Abstract
Background
Gamma-hydroxybutyrate (GHB) dependence is associated with a severe, potentially lethal, withdrawal syndrome and relapse rates as high as 60% within 3 months of detoxification. Baclofen has been shown to decrease self-administration of GHB in mice and reduce relapse in a case series of GHB-dependent patients. Controlled studies on the effectiveness of baclofen to prevent relapse in GHB-dependent patients are lacking.
Aim
The aim of this study was to assess effectiveness of baclofen in preventing relapse in GHB-dependent patients.
Methods
This was an out-patient, multicentre, open-label, non-randomized, controlled trial in GHB-dependent patients (n = 107) in the Netherlands. Treatment as usual (TAU, n = 70) was compared with TAU plus baclofen 45–60 mg/day for 3 months (n = 37). Outcome measures were rates of lapse (any use) and relapse (using GHB on average once a week or more), based on self-report. Side effects were monitored with a baclofen side-effects questionnaire. Treatment groups were compared using Chi square analyses, with both per-protocol (PP) and intention-to-treat (ITT) analyses.
Results
GHB-dependent patients treated with baclofen after detoxification showed no reduced lapse rates, but reduced relapse and dropout rates, compared with patients receiving TAU only (24 vs 50%). While both ITT and PP analyses revealed similar results, the effectiveness of baclofen prescribed PP was slightly higher than in ITT analysis. Patients reported overall limited side effects, with the most frequently reported being feeling tired (28%), sleepiness (14%) and feeling depressed (14%). No serious adverse events were reported.
Conclusions
This study showed potential effectiveness of baclofen in preventing relapse in patients with GHB dependence after detoxification. Though promising, future studies with longer follow-up and a randomized double-blind design should confirm these findings before recommendations for clinical practice can be made.
Clinical trial registration
Netherlands Trial Register with number NTR4528.
Electronic supplementary material
The online version of this article (10.1007/s40263-018-0516-6) contains supplementary material, which is available to authorized users.
Key Points
| Baclofen up to 60 mg daily might be effective in preventing relapse and increasing treatment adherence in patients with GHB use disorder. |
| The use of baclofen up to 60 mg daily in patients with GHB use disorder seems safe, when prescribed according to the protocol. |
Introduction
In Europe, misuse of gamma-hydroxybutyrate (GHB) has increased over the past decade, particularly in the Netherlands, Norway, Spain and the UK [1]. GHB originally emerged in the 1990s as an innocent party drug, but later proved to be highly addictive. Precise prevalence rates are unknown due to a lack of systematic surveillance on GHB use [2]. Physical dependence on GHB can develop within weeks, when used daily [3]. GHB dependence is associated with a severe, potentially lethal withdrawal syndrome and high relapse rates of 60% within 3 months of detoxification [4]. However, studies on relapse prevention in GHB dependence are lacking.
GHB is a short-chain fatty acid, which is biosynthetically derived from the inhibitory neurotransmitter GABA [5]. It occurs naturally in the brain, predominantly in the hypothalamus and basal ganglia [6, 7]. GHB binds to GABA-A receptors, GABA-B receptors and GHB receptors [8]. It has a rapid onset of action after ingestion, reaching maximum concentration (Cmax) in a short period (Tmax = 20–60 min), and a short half-life (T½ = 30–60 min). The clinical effects of GHB include sedation, euphoria and, in higher doses, hypoventilation and coma; see [9] for further details.
Baclofen might be an adequate substitute for GHB. It is a high-affinity GABA-B receptor agonist, similar to GHB [10, 11], but with a longer half-life (T½ = 2–6 h). This has the theoretical advantage of more stable drug-plasma levels, and subsequent GABA-B activation, with less frequent dosing (i.e. 3 times daily, instead of 12) [4]. Indeed, one animal study in mice showed that baclofen reduced GHB self-administration [12]. To date, only one case series on baclofen treatment (30–60 mg/day) in GHB dependence has been reported, showing 3-month abstinence in nine out of eleven cases [13]. Baclofen has also been shown to increase abstinence rates and reduce craving and anxiety in alcohol-dependent patients [14–18]. However, several studies failed to replicate these effects [19, 20].
