, Non-thermal processing, Brassica
Introduction
Consumers are nowadays more aware of the relationship between food, diet, and health, and this has led to increased interest in natural ingredients and in the consumption of foods that are tasty, nutritious, and healthy. Consumption of a diet rich in brassica vegetables has been associated with health effects such as neuroprotective effects (Angeloni et al. 2017) or reduced abundance of intestinal sulphate-reducing bacteria (Kellingray et al. 2017), cardiovascular diseases (Francisco et al. 2017), and some types of cancer (Mori et al. 2017).
The chemoprotective activities of cruciferous vegetables were first recognized in the early 1990s and are nowadays accepted after large amounts of scientific evidence in various cancer models including breast cancer (Lin et al. 2017). Follow-up studies have attributed this activity to the metabolic products of glucosinolates, a class of secondary sulphur-containing metabolites produced by crucifers (Watson et al. 2013). The enzymatic breakdown of glucosinolates is also of key importance for food quality as isothiocyanates, the main breakdown products, are responsible for the sharp taste of mustard, radish, or broccoli sprouts (Hanschen and Schreiner 2017). Although more than 120 different glucosinolates have been identified in cruciferous vegetables, only some of these are present in high quantities (Possenti et al. 2017). Glucoraphanin is the predominant glucosinolate in broccoli and broccoli sprouts (Westphal et al. 2017) followed by progoitrin, glucoiberin, and glucobrassicin (Possenti et al. 2017). However, the glucosinolates profile of broccoli is highly different from those of kale, cauliflower, or Brussel sprouts and can vary even between plants belonging to the same family and between different parts of the same plant (Possenti et al. 2017).
The conditions of post-harvest processing and cooking are important factors of food quality (Francisco et al. 2017). Brassica vegetables are generally eaten cooked after steaming, boiling, or microwaving. However, glucosinolates, vitamins, phenolic compounds, and other health-promoting compounds have been shown to be heavily lost during thermal processing (Kapusta-Duch et al. 2016; Soares et al. 2017). The food industry is very active in technological innovation and over the last 2 decades novel non-thermal processing technologies have been viewed as useful for microbial inactivation while maintaining quality of fresh and processed fruits and vegetables.
The objective of this paper was to review current knowledge on the effects of both thermal and non-thermal technologies on the content of glucosinolates and its derived products in cruciferous vegetables. Furthermore, the current paper also reviews the known phytochemicals found in cruciferous vegetables and highlights the importance of their inclusion into our diet.
Cruciferous vegetables: economic and health importance
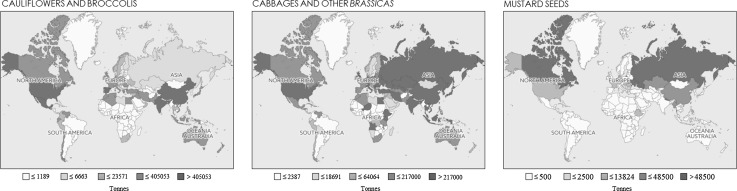
Brassica foods which have been identified as important components of a healthy diet are among the top 10 economic crops in the world (Francisco et al. 2017). Indeed, Brassica oilseed production has increased over the last 40 years and has become the most important source of vegetable oil after soybean and cotton seed (Rakow 2004). The genus Brassica, which contains over 37 species, is one of 51 genera in the tribe Brassiceae belonging to the crucifer family. Many crop species are included in the Brassica genus which includes edible roots, leaves, stems, buds, flowers, and seeds. Figure 1 shows the production quantities of some common brassica vegetables. Several studies have predicted the human population to grow by two to four billion people by 2050 (Cohen 2003). This expanded population is expected to consume twice as much food than currently consumed today and production of crucifers is likely to continue to grow. Indeed, according to data accessed from FAOSTAT, production quantities of common brassica vegetables such as cabbages and broccolis are increasing every year. The principal Brassica vegetable species is B. oleracea which includes a large range of unique cole and cabbage types that include Brussel sprouts, cauliflower, broccoli, and others. Much of the production is locally consumed. Top producers of cauliflowers and broccoli are China and India, with a total production of approximately 9.2 and 8.5 million tonnes per year (FAOSTAT 2017).
Fig. 1.
Brassica production quantities per country.