These findings warrant further studies on the potential efficacy of baclofen in the treatment of GHB use disorders. To our knowledge, no clinical studies on the effects of baclofen in GHB use disorders have been published. Here, we investigated the effectiveness of baclofen in recently detoxified GHB-dependent patients to prevent relapse in an open-label, non-randomized, controlled clinical trial. Specifically, we tested the hypothesis that patients receiving baclofen on top of treatment as usual (TAU) after GHB detoxification have decreased relapse rates compared with patients receiving TAU.
Methods
Study Design
The effectiveness of baclofen was assessed in a multicentre, open-label, non-randomized, controlled clinical trial (see protocol publication [21]). After detoxification from GHB, patients received TAU or TAU combined with baclofen, based on patient preference. Participants provided written informed consent. The study was approved by the Medical Ethics Committee, Twente Medical School (METC/14015.am) study number NL40321.044.13. The study was registered in the Netherlands Trial Register with number NTR4528.
Participants
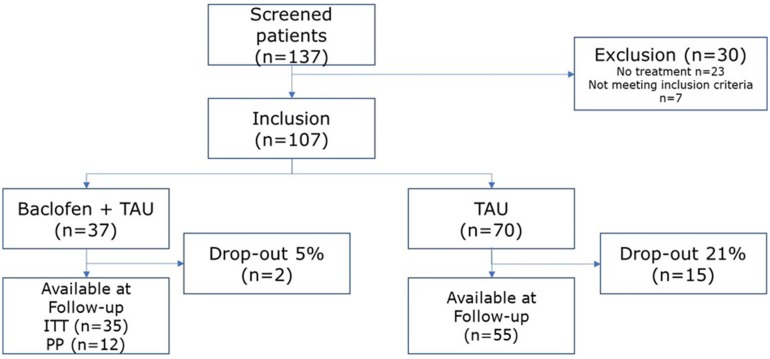
GHB-dependent patients (according to DSM-IV criteria of substance dependence) were recruited at six addiction care facilities (IrisZorg, Mondriaan, Novadic-Kentron, Tactus, Victas and VNN) in the Netherlands, when admitted for detoxification. Inclusion criteria comprised completed GHB detoxification, wish for abstinence, continuing out-patient treatment after detoxification, age between 18 and 40 years, and comprehension of Dutch. Exclusion criteria comprised physical contra-indications for baclofen (e.g. liver problems, renal impairment, hypertension, diabetes mellitus, seizure disorder and pregnancy), severe psychiatric conditions (e.g. bipolar disorder, major depression, psychotic disorders and suicidal ideations), use of anxiolytics, stimulants or hypnotics after detoxification, or previous misuse of baclofen. Of 137 GHB-dependent patients admitted for detoxification, 107 were eligible for participation. Thirty-seven patients received baclofen on top of TAU; 70 received TAU only. During admission, a physician informed patients about the baclofen study. A flowchart is shown in Fig. 1.
Fig. 1.
Flowchart of participants included in the study. ITT intention to treat, PP per protocol, TAU treatment as usual
Sample Size Calculation
Sample size calculation was based on the effectiveness of baclofen in alcohol use disorders. Though the literature on baclofen’s efficacy in alcohol use disorders is contradictory, several studies do suggest a beneficial response of baclofen versus placebo (abstinence rates 70% vs 20–30%) [18, 22]. Anticipating a smaller effectiveness in GHB dependence (3-month abstinence rates 60 vs 40%) based on our previous studies [4], approximately 30 patients per group are needed in order to detect any significant effects of baclofen, with α = 0.05 and β = 0.80 [21].
Treatment Intervention
Baclofen
Participants initially received 15 mg per day, divided over three doses, which was gradually increased over a period of 10 days to 45 mg daily. When patients reported no or limited effects of baclofen on anxiety and craving after 2 weeks without side effects, the dose was increased to a maximum of 60 mg daily. This dose was maintained for 10 weeks. In case of relapse or adverse events, immediate cessation of treatment was considered to avoid intoxication hazards. Compliance was assessed during weekly meetings between the prescribing physician and the patient.
Treatment as Usual (TAU)
All participants received TAU as provided by their addiction treatment centre, including cognitive behavioural therapy with additional treatment for social, psychiatric and medical problems if necessary.
Outcome Measures
Lapse and Relapse
Lapse and relapse were measured by self-report on a questionnaire at 3 months’ follow-up. Lapse was defined as any use of GHB and relapse as weekly use of GHB during the past 3 months. Patients who were no longer in care at follow-up were contacted via telephone and e-mail. Those unavailable for follow-up were considered relapsed.