Data accessed from the Food and Agriculture Organization Corporate Statistical Database (FAOSTAT) available at http://www.fao.org/faostat/en/
Cruciferous vegetables are rich sources of bioactive health promoting compounds including vitamin C and E, dietary fiber, and the glycosides of the flavonoids quercetin and kaempferol (Jeffery and Araya 2009). However, the components that set cruciferous vegetables apart from other vegetables are the glucosinolates. Glucosinolates are β-thioglucoside N-hydroxysulfates with a side chain and a sulfur linked β-d-glucopyranose moiety (Possenti et al. 2017). Glucosinolates can be divided into three major groups based on the structure of their amino acid precursors: (1) aliphatic glucosinolates derived from methionine, isoleucine, leucine, or valine; (2) aromatic glucosinolates, those derived from phenylalanine or tyrosine; and (3) indole glucosinolates which are those derived from tryptophan (Possenti et al. 2017). Myrosinase (EC 3.2.3.1), which is physically separated from the glucosinolates in the intact plant cells, catalyzes the hydrolysis of glucosinolates (Deng et al. 2015). When crucifers are processed, myrosinase can interact with glucosinolates and, based on reaction conditions namely pH and temperature, release either isothiocyanates, thiocyanates, or nitriles from their precursors (Wagner et al. 2013). Sulforaphane is the most widely studied isothiocyanate and is considered to be responsible for the major part of cancer prevention by broccoli (Jeffery and Araya 2009). In vitro and in vivo studies have reported that glucosinolate breakdown products can affect several stages of cancer development, including the inhibition of activation enzymes (phase I) and the induction of detoxification enzymes (phase II) (Possenti et al. 2017). In addition, isothiocyanates and indole products formed from glucosinolates may regulate cancer cell development by regulating target enzymes, controlling apoptosis, inhibiting angiogenesis, inhibiting metastasis and migration of cancer cells, and blocking the cell cycle (Possenti et al. 2017). Sulforaphane is derived from glucoraphine and sinigrin, glucotropaeolin, and gluconasturtiin are the precursors of allyl, benzyl, and phenethyl isothiocyanates respectively. Epithionitriles are important, but yet underestimated glucosinolate hydrolysis products generated instead of isothiocyanates (Hanschen et al. 2018). Epithionitriles including 1-cyano-2,3-epithiopropane have been suggested to possess cancer cell-killing properties involving both intrinsic and extrinsic apoptosic signaling pathways (Conde-Rioll et al. 2017).
Besides their anticarcinogenic properties, glucosinolates obtained from broccoli also showed antimicrobial activities against Gram positive and Gram negative bacterial strains and were suggested as potential antibacterial agents for use as such in food products (Hinds et al. 2017). As mentioned previously, cruciferous vegetables are also rich sources of vitamins, including those listed in Table 1, phenolic compounds, and flavonoids (Bhandari and Kwak 2015). Brassica vegetables contain high concentrations of vitamin C, which includes ascorbic acid and its oxidation product dehydroascorbic acid. Vitamin C which has several biological activities in the human body and is also thought to have cancer-protective capacities (Bakker et al. 2016) and a positive association has been made between dietary vitamin C and bone mineral density (Sahni et al. 2016). Brassica vegetables such as kale or mustard spinach are rich sources of minerals such as calcium and potassium—Table 1.
Table 1.
Nutritional composition of raw cruciferous vegetables per 100 g of fresh produce
| Broccoli | Kale | Cauliflower | Brussel sprouts | Mustard spinach | |
|---|---|---|---|---|---|
| Proximates | |||||
| Water (g) | 89.3 | 84.04 | 92.07 | 86 | 92.2 |
| Energy (kcal) | 34 | 49 | 25 | 43 | 22 |
| Protein (g) | 2.82 | 4.28 | 1.92 | 3.38 | 2.2 |
| Total lipid (g) | 0.37 | 0.93 | 0.28 | 0.3 | 0.3 |
| Carbohydrate (g) | 6.64 | 8.75 | 4.97 | 8.95 | 3.9 |
| Dietary fiber (g) | 2.6 | 3.6 | 2 | 3.8 | 2.8 |
| Sugars (g) | 1.7 | 2.26 | 1.91 | 2.2 | N/A |
| Minerals | |||||
| Calcium, Ca (mg) | 47 | 150 | 22 | 42 | 210 |
| Iron, Fe (mg) | 0.73 | 1.47 | 0.42 | 1.4 | 1.5 |
| Magnesium, Mg (mg) | 21 | 47 | 15 | 23 | 11 |
| Phosphorus, P (mg) | 66 | 92 | 44 | 69 | 28 |
| Potassium, K (mg) | 316 | 491 | 299 | 389 | 449 |
| Sodium, Na (mg) | 33 | 38 | 30 | 25 | 21 |
| Zinc, Zn (mg) | 0.41 | 0.56 | 0.27 | 0.42 | 0.17 |
| Vitamins | |||||
| Vitamin C (mg) | 89.2 | 120 | 48.2 | 85 | 130 |
| Thiamin (mg) | 0.071 | 0.11 | 0.05 | 0.139 | 0.068 |
| Riboflavin (mg) | 0.117 | 0.13 | 0.06 | 0.09 | 0.093 |
| Niacin (mg) | 0.639 | 1 | 0.507 | 0.745 | 0.678 |
| Vitamin B-6 (mg) | 0.175 | 0.271 | 0.184 | 0.219 | 0.153 |
| Folate, DFE (mg) | 0.063 | 0.141 | 0.057 | 0.061 | 0.159 |
| Vitamin B-12 (mg) | 0 | 0 | 0 | 0 | 0 |
| Vitamin A (mg) | 0.031 | 0.5 | 0 | 0.038 | 0.495 |
| Vitamin E (mg) | 0.78 | 1.54 | 0.08 | 0.88 | N/A |
| Vitamin D | 0 | 0 | 0 | 0 | 0 |
| Vitamin K (mg) | 0.101 | 0.704 | 0.015 | 0.177 | N/A |
| Lipids | |||||
| Saturated fatty acids (mg) | 0.039 | 0.091 | 0.13 | 0.062 | 0.015 |
| Monounsaturated fatty acids (mg) | 0.011 | 0.052 | 0.034 | 0.023 | 0.138 |
| Polyunsaturated fatty acids (mg) | 0.038 | 0.338 | 0.031 | 0.153 | 0.057 |
| Trans fatty acids (mg) | 0 | 0 | 0 | 0 | 0 |
| Cholesterol (mg) | 0 | 0 | 0 | 0 | 0 |
N/A: Data not available
Data accessed from the Food Composition Database of the United States Department of Agriculture (USDA) available at https://ndb.nal.usda.gov/ndb/
Thermal processing of brassica vegetables
Although some crucifers can be eaten fresh, these vegetables are most commonly eaten cooked after blanching, steaming, boiling, or microwaving. Thermal processing strategies have been used in the food industry since ancient times with the aim of not only making certain foods edible but delaying the inevitable deterioration of perishable foods between production and consumption. This is achieved by the destruction of microbial pathogens and the reduction of spoilage microorganisms as well as the inactivation of enzymes involved in food deterioration.
Thermal processing of brassica vegetables improves palatability and extends shelf-life. However, high temperatures also result in changes in the content of health-promoting compounds including glucosinolates, which intakes are associated with a reduced risk of several forms of cancer (Capuano et al. 2017). Indeed, Kapusta-Duch et al. (2016) recently reported a significant reduction in the content of glucosinolates and their derived products in green and purple cauliflower and rutabaga after boiling (100 °C, 15 min). Similar results were published by Cieślik et al. (2007), who evaluated the effects of blanching, boiling, and freezing on the glucosinolate content of a number of cruciferous vegetables including Brussel sprouts, white and green cauliflower, broccoli, and curly kale. The authors of this study observe considerable losses of total glucosinolates after blanching and cooking, from 2.7 to 30.0% and from 35.3 to 72.4%, respectively. Furthermore, no changes in the total glucosinolate content were found in vegetables that were blanched and frozen for 48 h. In addition, Tiwari et al. (2015) modeled and quantified the level of glucosinolates in broccoli, cabbage, cauliflower, and Brussel sprouts upon thermal processing and reported that thermal processing had a major impact on the level of glucosinolates in cruciferous vegetables. The authors of this study also evaluated subsequent human exposure to glucosinolates, based on dietary surveys, and concluded that consumption of processed crucifers indicated a low mean weakly intake. However, the model observed a higher level of exposure following consumption of steamed vegetables compared to boiling, sous-vide, and grilling processes. In a different study, Sarvan et al. (2014) modeled the degradation kinetics of glucosinolates during processing of four crucifers namely broccoli, red cabbage, white cabbage, and Brussels sprouts. This study demonstrated that glucosinolates are heavily lost after thermal processing and that their thermostability varied not only in different media such as the food matrix or the cooking water, but also with the vegetable variety in which the glucosinolates were present. Several studies suggested steaming as the most efficient process to retain glucosinolates in cruciferous vegetables when compared with blanching, boiling, or microwaving (Bongoni et al. 2014; Deng et al. 2015; Soares et al. 2017; Tiwari et al. 2015; Volden et al. 2008) and Florkiewicz et al. (2017) recently suggested sous-vide as an advantageous processing method of broccoli, Brussels sprouts, and cauliflower. Table 2 lists the predicted effects of blanching (3 min, 95 °C), cooking (40 min, 100 °C), and canning (40 min, 120 °C) on the residual percentage of glucosinolates in red cabbage as a result of thermal degradation. Results, previously reported by Oerlemans et al. (2006) showed that mild heat treatments, such as blanching, have little impact on glucosinolates. Furthermore, Giambanelli et al. (2015) reported that broccoli glucosinolates degradation can be reduced by performing the thermal treatment in binary systems with other food ingredients such as onion, pointing out that the interaction of different ingredients may not only improve the taste of a dish, but also its healthiness.
Table 2.
Predicted effects of three different heat treatments (blanching, cooking, and canning) on the residual percentage of glucosinolates in red cabbage as a result of thermal degradation.