Side Effects
Safety of baclofen was monitored using a baclofen side effects questionnaire, both self-monitored and observed by treating physicians. This questionnaire was based on the side effects of baclofen reported in the literature [13], containing 21 items with a five-point Likert scale (range: 0 = never to 4 = always). Examples are vomiting, nausea and diarrhoea. See Supplementary Table 1 in the electronic supplementary material for the complete list.
Analysis
Demographics were calculated using descriptive statistics and compared between groups using univariate analysis of variance (ANOVA) and Chi square analyses. Lapse, relapse and relapse including drop-out rates in each group were compared using Chi square analyses. In contrast to the original protocol publication [21], we only analysed primary outcomes using both intention to treat (ITT) and per protocol (PP) analyses due to the limited influx of patients receiving baclofen after a prolonged inclusion period (n = 37 instead of n = 80). In the more conservative ITT analyses, all participants receiving baclofen were compared with TAU. In the PP analyses, only those participants receiving baclofen according to the protocol were compared with TAU.
Though a historical control group was available for comparison [13], only the current control group was included in the analyses. First, the current control group is substantially larger than the intervention group, making addition of an extra control group redundant. Second, relapse rates in the current control group were substantially lower compared with our historical control group (50 vs 65%, respectively). Finally, the current control group was more comparable to the baclofen group in terms of received TAU. Therefore, adding a historical control group to the analyses was considered of no added value.
All analyses were carried out in SPSS version 21, with α < 0.05 considered significant.
Results
Demographics
Patients in the baclofen group were more often male than in the TAU group, but gender was not related to treatment outcome. There were no other differences in demographics, GHB use or psychiatric comorbidity, see Table 1. Of the 37 patients receiving baclofen (included in ITT analysis), 13 received baclofen according to protocol (included in PP analysis).
Table 1.
Demographics and GHB use per sub group
| Treatment as usual (N = 70) | Baclofen + treatment as usual (N = 37) | Test statistic | p value | |
|---|---|---|---|---|
| Male (%) | 54 | 74 | χ2 = 4.68 | 0.030 |
| Age, mean (SD) | 28.9 (7.8) | 29.5 (7.0) | F (1.98) = 0.014 | 0.905 |
| Employment (%) | 31 | 32 | χ2 = 0.01 | 0.916 |
| GHB use, mean (SD) | ||||
| Months using GHB | 58.3 (42.2) | 63.5 (43.0) | F (1.85) = 0.285 | 0.595 |
| Months using daily GHB | 27.4 (28.2) | 41.2 (43.0) | F (1.79) = 2.958 | 0.089 |
| GHB gram daily | 55.6 (53.8) | 46.1 (40.9) | F (1.93) = 0.797 | 0.374 |
| Interval between doses (h) | 1.8 (0.73) | 1.84 (0.64) | F (1.85) = 0.114 | 0.736 |
GHB Gamma-hydroxybutyrate
Effectiveness
ITT analysis showed no difference in lapse rates (χ2 = 0.20, p = 0.885) and relapse rates excluding drop-out (χ2 = 3.29, p = 0.069) in the baclofen-treated group compared with TAU, see Table 2. In the baclofen group, relapse rates including drop-out as relapse were lower compared with TAU (χ2 = 6.59, p = 0.010). PP analysis showed no difference in lapse rates (χ2 = 1.99. p = 0.158), but lower relapse rates in the baclofen group when drop-out rates were not included (χ2 = 3.97, p = 0.046) and included as relapse (χ2 = 5.31, p = 0.021) compared with TAU, see Table 3.
Table 2.
Comparison of (re)lapse in GHB use in the 3 months after detoxification between patients prescribed baclofen (ITT) and patients who received treatment as usual
| TAU | Baclofen + TAU | Test statistic | p value | |
|---|---|---|---|---|
| Patient completed follow-up | 55 | 35 | ||
| Lapse (any use) | 47% (n = 26) | 46% (n = 16) | χ2 = 0.20 | 0.885 |
| Relapse (weekly use) | 38% (n = 21) | 20% (n = 7) | χ2 = 3.29 | 0.069 |
| Patients including drop-out | 70 | 37 | ||
| Relapsea | 50% (n = 35) | 24% (n = 9) | χ2 = 6.59 | 0.010 |
GHB Gamma-hydroxybutyrate, ITT intention to treat, TAU treatment as usual
aDrop-out is considered relapse in GHB-dependent patients, therefore only relapse is mentioned
Table 3.