Data reprinted from Oerlemans et al. (2006) with permission from Elsevier
| Glucosinolate | Initial concentration set to 100% (µmol/100 g FW) | Blanching for 3 min at 95 °C (%) | Cooking for 40 min at 100 °C (%) | Canning for 40 min at 120 °C (%) |
|---|---|---|---|---|
| Glucoiberin | 14.8 | 100 | 94 | 18 |
| Progoitrin | 23.8 | 100 | 93 | 38 |
| Sinigrin | 14.7 | 100 | 91 | 12 |
| Glucoraphanin | 48.2 | 100 | 90 | 15 |
| Gluconapin | 36.9 | 100 | 93 | 53 |
| 4-Hydroxyglucobrassicin | 1.9 | 93 | 26 | 3 |
| Glucobrassicin | 8.8 | 99 | 72 | 1 |
| 4-Methoxyglucobrassicin | 1.6 | 97 | 48 | 1 |
| Total aliphatic glucosinolates | 138.4 | 100 | 92 | 29 |
| Total indole glucosinolates | 12.3 | 98 | 62 | 2 |
| Total glucosinolates | 150.8 | 100 | 89 | 27 |
Thermal processing does not affect all phytochemicals in the same way. Indeed, Oerlemans et al. (2006) demonstrated the conventional cooking did not affect aliphatic glucosinolates but significantly decreased the concentration of indole glucosinolates. However, in that study the more severe heat treatment significantly affected all glucosinolates and the authors suggested that those conditions would have a great impact on the health promoting compounds available in canned Brassica vegetables. In addition, Cieślik et al. (2007) reported how the glucoiberin content in broccoli decreased from 0.42 to 0.21 mg/100 g while the glucoraphanin and glucoalyssin content varied from 48.7 to 30.1 and 0.52 to 0.32 mg/100 g, respectively. Similarly, blanching and boiling of Brussel sprouts resulted in significant losses of sinigrin (25.4 and 58.6%, respectively) and glucobrassicin (22.3 and 72.8%, respectively). Cieślik et al. (2007) suggested that the relative stabilities of individual glucosinolates may be a function of their respective chemical structures as, for example, aliphatic glucosinolates are generally more stable than indole glucosinolates.
Previous studies reported that thermal processing could inactivate the enzyme myrosinase. Indeed, Verkerk and Dekker (2004) studied the effect of various microwave treatments on the activity of myrosinase in red cabbage (Brassica oleracea L. Var. Capitata f. rubra DC.) and reported that a substantial myrosinase activity was retained in cabbage treated at low (24 min, 180 W) and intermediate (8 min, 540 W) microwave powers while microwave cooking for 4.8 min at 900 W resulted in a complete loss of hydrolytic activity. Therefore, thermal processing can also affect the concentration of indoles, isothiocyanates, and other glucosinolate breakdown products. Kapusta-Duch et al. (2016) reported a decrease in the total indoles content of 48.5 and 75.8% and a reduction in the content of total isothiocyanates of 11.0 and 42.4% after processing of green and purple cauliflower at 100 °C during 15 min, when compared to vegetables before treatment. Boiling, frying, and microwaving of broccoli also resulted in losses of ascorbic acid, the predominant form of vitamin C, of 33, 24, and 16% respectively (Soares et al. 2017). High temperatures can result in the loss of nutritional quality attributes of cruciferous vegetables and for this reason, thermal treatment conditions namely temperature and duration should be kept at the least possible values.
Non-thermal technologies: beyond food safety
High-pressure processing (HPP) has been successfully applied to a large variety of foods during the last 2 decades. The potential of this technology to improve both safety, by eliminating pathogenic microorganisms, and health-promoting attributes of foods has been largely studied. Several studied have evaluated the impact of HPP on glucosinolates and their derived forms. For example, Westphal et al. (2017) studied the effects of HPP (100–600 MPa, 3 min, and 30 °C) on the glucosinolates content and conversion to isothiocyanates during storage in fresh broccoli sprouts. Myrosinase was active after HPP and a formation of isothiocyanates was observed in all HPP-treated sprouts. The degrees of conversion in the sprouts treated at 100–300 MPa ranged from 11 to 18% and from 400 MPa onward, the degree of conversion increased up to 85% for 600 MPa. Similar results were obtained by Wang et al. (2016) who observed a maximum degradation of glucosinolates in seedlings from Brussel sprouts at 600 MPa. This study also evaluated the effect of HPP on purified myrosinase and observed that although the enzyme was still active after processing at 600 MPa, a decrease in its activity upon increasing pressure to 800 MPa was detected. Alvarez-Jubete et al. (2014) observed higher concentrations of isothiocyanates after processing of white cabbage at 600 MPa when compared to blanching. HPP-treated samples also showed significantly higher levels of total phenols and a higher antioxidant capacity when compared to thermally-treated samples (Table 3). Overall, maximum degradation of glucosinolates in cruciferous vegetables has been observed at 600 MPa. Myrosinase is active after HPP at 100–600 MPa. However, a reduction in myrosinase activity has been observed at higher pressures.
Table 3.
Total phenolic content, antioxidant capacity and total isothiocyanate content of non-treated, blanched, and high pressure processed white cabbage.
Table modified from Alvarez-Jubete et al. (2014) with permission from Springer
| Untreated | Blanching | 200 MPa | 400 MPa | 600 MPa | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 40 °C | 20 °C | 40 °C | 20 °C | 40 °C | ||||
| Total phenols (mg/100 g DW) | 338.27 ± 17.93 abc | 282.47 ± 7.14 c | 310.04 ± 43.2 c | 340.09 ± 14.31 abc | 319.32 ± 34.84 bc | 340.97 ± 12.40 abc | 384.55 ± 18.57 ab | 401.47 ± 16.97 | < 0.001 |
| Antioxidant capacity (Trolox equivalents) | 354.16 ± 38.06 a | 258.39 ± 17.1 b | 82.63 ± 10.37 c | 100.37 ± 19.56 c | 80.95 ± 6.63 c | 79.77 ± 4.11 c | 322.42 ± 13.80 a | 303.76 ± 35.18 ab | < 0.001 |
| Total isothiocyanates (µmol/g DW) | 0.73 ± 0.03 bc | 0.41 ± 0.04 c | 2.34 ± 0.31 b | 1.38 ± 0.23 bc | 5.18 ± 0.08 a | 5.11 ± 0.49 | 5.07 ± 0.49 a | 5.12 ± 0.38 a | < 0.0001 |
Different letters within a row are used to indicate significant differences among treatments
Ultraviolet (UV) irradiation has emerged as a potential alternative to currently used post-harvest treatments. For example, UV-C irradiation has been efficiently used to reduce microbial contamination of foods and food contact surfaces (Lim and Harrison 2016) and it is known that UV-B irradiation enhances vitamin D production in mushrooms (Urbain et al. 2016). Although UV light has been shown to be effective in modifying the activity of certain enzymes commonly found in cruciferous vegetables such as peroxidase (Cruz et al. 2016), to promote the production of certain flavonoids (Neugart et al. 2014) or ascorbic acid (Topcu et al. 2015), and to increase antioxidant capacity (Darré et al. 2017) of different brassica vegetables, little is known about the effect of UV light on glucosinolates and isothiocyanates. Formica-Oliveira et al. (2017) recently observed that single or combined UV-B (5, 10, and 15 kJ/m2) and UV-C (9 kJ/m2) treatments could revalorize broccoli by-products by increasing their concentration of glucosinolates after a 3-day storage period. Supplementary UV radiation (2.2, 8.8, and 16.4 kJ/m2/day) during the vegetative period of broccoli also resulted in increased glucosinolates content (Topcu et al. 2015). Similar results were obtained by Mewis et al. (2012) who observed increased levels of glucosinolates in broccoli sprouts after pre-harvest UV-B radiation at 0.3–1.0 kJ/m2/day. However, other studies reported no differences in the glucosinolates content of cruciferous vegetables after UV-B exposure at 20 kJ/m2/day (Rybarczyk-Plonska et al. 2016). The majority of the paper published to date focused on broccoli and knowledge on the effect of UV processing on other crucifers is lacking. From the latest reports it can be observed that low intensity UV-B treatments seem to be more efficient in enhancing glucosinolates production in broccoli. However, further research studies are needed in order to optimize glucosinolate production in different brassica vegetables. Intense pulsed light (IPL) is a non-thermal food processing technology with potential for being used in the food industry. Although, up to the best of the authors knowledge, no studies have been published over the last couple of years on the effects of IPL on the glucosinolates content of cruciferous vegetables, results obtained using this technology on other foods are encouraging. For example, carrots treated with pulsed light doses of 2.26 J/cm2 showed increased falcarindiol and β-carotene content when compared to the control (Aguiló-Aguayo et al. 2017).
Pulsed electric field (PEF) is a novel technique able to permeabilize vegetable tissue without an important increase of the product temperature, avoiding an excessive deterioration of the product (Puértolas et al. 2016). PEFs have the ability to inactivate microorganisms and enzymes while preserving the nutritional quality of fresh and minimally processed foods (Odriozola-Serrano et al. 2016). Indeed, previous studies suggested that PEF processing at 15, 25, or 35 kV was efficient in preserving bioactive compounds and antioxidant activity of broccoli when compared to thermal processing at 90 °C during 1 min (Sánchez-Vega et al. 2015). Only few studies have assessed the effect of PEF processing on the glucosinolates content of broccoli and broccoli-derived products. It is believed that PEF processing, especially at moderate conditions, could be a suitable method to promote glucosinolates production in broccoli. Indeed, Aguiló-Aguayo et al. (2015) used a response surface methodology to calculate 4 kV/cm for 525 and 1000 µs as the optimum conditions to maximize glucosinolate levels in broccoli florets and stalks, which ranged from 187.1 to 212.5 and 110.6 to 203.0%, respectively. These results contrast to those obtained by Frandsen et al. (2014) who processed broccoli puree with either 3, 10, or 20 kV/cm and varied number of pulses and observed that, although most of the glucosinolates were degraded during pureeing, the PEF conditions studied did not negatively affect the activity of myrosinase as a further intensity-dependent degradation was observed. The observed degradation was especially high at stronger processing conditions. The authors of this study suggested that an initial myrosinase inactivation step would be needed if glucosinolates are intended to be kept intact while PEF processing. Overall, results obtained so far are contradictory and further research is needed to understand the effects of this technology on glucosinolates in cruciferous vegetables. Studying the effects of different PEF processing parameters on the glucosinolates content of crucifers as well as combinations of PEF with other non-thermal strategies is worthy studying.