Comparison of (re)lapse in GHB use in the 3 months after detoxification between patients prescribed baclofen according to the study protocol (PP) and patients who receive treatment as usual
| TAU | Baclofen + TAU | Test statistic | p value | |
|---|---|---|---|---|
| Patient completed follow-up | 55 | 12 | ||
| Lapse (any use) | 47% (n = 26) | 25% (n = 3) | χ2 = 1.99 | 0.158 |
| Relapse | 38% (n = 21) | 8% (n = 1) | χ2 = 3.97 | 0.046 |
| Patients including drop-out | 70 | 13 | ||
| Relapsea | 50% (n = 35) | 15% (n = 2) | χ2 = 5.31 | 0.021 |
GHB Gamma-hydroxybutyrate, PP per protocol, TAU treatment as usual
aDrop-out is considered relapse in GHB dependent patients, therefore only relapse is mentioned
Side Effects
Patients reported overall limited side effects, with the most frequently reported being feeling tired (28%), sleepiness (14%) and feeling depressed (14%). No serious adverse events were reported.
Discussion
This is the first case–control study evaluating the effectiveness of baclofen to prevent relapse in GHB-dependent patients. Patients receiving baclofen per protocol after detoxification showed reduced relapse rates compared with patients receiving TAU, supported by a similar trend towards beneficial effects of baclofen in the ITT analysis. Mild tiredness, sleepiness and depressed feelings were reported in the baclofen group as the most relevant side effects of baclofen.
These results are comparable with an earlier case series (n = 11) on baclofen treatment in GHB-dependent patients, showing 81% abstinence rates during 3 months’ follow-up, without significant side effects [13]. Similarly, Fattore et al. [12] showed prevention of self-administration of GHB in mice when treated with baclofen (0.625 and 1.25 mg/kg). Importantly, a lower dosage of baclofen (0.312 mg/kg) did not prevent GHB self-administration. There is currently no consensus on the most appropriate dose of baclofen in addiction treatment. In line with the previous case series, we prescribed a relatively low dosage of baclofen (45–60 mg daily) in comparison with studies on alcohol dependence (up to 300 mg daily [23]). As higher doses of baclofen might be more effective, future studies on baclofen effectiveness in GHB dependence should also study higher doses of baclofen. However, caution is warranted as data about safety of high-dose baclofen are limited.
GHB, baclofen and alcohol share a similar pharmacological profile. Studies on alcohol dependence have shown that GHB is effective in reducing alcohol craving and intake [24]. Therefore, it is conceivable that baclofen should be effective in reducing GHB dependence in view of its efficacy in reducing alcohol dependence [25]. In light of the longer half-life of baclofen compared with GHB, it can also be speculated that baclofen might be considered a substitute for GHB [26]. The currently poor prognosis in GHB dependence and severity of complications might justify a substitution therapy approach [4]. Recently, baclofen gained attention for its potential effectiveness in the detoxification of GHB [27]. One could also suggest using baclofen to ameliorate GHB withdrawal during detoxification, without tapering off completely, in order to prevent relapse. This would likely increase treatment adherence in some patients, preventing them from dropping out of treatment and relapse in GHB use.