Conclusion
Glucosinolates and its derived products have the potential for being used as ingredients in functional foods, which is one of the top trends in the food industry. The incorporation of cruciferous vegetables into out diet or the use of glucosinolates and their derived products as ingredients in functional foods is of special interest due to their anticancer properties. However, temperature processing degrades glucosinolates and other compounds such as vitamins, and phenolic compounds and this needs to be considered when calculating the dietary intake of these compounds from cooked crucifers. Thermal processing conditions namely temperature and duration should be kept at the least possible values. Several studies concluded that steaming is the most efficient process to retain glucosinolates in cruciferous vegetables when compared with blanching, boiling, microwaving, frying, or sous-vide processing. Furthermore, the conditions of post-harvest processing are essential to improve the nutritional and health-promoting properties of cruciferous vegetables. Overall, non-thermal processing technologies are promising strategies that could be used to promote the production of glucosinolates in cruciferous vegetables or to minimize their degradation during processing. For example, previous studies suggested that UV irradiation or PEF processing could promote glucosinolate production in cruciferous vegetables including broccoli. Further studies are needed in order to optimize the conditions needed to generate cruciferous vegetables enriched in glucosinolates and to assess their resistance to cooking and gastrointestinal degradation.
Acknowledgements
This work was supported by the CERCA Programme and the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya (FI-DGR-2015-0004). T. Lafarga is in receipt of a Juan de la Cierva contract awarded by the Spanish Ministry of Economy, Industry, and Competitiveness (FJCI-2016-29541). I. Aguiló-Aguayo thanks the National Programme for the Promotion of Talent and its Employability of the Spanish Ministry of Economy, Industry and Competitiveness and to the European Social Fund for the Postdoctoral Senior Grant Ramon y Cajal (RYC-2016-19949).
Abbreviations
- HPP
High pressure processing
- UV
Ultraviolet
- IPL
Intense pulsed light
- PEF
Pulsed electric field
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Aguiló-Aguayo I, Suarez M, Plaza L, Hossain MB, Brunton N, Lyng JG, Rai DK. Optimization of pulsed electric field pre-treatments to enhance health-promoting glucosinolates in broccoli flowers and stalk. J Sci Food Agric. 2015;95:1868–1875. doi: 10.1002/jsfa.6891. [DOI] [PubMed] [Google Scholar]
- Aguiló-Aguayo I, Gangopadhyay N, Lyng J, Brunton N, Rai D. Impact of pulsed light on colour, carotenoid, polyacetylene and sugar content of carrot slices. Innov Food Sci Emerg. 2017;42:49–55. doi: 10.1016/j.ifset.2017.05.006. [DOI] [Google Scholar]
- Alvarez-Jubete L, Valverde J, Patras A, Mullen AM, Marcos B. Assessing the impact of high-pressure processing on selected physical and biochemical attributes of white cabbage (Brassica oleracea L. var. capitata alba) Food Bioprocess Technol. 2014;7:682–692. doi: 10.1007/s11947-013-1060-5. [DOI] [Google Scholar]
- Angeloni C, Hrelia S, Malaguti M. Neuroprotective effects of glucosinolates. In: Mérillon JM, Ramawat KG, editors. Glucosinolates. Basel: Springer; 2017. pp. 275–299. [Google Scholar]
- Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A, Tjønneland A, Overvad K. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2016;103:454–464. doi: 10.3945/ajcn.114.101659. [DOI] [PubMed] [Google Scholar]
- Bhandari SR, Kwak JH. Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules. 2015;20:1228–1243. doi: 10.3390/molecules20011228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok Frandsen H, Ejdrup Markedal K, Martín-Belloso O, Sánchez-Vega R, Soliva-Fortuny R, Sørensen H, Sørensen S, Sørensen JC. Effects of novel processing techniques on glucosinolates and membrane associated myrosinases in broccoli. Pol J Food Nutr Sci. 2014;64:17–25. [Google Scholar]
- Bongoni R, Verkerk R, Steenbekkers B, Dekker M, Stieger M. Evaluation of different cooking conditions on broccoli (Brassica oleracea var. italica) to improve the nutritional value and consumer acceptance. Plant Food Hum Nutr. 2014;69:228–234. doi: 10.1007/s11130-014-0420-2. [DOI] [PubMed] [Google Scholar]
- Capuano E, Dekker M, Verkerk R, Oliviero T. Food as pharma? The case of glucosinolates. Curr Pharm Des. 2017;23:2697–2721. doi: 10.2174/1381612823666170120160832. [DOI] [PubMed] [Google Scholar]
- Cieślik E, Leszczyńska T, Filipiak-Florkiewicz A, Sikora E, Pisulewski PM. Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chem. 2007;105:976–981. doi: 10.1016/j.foodchem.2007.04.047. [DOI] [Google Scholar]
- Cohen JE. Human population: the next half a century. Science. 2003;302:1172–1175. doi: 10.1126/science.1088665. [DOI] [PubMed] [Google Scholar]
- Conde-Rioll M, Gajate C, Fernández JJ, Villa-Pulgarin JA, Napolitano JG, Norte M, Mollinedo F. Antitumor activity of Lepidium latifolium and identification of the epithionitrile 1-cyano-2, 3-epithiopropane as its major active component. Mol Carcinogen. 2017;57:1–14. doi: 10.1002/mc.22759. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Godinho AI, Aslan D, Koçak NF, Vieira MC. Modeling the kinetics of peroxidase inactivation, colour and texture changes of Portuguese cabbage (Brassica oleracea L. var. costata DC) during UV-C light and heat blanching. Int J Food Stud. 2016;5:180–192. doi: 10.7455/ijfs/5.2.2016.a6. [DOI] [Google Scholar]
- Darré M, Valerga L, Araque LCO, Lemoine ML, Demkura PV, Vicente AR, Concellón A. Role of UV-B irradiation dose and intensity on color retention and antioxidant elicitation in broccoli florets (Brassica oleracea var. Italica) Postharvest Biol Technol. 2017;128:76–82. doi: 10.1016/j.postharvbio.2017.02.003. [DOI] [Google Scholar]
- Deng Q, Zinoviadou KG, Galanakis CM, Orlien V, Grimi N, Vorobiev E, Lebovka N, Barba FJ. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- FAOSTAT (2017) The Food and Agriculture Organization Corporate Statistical Database. http://www.fao.org/faostat/en/#home
- Florkiewicz A, Ciska E, Filipiak-Florkiewicz A, Topolska K. Comparison of sous-vide methods and traditional hydrothermal treatment on GLS content in Brassica vegetables. Eur Food Res Technol. 2017;9:1–11. [Google Scholar]
- Formica-Oliveira AC, Martínez-Hernández GB, Díaz-López V, Artés F, Artés-Hernández F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov Food Sci Emerg. 2017;43:77–83. doi: 10.1016/j.ifset.2017.07.036. [DOI] [Google Scholar]
- Francisco M, Tortosa M, Martínez-Ballesta M, Velasco P, García-Viguera C, Moreno D. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann Appl Biol. 2017;170:273–285. doi: 10.1111/aab.12318. [DOI] [Google Scholar]
- Giambanelli E, Verkerk R, Fogliano V, Capuano E, D’Antuono L, Oliviero T. Broccoli glucosinolate degradation is reduced performing thermal treatment in binary systems with other food ingredients. RSC Adv. 2015;5:66894–66900. doi: 10.1039/C5RA11409H. [DOI] [Google Scholar]
- Hanschen FS, Schreiner M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front Plant Sci. 2017;8:1095. doi: 10.3389/fpls.2017.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen FS, Kaufmann M, Kupke F, Hackl T, Kroh LW, Rohn S, Schreiner M. Brassica vegetables as sources of epithionitriles: novel secondary products formed during cooking. Food Chem. 2018;245:564–569. doi: 10.1016/j.foodchem.2017.10.124. [DOI] [PubMed] [Google Scholar]
- Hinds L, Kenny O, Hossain M, Walsh D, Sheehy E, Evans P, Gaffney M, Rai D. Evaluating the antibacterial properties of polyacetylene and glucosinolate compounds with further identification of their presence within various carrot (Daucus carota) and Broccoli (Brassica oleracea) cultivars using high-performance liquid chromatography with a diode array detector and ultra performance liquid chromatography–tandem mass spectrometry analyses. J Agric Food Chem. 2017;65:7186–7191. doi: 10.1021/acs.jafc.7b02029. [DOI] [PubMed] [Google Scholar]
- Jeffery EH, Araya M. Physiological effects of broccoli consumption. Phytochem Rev. 2009;8:283–298. doi: 10.1007/s11101-008-9106-4. [DOI] [Google Scholar]
- Kapusta-Duch J, Kusznierewicz B, Leszczyńska T, Borczak B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J Funct Food. 2016;23:412–422. doi: 10.1016/j.jff.2016.03.006. [DOI] [Google Scholar]
- Kellingray L, Tapp HS, Saha S, Doleman JF, Narbad A, Mithen RF. Consumption of a diet rich in Brassica vegetables is associated with a reduced abundance of sulphate-reducing bacteria: a randomised crossover study. Mol Nutr Food Res. 2017;61:1600992. doi: 10.1002/mnfr.201600992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Harrison MA. Effectiveness of UV light as a means to reduce Salmonella contamination on tomatoes and food contact surfaces. Food Control. 2016;66:166–173. doi: 10.1016/j.foodcont.2016.01.043. [DOI] [Google Scholar]
- Lin T, Zirpoli GR, McCann SE, Moysich KB, Ambrosone CB, Tang L. Trends in cruciferous vegetable consumption and associations with breast cancer risk: a case-control study. Curr Dev Nutr. 2017;1:e000448. doi: 10.3945/cdn.117.000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012;53:1546–1560. doi: 10.1093/pcp/pcs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Shimazu T, Sasazuki S, Nozue M, Mutoh M, Sawada N, Iwasaki M, Yamaji T, Inoue M, Takachi R. Cruciferous vegetable intake is inversely associated with lung cancer risk among current nonsmoking men in the Japan Public Health Center Study. J Nutr. 2017;147:841–849. doi: 10.3945/jn.117.247494. [DOI] [PubMed] [Google Scholar]