Given the explorative, non-randomized, open-label design of our study, the results need to be interpreted with caution and further studies are needed in order to confirm our findings. Several limitations should be considered when interpreting the results. First, sample size was limited and lower than anticipated. Moreover, patients who chose baclofen treatment might have been more motivated to achieve full abstinence, adding to the chance of good outcome at follow-up. However, we did observe similar findings to previous animal work and a case series of GHB-dependent patients [12, 13]. The observed effectiveness of baclofen, despite a limited sample size, does suggest treatment potential of baclofen in patients with GHB use disorders. Second, TAU was not specified in the current study. Any variation in TAU between groups might confound the results. While we have no such indication when it comes to psychosocial treatment, it is, however, possible that some patients in the TAU group were prescribed benzodiazepines on top of their psychosocial treatment. Therefore, a potential confounding effect cannot be fully ruled out. Third, abstinence was not confirmed using systematic urine or blood tests, due to the narrow timeframe in which GHB can be detected as a result of its short half-life [28]. We relied on self-report measures, with potential recall bias, particularly given the open-label design of the study. Compliance with the baclofen treatment was also assessed by self-report, during weekly meetings between the prescribing physician and the patient. Pill count was not used. This is a potential confounder of the data, since compliance is considered highly relevant for the effectiveness of baclofen. Finally, follow-up duration was 3 months after detoxification, which makes drawing conclusions about long-term effects impossible. Fourth, side effects were not measured in the TAU group, therefore reported side effects cannot be solely attributed to baclofen. Many of the reported side effects are common in GHB-dependent patients in general after detoxification [4]. Future studies should address long-term efficacy of baclofen in GHB dependence, using placebo-controlled, randomized designs in substantially large samples.
Conclusion
This study showed that baclofen could be a potential candidate for preventing relapse in GHB-dependent patients after detoxification, particularly when administered strictly according to the protocol. Though promising, future studies with longer follow-up and a randomized double-blind design should be conducted to confirm these findings, before recommendations for clinical practice can be made.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
Harmen Beurmanjer, Rama M. Kamal, Cor A. J. de Jong, Boukje A. G. Dijkstra and Arnt F. A. Schellekens declare no conflicts of interest.
Funding
The Ministry of Health of The Netherlands provided funding for this study. The funding for open access is provided by the Radboud University Nijmegen.
Informed consent
Participants provided written informed consent.
Ethical approval
The study was approved by the Medical Ethics Committee, Twente Medical School (METC/14015.am) study number NL40321.044.13. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments, or comparable ethical standards.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40263-018-0516-6) contains supplementary material, which is available to authorized users.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2016: Trends and Developments [Internet]. European Monitoring of Drugs and Drugs Addiction. 2016; p 80. http://www.emcdda.europa.eu/edr2016.
- 2.Brunt T, Schrooten J. GHB-epidemiologie in Nederland en Vlaanderen. Verslav Tijdschr over Verslavingsproblematiek. 2014;10(3):20–32. [Google Scholar]
- 3.McDaniel CH, Miotto KA. Gamma hydroxybutyrate (GHB) and gamma butyrolactone (GBL) withdrawal: Five case studies. J Psychoact Drugs. 2001;33(2):143–149. doi: 10.1080/02791072.2001.10400479. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra BAG, Kamal R, van Noorden MS, de Haan H, Loonen AJM, De Jong CAJ. Detoxification with titration and tapering in gamma-hydroxybutyrate (GHB) dependent patients: the Dutch GHB monitor project. Drug Alcohol Depend. 2017;170:164–173. doi: 10.1016/j.drugalcdep.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Tarabar AF, Nelson LS. The gamma-hydroxybutyrate withdrawal syndrome. Toxicol Rev. 2004;23(1):45–49. doi: 10.2165/00139709-200423010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bessman SP, Fishbein WN. Gamma-hydroxybutyrate, a normal brain metabolite. Nature. 1963;200:1207–1208. doi: 10.1038/2001207a0. [DOI] [PubMed] [Google Scholar]
- 7.Snead OC, 3rd, Morley BJ. Ontogeny of gamma-hydroxybutyric acid. I. Regional concentration in developing rat, monkey and human brain. Brain Res. 1981;227(4):579–589. doi: 10.1016/0165-3806(81)90010-9. [DOI] [PubMed] [Google Scholar]