- Neugart S, Fiol M, Schreiner M, Rohn S, Zrenner R, Kroh LW, Krumbein A. Interaction of moderate UV-B exposure and temperature on the formation of structurally different flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica) J Agric Food Chem. 2014;62:4054–4062. doi: 10.1021/jf4054066. [DOI] [PubMed] [Google Scholar]
- Odriozola-Serrano I, Soliva-Fortuny R, Martín-Belloso O. Pulsed electric fields effects on health-related compounds and antioxidant capacity of tomato juice. In: Miklavcic D, editor. Handbook of electroporation. Cham: Springer; 2016. pp. 1–14. [Google Scholar]
- Oerlemans K, Barrett DM, Suades CB, Verkerk R, Dekker M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006;95:19–29. doi: 10.1016/j.foodchem.2004.12.013. [DOI] [Google Scholar]
- Possenti M, Baima S, Raffo A, Durazzo A, Giusti AM, Natella F. Glucosinolates in food. In: Mérillon JM, Ramawat KG, editors. Glucosinolates. Basel: Springer; 2017. pp. 87–132. [Google Scholar]
- Puértolas E, Saldaña G, Raso J. Pulsed electric field treatment for fruit and vegetable processing. In: Miklavcic D, editor. Handbook of electroporation. Cham: Springer; 2016. pp. 1–21. [Google Scholar]
- Rakow G. Species origin and economic importance of Brassica. In: Pua EC, Douglas CJ, editors. Brassica. Berlin: Springer; 2004. pp. 3–11. [Google Scholar]
- Rybarczyk-Plonska A, Hagen SF, Borge GIA, Bengtsson GB, Hansen MK, Wold AB. Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by postharvest temperature and radiation treatments. Postharvest Biol Technol. 2016;116:16–25. doi: 10.1016/j.postharvbio.2015.12.010. [DOI] [Google Scholar]
- Sahni S, Kiel DP, Hannan MT. Vitamin C and bone health. In: Weaver C, Dary R, Bischoff-Ferrari H, editors. Nutritional influences on bone health. Cham: Springer; 2016. pp. 87–98. [Google Scholar]
- Sánchez-Vega R, Elez-Martínez P, Martín-Belloso O. Influence of high-intensity pulsed electric field processing parameters on antioxidant compounds of broccoli juice. Innov Food Sci Emerg. 2015;29:70–77. doi: 10.1016/j.ifset.2014.12.002. [DOI] [Google Scholar]
- Sarvan I, Verkerk R, van Boekel M, Dekker M. Comparison of the degradation and leaching kinetics of glucosinolates during processing of four Brassicaceae (broccoli, red cabbage, white cabbage, Brussels sprouts) Innov Food Sci Emerg. 2014;25:58–66. doi: 10.1016/j.ifset.2014.01.007. [DOI] [Google Scholar]
- Soares A, Carrascosa C, Raposo A. Influence of different cooking methods on the concentration of glucosinolates and vitamin C in broccoli. Food Bioprocess Technol. 2017;10:1–25. doi: 10.1007/s11947-017-1930-3. [DOI] [Google Scholar]
- Tiwari U, Sheehy E, Rai D, Gaffney M, Evans P, Cummins E. Quantitative human exposure model to assess the level of glucosinolates upon thermal processing of cruciferous vegetables. LWT Food Sci Technol. 2015;63:253–261. doi: 10.1016/j.lwt.2015.03.088. [DOI] [Google Scholar]
- Topcu Y, Dogan A, Kasimoglu Z, Sahin-Nadeem H, Polat E, Erkan M. The effects of UV radiation during the vegetative period on antioxidant compounds and postharvest quality of broccoli (Brassica oleracea L.) Plant Physiol Biochem. 2015;93:56–65. doi: 10.1016/j.plaphy.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Urbain P, Valverde J, Jakobsen J. Impact on vitamin D2, vitamin D4 and Agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Food Hum Nutr. 2016;71:314–321. doi: 10.1007/s11130-016-0562-5. [DOI] [PubMed] [Google Scholar]
- Verkerk R, Dekker M. Glucosinolates and myrosinase activity in red cabbage (Brassica oleracea L. var. Capitata f. rubra DC.) after various microwave treatments. J Agric Food Chem. 2004;52:7318–7323. doi: 10.1021/jf0493268. [DOI] [PubMed] [Google Scholar]
- Volden J, Borge GIA, Bengtsson GB, Hansen M, Thygesen IE, Wicklund T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra) Food Chem. 2008;109:595–605. doi: 10.1016/j.foodchem.2008.01.010. [DOI] [Google Scholar]
- Wagner AE, Terschluesen AM, Rimbach G. Health promoting effects of brassica-derived phytochemicals: from chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxidative Med Cell Longev. 2013;2013:964539. doi: 10.1155/2013/964539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barba FJ, Frandsen HB, Sørensen S, Olsen K, Sørensen JC, Orlien V. The impact of high pressure on glucosinolate profile and myrosinase activity in seedlings from Brussels sprouts. Innov Food Sci Emerg. 2016;38:342–348. doi: 10.1016/j.ifset.2016.06.020. [DOI] [Google Scholar]
- Watson GW, Beaver LM, Williams DE, Dashwood RH, Ho E. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J. 2013;15:951–961. doi: 10.1208/s12248-013-9504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A, Riedl KM, Cooperstone JL, Kamat S, Balasubramaniam V, Schwartz SJ, Böhm V. High-pressure processing of broccoli sprouts: influence on bioactivation of glucosinolates to isothiocyanates. J Agric Food Chem. 2017;65:8578–8585. doi: 10.1021/acs.jafc.7b01380. [DOI] [PMC free article] [PubMed] [Google Scholar]