- 8.Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol. 1964;3(4):433–IN8. http://www.sciencedirect.com/science/article/pii/0028390864900747. [DOI] [PubMed]
- 9.Kamal RM, van Noorden MS, Wannet W, Beurmanjer H, Dijkstra BAG, Schellekens A. Pharmacological Treatment in gamma-Hydroxybutyrate (GHB) and gamma-Butyrolactone (GBL) Dependence: detoxification and Relapse Prevention. CNS Drugs. 2017;31(1):51–64. doi: 10.1007/s40263-016-0402-z. [DOI] [PubMed] [Google Scholar]
- 10.Cruz HG, Ivanova T, Lunn M-L, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7(2):153–9. http://www.nature.com/doifinder/10.1038/nn1181. [DOI] [PubMed]
- 11.Crunelli V, Emri Z, Leresche N. Unravelling the brain targets of γ-hydroxybutyric acid. Curr Opin Pharmacol. 2006;6:44–52. doi: 10.1016/j.coph.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattore L, Cossu G, Martellotta MC, Deiana S, Fratta W. Baclofen antagonises intravenous self-administration of gamma-hydroxybutyric acid in mice. NeuroReport. 2001;12(10):2243–2246. doi: 10.1097/00001756-200107200-00039. [DOI] [PubMed] [Google Scholar]
- 13.Kamal RM, Loonen AJM, Dijkstra BAG, De Jong CAJ. Baclofen as Relapse Prevention in the Treatment of Gamma-Hydroxybutyrate Dependence. J Clin Psychopharmacol. 2015;35(3):313–8. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-201506000-00017. [DOI] [PubMed]
- 14.Cryan JF, Kaupmann K. Don’t worry “B” happy!: A role for GABA B receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. [DOI] [PubMed]
- 15.Terrier J, Ort A, Yvon C, Saj A, Vuilleumier P, Lüscher C. Bi-directional effect of increasing doses of baclofen on reinforcement learning. Front Behav Neurosci. 2011;5(July):40. doi: 10.3389/fnbeh.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agabio R, Preti A, Gessa GL. Efficacy and tolerability of baclofen in substance use disorders: a systematic review. Eur Addict Res. 2013;19:325–45. [DOI] [PubMed]
- 17.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 18.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 19.Muller CA, Geisel O, Pelz P, Higl V, Kruger J, Stickel A, et al. High-dose baclofen for the treatment of alcohol dependence (BACLAD study): a randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2015;25(8):1167–77. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=psyc11&NEWS=N&AN=2015-36592-009. [DOI] [PubMed]
- 20.Beraha EM, Salemink E, Goudriaan AE, Bakker A, de Jong D, Smits N, et al. Efficacy and safety of high-dose baclofen for the treatment of alcohol dependence: a multicentre, randomised, double-blind controlled trial. Eur Neuropsychopharmacol. 2016;26(12):1950–1959. doi: 10.1016/j.euroneuro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Kamal RM, Schellekens A, De Jong CA, Dijkstra BA. Baclofen as relapse prevention in the treatment of Gamma—Hydroxybutyrate (GHB) dependence: an open label study. BMC Psychiatry. 2015;15(1):91. http://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-015-0471-4. [DOI] [PMC free article] [PubMed]
- 22.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 23.Rolland B, Labreuche J, Duhamel A, Deheul S, Gautier S, Auffret M, et al. Baclofen for alcohol dependence: relationships between baclofen and alcohol dosing and the occurrence of major sedation. Eur Neuropsychopharmacol. 2015;25(10):1631–1636. doi: 10.1016/j.euroneuro.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Addolorato G, Leggio L, Ferrulli A, Caputo F, Gasbarrini A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert Opin Investig Drugs. 2009;18(5):675–86. http://www.tandfonline.com/doi/full/10.1517/13543780902905855. [DOI] [PubMed]
- 25.Mirijello A, Caputo F, Vassallo G, Rolland B, Tarli C, Gasbarrini A, et al. Gaba < inf > B</inf > agonists for the treatment of alcohol use disorder. Curr Pharm Des. 2015;21(23):3367–3372. doi: 10.2174/1381612821666150619091858. [DOI] [PubMed] [Google Scholar]
- 26.Rolland B, Jaillette E, Carton L, Bence C, Deheul S, Saulnier F, et al. Assessing alcohol versus baclofen withdrawal syndrome in patients treated with baclofen for alcohol use disorder. J Clin Psychopharmacol. 2014;34(1):153–6. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-201402000-00026. [DOI] [PubMed]
- 27.Lingford-Hughes A, Patel Y, Bowden-Jones O, Crawford MJ, Dargan PI, Gordon F, et al. Improving GHB withdrawal with baclofen: study protocol for a feasibility study for a randomised controlled trial. Trials. 2016;17(1):472. http://trialsjournal.biomedcentral.com/articles/10.1186/s13063-016-1593-9. [DOI] [PMC free article] [PubMed]
- 28.Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B. The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol. Clin Toxicol. 2012;50(6):458–70. http://www.tandfonline.com/doi/full/10.3109/15563650.2012.702218. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.